Abstract
Although wild boar can act as a persistent Aujeszky’s disease (AD) reservoir, limited data are available on long-term epidemiology in free-ranging wild boar living in areas where industrial swine herds are limited. Hence, this study provides crucial information, which fills this knowledge gap, on the natural dynamics of AD infection. From 3260 sera sampled during eight hunting seasons, 162 (4.97%) were tested positive. Factors, including the animal’s age class, and the sampling year, had significant effects on the probability of the wild boar being seropositive, while wild boar mean abundance per area, yearly abundance and the total number of pig farms, as well as interactions among age, year and sex, were not significant. In particular, a positive trend of seroprevalence was observed over the years, with values ranging from 2.1 to 10.8%. This long-term surveillance showed an increase in seroprevalence with a higher probability of being seropositive in older individuals and the independence of wild boar seropositivity from the likelihood of contact with pigs in the area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudorabies virus (PRV) or suid herpesvirus 1 (SHV-1), a member of the Alphaherpesvirinae subfamily, is the causative agent of Aujeszky’s disease (AD), an economically important disease of pigs (Aujeszky 1902). Its host range includes a wide spectrum of mammals, although domestic and wild members of the Suidae family are the only hosts capable of surviving a productive infection and can serve as reservoirs for the virus (Pensaert and Kluge 1989).
As a general pattern, wildlife can both maintain and spread infections to domestic species (Gortazar et al. 2007), and the wild boar–domestic pig interface represents an example of this interaction, as both species have mutual transmission risks for their parasitic and infectious diseases (Boadella et al. 2012). AD is such a disease, since the presence of the PRV infection in wild boar populations has been reported worldwide with variable prevalence rates (Ruiz-Fonset al. 2008a). Although reports of PRV transmission from wild boar to domestic pigs are surprisingly rare, the success of disease eradication programmes in the domestic species could be influenced by wildlife reservoirs (Müller et al. 2011).
Since the early 1980s, AD has spread globally due to the appearance of more virulent PRV strains and to changes in swine production systems, such as increases in animal density and the total confinement of the animals (Müller et al. 2011). Today, the virus has spread worldwide and causes economic losses in the pig industry due to increased mortality rates, depending on the age of the host and the virulence of the virus strain involved. PRV is currently the focus of eradication programmes almost worldwide, which include large-scale vaccination with gE-deleted vaccines. This strategy, together with increased control efforts, has decreased the incidence of the disease in several European Union (EU) member states (Pannwitz et al. 2012). In Italy, an AD national monitoring programme has begun in 1997 (Decreto Ministeriale 1997); it includes the application of direct prophylaxis, biosecurity measures and vaccination programmes. Although AD has not yet been eradicated from Italian pig herds, a considerable reduction in the spread of the virus has occurred. Similar to the observations in many European countries, where AD was eradicated in domestic pigs but not in free-living wild boar populations (Boadella et al. 2012), PRV has been continuously detected in wild boar in Italy (Lari et al. 2006; Montagnaro et al. 2010; Verin et al. 2014).
Although wild boar can serve as reservoirs for PRV (Ruiz-Fonset al. 2008b), limited data are available on the long-term epidemiology of PRV in free-ranging populations in areas without industrial swine herds. The analysis of these data may provide baseline information on PRV infection dynamics under natural conditions indicating those factors most influential on the spread and maintenance of the virus into the wild populations. Therefore, the aim of this study, through targeted surveillance, and using serological and molecular testing, was to describe the temporal dynamics of PRV infection and to define the role of wild boar population structure and the presence of domestic pig farms on spread and maintenance of AD in free-ranging wild boar populations.
Materials and Methods
Study Area and Wild Boar Sampling
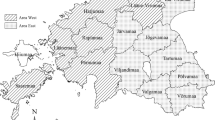
A total of 3260 sera samples were collected during eight hunting seasons (from 2006–2007 to 2013–2014) in Brescia Province (45°32′20″N, 10°13′10″E), Northern Italy, from 4007 hunted free-living wild boars coming from six distinct hunting districts (Table 1).
All of these districts are located in the alpine footstep mountains, characterised by the presence of small, but not free-ranging, pig farms (Table 2). The features of swine herds were obtained from the Official National Livestock Registry. In particular, two variables were available for analysis of each hunting district: the total number of pig farms and the density of pig farms. The density of pig farms was computed by dividing the total number of farms by the corresponding hunting district area (No of farms/km2).
The overall numbers of blood samples in the six hunting districts during eight hunting seasons were 787, 422, 142, 217, 1163 and 181, respectively. For 348 samples, the district of origin was not recorded (Table 1). The numbers of sera collected during each hunting season (from 2006/2007 to 2013/2014) were 233, 444, 519, 445, 476, 373, 355 and 415, respectively. The age class and sex of the wild boar were registered from each hunting district since 2008/09 (total = 2392). The age of the animals was determined based on the tooth eruption pattern (Saez-Royuela et al. 1989): individuals were considered “young” at <12 months of age, “sub-adult” at 13–24 months of age and “adult” at >24 months of age. Tested sera were obtained from “young” (n = 517), “sub-adult” (n = 698) and “adult” (n = 1177) wild boar, and the sex composition was 1201 males and 1129 females (62 not recorded). Sera were collected and conserved at −20°C until the analysis, which was performed at the end of each hunting season.
In the last decade, a variable trend in the wild boar population has been registered in the study area, characterised by different population growth intensities in the different hunting districts. In contrast to some other European wild boar populations, in this large territory, wild boar is completely free-living (i.e., not restricted to fenced areas) and it is not specifically managed for hunting (i.e., supplementary feeding). Indeed, the wild boar harvest regime has no hunting bag restrictions since the aim is to keep the populations under control. The harvest regime is regulated by hunting efforts, which depend on the number of hunters and hunting days. These were constant over the years during the study and equivalent between each district.
Studies carried on long temporal trend and on wide area often face the problem to have consistent data between time and area. This issue is particularly relevant to estimates on animal abundance in species as wild boar which presents logistic problems in censusing. From the official data on hunting activity provided by the local hunting office (data not showed), we assumed a similar and constant hunting efforts were spent among hunting districts and years. For this reasons, we used the total number of wild boar hunted per year as an approximation of the wild boar abundance. To take into account the different sizes of the hunting districts, a relative index of abundance was calculated, scaling the animal abundance to its district’s area, expressed in km2 as described in Chiari et al. 2015 (Table 2). The values computed are varying over years and districts and they are referred with the variable “yearly abundance”, whereas the mean values over years for each district have been computed returning the variable “mean area-abundance over 7 years”.
During the 2011–2012 and 2012–2013 hunting seasons, 534 and 326 wild boar amygdalae were collected after necropsy, respectively. Tonsil samples were immediately processed and analysed for the presence of PRV DNA.
Laboratory Analysis
Serological analyses were performed using an ELISA test for the detection of anti-gE antibodies with the Pseudorabies Virus gpI Antibody test kit (IDEXX PRV/ADV gI). The ELISA test was carried out according to the manufacturer’s instructions (Idexx, EK Hoofddorp, The Netherlands).
Genomic DNA extractions from wild boar samples were performed using an RNeasy kit (Qiagen, Hilden, Germany). The presence of PRV DNA was routinely determined using real-time PCR tests based on the specific detection of the gB and gE genes (Ma et al. 2008; Yoon et al. 2005).
Statistical Analysis
Three analytical approaches were used to assess in order: difference of composition of wild boar population between districts, evaluation of extent of the previous differences observed and finally the effect of wild boar and district factors on AD seroprevalence. In particular, differences in the compositions of wild boar populations among districts were investigated using a multinomial logistic regression that considered sex, age, year and serological status as the explanatory variables and district as the dependent variable. This model evaluated the effects of the explanatory variables on the probability of each individual wild boar to belong to each district.
In order to quantify the previous difference among districts, further analyses were carried out using binomial confidence intervals for proportions. Moreover, for the areas that showed a relevant difference in temporal trends from multinomial model, Spearman’s coefficient was performed to assess the correlation between the year and the number of samplings (Agresti 2007).
To investigate factors affecting seropositivity, a generalised linear mixed model (GLMM) for binomial data was performed including sex, age and year, and their first order interactions, as first-level variables (Goldstein 2011). We included the hunting districts as a random factor in order to overcome the non-independence of data coming from the same sampling areas. The inclusion of hunting district as random factor takes into account the effect of spatial differences on response variable, but evaluating this effect is not direct purpose of the GLMM. After identifying the minimal adequate model for first-level variables, the second level variables, which are not varying for each individual wild boar, but are measured on district-level, were added to the model to determine whether any of these district’s features were influential. These variables are yearly abundance, mean district-abundance over 7 year, total number of pigs and density of pig farms. The likelihood ratio test was used to ascertain any differences between models (Bliese 2013). All of the statistical analyses were performed using the software R, version 3.1.2 (R Foundation for Statistical Computing 2014).
Results
From 4007 hunted wild boars during the eight hunting seasons, 3260 valid sera were examined and 162 samples (4.97%; 95% CI 4.25–5.77%) tested positive for AD (Tables 1, 2; Fig. 1). Furthermore, different values of seroprevalence were observed in the six hunting districts during the different years, with the highest overall values recorded in District 1 (14.61%) (Table 1;).
Plot of the 95% CI’s for the mean seroprevalence and number of culled wild boar for the total study area. The grey line represents an increasing trend of seroprevalence with a possible and not demonstrated cyclicity, the dashed line refers to the number of culled animals as proxy of wild boar abundance.
Based on sex, 75 samples out of 1201 (6.2%) females tested positive, whereas 64 samples out of 1129 (5.6%) males tested positive. Out of 517 “young”, 698 “sub-adult” and 1177 “adult” samples tested, 14 (2.7%), 37 (5.3%) and 92 (7.8%), respectively, were positive. Eight (0.94%; CI 0.41–1.85%) wild boars, out of 847 sampled, tested PCR positive from the amygdalae. These positive animals were hunted in District 1 (3 wild boars), District 2 (1 boar), District 3 (2 wild boars) and District 5 (2 wild boars), six were adults and two were juveniles, one from District 1 and the other from District 3.
In terms of structure of the wild boar populations, hunting districts differed in seroprevalence values, in the sex and age composition and in hunted wild boar between years (Table 3). In particular, the population of District 6 had the highest proportion of males (59%) and the District 4 the lowest (43%), while the others ranged between 45 and 51%. District 1 had the highest proportion of old animals with 85% of the population in the age classes “adult” and “sub-adult” (CI 82–88%), while District 2, 5 and 6 had significantly younger populations containing 72% (CI 67–77%), 76% (CI 73–79%) and 71% (CI 63–77%) of “adult” and “sub-adults”, respectively (Table 3).
The analysis of trends using Spearman’s coefficient showed that the number of wild boar hunted increased over the years in District 5 (Spearman’s ρ = 0.75, P = 0.02), while in District 1, the number did not vary significantly over the years (Spearman’s ρ = −0.68, P = 0.11).
The best model describing the probability of being seropositive included wild boar age and year as significant variables, while wild boar yearly abundance, mean district-abundance over 7 years, total number and density of pig farms, as well as interactions among age, year and sex, were not significant (Table 4).
In particular, the odds of testing positive for AD were almost three times higher for adults compared with young boar (OR = 2.7), once the other variables were fixed (Table 5). A generalised positive trend in seroprevalence was observed over the years, with an odds ratio of 1.2 corresponding to a relative increase in seropositivity of 20% each year (Table 5).
Discussion
The present long-term surveillance of AD in the wild boar of the central Italian Alps, based on an estimated index of abundance, showed an increase in seroprevalence during the years, with a higher probability of being seropositive in older individuals, while the presence of domestic pigs had no effect on seropositivity. In particular, the total AD seroprevalence in the study area increased 1.2 times each year, showing that the infection could persist as an endemic disease at low prevalence values in wild boar populations.
The absence of the effect of wild boar abundance on seroprevalence emerged in the current study, revealing that, in this context, other factors were more influential on the spread and maintenance of AD. As expected, the seroprevalence was influenced by age rather than sex, with a significantly higher percentage of positive animals being adults. This was also supported by the results of the molecular analyses, where six of the eight PCR positive animals were adults, confirming the presence of ADV, even with a low diffusion, inside the adult population. The influence of age resulted to be the principal factor affecting seroprevalence, as reflected in the District 1 with highest seroprevalence (14.61%) and PCR positive animals (37.5%), where the population structure shows the highest proportion of adult animals.
The present results showed that AD seropositivity in wild boar did not correlate to the likelihood of contact with pigs in an area. It should be emphasised that the pig farms in the study area are very small (<50 heads/unit) usually with a restricted number of fattening pigs bred for a limited period of the year. Although these animals are not kept outdoors, the farms’ biosecurity measures could be lower than those of industrial swine herds, posing sanitation concerns. However, the total number and the density of pig farms were not significant, indicating a possibly distinct epidemiological evolution of the disease inside the wild boar and domestic pig populations. Since a clear differentiation between the strains isolated from hunting dogs, which are related to the wild boar strains, and those originating from domestic pigs was demonstrated (Sozzi et al. 2013; Moreno et al. 2015), the relationship between AD prevalence in pig farms and wild boar populations could be indirect and based on the aggregation of individuals and contact rates between them.
Domestic pigs and wild boar have a reciprocal transmission risk for their infections, including AD, as demonstrated by experimentally infecting domestic pigs with AD strains of wild boar origin, suggesting the possible AD transmission between both sides (Müller e al. 2001). Although the presence of ADV in wild boar already posed concerns for AD control in pigs (Corn et al. 2009), it has been shown that the AD prevalence in wild boar was not a significant risk factor for the AD prevalence in the coexisting pig farms (Ruiz-Fons et al. 2008b).
The overall prevalence found in our study (4.97%) is lower than values previously reported in central-southern Italy, which ranged from 10 to 30% (Lari et al. 2006; Montagnaro et al. 2010; Müller et al. 2011; Verin et al. 2014). Since wild boar density has been suggested to be the main risk factor for AD prevalence in wild boar (Acevedo et al. 2007; Ruiz-Fons et al. 2008a), the observed seroprevalence could be a consequence of the lower wild boar densities in our study area compared with other areas of Italy. In fact, in the central Alpine areas where our study area is located, the wild boar population densities are lower than in the central and southern regions of Italy, with a discontinuous and fragmented population distribution (Santilli et al. 2013). The effect of this geographical distinction seems to be supported by comparisons with other Alpine areas, which showed similar results (Müller et al. 2011). In fact in Alpine countries, such as Switzerland, the prevalence ranged from 0 to 6.88% (Köppel et al. 2007; Leuenberger et al. 2007).
In addition to density, supplementary feeding and spatial aggregation within fenced hunting areas have been identified as risk factors for increases in the ADV prevalence in wild boar in Spain (Boadella et al. 2012; Ruiz-Fons et al. 2008b). In contrast to the Spanish wild boar management system, in our study area, wild boar is completely free-living and is not specifically managed for hunting (such as supplementary feeding in fenced areas). The differences in wild boar management strategies could explain the lower wild boar density and ADV seroprevalence. At this low abundance value, the mean age of the wild boar populations seems to influence the transmission of ADV more than the wild boar density, as already reported in different European studies (Müller et al. 2011; Lutz et al. 2003; Montagnaro et al. 2010). In fact, just the age of wild boar seems to significantly influence the seroprevalence in our populations, and while the population of District 5 had an increasing abundance trend during the study period showing the lowest prevalence, the population of District 1, where the highest seroprevalence value was registered, was significantly older than the populations in other districts. Our results corroborate those of other studies that recorded stable or even increasing trends of ADV infections in wild boar and feral pig populations (Albina et al. 2000; Lutz et al. 2003; Corn et al. 2009; Boadella et al. 2012; Pannwitz et al. 2012).
Conclusion
The present long-term surveillance may provide baseline information on the dynamics of AD infections under natural conditions. The study was conducted in an area where dynamics of ADV transmission were determined as independent from the likelihood of contact between the wild boar and pigs. In addition, our results together with the absence of evidence of epidemiological association of ADV between the domestic pig and the wild boar in the study area (Moreno et al. 2015) corroborate the hypothesis that AD maintenance in the wild boar population is independent of the occurrence of AD in pigs (Müller et al. 1998; Ruiz-Fons et al. 2008b; Pannwitz et al. 2012). Nevertheless, since spillovers cannot completely be ruled out and due to the fact that our results reinforce the idea that wild boar is able to maintain the virus at natural condition also at low density values, open-air pig systems, in particular, might be at risk if preventive measures are disregarded (Pannwitz et al. 2012; Ruiz-Fons et al. 2008a, b). These findings may be considered when implementing ADV eradication programmes in livestock, in particular, in areas where the wild boar population is maintained at a low density, as in the Alps.
References
Acevedo P, Vicente J, Hofle U, Cassinello J, Ruiz-Fons F, Gortazar C (2007) Estimation of European wild boar relative abundance and aggregation: a novel method in epidemiological risk assessment. Epidemiology and Infection 135:519–527
Agresti A (2007) An introduction to categorical data analysis. 2nd ed., Wiley, London
Albina E, Mesplède A, Chenut G, Le Potier MF, Bourbao G, Le Gal S, Leforban Y (2000). A serological survey on classical swine fever (CSF), Aujeszky’s disease (AD) and porcine reproductive and respiratory syndrome (PRRS) virus infections in French wild boar from 1991 to 1998. Veterinary microbiology 77:43–57
Aujeszky A (1902). Uber ein neue Infektionskrankheit bei Haustieren. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt. 32:353–357
Bliese P (2013). Multilevel modeling in R (2.5). A brief introduction to R, the multilevel package and the nlme package. http://cran.r-project.org/doc/contrib/Bliese_Multilevel.pdf. Accessed 26 Jan 2015
Boadella M, Gortázar C, Vicente J, Ruiz-Fons F (2012). Wild boar: an increasing concern for Aujeszky’s disease control in pigs? BMC Veterinary Research 8:7
Corn JL, Cumbee JC, Barfoot R, Erickson GA (2009). Pathogen exposure in feral swine populations geographically associated with high densities of transitional swine premises and commercial swine production. Journal of Wildlife Disease 45:713–721
Decreto Ministeriale 01 aprile 1997 Piano nazionale di controllo della malattia di Aujeszky nella specie suina. (G.U. Serie generale, n. 103 del 06 maggio 1997)
Goldstein H (2011). Multilevel Statistical Models. 4th ed., Wiley Press, London
Gortazar C, Ferroglio E, Hofle U, Frolich K, Vicente J (2007). Diseases shared between wildlife and livestock: a European perspective. European Journal of Wildlife Research. 53:241–256
Köppel C, Knopf L, Ryser MP, Miserez R, Thur B, Stark KDC (2007). Serosurveillance for selected infectious disease agents in wild boars (Sus scrofa) and outdoor pigs in Switzerland. European Journal of Wildlife Research 53:212–220
Lari A, Lorenzi D, Nigrelli D, Brocchi E, Faccini S, Poli A (2006). Pseudorabies virus in European wild boar from Central Italy. Journal of Wildlife Disease 42:319–324
Leuenberger R, Boujon P, Thur B, Miserez R, Garin-Bastuji B, Rufenacht J, Stark KDC (2007). Prevalence of classical swine fever, Aujeszky’s disease and brucellosis in a population of wild boar in Switzerland. Veterinary Record 160:362–368
Lutz W, Junghans D, Schmitz D, Müller T (2003). A long-term survey of pseudorabies virus infections in European wild boar of western Germany. Zeitschrift für Jagdwissenschaft 49:130–140
Ma W, Lager KM, Richt JA, Stoffregen WC, Zhou F, Yoon KJ (2008). Development of real-time polymerase chain reaction assays for rapid detection and differentiation of wild-type pseudorabies and gene-deleted vaccine viruses. Journal of veterinary diagnostic investigation 20:440–447
Montagnaro S, Sasso S, De Martino L, Longo M, Lovane V, Ghlurmino G, Plsanelli G, Nava D, Baldl L, Pagninl U (2010). Prevalence of antibodies to selected viral and bacterial pathogens in wild boar (Sus scrofa) in Campania Region, Italy. Journal of Wildlife Disease 46:316–319
Moreno A, Sozzi E, Grilli G, Gibelli LR, Gelmetti D, Lelli D, Chiari M, Prati P, Alborali GL, Boniotti MB, Lavazza A, Cordioli P (2015). Detection and molecular analysis of Pseudorabies virus strains isolated from dogs and a wild boar in Italy. Veterinary microbiology, 177:359-365
Müller T, Hahn EC, Tottewitz F, Kramer M, Klupp BG, Mettenleiter TC, Freuling C (2011). Pseudorabies virus in wild swine: a global perspective. Archives of virology 156:1691–1705
Müller T, Teuffert J, Zellmer R, Conraths FJ (2001). Experimental infection of European wild boars and domestic pigs with pseudorabies viruses with differing virulence. American journal of veterinary research 62:252–258
Müller T, Teuffert J, Ziedler K, Possardt C, Kramer M, Staubach C, Conraths FJ (1998). Pseudorabies in the European wild boar from Eastern Germany. Journal of Wildlife Disease 34:251–258
Pannwitz G, Freuling C, Denzin N, Schaarschmidt U, Nieper H, Hlinak A, Burkhardt S, Klopries M, Dedek J, Hoffmann L, Kramer M, Selhorst T, Conraths FJ, Mettenleiter T, Müller T (2012). A long-term serological survey on Aujeszky’s disease virus infections in wild boar in East Germany. Epidemiology and Infection 140:348–358
Pensaert MB, Kluge P (1989). Pseudorabies virus (Aujeszky’s disease). In: Pensaert MB (ed) Virus infections of porcines. Elsevier Science Publishers, New York
R Foundation for Statistical Computing (2014) R: a language and environment for statistical computing. http://www.R-project.org/. Accessed 26 Jan 2015
Ruiz-Fons F, Segalés J, Gortázar C (2008a). A review of viral diseases of the European wild boar: effects of population dynamics and reservoir role. The Veterinary Journal 176:158–169
Ruiz-Fons F, Vidal D, Vicente J, Acevedo P, Fernandez-de-Mera IG, Montoro V, Gortazar C (2008b). Epidemiological risk factors of Aujeszky’s disease in wild boars (Sus scrofa) and domestic pigs in Spain. European Journal of Wildlife Research 54:549–555
Saez-Royuela C, Gomariz C, Telleria JL (1989). Age determination of European wild boar (Sus scrofa). Wildlife Society Bulletin 17:326–329
Santilli F, Varuzza P (2013). Factors affecting wild boar (Sus scrofa) abundance in southern Tuscany. Hystrix, the Italian Journal of Mammalogy 24(2):169–173
Sozzi E, Moreno A, Lelli D, Cinotti S, Alborali GL, Nigrelli A, Luppi A, Bresaola M, Catella A, Cordioli P (2013). Genomic characterization of pseudorabies virus strains isolated in Italy. Transboundary and emerging diseases 61:334–340
Verin R, Varuzza P, Mazzei M, Poli A (2014). Serologic, molecular, and pathologic survey of pseudorabies virus infection in hunted wild boars (Sus Scrofa) in Italy. Journal of wildlife diseases 50:559–565
Yoon HA, Eo SK, Aleyas AG, Park SO, Lee JH, Chae JS, Cho JG, Song HJ (2005). Molecular survey of latent pseudorabies virus infection in nervous tissues of slaughtered pigs by nested and real-time PCR. Journal of Microbiology-Seoul 43:430–436
Acknowledgments
This paper is dedicated to the memory of our friend and colleague, Paolo Cordioli, who prematurely passed away in August 2014. He was the head of the Virology Department and responsible for the National reference Laboratory for Aujeszky Disease at IZSLER for over 20 years. This study is a contribution to the European project APHAEA (EMIDA ERA-NET). The authors gratefully acknowledge the local hunters (Brescia Province, Northern Italy); without their cooperation, these studies would not have been possible. The authors would like to thank Daniel Bresciani and Michela Fazio for their excellent technical support. The authors wish to thank Lesley S. Benyon (ScienceDocs) for the editing of this manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
MC, DA, AL and GLA conceived and designed the study. AMM, DL and AL carried out the laboratory work. MC, MZ and GLA participated in sampling and field work. MB, NF, MC and PL analysed the data. All authors participated in drafting the manuscript. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiari, M., Ferrari, N., Bertoletti, M. et al. Long-Term Surveillance of Aujeszky’s Disease in the Alpine Wild Boar (Sus scrofa). EcoHealth 12, 563–570 (2015). https://doi.org/10.1007/s10393-015-1064-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-015-1064-x