Abstract
Oral vaccination is an emerging management strategy to reduce the prevalence of high impact infectious diseases within wild animal populations. Plague is a flea-borne zoonosis of rodents that often decimates prairie dog (Cynomys spp.) colonies in the western USA. Recently, an oral sylvatic plague vaccine (SPV) was developed to protect prairie dogs from plague and aid recovery of the endangered black-footed ferret (Mustela nigripes). Although oral vaccination programs are targeted toward specific species, field distribution of vaccine-laden baits can result in vaccine uptake by non-target animals and unintended indirect effects. We assessed the impact of SPV on non-target rodents at paired vaccine and placebo-treated prairie dog colonies in four US states from 2013 to 2015. Bait consumption by non-target rodents was high (70.8%, n = 3113), but anti-plague antibody development on vaccine plots was low (23.7%, n = 266). In addition, no significant differences were noted in combined deer mice (Peromyscus maniculatus) and western harvest mouse (Reithrodontomys megalotis) abundance or community evenness and richness of non-target rodents between vaccine-treated and placebo plots. In our 3-year field study, we could not detect a significant positive or negative effect of SPV application on non-target rodents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An emerging management strategy to reduce pathogen prevalence in wildlife and decrease disease risk to humans and animals is oral vaccination of wild animal populations. Using this method, carnivores have been immunized for rabies control (Slate et al. 2005), and more recently vaccines have been developed for plague in prairie dogs (Cynomys spp.) (Rocke et al. 2014; Tripp et al. 2015) and for Lyme disease in white-footed mice (Peromyscus leucopus) (Tsao et al. 2004). Although oral vaccination programs are targeted toward specific species, field distribution of vaccine-laden baits can result in vaccine uptake by non-target animals. Both direct and indirect effects of vaccines on non-target animals should be considered in the evaluation of a vaccine’s potential ecological impact. Here we assess the impact of the prairie dog oral sylvatic plague vaccine (SPV) on non-target small rodent community dynamics.
Many wild rodent populations have been severely impacted by plague since Yersinia pestis was introduced in the western USA in the early twentieth century (Eskey and Haas 1939; Gage and Kosoy 2005). Small rodents such as deer mice (Peromyscus maniculatus), northern grasshopper mice (Onychomys leucogaster) and voles (Microtus spp.) have variable susceptibility to infection by Y. pestis (McCoy and Smith 1910; Holdenried and Quan 1956; Thomas et al. 1988) and might be involved in the maintenance (Kartman et al. 1958; Cully and Williams 2001) and spread of this primarily flea-borne pathogen during plague outbreaks (Stapp et al. 2008, 2009; Salkeld et al. 2010). Most prairie dogs (Cynomys spp.) are highly susceptible to plague (McCoy and Smith 1910; Rocke et al. 2011), and the disease is considered a major contributor to the declines in their abundance and distribution (Anderson and Williams 1997; Antolin et al. 2002; Cully et al. 1997; Eads and Biggins 2015; Pauli et al. 2006). Prairie dogs are a keystone species in the grassland ecosystem (Kotliar et al. 1999). Their burrows and feeding behavior create a unique habitat of short grasses and shrubs that support numerous species (Whicker and Detling 1988). Importantly, they are prey for many predatory species, especially the endangered black-footed ferret (Mustela nigripes) (Brickner et al. 2014), which is also highly susceptible to plague (Rocke et al. 2004). Plague outbreaks can decimate entire prairie dog colonies, with concurrent losses in associated species and disruptions to grassland ecosystems (Anderson and Williams 1997; Cully et al. 1997; Pauli et al. 2006). Thus, plague management is of great interest for conservationists and wildlife managers.
Recently, field studies were initiated to assess the safety and efficacy of SPV as a plague management tool in prairie dog colonies (Tripp et al. 2015; Rocke et al. 2017). SPV is a recombinant raccoon poxvirus (RCN-F1/V307; unlicensed Y. pestis Vaccine Live Raccoon Poxvirus Vector, Code 11Y2.R0) engineered to express two protective antigens from Y. pestis, F1 and a truncated V protein (Rocke et al. 2014). The vaccine effectively protected prairie dogs against Y. pestis in laboratory challenge trials (Rocke et al. 2008, 2010a, b, 2014, 2015). Palatable baits were developed to deliver SPV to prairie dogs (Fernandez and Rocke 2011), and they were readily consumed by all four US species: black-tailed (C. ludovicianus), Utah (C. parvidens), Gunnison’s (C. gunnisoni) and white-tailed prairie dogs (C. leucurus), as well as by non-target small rodents (Tripp et al. 2014). In a small field trial in Colorado, no pox lesions or short-term mortalities were observed in prairie dogs and non-target species after SPV bait distribution (Tripp et al. 2015), confirming vaccine safety and paving the way for further studies.
From 2013 to 2015, an extensive field study was conducted in seven western states to assess SPV effectiveness in prairie dogs by distributing vaccine-laden and placebo baits on 58 paired plots (Rocke et al. 2017). In this study, we evaluated the impact of annual SPV application on non-target small rodents in 34 of these study plots across four states. Although we expected non-target small rodents would readily consume bait during this study, the direct and indirect effects of SPV application on their populations had not been evaluated. We hypothesized that non-target small rodent communities could be affected by vaccine distribution via two different mechanisms. First, consumption of SPV baits could have a direct effect by increasing survival of some non-target species that are exposed to plague. Second, SPV application could have an indirect effect on non-target species due to changes in plague dynamics, rodent abundance and density on prairie dog colonies. This indirect effect could be positive or negative. Specifically, we assessed which of the most common non-target small rodents consumed bait and developed antibodies against Y. pestis antigens, and if SPV distribution influenced their population dynamics (abundance) and community structure (richness—the number of species per plot, and evenness—a measure of the similarity in abundance of each species per plot).
Materials and Methods
Ethics Statement Animal trapping and sampling procedures (EP130214) were reviewed and approved by the Institutional Animal Care and Use Committee of the USGS National Wildlife Health Center (NWHC).
Field Trials
Study Plots and Treatment
We trapped small rodents on 17 study pairs (34 plots on colonies of three prairie dog species) included in the SPV field trial during the summers of 2013–2015. Plots spanned four western states and were grouped in six study areas; Montana, South Dakota, Wyoming and three study areas in Utah (one study area on white-tailed prairie dog colonies CBUT, two study areas on Utah prairie dog colonies, HEUT at high elevation and CCUT at low elevation; see Fig. 1 and Table 1). Briefly, plots were paired by prairie dog species, distance between them, colony size and habitat type (Rocke et al. 2017). Paired study plots were randomly assigned to treatments (vaccine-laden bait or placebo bait), and assignments were not disclosed to study participants or collaborators (i.e., blinded). Baits were distributed prior to small rodent trapping (1–6 weeks, see Table 1) at 100 baits per hectare, except on black-tailed prairie dog plots in 2014 and 2015; these plots received 125 baits per hectare. Baits were peanut butter-flavored, contained the vaccine or an equivalent volume in buffer solution, and included 0.25% rhodamine B (RB), a biomarker which can be visualized under UV light or by fluorescent microscopy in excreta, hair and whiskers (Fernandez and Rocke 2011). In red-backed voles (Myodes gapperi), fluorescence was reliably visible for 5 weeks after consumption of ~ 100 mg RB/kg (Bron 2017); for a 15-g mouse this equates to consumption of 10–20% of a bait.
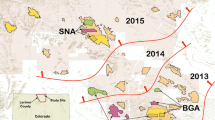
Map of the non-target species study areas in the prairie dog ranges. Pair abbreviations are included: CMR, Charles M. Russell National Wildlife Refuge, Montana; BGSD, Buffalo Gap, South Dakota; HEUT, high elevation, Awapa Plateau, Utah; CCUT, north of Cedar City, Utah; CBUT, northeast of Coyote Basin Utah; PRWY, Pitchfork Ranch, Wyoming.
Trapping
We trapped small rodents simultaneously on both members of a pair annually in July or August, within 1–5 weeks of baiting (Table 1) for 3–5 consecutive nights using Sherman traps (7.6 × 8.9 × 22.9 cm) with bedding (e.g., large cotton balls) and food (e.g., mixed oatmeal and peanut butter). Trapping grids were 150 m (HEUT-1 and 2) to nearly 7 km (CMR-5) apart (Table 1). At most locations, grids of 10 by 10 traps were centered on the baited area of the prairie dog plots, with 15 m between traps. In Wyoming, traps were placed in 11 by 12 grids, with 16 m between traps. Traps were set at sunset and checked at sunrise. We transported occupied traps to a central processing location and left unoccupied traps open until noon to trap diurnal animals, when weather conditions (dry, < 21°C) and available personnel allowed. Adverse weather and logistics only allowed for two trap nights in Buffalo Gap National Grasslands, SD (BGSD-1) in 2015, so data from this trapping effort were not included in abundance and community structure analysis.
Sampling
Measurements (weight, tail, hindfoot and body length) and demographic characteristics (species, sex and age) from each individual were recorded. Incisors were not inspected. Animals were shaken into a plastic bag (S1340, Uline) and anesthetized using a vaporizer with 2–3% isoflurane (Piramal Healthcare, India) at 1–2 l oxygen per minute. Hair and whiskers were plucked and stored in a manila coin envelope (Uline). Blood was collected from the saphenous or facial vein on Nobuto blood filter strips (Advantec, maximum capacity 100 µl), and strips were stored in manila coin envelopes (Uline).
Laboratory Assessments and Data Analyses
Bait Uptake
Hair and whisker samples were examined for RB fluorescence using a stereomicroscope (Nikon SMZ-1270) with a CY3 filter and categorized as negative or positive (Fernandez and Rocke 2011). Bait uptake scores from individuals of the most common species with known sex, age and trap night were used for statistical analysis [deer mice combined with western harvest mice (Reithrodontomys megalotis), northern grasshopper mice and Ord’s kangaroo rats (Dipodomys ordii)]. The probability of bait uptake by small rodents on vaccine and placebo plots (Treatment) was assessed using generalized linear mixed models in R (R Core Team 2017). Models had a Bernoulli distribution and logit link with a hierarchical random effect using the glmer function of the lme4 package (Bates et al. 2015). For consistency, pair was nested within study area. Although glmer does not use classical hierarchical analysis, nested observations are treated as correlated instead of independent (Bates et al. 2015). We ran single-covariate models with plot-level and individual-level effects. Plot-level effects included: year, treatment (placebo or vaccine bait), bait density (baits per hectare in the small rodent grid), bait date (as a number with June 4 being day 1), time since baiting until the first trap night (in days), plague status; non-detected, suspect (positive prairie dog fleas collected), confirmed (plague-positive prairie dog carcasses), and covariates reported in Abbott et al. (2017); prairie dog catch per unit effort (CPUE); the number of unique prairie dogs caught per 100 trap days which is an estimate of relative abundance of prairie dogs within plots (Rocke et al. 2017), prairie dog bait uptake (in percent, Abbott et al. 2017), and a normalized difference vegetation index; an estimate of the relative density and greenness of vegetation at each plot at the time of bait distribution (7-day Moderate Resolution Imaging Spectroradiometer (MODIS) composites for the center point of each plot were extracted from the US Geological Survey, https://lta.cr.usgs.gov/emodis; Jenkerson et al. 2010). We also included the interaction between CPUE and prairie dog bait uptake. Individual-level covariates included: species, sex, age and trap night (the night within a trap session on which an animal was captured and sampled for hair and whiskers, range 1–5) and time since baiting for each individual (the number of days between sample collection and bait distribution). We also included the interaction between sex and age. Models were assessed by Akaike information criteria (AIC) (Burnham and Anderson 2004); the covariates of the models that improved the AIC by 2 or more compared to the intercept model were used in the multi-variate model. Interaction terms were dropped if the individual model had a similar (within 2 AIC) or lower AIC value than the intercept model. Because treatment was our variable of interest it was kept in the multi-variate model, independent of its contribution to the model. Backward elimination was used to reduce the multi-variate model to the most parsimonious model; the covariate that contributed least to the model based on the likelihood ratio test (function drop 1 with Chi-squared test was used) was removed until all covariates were significant at the 0.05 level. In other words, the removal of covariates would increase the AIC of the model by 2 or more. We used area under the receiver operator curve [AUC, package pROC (Robin et al. 2011)] as a measure of model fit. To examine effect size, odds ratios were calculated by eparameter estimate and confidence intervals were estimated by exponentiation of the upper and lower 95% confidence limits of the parameter estimates.
Serology
Lateral flow tests (Abbott et al. 2014) were used to detect antibodies against Y. pestis F1 and V antigens. This test was compared to serology by enzyme linked immuno-sorbent assays (Bron 2017). Blood-filled Nobuto strips were stored at − 20°C until elution and testing. Strips were cut into 5–6 pieces, placed into the recommended volume (400 μl for a full strip, i.e., a ¼ filled strip was placed in 100 µl of phosphate-buffered saline (PBS), and shaken overnight at 4°C). Eluates were transferred to a clean tube and stored at − 20°C until testing. We combined 5 μl of eluate with 95 μl diluent for the protein G-based lateral flow test for F1 and V. Optical scores of the test ranged from 0 (negative) to 4 (strong positive). To distinguish vaccine-induced antibodies from exposure to Y. pestis, we only tested sera from plots where plague was not detected. We included blood samples from deer mice collected in 2015 and Ord’s kangaroo rat and northern grasshopper mice from all years.
Vaccine Effect on Abundance, Richness and Evenness
We standardized for trap effort by using the first three nights a pair was trapped each year. Rodent abundance was estimated as the number of unique individuals captured during the trap session (minimum number known alive, MNA) per plot per year. Thus, recapture events within a trap session were not counted (MNA is Mt+1 in Slade and Blair 2000). If a species was not captured on one plot, 0 was used. If the species was not present on both plots of a pair, the observation was not included in the analysis. Deer mice and western harvest mice observations were pooled for analysis because these species can be difficult to distinguish in the field, and it appears that western harvest mice were not consistently identified between years at CMR. To assess the vaccine effect on abundance, we calculated the difference between vaccine and placebo plot MNA (ΔMNA). The use of ΔMNA removes the effects of variable trap success between different pairs. A positive difference in MNA represents higher abundance on vaccine plots and a negative ΔMNA represents higher abundance on placebo plots. We used ΔMNA in linear mixed effects models using the function lmer of package lme4 (Bates et al. 2015) in R (R Core Team 2017). Pair was used as a random effect to allow for repeated measures, and pairs were nested within each study area to account for non-independence within an area. Year and plague status per pair were explanatory variables. Plague status was “confirmed” on plots where Y. pestis was isolated from prairie dog carcasses, “suspect” on plots where Y. pestis positive prairie dog fleas were found by PCR and “not detected” on the remaining plots (no Y. pestis was isolated and no Y. pestis was amplified from prairie dog fleas on those plots). The analyses were run on a subset of the data; three pairs were removed (HEUT-1, 2 and 4) due to significant prairie dog movement between vaccine and placebo plots thereby diluting treatment effects (similar to Rocke et al. 2017) that could affect a potential indirect vaccine effect on the non-target species. The best model was selected based on lowest AICc; models within 2 AICc were considered to be similar (Burnham and Anderson 2004); and the most parsimonious model was used. Models were assessed using standard diagnostic plots and pseudo R2 (package MuMIn, Barton 2016) to assess goodness of model fit (Nakagawa and Schielzeth 2013). Similar analyses were performed for the difference in richness (number of rodent species per plot per year) and Pielou’s evenness (Shannon’s diversity index/log richness). When only one species was present, Shannon’s diversity index could not be used, so evenness was assumed to be 1.0 (complete evenness). In R, we used the package vegan (Oksanen et al. 2015) and its function diversity for the calculation of diversity metrics.
Results
Bait Uptake
During ~ 34,900 trap nights and 10,654 trap days, there were 7763 captures of 3480 individuals of 15 non-target small rodent species. The mean detected bait uptake by non-target species (3113 hair samples examined) was 70.8% (95% confidence interval (CI) 69.2%, 72.4%), and multiple fluorescent bands per whisker were observed. Uptake in the most abundant species, deer mice combined with western harvest mice (n = 2564), was 72.5% (95% CI 70.7%, 74.2%). Bait uptake varied among common species, and northern grasshopper mice had the highest bait uptake (mean = 86.6%, 95% CI 80.7%, 90.9%).
Plot-level and individual-level models that were better than the intercept model included the following covariates: prairie dog bait uptake (ΔAIC = −6.5), plague status (ΔAIC = −2.9), trap night (ΔAIC = −61.3), species (ΔAIC = −14.3), age (ΔAIC = −7.9) and time since baiting for the individual animal (ΔAIC = −7.7). Sex, age and trap night were known for 2896 individuals; these observations were included in the models. The best reduced multi-variate model, with treatment forced in as an explanatory variable, included: prairie dog bait uptake, species, age and trap night (ΔAIC from intercept-only model = −83.5, AUC = 0.72, see Table S1 and Fig. 2). Bait uptake varied among species (likelihood ratio test P < 0.001), was marginally lower in juvenile animals [odds ratio (OR) 0.79, 95% CI 0.63–0.98, P = 0.037], and hair and whiskers from animals caught on the first trap nights were more frequently RB positive than those caught on later nights (OR 0.73, 95% CI 0.67–0.79, P < 0.001). Prairie dog bait uptake and small rodent bait uptake were also marginally correlated with lower odds of bait consumption by small rodents when bait uptake in prairie dogs was higher (OR 0.87, 95% CI 0.76–0.98, P = 0.027). Lastly, bait uptake was not significantly different between vaccine and placebo plots (OR 0.90, 95% CI 0.75–1.08, P = 0.268).
Serology
Deer mice (n = 177) on vaccine plots had significantly more frequent positive anti-F1 or anti-V antibody scores than tested deer mice (n = 96) on placebo plots (Table 2). Anti-F1 and anti-V antibody development in grasshopper mice (n = 97) and Ord’s kangaroo rats (n = 47) was not significantly different (P > 0.05) between treatments (Table 2 and Table S2). None of the Ord’s kangaroo rats had detectable anti-V antibodies (Table S2). Positive optical scores on placebo plots can be due to enzootic plague exposure, animal movement from vaccine plots or an epizootic plague area, or a score of 1 could be due to non-specific binding on the test strip (Table S2).
Vaccine Effect on Abundance
During the first three nights, 3018 individuals were caught (Table 3). The combined 3-night MNA of deer mice and western harvest mice included in the analysis ranged from 0 to 89 (median = 24, mean = 26.36, 95% CI 21.87–30.86). The difference between vaccine and placebo plots in the 3-night dataset was 2.44 animals (95% CI of mean − 3.08 to 7.96, median = −2) (Fig. 3a); this difference was not significantly different from 0. The intercept model had the lowest AICc value (pseudo R2 random effects = 57.0%) (Table S3), and the model intercept includes zero (intercept estimate 95% CI −5.5 to 9.2), indicating that no significant effect of vaccine treatment on abundance could be detected in our study.
The absolute differences between paired vaccine and placebo plots (n = 34 for abundance and n = 35 for richness and evenness, HEUT-1, 2 and 4 were excluded) during three trap nights per plot per year (2013–2015). Plots where plague was detected, either in a prairie dog flea or in a prairie dog carcass, are shaded black. Cumulative deer mice and western harvest mouse unique individuals caught (a), richness (singletons included, b) and evenness (c).
Vaccine Effect on Richness and Evenness
Richness per plot during the 3-night trap session, ranged from 1 to 4 small rodent species per year (mean 2.5, 95% CI 2.39–2.61). Our best model explaining the difference in species richness between vaccine and placebo plot was the intercept-only model and the intercept contained 0 (95% CI of estimate − 0.344 to 0.344). Thus, richness was not significantly different between vaccine and placebo plots. Pielou’s evenness ranged from 0.08 to 1.0. Our best model describing the difference between vaccine and placebo plots was our intercept-only model and the intercept contained zero (95% CI of estimate − 0.176 to 0.008; Fig. 3b, c). Thus, evenness was not significantly different between vaccine and placebo plots.
Discussion
Although SPV had a positive effect on survival and abundance of prairie dogs, the target species of plague vaccination (Rocke et al. 2017), we did not detect a significant positive or negative effect on non-target rodents on our study pairs. Despite high bait consumption by non-target species (70.8%), the combined abundance of deer mice and western harvest mice was not significantly different between vaccine and placebo plots, and anti-plague antibody prevalence in non-target species on vaccine plots was low (23.7%). Rodent richness and evenness were also not significantly different between vaccine and placebo plots in our study.
The assessment of bait uptake revealed similar rates of consumption in small rodents between placebo and vaccine plots. The weak correlation between prairie dog bait uptake and small rodent bait uptake is logical but the causality cannot be assessed; low uptake by prairie dogs leaves a lot of bait for the small rodents to eat; alternatively, a lot of bait consumption by the small rodents leaves less bait for the prairie dogs to eat. However, it should be noted that prairie dogs likely consume at least a whole bait, whereas small rodent hair samples will have detectable RB after consumption of ~ 10% of a bait. Either way, bait distribution around sunrise will provide prairie dogs with the prime opportunity to consume bait.
Interestingly, detection of RB was associated with trap night of individual small rodents but not time since bait distribution. It is well known that some animals are trap-prone and others are trap-shy; this has been weakly linked to home range and more convincing to intra-specific competition and individual traits (Crowcroft and Jeffers 1961; Réale et al. 2007). Although, Biro and Stamps (2008) suggest in their opinion piece that food-intake and boldness/activity levels are related, it has not been reported previously that trap-prone rodents acquired novel food resources in the field more frequently, as suggested by our data and the larger prairie dog study (Abbott et al. 2017). Further recapture and trap location analysis could possibly elucidate this, but no true behavioral assessment and effective trap area calculation can be performed.
A direct protective effect of the vaccine on non-target small rodent community is unlikely. The prevalence of anti-Y. pestis antibody was low in our study, and in a concurrent laboratory study, consumption of SPV baits did not increase the survival of deer mice and grasshopper mice upon plague challenge (Bron 2017). The absence of a protective immune response after consumption of SPV could be due to low susceptibility to infection by the vaccine vector, raccoon poxvirus, or due to an inefficient immune response to Y. pestis antigens. Susceptibility to raccoon poxvirus varies among laboratory mice strains (Rocke et al. 2010a), as could be true in the field. In general, poxviruses may induce a stronger immune response in younger individuals (Lane et al. 1970; Domenico et al. 2012). For example, young Gunnison’s prairie dogs (C. gunnisoni) were more likely to survive plague challenge after SPV consumption than adults, while plague susceptibility was similar among ages (Rocke et al. 2015). Prairie dogs are not fully mature until about 2 years old, but mice mature very quickly and are capable of reproducing at 5–6 weeks of age. Even if a direct vaccine effect was present, the protective effect in mice would only have a short impact on plague dynamics on the colony, because of the rapid turnover and short lifespan of the most common species, deer mice. In contrast to longer-lived prairie dogs, for most mice, vaccine effects would not accumulate over years. This was illustrated in our study where only one deer mouse was recaptured between years (adult female from 2013 to 2014 on HEUT-3A).
We expected that successful vaccination of prairie dogs could lead to indirect vaccine effects on non-target species due to higher prairie dog densities and lower Y. pestis abundance. Higher prairie dog densities could lead to increased competition for resources among rodent species, but more prairie dog burrows may also provide additional shelter for mice. We hypothesized that non-target rodent abundance could be higher on vaccine plots than on placebo plots when an active plague outbreak occurred, because more prairie dogs would survive due to vaccination, limiting pathogen amplification and leading to fewer infectious fleas. This reduced force of infection could also reduce plague-induced mortality in other species, resulting in higher non-target rodent abundance on vaccine plots. Deer mice and western harvest mice tended to have higher abundance on vaccine plots that experienced plague (Fig. 3a and supplemental Table S4). However, only a few plots experienced plague outbreaks during the course of our study and the increased abundance was not statistically significant.
In general, rodent population cycles and fluctuations are common due to numerous extrinsic and intrinsic factors (Krebs et al. 1973). During other studies of plague epizootics on black-tailed prairie dog colonies in northern Colorado, the abundance of grasshopper mice decreased, while abundance of deer mice was not correlated with plague mortality (Stapp et al. 2009). This suggests that deer mice are not affected by plague or that their natural population cycles and demographic patterns obscure possible plague effects. We believe that additional collection of environment factors and increased trapping effort (longer trap sessions, repeated sessions, larger grids and continuation of the study over additional years) might reveal more subtle patterns of potential indirect vaccine effects on non-target small rodents More specifically, survival and serological studies would discern if non-target species are affected during plague outbreaks and could indicate if these species were exposed to plague and survived infection.
Although our current understanding of plague dynamics on prairie dog colonies is incomplete, the application of SPV to prairie dog colonies at landscape levels could benefit prairie dog conservation (Rocke et al. 2017). The reservoir of the bacterium remains uncertain, and the mechanism(s) facilitating its rapid spread through some prairie dog colonies are unexplained. Thus, assessment of the long-term effect (> 5 years) and the impact of larger-scale application of SPV on both target and non-target species is an important area for future research.
References
Abbott RC, Hudak R, Mondesire R, Baeten LA, Russell RE, Rocke TE (2014) A rapid field test for sylvatic plague exposure in wild animals. Journal of Wildlife Diseases 50:384-388. https://doi.org/10.7589/2013-07-174
Abbott RC, Russell RE, Richgels KLD, Tripp DW, Matchett MR, Biggins DE, Rocke TE (2017) Factors influencing uptake of sylvatic plague vaccine baits by prairie dogs. Ecohealth https://doi.org/10.1007/s10393-017-1294-1
Anderson SH, Williams ES (1997) Plague in a complex of white-tailed prairie dogs and associated small mammals in Wyoming. Journal of Wildlife Diseases 33:720–732. https://doi.org/10.7589/0090-3558-33.4.720
Antolin MF, Gober P, Dakota S, Biggins DE, Collins F, Lockhart M, Ball M (2002) The influence of sylvatic plague on North American wildlife at the landscape level with special emphasis on black-footed ferret and prairie dog conservation. In: Transactions of the Sixty-Seventh North American Wildlife and Natural Resources Conference, pp 104–127
Barton K (2016) MuMIn: Multi-Model Inference. R package version 1.15.6. https://CRAN.R-project.org/package=MuMIn
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67:1–48. https://doi.org/10.1177/009286150103500418
Biro PA, Stamps JA (2008) Are animal personality traits linked to life-history productivity? Trends in Ecology and Evolution 23:361–368
Brickner, KM, Grenier, MB, Crosier, AE & Pauli, JN (2014) Foraging plasticity in a highly specialized carnivore, the endangered black-footed ferret. Biological Conservation 169: 1–5.
Bron GM (2017) The Role of Short-Lived Rodent in Plague Ecology on Prairie Dog Colonies, Ph.D. Thesis, University of Wisconsin-Madison
Burnham KP, Anderson RP (2004) Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociological Methods & Research 33:261–304. https://doi.org/10.1177/0049124104268644
Crowcroft P, Jeffers JNR (1961) Variability in the behaviour of wild house mice (mus musculus) towards live traps. Journal of Zoology 137:573–582.
Cully Jr, JF, Barnes, AM, Quan, TJ, & Maupln, G (1997) Dynamics of plague in a Gunnison’s prairie dog colony complex from New Mexico. Journal of Wildlife Diseases, 33: 706-719
Cully JF, Williams ES (2001) Interspecific comparison of sylvatic plague in prairie dogs. Journal of Mammalogy 82:894–905.
Domenico J, Lucas JJ, Fujita M, Gelfand EW (2012) Susceptibility to vaccinia virus infection and spread in mice is determined by age at infection, allergen sensitization and mast cell status. International Archives Allergy Immunology 158:196–205.
Eads DA, Biggins DE (2015) Plague bacterium as a transformer species in prairie dogs and the grasslands of western North America. Conservation Biology 29: 1086-1093. https://doi.org/10.1111/cobi.12498
Eskey CR, Haas VH (1939) Plague in the western part of the United States. Public Health Reports 54:1467–1481.
Fernandez JR-R, Rocke TE (2011) Use of rhodamine B as a biomarker for oral plague vaccination of prairie dogs. Journal of Wildlife Diseases 47:765–8.
Gage KL, Kosoy MY (2005) Natural history of plague: perspectives from more than a century of research. Annual Review of Entomology 50:505–28. https://doi.org/10.1146/annurev.ento.50.071803.130337
Holdenried R, Quan SF (1956) Susceptibility of New Mexico Rodents to Experimental Plague. Public Health Reports 71:979–984.
Jenkerson CB, Maiersperger T, Schmidt G (2010) eMODIS: A user-friendly data source: U.S. Geological Survey Open-File Report 2010–1055
Kartman, L, Prince, FM, Quan, SF, & Stark, HE (1958). New knowledge on the ecology of sylvatic plague. Annals of the New York Academy of Sciences, 70:668-711.
Kotliar N, Baker B, Whicker A, Plumb G (1999) A critical review of assumptions about the prairie dog as a keystone species. Environmental Management 24:177–192.
Krebs, CJ, Gaines, MS, Keller, BL, Myers, JH, & Tamarin, RH (1973) Population cycles in small rodents. Science, 179:35-41.
Lane J. M, Ruben FL., Neff JM., Millar J. D. (1970) Complications of smallpox vaccination, 1968: results of ten statewide surveys. Journal of Infectious Diseases 122:303–309.
McCoy GW, Smith FC (1910) The susceptibility to plague of the prairie dog, the desert wood rat, and the rock squirrel. The Journal of Infectious Diseases 7:374–376.
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecology and Evolution 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Oksanen AJ, Blanchet FG, Kindt R, Legendre P, Minchin PR, Hara RBO, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) Package “vegan”. ISBN 0-387-95457-0
Pauli JN, Buskirk SW, Williams ES, Edwards WH (2006) A plague epizootic in the black-tailed prairie dog (Cynomys ludovicianus). Journal of Wildlife Diseases 42:74–80.
R Core Team (2017) R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biological Reviews 82:291–318
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, Müller M (2011) pROC: an open-source package for R and S + to analyze and compare ROC curves. BMC Bioinformatics 12:77. https://doi.org/10.1186/1471-2105-12-77
Rocke TE, Kingstad-Bakke B, Berlier W, Osorio JE (2014) A recombinant raccoon poxvirus vaccine expressing both Yersinia pestis F1 and truncated V antigens protects animals against lethal plague. Vaccines 2:772–784. https://doi.org/10.3390/vaccines2040772
Rocke TE, Iams KP, Dawe S, Smith SR, Williamson JL, Heisey DM, Osorio JE (2010a) Further development of raccoon poxvirus-vectored vaccines against plague (Yersinia pestis). Vaccine 28:338–344. https://doi.org/10.1016/j.vaccine.2009.10.043
Rocke, T. E., Mencher, J., Smith, S. R., Friedlander, A. M., Andrews, G. P., Baeten, L. A. (2004). Recombinant F1-V fusion protein protects black-footed ferrets (Mustela nigripes) against virulent Yersinia pestis infection. Journal of Zoo and Wildlife Medicine 35: 142-146.
Rocke TE, Pussini N, Smith SR, Williamson J, Powell B, Osorio JE (2010b) Consumption of baits containing raccoon pox-based plague vaccines protects black-tailed prairie dogs (Cynomys ludovicianus). Vector-Borne and Zoonotic Diseases 10: 53–8. https://doi.org/10.1089/vbz.2009.0050
Rocke TE, Smith SR, Stinchcomb DT, Osorio JE (2008) Immunization of black-tailed prairie dog against plague through consumption of vaccine-laden baits. Journal of Wildlife Diseases 44:930–937.
Rocke TE, Tripp D, Lorenzsonn F, Falendysz E, Smith S, Williamson J, Abbott R (2015) Age at vaccination may influence response to sylvatic plague vaccine (SPV) in Gunnison’s prairie dogs (Cynomys gunnisoni). EcoHealth 278–287. https://doi.org/10.1007/s10393-014-1002-3
Rocke, TE, Tripp, DW, Russell, RE, Abbott, RC, Richgels, KLD, Matchett, MR, Biggins, DE, Griebel, R, Schroeder, G, Grassel, SM, Pipkin, DR, Cordova, J, Kavalunas, A, Maxfield, B, Boulerice, J, Miller, MW (2017) Sylvatic plague vaccine partially protects prairie dogs (Cynomys spp.) in field trials. EcoHealth 14:438-450. https://doi.org/10.1007/s10393-017-1253-x
Rocke TE, Williamson J, Cobble KR, Busch JD, Antolin MF, Wagner DM (2011) Resistance to plague among black-tailed prairie dog populations. Vector-Borne and Zoonotic Diseases 12: 111-116. https://doi.org/10.1089/vbz.2011.0602
Salkeld DJ, Salathé M, Stapp P, Jones JH (2010) Plague outbreaks in prairie dog populations explained by percolation thresholds of alternate host abundance. Proceedings of the National Academy of Sciences 107:14247–50. https://doi.org/10.1073/pnas.1002826107
Slade, NA, Blair, SM (2000) An empirical test of using counts of individuals captured as indices of population size. Journal of Mammalogy, 81:1035-1045.
Slate D, Rupprecht CE, Rooney JA., Donovan D, Lein DH, Chipman RB (2005) Status of oral rabies vaccination in wild carnivores in the United States. Virus Research 111:68–76. https://doi.org/10.1016/j.virusres.2005.03.012
Stapp P, Salkeld DJ, Eisen RJ, Pappert R, Young J, Carter LG, Gage KL, Tripp DW, Antolin MF (2008) Exposure of small rodents to plague during epizootics in black-tailed prairie dogs. Journal of Wildlife Diseases 44:724–30.
Stapp P, Salkeld DJ, Franklin HA, Kraft JP, Tripp DW, Antolin MF, Gage KL (2009) Evidence for the involvement of an alternate rodent host in the dynamics of introduced plague in prairie dogs. Journal of Animal Ecology 78:807–17. https://doi.org/10.1111/j.1365-2656.2009.01541.x
Thomas, RE, Barnes, AM, Quan, TJ, Beard, ML, Carter, LG, & Hopla, CE (1988) Susceptibility to Yersinia pestis in the northern grasshopper mouse (Onychomys leucogaster). Journal of Wildlife Diseases, 24:327-333.
Tripp DW, Rocke TE, Streich SP, Abbott RC, Osorio JE, Miller MW (2015) Apparent field safety of raccoon poxvirus-vectored plague vaccine in free-ranging prairie dogs (Cynomys Spp.), Colorado, USA. Journal of Wildlife Diseases 51:401–410. https://doi.org/10.7589/2014-02-051
Tripp DW, Rocke TE, Streich SP, Brown NL, Fernandez JR-R, Miller MW (2014) Season and application rates affect vaccine bait consumption by prairie dogs in Colorado and Utah, USA. Journal of Wildlife Diseases. 50: 224-234: https://doi.org/10.7589/2013-04-100
Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG (2004) An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proceedings of the National Academy of Sciences 101:18159–64. https://doi.org/10.1073/pnas.0405763102
Whicker, A., Detling, J.K. (1988) Ecological consequences of prairie dog disturbances. BioScience 38, 778–785
Acknowledgements
This research and GMB were supported by the Morris Animal Foundation (#D14ZO-031 and #D14ZO-412) and the U.S. Geological Survey. GMB performed this work with the USGS while she was a graduate student at the University of Wisconsin. We thank J. Cordova and G. Schroeder for trapping and sampling small rodents and sharing their samples and data. We also thank R. Russell for quantitative insight and expertise, D. Biggins and D. Tripp for methodological advice and D. Biggins, D. Eads, M.R. Matchett, M. McCollister, R. Griebel, A. Kavalunas and B. Maxfield for field support. We are very grateful to C. Crill, C. Tremper, K. Bach, Z. Vizer, N. Pawlikovsky, L. Brenner, A. Andrews, T. Brown, M. Murphy, K. Heitkamp, K. Palframan, J. Gruel, C. Poje, R. Larson, K. Murphy, C. Salas-Quinchucua, C. Malavé, E. Falendysz, J. Williamson, S. Smith, R. Abbott and others for field and laboratory assistance. This manuscript was greatly improved by comments from B. Christensen, B. Connely, R. Abbott and two anonymous reviewers. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bron, G.M., Richgels, K.L.D., Samuel, M.D. et al. Impact of Sylvatic Plague Vaccine on Non-target Small Rodents in Grassland Ecosystems. EcoHealth 15, 555–565 (2018). https://doi.org/10.1007/s10393-018-1334-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-018-1334-5