Abstract
The cricetid rodent Oligoryzomys longicaudatus is the species host of Andes virus (ANDV) which causes hantavirus pulmonary syndrome in southern Argentina and Chile. Population density, behavioral interactions, and spacing patterns are factors that affect viral transmission among wild rodents. We predict that the highest prevalence of hantavirus antibody positive would be found among wounded, reproductive males and that, at high population densities, wounded, reproductive males would be dispersers rather than resident individuals. The study was conducted seasonally from October (spring) 2011 to October (spring) 2013 in a shrubland habitat of Cholila, Argentina. During each trapping session, we classified captured O. longicaudatus as resident or disperser individuals, estimated population density, and recorded wounds as an indicator of aggression among individuals. We obtained blood samples from each individual for serological testing. We used generalized linear models to test the statistical significance of association between antibody prevalence, and sex, resident/dispersal status, wounds and trapping session. The highest proportion of seropositive O. longicaudatus individuals was among wounded reproductive males during periods of the greatest population density, and the characteristics of seroconverted individuals support that transmission is horizontal through male intrasexual competition. A positive association between dispersing individuals and hantavirus antibody was detected at high population density. Our study design allowed us to obtain data on a large number of individuals that are seroconverted, enabling a better understanding of the ecology and epidemiology of the ANDV host system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aim of this study was to investigate whether there is a relationship between sexes, resident or transient status, presence of wounds, population density and ANDV antibody prevalence in O. longicaudatus. On the American continent, the genus Orthohantavirus (Family Hantaviridae) is a group of rodent-borne viruses that cause the human disease, hantavirus pulmonary syndrome (HPS) (Levis et al. 1997; Mills et al. 2010; Maes et al. 2019). Infection by hantaviruses usually results in an asymptomatic chronic infection in their rodent hosts and is transmitted to humans through inhalation of aerosolized particles from virus contaminated rodent excreta (Glass 1997; Schmaljohn and Hjelle 1997). It has been suggested that changes in climatic conditions may cause the rapid spread of the virus from focal “safe havens” (Mills et al. 1999; Abbot et al. 1999; Glass et al. 2007). Besides, both field and laboratory studies suggest that nearly all hantavirus transmission is via horizontal routes (Young et al. 1998; Padula et al. 2004). According to Young et al. (1998), the virus would be transmitted among individuals in rodent populations through fighting and social grooming. Several researchers have found that the presence of hantavirus antibodies is more frequent in adult males and that it is correlated with the presence of scars or wounds (Glass et al. 1988; Hinson et al. 2004; Bagamian et al. 2012).
In the southern regions of Argentina and Chile, Oligoryzomys longicaudatus has been identified as the principal host of Andes virus (ANDV), which is the main cause of HPS in the region (Levis et al. 1998; Calderón et al. 1999; Cantoni et al. 2001; Polop et al. 2010; Andreo et al. 2011, 2014). In Argentina, this rodent species inhabits multiple habitat types such as pastures, shrublands, Andean Patagonian forest and peridomestic settings (Piudo et al. 2005, 2011; Polop et al. 2010, 2014a; Andreo et al. 2012). Although the mechanism of transmission for ANDV infection is not fully understood, both laboratory and field studies indicate that infection is chronic in the host who excretes the virus in its urine, feces and saliva (Padula et al. 2004; Lázaro et al. 2007). Several studies have documented a positive relationship between O. longicaudatus population abundance and the prevalence of ANDV (Pearson 2002; Murúa et al. 2003; Murúa and Briones 2005; Sage et al. 2007; Polop et al. 2010). In this virus-host system, Piudo et al. (2012) and Polop et al. (2018) found that population structure (age and sex), the reproductive status of the host, and the presence of wounds were fundamental predictors when modelling the probability that the virus enters and/or persists in a population. However, Padula et al. (2004) expressed that it is likely that other factors, such as grooming or aerosol transmission, are required for efficient virus transmission. Recently, in a study of space use, Juan et al. (2018) found that O. longicaudatus males and females use space differently: Males have mutually exclusive home ranges which are not only larger than females’ but also extensively overlap with several female home ranges. Based on these space patterns, Juan et al. (2018) suggested that this species may follow a polygynous mating system, in which dispersion is strongly biased toward males due to the avoidance of intrasexual local competition for breeding opportunities (Emlen and Oring 1977; Agrell et al. 1996; Waterman 2007; Wolff 2007). Thus, male-biased reproductive dispersion in O. longicaudatus would be primarily related to its mating system, beyond that infection may increase dispersion, as suggested by Douglass et al. (2007) and Lonner et al. (2008) for Peromyscus maniculatus.
The proliferation of the virus in an environment can have two phases: the spread within the host population and the dispersal between populations. Dispersal may occur more quickly in some populations, possibly because of differences in the early stages of infection, the population composition or the dispersal behavior of individuals (Abbot et al. 1999; Mills et al. 1999; Glass et al. 2007; Polop et al. 2018). Increasing the understanding of wild population dynamics and host dispersal are crucial to better characterize disease dynamics and devise ways to protect human health.
Dispersal depends on the degree of habitat saturation and the food, refuge and vacant reproductive territories available (Bondrup-Nielsen 1985; Lambin et al. 2001). Thus, high population density can lead to increased dispersal of voles (Lidicker 1975; Wolff 1999) and mice (Wolff 2003; Austrich et al. 2014). Many researchers study animal dispersal through the identification and quantification of resident and transient individuals present in the study population (Douglass et al. 2003; Priotto et al. 2004; Steinmann et al. 2006a, b; Lonner et al. 2008; Austrich et al. 2014). Transients or dispersers are individuals that do not establish a home range and move a minimum unidirectional distance equal to the average home range diameter of the population, both within and between populations. Residents are individuals that settle in a patch area and keep their movements within their home range’s area (Stickel 1968a, b).
Several authors propose that underlying causes of dispersal are linked to mating systems and intrasexual competition and have important ecological consequences that affect both population dynamics and disease transmission (Lidicker and Stenseth 1992; Stenseth and Lidicker 1992; Clobert et al. 2012). According to Farias et al. (2006) and Schooley and Branch (2006), both behavioral interactions and patterns of space use are factors that affect viral transmission. Thus, under the hypothesis that ANDV transmission is horizontal through O. longicaudatus male intrasexual competition we expect that the highest proportion of antibody-positive individuals: (1) is more likely to be males, (2) is more likely to be reproductively active, (3) is more likely to have wounds. Also, we expect that individuals with these features would be more common at higher population densities. Regarding the maintenance of ANDV in relation to dispersal, we expect a greater number of transient or disperser individuals to be hantavirus antibody-positive than residents at high population densities.
Materials and Methods
Study Area
We conducted this study at Rivadavia Lake valley (42°31 S; 71°27 W), Cholila, Andean region, Chubut Province. This province has been associated with human cases of HPS attributed to ANDV infection (Levis et al. 1998; Enría and Pinheiro 2000; Padula et al. 2000). The study area is a steppe-rainforest transition zone (for a detailed description of the study area, see Juan et al. 2018). Rodent populations were sampled in a shrubland habitat, characterized by native species, such as calafate (Berberis buxifolia), romerillo (Acanthostyles buniifolius), espino negro (Rhamnus lycioides) and Laura (Schinus patagonicus), and exotic species, such as rosa mosqueta (Rosa spp.). In this habitat, Rosa spp. prevails and high population abundances of O. longicaudatus occurred (Polop et al. 2010, 2014b; Andreo et al. 2012).
Rodent Trapping and Processing
We performed rodent trapping sessions in spring, summer, autumn and winter from spring 2011 (October) to spring 2013 (October), except in winter 2012 (due to weather conditions), on two capture-marked-recapture (CMR) grids of 10 × 10 traps, with an interstation distance of 20 m. Each grid was 3.24 ha and they were 150 m away from each other. Two live traps (8 × 9 × 23 cm) similar to a Sherman trap (Alejandro Möller manufacturer, Argentina) were placed at each station and baited with rolled oats, bovine fat and vanilla essence. To obtain enough recaptures to estimate individual’s home range, each trapping session included ten consecutive nights (Juan et al. 2018). Traps were checked each morning. To identify captured animals, individuals were foot tagged with numbered metal rings. Trapped animals were weighed, and body and tail length were recorded. Sex and reproductive status (for males, scrotal or abdominal testicles; for females, perforate or non-perforate vagina, nipples visible or not and evidence of pregnancy) were recorded.
In this study, individuals without evidence of reproductive activity and a body mass less than 14 g, or between 14 and 19.9 g were considered juveniles and subadults, respectively; those individuals with evidence of reproductive activity (males with scrotal testes, and females with perforate vagina, visible nipples and/or evidence of pregnancy) and with a body mass greater or equal than 20 g were considered adults.
Wounds were considered an indicator of aggression among individuals. Thus, in each trapping session and in all captured O. longicaudatus, the presence of any peeling on the body surface, bites in ears, at the base of the tail, and/or at snout and flanks was recorded. For serological testing, blood samples were obtained from the retro-orbital sinus from each individual (only once per session trapping) using capillary tubes. Rodent blood samples were collected in cryovials, which were then centrifuged in the field, and stored in liquid nitrogen until further testing at the Instituto Nacional de Enfermedades Virales Humanas “Dr. Julio Maiztegui” (INEVH).
In each trapping session captured O. longicaudatus were classified as residents or transients. Residents were those individuals who maintained a home range during a trapping session without exploratory displacements. Transients were those individuals who did not maintained a home range (animals caught less than 3 times in the same trapping session) or moved a minimum unidirectional distance equal to the average home range diameter of the population. Home range configuration of each animal was calculated following the boundary strip methods (Stickel 1954). Transient and resident status of individuals was determined for each trapping session (Priotto et al. 2004).
Oligoryzomys longicaudatus population density was expressed for each trapping session as the number of unique individuals captured per hectare. Handling of rodents followed standardized safety guidelines recommended by the Centers for Disease Control and Prevention (Mills et al. 1995). The animals were treated in a humane manner according to the current Argentinean Laws (National Law 14346).
Serology
Hantavirus IgG antibodies were detected by an ELISA test using whole Maciel virus antigen (Guzman et al. 2013). Briefly, 96-well polyvinyl microplates were coated with Maciel hantavirus Vero E6 cell lysate and control antigens overnight; then, serum samples and positive and negative controls were applied, followed by a mix of peroxidase-conjugated anti-Rattus norvegicus and anti-P. maniculatus IgG. Additionally, in 8 of the MACV IgG ELISA positive O. longicaudatus samples, the ANDV genome was characterized by using RT-PCR and sequencing the amplicons of 303 nucleotides from the viral M segment obtained by Levis et al. (1998). The substrate applied was 2.20-azino-di(3-etilbentiazolin sulfonate) (ABTS, Kierkegaard & Perry Laboratories, Inc., Gaithersburg, MD). Absorbance was measured at 405 and 450 nm. Serum dilutions were considered positive if the optical density was > 0.2 after subtraction of the corresponding negative-antigen optical density. Serum samples with titers ≥ 400 were considered positive; cross-reactive IgG antibodies to Maciel virus were considered as a marker of infection with ANDV in O. longicaudatus, the primary ANDV reservoir in Southern Argentina.
Data Analysis
Prevalence of hantavirus antibodies over the course of the entire study and also by each trapping session was calculated as the number of antibody-positive animals divided by the total number tested, and it was expressed as a percentage. We considered seroconversion to have occurred when an antibody-negative individual captured during one trapping session was antibody-positive at a following trapping session. To assess the relationship between seroprevalence and sex, resident/transient status, or trapping session, a χ2 test was used (Zar 1996). We used generalized linear models (GLM) (binomial distribution) to determine which variables are most associated with the condition of being infected or not. First, we evaluated all possible additive models and a null model using the R package MuMIn (Barton 2018). The response variable was seroprevalence status (positive or negative) and the predictor variables were: sex (fixed factor with two levels; male or female), population density by trapping session (fixed factor with two levels; high or low density), wounds (fixed factor with two levels; presence or absence) and status (fixed factor with two levels; resident or transient). Akaike information criterion was used as a measure of a model fit (the smaller the AIC, the better the fit). Weights of each model were considered to select the best in the set of candidate models (Burnham and Anderson 2002). Statistical analyses were carried out using R version 3.0.3 library (nlme) (R Development Core Team 2009, www.r-project.org).
Results
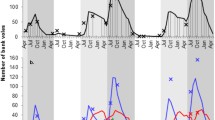
During the study, 1411 different individuals were captured with a total effort of 26,517 trap nights. The assemblage was comprised of 8 small rodent species: Oligoryzomys longicaudatus (1072), Abrothrix longipilis (188), A. olivaceus (109), Loxodontomys micropus (20), Reithrodon auritus (11), Chelemys macronyx (7), Irenomys tarsalis (3) and Geoxus valdivianus (1). Eighty-eight percent of the total O. longicaudatus captured were adults. During the study period, population density fluctuated between 5.00 and 75.46 individuals/hectare (Fig. 1). For the first year (2012), the lowest density occurred during the summer (5.00 ind/ha) and the highest was detected in the autumn (24.69 ind/ha), while for the second year (2013) the lowest density was in summer (5.71 ind/ha), and the maximum density was in spring (75.46 ind/ha). High percentages of transient individuals of both sexes of O. longicaudatus were recorded, reaching values higher than 70%.
Seroprevalence of IgG antibodies specific for Hantavirus (Andes), detected by ELISA tests for rodent assembly in a shrubland habitat (valley of the Rivadavia Lake, Chubut-Argentina Province), registered in each of the trapping sessions, from spring 2011 to spring 2013. The absence of values corresponding to winter 2012 is due to the fact that there was no sampling at that station. Line gray and discontinuous indicates seroprevalence; line black and continuous indicates population density values.
We analyzed 1327 blood samples from captured and recaptured rodents, and specific IgG antibodies to hantavirus were detected in 98 individuals of three species (96/1115 O. longicaudatus, 1/164 Abrothrix olivaceus and 1/48 A. longipilis). The total prevalence of hantavirus antibodies in O. longicaudatus for the study period was 8.61% (83 males and 13 females/1115 individuals), with minimum prevalence in spring 2011 and summer 2013, and maximum in spring 2013 (62/413 = 15.38%) (Fig. 1). In this study, we found that the highest seroprevalence values were obtained at highest population densities (autumn 2012; spring 2013) (Fig. 1).
Models were obtained to determine which variables had a stronger relationship with the condition of being infected are detailed in Table 1, and those that showed the lowest AICc values were selected to draw inferences. Wounds and sex were present in the 3 most parsimonious models. Trapping session (model 14) and resident/transient status (model 8) also influence serologic condition increasing the probability for O. longicaudatus to be seropositive (Table 1). The coefficients for the best models are shown in Table 2.
Prevalence of hantavirus antibodies was more frequent in adult males (86.7% infected males) (χ2 = 49.6, df = 1, p < 0.001). No association was found regarding resident or transient status (χ2 = 0.42, d.f = 1, p = 0.52). Nevertheless, during the study period, the percentage of seropositive transient males (66%) was greater than resident males (Fig. 2).
Percentage of males of Oligoryzomys longicaudatus with presence of IgG antibodies specific for Hantavirus (Andes) discriminated by their condition of resident or transient, registered in each of the trapping sessions, between spring 2011 and spring 2013, in a shrubland habitat in the valley of Lake Rivadavia (Province of Chubut, Argentina). The numbers within the columns represent the totality of individuals of each condition.
However, when trapping sessions were included in the analysis, there was a significant association between antibody prevalence and being male in winter 2013 (χ2 = 10.62, df = 1, p = 0.0011) and spring 2013 (χ2 = 11.72, df = 1, p = 0.0006). Although both the number of seropositive transient and resident males increased during these trapping sessions, the increase in transients was much greater (Fig. 2). Considering all O. longicaudatus individuals, our results showed that although both sexes exhibited wounds, the percentage of males with wounds was almost double compared to females (45.09 ± 21.60 and 25.87 ± 9.82%, respectively).
The highest percentages of seroconversions were among males captured in winter and spring of 2013 (85.7% seroconverted individuals) (Table 3), coinciding both with the highest population densities (59.88–75.5 ind/ha, respectively) and a greater percentage of males with wounds (> 40%). The average weight of seroconverted males captured in autumn and spring 2013 (37.68 ± 4.38 g) was greater than the mean weight value for O. longicaudatus adult males (33 ± 8 g).
Discussion
Our results showed that reproductively active males with wounds constitute the highest proportion of hantavirus antibody-positive O. longicaudatus, supporting our hypothesis that ANDV transmission is horizontal through male intrasexual competition. However, other indirect transmission routes were not tested in this study. The average prevalence for hantavirus antibody found in this study was 8.79% (range 0–15%), which is within the range reported by other authors for sigmodontine rodents from Argentina and other areas of America (3–15%) (Levis et al. 1997; Williams et al.1997; Cantoni et al. 2001; Padula et al. 2004; Enría and Levis 2004; Torres-Perez et al. 2004; Mills et al. 2007). Although other studies found an association between seasonal and annual seroprevalence values and population density in O. longicaudatus (Piudo et al. 2005; Polop et al. 2010), O. flavescens (Mills et al. 2007) and Peromyscus maniculatus (Kuenzi et al. 1999), in ours this association was only evident in 2013. In our study, a larger percentage of adult males were antibody-positive. This could be explained by male intrasexual competition of O. longicaudatus mating system (Juan et al. 2018), in which a minority of males monopolizes several fertile females leaving other males without access to them. Several authors have found that the presence of wounds is a good indicator of aggressive interactions in voles and mice (Blanchard and Blanchard 1977; Van Zegeren 1980; Dewsbury 1988; Wolff and Summerlin 1993; Steinmann et al. 2009; Steinmann and Priotto 2011). In O. longicaudatus, the high percentage of males with wounds could indicate agonistic interactions between active reproductively males. The association between the presence of wounds and infections has been observed in different species: SNV, in P. maniculatus (Mills et al. 1997), Seoul Virus, in Rattus norvergicus (Glass et al. 1988) and Oliveros Virus (an arenavirus), in Akodon azarae (Mills et al. 1994). Biggs et al. (2000) proposed that wounding would occur because at high population densities the probability of contact between individuals increases, increasing the chances of transmission of the virus to susceptible rodents. This is also consistent with a polygynous mating system in which intrasexual competition between males to gain access to receptive females increase with population density (Clutton-Brock 1989; Loughran 2007; Korpela et al. 2011; Bonatto et al. 2013, 2015). Other studies have suggested that the frequent association between wounds and seroprevalence could be the result of a post-infection phenomenon (Glass et al. 1988; Calisher et al. 1999; Douglas et al. 2001; Hinson et al. 2004; Kallio et al. 2006), that at high population density could increase transmission by viral contamination of the environment (spill-over), more than by intraspecific behavioral interactions. In our study, this last mechanism could be proposed for the other two positive species of Abrothrix recorded.
Our results indicate that O. longicaudatus adult males are more like to be dispersers and seropositive. We captured few (13) seropositive O. longicaudatus adult females as compared to adult males (83). Seropositive disperser males, together with the high rate of exogamy found for this species by Ortiz et al. (unpublished data), would favor an increase in the contact rate between males, and therefore, increase the probability of dispersion of the virus among populations. These authors found that males did not show significant genetic structuring in any of the years compared (the distribution of their genotypes was always random) and proposed that O. longicaudatus females have a philopatric behavior, while males represent the dispersing sex. This is consistent with O. longicaudatus’ polygynous mating system, in which, faced with an increase in intrasexual competition, males that fail in the competitive interactions, would disperse from their original area in search of exclusive reproductive spaces to monopolize receptive females (Emlen and Oring 1977; Clutton-Brock 1989). Thus, because adult O. longicaudatus males have higher infection rates of ANDV compared with females, those males are more likely to transmit the virus as they disperse. On the other hand, seropositive resident males of O. longicuadatus would be responsible for maintaining the infection within the population. Calisher et al. (1999) found that long-lived, persistently infected P. maniculatus by SNV serve as reservoirs within populations, reintroducing the virus into susceptible animals every spring.
In our study, body mass of adult males O. longicaudatus that seroconverted were similar to those reported by Piudo et al. (2012) and Polop et al. (2018). Piudo et al. (2012) found that adult males with a body mass greater than 37 g had a probability greater than 50% of presenting IgG antibodies specific for ANDV, increasing this probability to 80% in individuals with a body mass greater than 44 g. The highest number of seroconversions was between August and October 2013 (late winter–spring), when there was a higher population density and a greater percentage of males with wounds. Douglass et al. (2007) found that the seroconversion rate for P. maniculatus began to increase in late winter through spring, but contrary to our results, they found that seroconversion rates remained constant through summer. This difference in seroconversion rate between P. maniculatus and O. longicaudatus could be the result of differing sampling frequencies between both studies, being monthly that carried out by Douglass et al. (2007) and seasonal ours. We acknowledge that a more intense sampling frequency would be optimal, but the distances between the area of study and our University, in addition to the logistics involved, prevented it. In relation to seroconversion and the presence of wounds, these authors did not find a strong association between them. Instead they suggested that wounds might be inflicted by infected rodents whose behaviors were influenced by the virus. In our study, the high percentage of seroconverted adult males with wounds at high population density periods could be related to an increase in intrasexual competition associated with their mating strategies.
Conclusion
Our study supports the hypothesis that ANDV transmission is horizontal through male intrasexual competition, similar to what has been found for other hantaviruses, although alternatives indirect transmission routes were not tested in this study. Our data also allow us to support the hypothesis that hantavirus antibody-positive transient individuals disperse the virus between populations; meanwhile, seropositive resident males of O. longicuadatus would be responsible for maintaining the infection within a population. Our study was able to follow many more individuals that seroconverted as compared with previous ANDV CMR studies and thus enabled a better understanding of the ecology and epidemiology of the ANDV host system.
References
Abbot KD, Ksiazek TG, Mills J (1999) Long term hantavirus persistence in rodent populations in Central Arizona. Emerging Infectious Diseases 5:8–18
Agrell J, Erlinge S, Nelson S, Sandell M (1996) Shifting spacing behaviour of male field voles (Microtus agrestis) over the reproductive season. Annales Zoologici Fennici 33:243–248
Andreo V, Glass G, Shields T, Provensal C, Polop J (2011) Modeling potential distribution of Oligoryzomys longicaudatus, the Andes virus reservoir, in Argentina. EcoHealth 8 (3): 332–348. https://doi.org/10.1007/s10393-011-0719-5
Andreo V, Provensal C, Levis S, Pini N, Enría D, Polop J (2012) Summer–autumn distribution and abundance of the hantavirus host, Oligoryzomys longicaudatus, in northwestern Chubut, Argentina. Journal of Mammalogy 93:1559–1568
Andreo V, Neteler M, Rocchini D, Provensal C, Levis S, Porcasi X, Rizzoli A, Lanfri M, Scavuzzo M, Pini N, Enria D, Polop J. (2014) Estimating Hantavirus Risk in Southern Argentina: A GIS-Based Approach Combining Human Cases and Host Distribution. Viruses 6:201–222; https://doi.org/10.3390/v6010201
Austrich A, Steinmann AR, Bonatto F, Gomez D (2014) Efecto de adultos en el establecimiento de juveniles de Calomys musculinus. Mastozoología Neotropical 21:101–107
Bagamian KH, Towner JS, Kuenzi AJ, Douglass RJ, Rollin PE, Waller LA, Mills JN (2012) Transmission ecology of Sin Nombre hantavirus in naturally infected North American deer mouse. Populations in outdoor enclosures. PLOS ONE. www.plosone.org
Barton K (2018) R package multi-model inference, Version 1.42. Encoding UTF-8. https://CRAN.R-project.org/package=MuMIn
Biggs JR, Bennett KD, Mullen MA, et al. (2000) Relationship of ecological variables to Sin Nombre Virus antibody seroprevalence in populations of deer mice. Journal of Mammalogy 81:676–682
Blanchard R, Blanchard C (1977) Aggressive Behavior in Rat. Behavioral Biology 21:197–224
Bonatto F, Coda J, Gomez D, Priotto J, Steinmann AR (2013) Inter-male aggression with regard to polygynous mating system in Pampean grassland mouse, Akodon azarae (Cricetidae: Sigmodontinae). Journal of Ethology 31:223–231
Bonatto F, Steinmann AR, Gomez D, Priotto J (2015) Do polygynous males of Akodon azarae (Rodentia: Sigmodontinae) vary their mating tactics at low availability of females? Mammalia 79:159–168
Bondrup-Nielsen S (1985) An evaluation of the effects of space use and habitat patterns on dispersal in small mammals. Annales Zoologici Fennici 22:373–383
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information–theoretic approach. Springer, Berlin
Calderón G, Pini N, Bolpe J, Levis S, Mills J, Segura E, Guthmann N, Cantón G, Bécker J, Fonollat A, Ripoll C, Bortman M, Benedetti R, Sabattini M, Enria D (1999) Hantavirus reservoir hosts associated with peridomestic habitats in Argentina. Emerging Infectious Diseases 5:792–797
Calisher CH, Sweeney WP, Mills JN, Beaty BJ (1999) Natural history of Sin Nombre virus in western Colorado. Emerging Infectious Diseases 5:126–134
Cantoni G, Padula P, Calderón G, Mills J, Herrero E, Sandoval P, et al. (2001) Seasonal variation in prevalence of antibody to hantaviruses in rodents from southern Argentina. Tropical Medicine and International Health 6:811–846
Clobert J, Baguette M, Benton T, Bullock J, Ducatez S (2012) Dispersal ecology and evolution. Oxford University Press, Oxford.
Clutton-Brock TH (1989) Mammalian mating systems. Proceedings of the Royal Society of London Biological Sciences 236:339–372
Dewsbury DA (1988) Kinship, Familiarity, Agression, and Dominance in Deer Mice (Peromyscus maniculatus) in Seminatural Enclosures. Journal of Comparative Psychology 102:124–128
Douglas RJ, Wilson T, Semmens WJ, Santo SN, Bond CW, Van Horn RC, Mills JN (2001) Longitudinal studies of Sin Nombre virus in deermouse-dominated ecosystems of Montana. American Journal of Tropical Medicine and Hygiene 65:33–392
Douglass RJ, Kuenzi AJ, Williams CY, Douglass SJ (2003) Removing deer mice from buildings: potential effects on risk of human exposure to Sin Nombre virus. Emerging Infectious Diseases 9:390–392
Douglass RJ, Calisher CH, Wagoner KD, Mills JN (2007) Sin nombre virus infection of deer mice in montana: characteristics of newly infected mice, incidence, and temporal pattern of infection. Journal of Wildlife Diseases 43:12–22
Emlen ST, Oring LW (1977) Ecology, sexual selection and the evolution of mating systems. Science 197:215–223
Enría D, Levis S (2004) Zoonosis virales emergentes: las infecciones por hantavirus. Revue Scientifique et Technique (International Office of Epizootics) 23:595–611
Enría DA, Pinheiro F (2000) Rodent-borne emerging viral zoonosis: hemorrhagic fevers and hantavirus infections in South America. In: Infectious disease clinics of North America. Emerging and reemerging diseases in Latin America, Gotuzzo E, Istúriz R (editors), Philadelphia, PA: WB Saunders, Vol. 14(1), pp 167–184
Farias V, Fuller TK, Cervantes FA, Lorenzo C (2006) Home range and social behavior of the endangered Tehuantepec jackrabbit (Lepus flavigularis) in Oaxaca, México. Journal of Mammalogy 87:748–756
Glass GE (1997) Hantaviruses. Current Opinion in Infectious Diseases 10:362–366
Glass GE, Childs JE, Korch, GW, LeDuc JW (1988) Association of intraspecific wounding with hantaviral infection in wild rats (Rattus norvergicus). Epidemiology and Infection 101:459–72
Glass GE, Shields T, Cai B, Yates TL, et al. (2007) Persistently highest risk areas for hantavirus pulmonary syndrome: potential sites for refugia. Ecological Applications 17:129–139
Guzman C, Mattar S, Levis S, Pini N, Figueiredo T, Mills J, Salazar-Bravo J (2013) Prevalence of antibody to hantaviruses in humans and rodents in the Caribbean region of Colombia determined using Araraquara and Maciel virus antigens. Memórias do Instituto Oswaldo Cruz 108:167–171.
Hinson ER, Shon SM, Zink MC, Glass GE, Klein SL (2004) Wounding: the primary mode of Seoul virus transmission among male Norway rats. American Journal of Tropical Medicine and Hygiene 70:310–317
Juan EE, Provensal MC, Steinmann AR (2018) Space Use and Social Mating System of the Hantavirus Host, Oligoryzomys longicaudatus. EcoHealth 15:96–108
Kallio ER, Klingström J, Gustafsson E, Manni T, Vaheri A, Henttonen H, Lundkvist A (2006) Prolonged survival of Puumala hantavirus outside the host: evidence for indirect transmission via the environment. Journal of General Virology 87:2127–2134
Korpela K, Sundell J., Ylönen H (2011) Does personality in small rodents vary depending on population density? Oecologia 165:67–77
Kuenzi AJ, Morrison ML, Swann DE, Hardy PC, Downard GT (1999) A longitudinal study of Sin Nombre Virus prevalence in rodents, Southeastern Arizona. Emerging Infectious Diseases 5:113–117
Lambin X, Aars J, Piertney SB (2001) Dispersal, intraspecific competition, kin competition and kin facilitation: A review of the empirical evidence. In: Dispersal, Clobert J, Danchin E, Dhondt A, Nichols JD (editors), Oxford: Oxford University Press, pp 110–22
Lázaro ME, Cantoni GE, Calanni LM, Resa AJ, Herrero ER, Iacono MA, Enría DA, González Cappa SM (2007) Clusters of hantavirus infection, southern Argentina. Emerging Infectious Diseases 13:104–110
Levis S, Rowe JE, Morzunov S, Enría DA, St. Jeor S (1997) New hantaviruses causing hantavirus pulmonary syndrome in central Argentina. The Lancet 349: 998–999
Levis S, Morzunov S, Rowe J, Enria D, Pini N, Calderón G, Sabattini M, St Jeor S (1998) Genetic diversity and epidemiology of hantaviruses in Argentina. Journal of Infectious Diseases 177:529–538
Lidicker WZ (1975) The role of dispersal in the demography of small mammals. In: Golley FB, Petrusewicz K, Ryszkowski L (eds) Small Mammals: Their Productivity and Population Dynamics. Cambridge University Press, London, pp 103–128
Lidicker WZ, Stenseth NC (1992) To disperse or not to disperse: who does it and why? In: Animal dispersal: small mammals as a model, Stenseth NC, Lidicker WZ (editors), London: Chapman and Hall, pp 21–36
Lonner BN, Douglass RJ, Kuenzi AJ, Hughes K (2008) Seroprevalence against Sin Nombre Virus in resident and dispersing deer mice. Vector Borne and Zoonotic Diseases 8:433– 441
Loughran MFE (2007) Social organization of the male field vole (Microtus agrestis): a case of transient territoriality?. Annals of Zoologici Fennici 44:97–106
Maes P, Adkins S, Alkhovsky SV, Avšič‑Županc T, Ballinger MJ (2019) Taxonomy of the order Bunyavirales: second update 2018. Archives of Virology 164:927–941
Mills JN, Childs JE, Enria DA, Bowen MD, Peters CJ, Ksiazek TG, Jahrling PB (1994) Oliveros virus: seroprevalence of a new arenavirus in rodents and humans in central Argentina. American Journal of Tropical Medicine and Hygiene 51:96–97
Mills JN, Yates TL, Childs JE, Parmenter RR, Ksiazek TG, et al. (1995) Guidelines for working with rodents potential infected with hantavirus. Journal of Mammalogy 76:716–722
Mills JN, Ksiazek TG, Ellis BA, Rollin PE, Nichol ST, Yates TL, Gannon WL, Levy CE, Engelthaler DM, Davis T, Tanda DT, Frampton JW, Nichols CR, Peters CJ, Childs JE. et al. (1997) Patterns of association with host and habitat: antibody reactive with Sin Nombre virus in small mammals in the major biotic communities of the Southwestern United States. American Journal of Tropical Medicine and Hygiene 56:273–84
Mills JN, Ksiazek TG, Peters CJ, Childs JE (1999) Long-term studies of hantavirus reservoir populations in the southwestern United States: a synthesis. Emerging Infectious Diseases 5:135–142
Mills JN, Schmidt K, Ellis BA, Calderón G, Enría DA, Ksiazek TG (2007) A longitudinal study of hantavirus infection in three sympatric reservoir species in agroecosystems on the Argentine Pampa. Vector-Borne and Zoonotic Diseases 7:229–240
Mills JN, Amman BR, Glass GE (2010) Ecology of Hantaviruses and their hosts in North America. Vector-Borne and Zoonotic Diseases 10, No. 6, published Online-2010. https://doi.org/10.1098/vbz.2009.0018
Murúa R, Briones M (2005) Abundance of the sigmodont mouse Oligoryzomys longicaudatus and patterns of tree seeding in Chilean temperate forest. Mammalian Biology 70:321–326
Murúa R, Navarrete M, Cadiz R, Figueroa R, Padula, Zaror L, Mancilla R, González L, Muñoz-Pedreros A (2003) Hantavirus pulmonary syndrome: Current situation among rodent reservoirs and human population in the Xth Region, Chile. Revista Médica de Chile 131:169–176
Padula P, Colavecchia SB, Martínez VP, Gonzalez Della Valle MO, Edelstein A, et al. (2000) Genetic diversity, distribution, and serological features of hantavirus infection in five countries in South America. Journal of Clinical Microbiology 38:3029–3035
Padula P, Figueroa R, Navarrete M, Pizarro E, Cadiz R, Bellomo C, Jofre C, Zaror L, Rodriguez E, Murúa R (2004) Transmission study of andes hantavirus infection in wild sigmodontine rodents. Journal of Virology 90:11972–11979
Pearson OP (2002) A perplexing outbreak of mice in Patagonia, Argentina. Studies on Neotropical Fauna and Environment 37:187–200
Piudo L, Monteverde M, Gonzalez Capria S, Padula P, Carmanchahi P (2005) Distribution and abundance of sigmodontine rodents in relation to hantavirus in Neuquén, Argentina. Journal of Vector Ecology 30:119–125
Piudo L, Monteverde MJ, Walker RS, Douglass RJ (2011) Rodent community structure and Andes virus infection in sylvan and peridomestic habitats in Northwestern Patagonia, Argentina. Vector-borne and Zoonotic Diseases 11:315–324
Piudo L, Monteverde MJ, Walker RS, Douglass RJ (2012) Características de Oligoryzomys longicaudatus asociadas a la presencia del virus Andes (Hantavirus). Revista Chilena de Infectología 29:200–206
Polop F, Provensal C, Pini N, Levis S, Priotto JW, Enría D, Calderón GE, Costa F, Polop JJ (2010) Temporal and spatial host abundance and prevalence ofAndes Hantavirus in southern Argentina. EcoHealth 7:176–184
Polop F, Juan J, Polop J, Provensal MC (2014a) Spatial and temporal variation of terrestrial rodent assemblages in Cholila, Chubut Province, Argentina. Studies on Neotropical Fauna and Environment 49:151–157
Polop F, Sepúlveda L, Pelliza Sbriller A, Polop J, Provensal MC (2014b) Food habits of Oligoryzomys longicaudatus (Rodentia) in a steppe-forest transitional area of Argentinean Patagonia. Ecología Austral 24:304–310
Polop F, Levis S, Pini N, Enría D, Polop J, Provensal MC (2018) Factors associated with hantavirus infection in a wild host rodent from Cholila, Chubut Province, Argentina. Mammalian Biology 88:107–113
Priotto JW, Steinmann AR, Provensal C, Polop J (2004) Juvenile dispersal in Calomys venustus (Muridae: Sigmodontinae). Acta Oecologica 25:205–210
R Development Core Team (2010) R: a language and environment for statistical computing, Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/. Accessed March 2011
Sage RD, Pearson OP, Sanguinetti J, Pearson AK (2007) Ratada 2001: a rodent outbreak following the flowering of bamboo (Chusquea culeou) in southwestern Argentina. In: The Quintessential Naturalist: Honoring the Life and Legacy of Oliver P. Pearson, Kelt DA, Lessa EP, Salazar-Bravo J, Patton JL (editors), California: University of California Publications in Zoology, pp 177–224
Schmaljohn C, Hjelle B (1997) Hantaviruses: a global disease problem. Emerging Infectious Diseases 3:95–104
Schooley RL, Branch LC (2006) Space use by round-tailed muskrats in isolated wetlands. Journal of Mammalogy 87:495–500
Steinmann AR, Priotto J (2011) Inter-male aggression in relation to female availability and residence status in corn mice, Calomys musculinus. Acta Theriologica 56: 81–89
Steinmann AR, Priotto JW, Sommaro L, Polop J (2006a) Spacing behaviour of juveniles corn mice Calomys musculinus at the beginning of the breeding period, in absence of adult males. Acta Oecologica 29:305–310
Steinmann AR, Priotto JW, Sommaro L, Polop J (2006b) The influence of adult female absence on the spacing behavior of juvenile corn mice, Calomys musculinus: a removal experiment. Annales Zoologici Fennici 43:366–372
Steinmann AR, Priotto J, Polop J (2009) Territorial behaviour in corn mice, Calomys musculinus (Muridae: Sigmodontinae), with regard to mating system. Journal of Ethology 27: 51–58
Stenseth N, Lidicker W Jr. (1992) Animal Dispersal. Small mammals as a model. Chapman and Hall.
Stickel L (1954) A comparison of certain methods of measuring ranges of small mammals. Journal of Mammalogy 35:1–5
Stickel L (1968a) Dispersal of the old field mouse. In: The Biology of Peromyscus, King JA (editor), Spec. Publ. No. 2, American Society of Mammalogists 373–411
Stickel L (1968b) Home range and travels. In: The Biology of Peromyscus, King JA (editor), Spec. Publ. No. 2, American Society of Mammalogists 412–456
Torres-Perez FJ, Navarrete-Droguett J, Aldunate R, Yates TL, Mertz GJ, Vial PA, et al. (2004) Peridomestic small mammals associated with confirmed cases of human hantavirus disease in southcentral Chile. American Journal of Tropical Medicine and Hygiene 70:305–309
Van Zegeren K (1980) Variation in aggressiveness and the regulation of numbers in house mouse populations. Netherlands Journal of Zoology 30:635–770
Waterman J (2007) Male Mating Strategies in rodents. In: Rodent Societies. An Ecological, Evolutionary Perspective, Wolff JO, Sherman PW (editors), University of Chicago Press, pp 27–41
Williams J, Bryan R, Mills J, Palma E, Vera I, Peters C, Zaki S, Khan A, Ksiazek T (1997) An outbreak of hantavirus pulmonary syndrome in western Paraguay. American Journal of Tropical Medicine and Hygiene 57:274–282
Wolff JO (1999) Behavioral model systems. In: Barret GW, Peles JD (eds) Landscape Ecology of Small Mammals. Springer, Berlin, pp 11–40
Wolff JO (2003) Density-dependence and the socioecology of space use in rodents. In: Rats, mice and people: rodent biology and management, Singleton GR, Hinds LA, Krebs C, Spratt D (editors), Australian Centre for International Agricultural Research, Canberra, pp 124–130
Wolff JO (2007) Social biology of rodents. Integrative Zoology 2:193–204
Wolff JO, Summerlin CT (1993) Agonistic behavior in organized and disorganized cotton rat populations. Science 160:98–99
Young JC, Mills JN, Enria DA, Dolan NE, Khan AS, Ksiazek TG (1998) Newworld hantaviruses. British Medical Bulletin 54:659–673
Zar JH (1996) Biostatistical analysis. Third ed. Prentice-Hall, Upper Saddle River, New Jersey, USA.
Acknowledgements
This study was supported by Fondo para la Investigación Científica y Tecnológica (FONCYT), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Fundación Mundo Sano. We thank Simon E. Gutiérrez-Brida for assistance with revision of the English version and the associate editor and the anonymous reviewers for helpful comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Juan, E., Levis, S., Pini, N. et al. Mechanisms of Hantavirus Transmission in Oligoryzomys longicaudatus. EcoHealth 16, 671–681 (2019). https://doi.org/10.1007/s10393-019-01454-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-019-01454-y