Abstract
Objectives
To identify the maternal and social characteristics associated with age standardised birthweight in a modern developed setting.
Methods
Birth records (n = 414,478) were obtained for live, singleton births in the period 2007–2015 in Queensland, Australia. Age-standardised birth weights were calculated and a multinomial logistic regression was performed to obtain odds ratios and 95% confidence intervals for a range of maternal and social characteristics.
Results
Mothers who smoke (OR 2.82, 95% CI 2.72–2.93), and mothers from Southern and Central Asia (OR 3.30, 95% CI 3.08–3.53) had the highest odds of delivering small for gestational age babies. Smoking alone accounted for 21% of low birthweight. Pre-existing diabetes (OR 5.98, 95% CI 5.12–6.99) had the highest odds ratio for large for gestational age births; however, maternal overweight and obesity accounted for 24% of all cases due to its greater prevalence in the population.
Conclusion for practice
Smoking continues to be an important modifiable predictor of low birthweight. The predictors associated with large for gestational age are modifiable, with maternal overweight and obesity the largest contributor to high birthweight.
Significance
Maternal characteristics are changing alongside broader population change, with mothers often older and heavier than in previous decades. This study provides an update to the role of maternal and social characteristics in optimal birthweight within a large developed population. The present study finds a range of traditional and emerging risk factors remain important. Population attributable risk fractions show that maternal overweight and smoking are the most important modifiable risk factors for birthweight extremes (foetal macrosomia and small for gestational age, respectively). Public health efforts to address these risk factors could reduce up to 20% of birth weight extremes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Impacts of birth weight extremes
Birth weight remains a practical and useful indicator of neonatal health (Malin et al. 2014). Low birth weight has long been associated with increased risk of mortality and morbidity for the neonate; however, with the advent of life course studies there is an increasing interest in the longer term effects of sub-optimal birthweights. Recent studies have associated low birth weight with premature mortality (Risnes et al. 2011), cardiovascular disease (Johnson and Schoeni 2011), cancer (Caughey and Michels 2009; Risnes et al. 2011), poorer mental health (Westrupp et al. 2011), diabetes (Johnson and Schoeni 2011), wheezing (Mebrahtu et al. 2015), increased susceptibility to infection (Villamor et al. 2010), and lower cognitive performance (Nakamuro et al. 2013). Further, there is an increasing focus on the opposite end of the spectrum, with evidence suggesting short- and long-term health effects for foetal macrosomia. Longer hospital stays (Australian Institute of Health and Welfare 2015), increased risk of birth trauma, hypoglycaemia, polycythaemia, increased risk of dental caries (Yamagata et al. 2015), leukaemia (Belbasis et al. 2016; Caughey and Michels 2009), and obesity (Belbasis et al. 2016; Zhao et al. 2012) have all been associated with high birthweights.
Maternal and social characteristics that can influence birthweight
A range of maternal characteristics are associated with sub-optimal birth weights. Low birth weight has been linked with underweight mothers (Yadav and Lee 2013), younger or older maternal age (Chiavarini et al. 2012; Yadav and Lee 2013), ethnicity or immigrant status (Chiavarini et al. 2012; Poon et al. 2012; Yadav and Lee 2013), low socio-economic status (Yadav and Lee 2013), reduced educational attainment (Chiavarini et al. 2012), being unmarried (Chiavarini et al. 2012), primiparity (Chiavarini et al. 2012), low or high blood pressure (Yadav and Lee 2013), and pregnancy complications (Chiavarini et al. 2012). High birthweights have been associated with increased maternal weight or BMI (McGrath et al. 2018; Shin and Song 2015), higher parity (Poon et al. 2012), and type 1 diabetes (McGrath et al. 2018).
While much is known about birthweight, changing patterns of maternal demographics in developed countries, such as increasing maternal age, increasing BMI, and reduction in smoking rates requires regular review of the characteristics associated with sub-optimal birthweight in neonates. The present study aims to identify the maternal and social characteristics that are most important in a developed nation with a modern and accessible health care system in influencing offspring birth weight.
Methodology
The current study uses a cohort of babies born in Queensland, Australia and retrospectively examines the birth records. Australia is a developed nation, and provides an accessible health care system that pregnant women can access. The Perinatal Data Collection (PDC) is a collection of birth records, compiled by the Statistical Services Branch of Queensland Health. Virtually all babies in Queensland that are live born, and any stillborn babies that reached 20 weeks of gestation and/or 400 g of weight, are captured in the PDC, along with a range of maternal characteristics. Queensland is a large state on the eastern side of Australia, with a population of around 5 million. It is a diverse state, with a climate ranging from tropical, to warm temperate, and hot arid. More than 50% of the population live outside the capital city, and a significant amount of the state qualifies as rural or remote (Queensland Government 2018). The PDC contains detailed birth records for all births in Queensland attended by a midwife or doctor. All records from the period of 1st Jan 2007 to 30th June 2015 were obtained. Variables on infant characteristics [birth date, weeks of completed gestation, birthweight, birth length, head circumference, plurality, sex, birth status (alive/stillborn), presence of congenital anomalies (Y/N flag) and type of anomaly (ICD code)], maternal demographics [region of birth, Indigenous status, age (5 year bands), previous pregnancies (Y/N flag), maternal pre-pregnancy BMI, pre-existing diabetes (Y/N flag)], the presence of pregnancy complications (maternal hypertension, gestational diabetes), smoking status (Y/N flag) and locality (maternal residential locality, postcode, SLA2) were available. Variable section was based on available data and previous research in the field, as well as the expertise of the research team. The dataset contained 529,860 individual birth records, and the number of eligible records available determined the sample size. Data on socioeconomic status were obtained from the Australian Bureau of Statistics. The Index of Relative Socioeconomic Disadvantage and Advantage is a composite index of several predictor variables that allocates each postcode area to a decile of socioeconomic standing (Pink 2011). Each record was allocated a decile score based on maternal residential postcode. Information on remoteness was acquired from the Australian Bureau of Statistics Remoteness Structure. This structure outlines five levels of remoteness areas, based on the relative access to services in each area (Australian Bureau of Statistics 2018). Each record was allocated a remoteness area based on maternal residential postcode. Births were excluded from the analysis if the mother’s usual residential postcode was outside of Queensland (n = 5158), and also if the baby (2) had a congenital anomaly that independently affects birth weight (n = 15,397), (3) was of indeterminate sex (n = 120), (4) lived in a postcode that could not be allocated a socioeconomic score (n = 1054), (5) was part of a multiple birth (n = 17,272), or (6) was stillborn (n = 3572). After exclusions, 494,126 birth records remained. Missing data was present in 79,648 records. The largest contributor to missing values was maternal BMI (n = 41,259), and remoteness category (n = 39,808). Analysis was restricted to records with complete data for all variables. Thus, the final sample size was 414, 478. Ethical approval for this study was granted by the Children’s Health Queensland Human Research Ethics Committee and the University of Queensland Human Research Ethics Committee.
Informed by previous research and expert knowledge, a directed acyclic graph (DAG) was developed to explore the potential causal relationships between the variables using the software DAGitty (Textor et al. 2016). The DAG illustrates the predicted relationships between variables, and identifies maternal Indigenous status as a potential confounder that required adjustment (see Fig. 1).
Directed acyclic graph depicting the predicted relationships between birthweight and explanatory variables. Blue circles with a line indicate outcomes, in this case birthweight. Plain blue circles indicate an ancestor of the outcome. Green circles with triangles indicate an exposure. Dark grey circles are observed variables that are not on an exposure pathway to the outcome. Light grey circles are unobserved variables, which play a role in the exposure pathway. Green arrows are exposure pathways related to the outcome. Black arrows are pathways that are not directly related to the outcome. Lastly, red arrows indicate a biasing pathway, with red circles the biasing variable
Birthweight in grams, gestational age, and sex were used to calculate age-standardised birthweight percentiles using the ‘childsds’ package (Vogel 2017) for R as the primary outcome variable. This was categorised into small for gestational age (SGA), appropriate for gestational age, and large for gestational age (LGA) using 10% cut-offs; thus, babies under the 10th percentile were categorised as SGA, and babies greater than the 90% percentile were categorised as LGA. The following variables were retained for use as predictors: maternal age (in categories), smoking (y/n), maternal Indigenous status, congenital anomalies (not independently linked to birthweight), maternal BMI category, previous pregnancies (y/n), maternal remoteness status, maternal country of birth, year of birth, month of birth, season of the baby’s birth, the presence of hypertension in the pregnancy, gestational diabetes, pre-existing diabetes, and socioeconomic status. Tests of multicollinearity between variables found a relationship between month of birth and year of birth, thus month was removed. All other variables remained.
Multinomial logistic regression was performed using the ‘nnet’ package (Venables and Ripley 2002) in R. This package fits a log-linear model via neural networks. The reference category was appropriate for gestational age. Odds ratios (OR) and 95% confidence intervals were calculated. Model selection was performed by stepwise regression, with AIC as the criteria for variable selection, using the ‘MASS’ package (Venables and Ripley 2002). Further testing was carried out on a range of models, using the change in deviance to assess model fit. The final model selected was identified as the best model within both methods. Attributable risk was estimated using the package ‘attribrisk’ (Schenck et al. 2014).
Some BMI values were identified as extreme outliers (BMI > 75). Sensitivity analysis was performed by fitting models both with and without these values. Results in both models were the same which was most likely due to the very small numbers of records exceeding the cut-off (n = 5). Further testing was performed with BMI observations that lie outside 1.5 times the interquartile range removed. This led to small changes in the estimates, that were not biologically significant. Therefore, outliers have been retained given the small change in estimate, and the fact that many of the values removed were biologically plausible. A further sensitivity analysis was performed on the data, removing BMI as a predictor variable. Changes in the estimates were minimal, and thus BMI was retained as a predictor, due to its importance in modern health-care management.
All analysis was performed in R version 3.5.1 “Feather Spray”. This manuscript was prepared following the guidelines of the STROBE statement (Vandenbroucke et al. 2007).
Results
The majority of babies in the cohort were born at full term (94%) and at an appropriate birthweight for their gestational age and sex (80%). The proportion of babies born small for gestational age and large for gestational age was 5% and 15% respectively. The majority of mothers were aged 25–34 years (59%), did not identify as Indigenous and/or Torres Strait Islanders (95%), were non-smokers (85%), had given birth previously (70%), and lived in major cities (66%) The characteristics of the babies are described in Table 1.
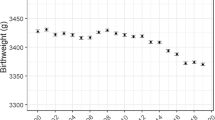
The mean birthweight in grams was slightly reduced across time in this cohort, from a high of 3446 g in 2008 to the lowest value of 3399 g in 2015. Similarly, mean gestational age decreased by around 1.5 days, from 39 weeks in 2000 to 38.8 weeks in 2015. Mean birthweights were highest in mothers aged 25–34 years, with mothers under the age of 19 years and over the age of 45 years having the lowest birthweight babies. Mean birthweight increased as maternal BMI category increased (Fig. 2). The lower values seen for underweight mothers was not explained by lower gestational age. The proportion of early pre-term births remained steady throughout the study period, whilst the proportion of babies born late pre-term increased from 5% in 2007 to 5.5% in 2015. There was a small increase in the proportion of underweight mothers and a decrease in the proportion of overweight and obese mothers.
Within the outcome variable of age-standardised birth weight, the majority of babies (79.7%, n = 330,462) in our cohort were born at an appropriate weight for their gestational age and sex: 4.6% of babies were small for gestational age (n = 19,167), while 15.6% were large for gestational age (64,849). These rates stayed fairly steady across the time period (data not shown).
Main results
Unadjusted odds ratios are presented in Table 2. The full model contains year and season as potential confounders, alongside the range of predictors.
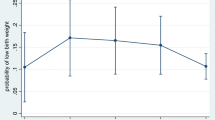
Adjusted analysis shows large increases in the odds of delivering a small for gestational age baby for smokers (OR 2.82, 95% CI 2.72–2.93, p < 0.001), mothers from North African or Middle Eastern countries (OR 2.21, 95% CI 1.95–2.50, p < 0.001), and mothers from Southern and Central Asian countries (OR 3.30, 95% CI 3.08–3.53, p < 0.001). Smaller but significant effects were seen for Indigenous mothers (OR 1.28, 95% CI 1.21–1.36, p < 0.001), living in rural (inner regional OR 1.05, 95% CI 1.00–1.09, p = 0.037; outer regional OR 1.09, 95% CI 1.04–1.13, p < 0.001) or remote areas (OR 1.13, 95% CI 1.02–1.25, p = 0.022), congenital anomalies (OR 1.39, 95% CI 1.29–1.48, p < 0.001), mothers born on the American continent (OR 1.15, 95% CI 1.00–1.32, p = 0.044), sub-Saharan Africa (OR 1.51, 95% CI 1.36–1.69, p < 0.001), North-East Asia (OR 1.71, 95% CI 1.58–1.85, p < 0.001) and South-East Asia (OR 1.75, 95% CI 1.62–1.89, p < 0.001, maternal hypertension (OR 1.83, 95% CI 1.72–1.94, p < 0.001), underweight mothers (OR 1.68, 95% CI 1.58–1.79, p < 0.001), and living in an area with the highest level of disadvantage (OR 1.13, 95% CI 1.06–1.20, p < 0.001).
A range of factors were also associated with increased odds of LGA babies. The adjusted model shows a nearly 6-fold increase in the odds of delivering LGA babies for mothers with pre-existing diabetes (OR 5.98, 95% CI 5.12–6.99, p < 0.001). Increased odds were also seen for mothers with gestational diabetes (OR 1.10, 95% CI 1.03–1.17, p = 0.005), mothers who were overweight (OR 1.48, 95% CI 1.44–1.51, p < 0.001) or obese (OR 1.91, 95% CI 1.87–1.96, p < 0.001), and mothers who had been pregnant previously (OR 1.62, 95% CI 1.59–1.66, p < 0.001).
Interactions
Effect modification between diabetes and BMI categories was included in the final model. For SGA babies, the interaction between maternal obesity and gestational diabetes was slightly protective (OR 0.83, 95% CI 0.71–0.97, p = 0.017). This is similar to the reduction in odds seen in the main effects model. For LGA babies, maternal underweight and gestational diabetes led to a larger increase in in odds compared with gestational diabetes alone (OR 1.46, 95% CI 1.07–2.00, p = 0.018). Obese mothers with gestational diabetes had higher odds of LGA babies (OR 1.37, 95% CI 1.27–1.49, p < 0.001), but this was reduced compared with the main effects model. Interestingly the odds changed significantly for obese mothers with pre-existing diabetes, with mothers in both categories having reduced odds of having a large for gestational age baby (OR 0.61, 95% CI 0.50–0.75, p < 0.001).
Attributable risk
Attributable risk was estimated for predictor variables considered to be modifiable. Removing all maternal smoking would lead to a 21% reduction in SGA (coeff = 0.209, 95% CI 0.201–0.218), whilst removing all maternal hypertension would give a reduction of 3% (coeff = 0.030, 95% CI 0.027–0.034).
Despite the large OR for pre-existing diabetes and LGA, the attributable risk was only 1% (coeff = 0.014, 95% CI 0.012–0.015). Prevention of all cases of gestational diabetes would lead to a 2% reduction in SGA cases (coeff = 0.022, 95% CI 0.019–0.0244). Maternal BMI is responsible for the largest fraction of attributable risk, with a 24% reduction (coeff = 0.240, 95% CI 0.232–0.249) possible if all mothers had a BMI in the normal range.
Discussion
The results of the present study show that traditional risk factors, such as smoking and low maternal BMI continue to be important predictors of low birthweight. In our study of an Australian population, we also find that women from African, Asian, and American backgrounds are more likely to deliver SGA babies. Characteristics of the maternal residential area, such as living in an area with the lowest socio-economic ranking or rural areas, may also increase the odds of delivering SGA babies. Most of the factors associated with SGA remain non-modifiable, with only smoking, hypertension, and BMI falling into the modifiable category, with smoking producing the largest attributable risk fraction.
The current study finds a nearly 3-fold increase in the odds for SGA for women who smoke during pregnancy. This finding is with the presence of any maternal smoking, suggesting that even low maternal use of cigarettes may contribute to this risk. As maternal smoking represents a preventable exposure, efforts to support women to quit smoking during pregnancy should continue.
Perhaps one of the more important findings of this study is the large increases in the odds for SGA for women from Asian, North African, or Middle Eastern countries. Not all of these cases will be due to pathological growth restriction (Urquia and Sørbye 2016), nor will they mean there is an adverse outcome associated with the child’s health. Nonetheless, these results highlight a potential intervention area for improved neonatal outcomes. The increased odds for SGA for women from migrant backgrounds could not be attributed to smoking rates, which were lower in these women, compared with Australian and European women (data not shown). Given that the regions with the highest odds ratios for SGA overlapped with regions with historically high levels of endemic stunting (World Health Organization 2018), it is likely that some of this effect is a long-term effect of childhood stunting on body composition and reproductive outcomes (Martorell and Zongrone 2012). A Swedish study also found this tendency for lighter babies in migrant women, and found the effect remains even when accounting for time since migration and other social factors (Juárez and Hjern 2017). The growth centiles used in the current study are based on the WHO Child Growth Standards; therefore, the risk of misclassification due to use of a local reference is reduced. The magnitude of effect is as large as for maternal smoking, suggesting that exploring care practices to reduce the prevalence of SGA amongst these groups should be a priority area for health providers. While the current study finds a potential link between maternal body composition, notably short stature, and SGA there is also a role for maternal prenatal care. There has been several complex reasons identified for sub-optimal sexual and reproductive healthcare amongst migrant women, ranging from language barriers, gender roles, cultural norms, and difficulty accessing the Australian medical system (Mengesha et al. 2016; Mengesha et al. 2017)
For large for gestational age babies, our key finding is the importance of maternal BMI to attributable risk fraction. Whilst pre-existing diabetes greatly increased the odds of a LGA offspring, and gestational diabetes was also associated with macrosomia, BMI was associated with the greatest risk contribution. Being overweight or obese was also associated with increased odds of delivering a LGA baby. Unlike SGA, all of the factors associated with LGA in this study are modifiable, and represent an action area for public health.
Body mass index (BMI) is associated with adverse maternal and neonatal outcomes. Existing studies have shown that being underweight decreases the odds for gestational hypertension, gestation diabetes, and high birth weight, while increasing the odds of delivering a SGA baby (Shin and Song 2015). A recent review highlighted the importance of diabetes and foetal macrosomia, focussing on the link between the two even where appropriate glycaemic control was achieved (McGrath et al. 2018). The interaction model found interesting reductions in the odds for obese and overweight women with diabetes, especially the protective effect of obesity and pre-existing diabetes. It is possible that this effect arises from care-giving practices during the pregnancy, with well-established care protocols available for diabetes in pregnancy (Kitzmiller et al. 2008)
This study utilised a large population-based cohort that comprised all births within the region for a 9-year period. However, there are limitations to our design. It is possible that some births were not registered in the PDC. Data on smoking was limited to a yes/no flag, and did not take into account the amount smoked. The population in this study was ideal for adding to the information about maternal and social risk factors in developed settings.
Conclusions for practice
There remain modifiable maternal risk factors associated with sub-optimal birthweight outcomes. Maternal smoking, hypertension, and a pre-pregnancy BMI in the underweight category are all modifiable risk factors associated with increased odds of delivering a SGA baby. Maternal overweight and obesity, as well as gestational diabetes, are modifiable risk factors associated with LGA offspring. While not strictly modifiable, women who have migrated from African, Asian, and American countries may require prenatal care that is more carefully targeted to their needs. These findings highlight the need for improved medical care guidelines to address these factors. Policy makers and healthcare providers should continue to focus their efforts towards smoking reduction for pregnant women, weight management and reduction programs for women of child bearing age, and culturally appropriate reproductive health care for migrant women.
References
Australian Bureau of Statistics (2018) Australian statistical geography standard (ASGS): volume 5 — remoteness structure. Australian Bureau of Statistics, Canberra. http://www.abs.gov.au/ausstats/abs@.nsf/mf/1270.0.55.005. Accessed 19/12/2018
Australian Institute of Health and Welfare (2015) Australia's mothers and babies 2013 — in brief. AIHW, Canberra
Belbasis L, Savvidou MD, Kanu C, Evangelou E, Tzoulaki I (2016) Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-analyses. BMC Med 14:147. https://doi.org/10.1186/s12916-016-0692-5
Caughey RW, Michels KB (2009) Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer 124:2658–2670. https://doi.org/10.1002/ijc.24225
Chiavarini M, Bartolucci F, Gili A, Pieroni L, Minelli L (2012) Effects of individual and social factors on preterm birth and low birth weight: empirical evidence from regional data in Italy. Int J Public Health 57:261–268. https://doi.org/10.1007/s00038-011-0311-3
Johnson RC, Schoeni RF (2011) Early-life origins of adult disease: national longitudinal population-based study of the United States. Am J Public Health 101:2317–2324. https://doi.org/10.2105/AJPH.2011.300252
Juárez SP, Hjern A (2017) The weight of inequalities: duration of residence and offspring's birthweight among migrant mothers in Sweden. Soc Sci Med 175:81–90. https://doi.org/10.1016/j.socscimed.2016.12.045
Kitzmiller JL, Block JM, Brown FM, Catalano PM, Conway DL, Coustan DR, Gunderson EP, Herman WH, Hoffman LD, Inturrisi M, Jovanovic LB, Kjos SI, Knopp RH, Montoro MN, Ogata ES, Paramsothy P, Reader DM, Rosenn BM, Thomas AM, Kirkman MS (2008) Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care 31:1060–1079. https://doi.org/10.2337/dc08-9020
Malin GL, Morris RK, Riley R, Teune MJ, Khan KS (2014) When is birthweight at term abnormally low? A systematic review and meta-analysis of the association and predictive ability of current birthweight standards for neonatal outcomes. BJOG 121:515–526. https://doi.org/10.1111/1471-0528.12517
Martorell R, Zongrone A (2012) Intergenerational influences on child growth and undernutrition. Paediatr Perinat Epidemiol 26:302–314. https://doi.org/10.1111/j.1365-3016.2012.01298.x
McGrath RT, Glastras SJ, Hocking SL, Fulcher GR (2018) Large-for-gestational-age neonates in type 1 diabetes and pregnancy: contribution of factors beyond hyperglycemia. Diabetes Care 41:1821–1828. https://doi.org/10.2337/dc18-0551
Mebrahtu TF, Feltbower RG, Greenwood DC, Parslow RC (2015) Birth weight and childhood wheezing disorders: a systematic review and meta-analysis. J Epidemiol Community Health 69:500–508. https://doi.org/10.1136/jech-2014-204783
Mengesha ZB, Dune T, Perz J (2016) Culturally and linguistically diverse women’s views and experiences of accessing sexual and reproductive health care in Australia: a systematic review. Sex Health 13:299–310. https://doi.org/10.1071/SH15235
Mengesha ZB, Perz J, Dune T, Ussher J (2017) Refugee and migrant women's engagement with sexual and reproductive health care in Australia: a socio-ecological analysis of health care professional perspectives. PLoS One 12:e0181421. https://doi.org/10.1371/journal.pone.0181421
Nakamuro M, Uzuki Y, Inui T (2013) The effects of birth weight: does fetal origin really matter for long-run outcomes? Econ Lett 121:53–58. https://doi.org/10.1016/j.econlet.2013.07.003
Pink B (2011) Socio-economic indexes for areas (SEIFA) 2011 technical paper. Australian Bureau of Statistics, Canberra. http://www.ausstats.abs.gov.au/Ausstats/subscriber.nsf/0/22CEDA8038AF7A0DCA257B3B00116E34/$File/2033.0.55.001seifa2011technicalpaper.pdf. Accessed 1/12/18
Poon LCY, Volpe N, Muto B, Syngelaki A, Nicolaides KH (2012) Birthweight with gestation and maternal characteristics in live births and stillbirths. Fetal Diagn Ther 32:156–165. https://doi.org/10.1159/000338655
Queensland Government (2018) Statistics and Facts — Queensland Government. Queensland Government, Brisbane https://www.qld.gov.au/about/about-queensland/statistics-facts. Accessed 10/10/18
Risnes KR, Vatten LJ, Baker JL, Jameson K, Sovio U, Kajantie E, Osler M, Morley R, Jokela M, Painter RC, Sundh V, Jacobsen GW, Eriksson JG, Sorensen TI, Bracken MB (2011) Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol 40:647–661. https://doi.org/10.1093/ije/dyq267
Schenck L, Atkinson E, Crowson C, Therneau T (2014) Attributable risk: .stimates population attributable risk and confidence intervals. Mayo Clinic, Rochester MI
Shin D, Song WO (2015) Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J Matern Fetal Neonatal Med 28:1679–1686. https://doi.org/10.3109/14767058.2014.964675
Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, GTH E (2016) Robust causal inference using directed acyclic graphs: the R package 'dagitty'. Int J Epidemiol 45:1887–1894. https://doi.org/10.1093/ije/dyw341
Urquia ML, Sørbye IK, Wanigaratne S (2016) Birth-weight charts and immigrant populations: a critical review. Best Pract Res Clin Obstet Gynaecol 32:69–76. https://doi.org/10.1016/j.bpobgyn.2015.09.001
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M (2007) Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology 18:805–835. https://doi.org/10.1097/EDE.0b013e3181577511
Venables W, Ripley B (2002) Modern applied statistics with S, Fourth edn. Springer, New York
Villamor E, Iliadou A, Cnattingius S (2010) Evidence for an effect of fetal growth on the risk of tuberculosis. J Infect Dis 201:409–413. https://doi.org/10.1086/650313
Vogel M (2017) Package ‘childsds’: Data and methods around reference values in pediatrics: R package version 0.6.5. CRAN repository
Westrupp EM, Northam E, Doyle LW, Callanan C, Anderson PJ (2011) Adult psychiatric outcomes of very low birth weight survivors. Aust N Z J Psychiatry 45:1069–1077. https://doi.org/10.3109/00048674.2011.620561
World Health Organization (2018) Joint child malnutrition estimates - Levels and trends (2018 edition). WHO, Geneva https://www.who.int/nutgrowthdb/estimates2017/en/
Yadav H, Lee N (2013) Maternal factors in predicting low birth weight babies. Med J Malaysia 68:44–47
Yamagata Z, Yokomichi H, Suzuki K, Tanaka T (2015) Macrosomia is one of risk factors for dental caries in 3-year-old infants in Japan. Int J Epidemiol 44:i77–i78. https://doi.org/10.1093/ije/dyv097.287
Zhao Y, Wang S-F, Mu M, Sheng J (2012) Birth weight and overweight/obesity in adults: a meta-analysis. Eur J Pediatr 171:1737–1746. https://doi.org/10.1007/s00431-012-1701-0
Acknowledgements
D Vilcins was supported by an Australian Government Research Training Program Scholarship.
Funding
There are no funding sources to declare for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and manuscript. Data acquisition and management was performed by Dwan Vilcins and Paul Jagals. Study design was developed by Dwan Vilcins, Peter Baker, and Peter Sly. Data analysis was performed by Dwan Vilcins, with support and checking by Peter Baker and Peter Sly. All authors contributed to the editing and checking of manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare they have no conflict of interest.
Ethical approval
This study was a secondary analysis of anonymised data. As such, there was no requirement to gain informed consent, and the study was deemed a low-risk study. Ethical approval was sought and granted from the Children’s Health Queensland Human Research Ethics Committee and The University of Queensland Human Research Ethics Committee. The ethical approval number is HREC/16/QRCH/320.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vilcins, D., Baker, P., Jagals, P. et al. Association of maternal and social characteristics with age-standardised birthweight. J Public Health (Berl.) 30, 373–383 (2022). https://doi.org/10.1007/s10389-020-01292-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-020-01292-1