Abstract
Objective
The authors investigated the effectiveness of a functionalized tricalcium phosphate (fTCP) combined with a low fluoride level in a mouthrinse to reharden eroded enamel lesions.
Methods
Ninety enamel slabs attached in pairs to removable appliances were randomly assigned to three treatment groups (n = 30/group): (T1) NaF rinse (225 ppm F + 40 ppm fTCP), (T2) NaF rinse (225 ppm F; ACT®), and (T3) no mouthrinse (saliva). While wearing the in situ appliance for 14 days, subjects brushed their teeth with 1100 ppm F toothpaste (Crest©) for 2 min, rinsed with 15 ml of water for 10s, and then rinsed with 15 ml of their assigned treatment mouthrinse. Treatment efficacy was evaluated using surface microhardness (SMH) and transverse microradiography (TMR). Intra- and intergroup comparisons (α = 0.05) were performed using the t-test and ANOVA followed by the Tukey test (HSD).
Results
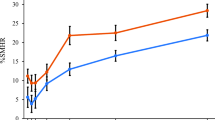
With SMH, intragroup comparison (t-test) indicated significant rehardening of the eroded lesion with exposure to T1 (p < 0.001) and T2 (p < 0.01) but not with T3 However, with TMR, remineralization was only significant (p = 0.01) with T1, but not with T2 and T3. In the intergroup comparison with percentage change in SMH, T1 was significantly different from T3 (p < 0.01; Tukey HSD) but not from T2, and T2 was significantly different from T3. Intergroup comparison based on percentage mineral gain showed that T2 (p = 0.02) and T3 (p = 0.01) differed significantly from fTCP, but not between each other.
Conclusion
Addition of low level fluoride to functionalized β-tricalcium phosphate promoted rehardening of eroded enamel lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental erosion is defined as a loss of tooth structure by acid dissolution of non-microbiological origin (Imfeld 1996). With regards to etiology, erosion has been associated with increasing consumptions of acidic food (e.g., apples and oranges) and beverages (e.g., sports drinks, orange juice, soft drinks, coffee, etc.) or the attack of stomach acids during acid reflux and/or vomiting (Lussi 2006). The continuous and repeated acid insults result in complete removal of the minerals and irreversible tissue loss (Voronets et al. 2008). It is a relatively difficult lesion to treat, considering that while relatively lower fluoride concentrations may provide anticaries benefits, higher fluoride concentrations are needed to address dental erosion (Amaechi and Higham 2001; Cochrane et al 2010). Materials or substances that are extraneous to dental tissue can be provided as reparative action (Amaechi and Higham 2001; Poggio et al. 2010). Many studies have reported possible remineralization of eroded lesions by fluoride products, but other anticaries agents, such as calcium and phosphate ions, may also have an important role in this process (Magio et al. 2010; Mathews et al. 2012; Srinivasan et al. 2010). Other studies demonstrated that products with a high concentration of both calcium and fluoride may not result in greater protection because of premature reaction of these two ions during storage to form calcium fluoride. This premature reaction reduces the bioavailability of these ions, thus compromising the therapeutic efficacy of the product (Karlinsey et al. 2010a, b; Vogel et al. 2006). Therefore, the development of a calcium-based technology that can permit the coexistence of fluoride and calcium ions without premature reaction, offering high bioavailability of these ions to provide greater efficacy in enamel remineralization, would enhance dental health benefits with regards to dental erosion management (Amaechi and Higham 2001; Cochrane et al 2010).
One advance towards erosion prevention and treatment has involved the development of functionalized tricalcium phosphate (fTCP), a combination of a low-dose fluoride and functionalized β-tricalcium phosphate (β-TCP) (Karlinsey and Pfarrer 2012; Mathews et al. 2012). The β-TCP serves as a bioactive source of mineralizing components, and it is an ideal choice of calcium phosphate system due in part to its limited solubility relative to other calcium salts and minerals, which has implications for fluoride compatibility in water-based preparations (Karlinsey et al. 2006). β-TCP is biocompatible and bioactive (Ghosh et al. 2008). The possibility of functionalizing the β-TCP (fTCP) provides a prospective finding in the research of bioactive and fluoride-compatible mineralizing agents: first, it creates barriers that prevent premature fluoride-calcium interactions; second, it facilitates targeted delivery (Karlinsey et al. 2008) of calcium and fluoride ions when applied to the teeth via common dental preparations (e.g., dentifrices, mouthrinse, etc.). Studies have demonstrated that fTCP enhances the benefits of fluoride by offering statistically greater remineralization of dental erosion (Amaechi et al. 2010; Karlinsey et al. 2009; Karlinsey et al. 2010a, b; Karlinsey and Pfarrer 2012; Mathews et al. 2012).
To further evaluate the benefits of the combined fluoride plus fTCP, this study aimed to determine the re-hardening potential of a prototype NaF rinse containing fTCP on eroded enamel in an in situ clinical model. After each of the following treatments, (1) saliva, (2) mouthrinse with 225 ppm F, and (3) mouthrinse with 225 ppm F plus fTCP, the enamel surface changes were assessed using surface microhardness (SMH) and transverse microradiography (TMR).
Methods
This was a double blind, randomized, and controlled in situ trial to assess the effect of two different mouthrinses on enamel surface rehardening. The study protocol was reviewed and approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio (UTHSCSA) and conducted according to Good Clinical Practice.
Subject selection
Forty-five healthy adult volunteers (between 18–40 years old; 11 males and 34 females) from different ethnic origins and socioeconomic statuses, who fulfilled the inclusion criteria without violating the exclusion criteria, were enrolled. To be included in the study subjects must have experienced dental caries but no active untreated caries, no significant gingivitis/periodontitis, physiological saliva flow rates (unstimulated >0.2 ml/min, stimulated >0.7 ml/min), and a mandibular first molar with a sound unrestored buccal surface for attachment of the appliance. Subjects were excluded if they had general/systemic illness or known allergy to the test products, or were using fixed or removable orthodontic appliances. Pregnant or breastfeeding mothers were also excluded. The subjects were identified with code numbers. All participants were given oral and written information about the aim of the study and test products. All volunteers gave their informed consent.
The power analysis and sample size calculations were performed using nQuery Advisor software (Statistical Solutions, Cork, Ireland) and were based on results of previous related studies (Amaechi et al. 1998; Amaechi et al. 2012; Mathews et al. 2012). The mean pretreatment %ΔZ was equal to 28.5 with a standard deviation of 31.2. For our null hypothesis that eroded lesion rehardening will be significantly greater than zero, the proposed sample size of 30 (n = 30 with each subject wearing two sample blocks) will have a power greater than 0.80 with a 0.05 one-sided significance level to detect a difference between a null hypothesis mean of zero and a sample mean %ΔZ ≥10 %.
Specimen preparation
Freshly extracted human molar teeth were collected and examined by transillumination before storing in 0.1 % thymol solution prior to use. Forty-five teeth without caries, cracks, or enamel malformations were selected and cleaned with pumice to remove remnants of the pellicle and debris/stains from the buccal surface. Using a water-cooled diamond saw (Well, Ebner, Switzland), a tooth block, approximately 6 mm long × 3 mm wide × 2 mm thick, was cut from the buccal surface of each tooth. Using plain back diamond lapping film (30 μm) in a MultiPrep™ Precision Polishing machine (Allied High Tech, USA), both the enamel and dentin (bottom) surfaces of each block were polished to achieve flat and planoparallel surfaces. Following this, two pieces of an adhesive UVPC tape were used to cover the polished enamel surface of each block, leaving a central area of 1 × 3 mm exposed. An early eroded enamel lesion was created on each exposed area by 2-h immersion in a static 0.3 % citric acid solution (pH 3.75) at room temperature (25 °C). Following the exposure, the UVPC tape was carefully removed, and each was cut to produce two blocks of approximately 3 mm length × 3 mm width × 2 mm thick. The two blocks from each tooth were assigned to one study subject.
Baseline measurements
Baseline surface microhardness (SMH) of the sound and eroded enamel surfaces was examined using a Knoop diamond indenter with a load of 50 g applied for 15 s (Shimadzu HMV AD Easy Test Version 3.0.00). For each SMH measurement, three indentations were made at the middle, upper, and lower ends of the eroded lesion in each block, and the average value of the Knoop hardness number was recorded for the sound (SMHs) and eroded (SMHe) enamel surfaces. Following this, one tooth slice (control) of approximately 100 μm thickness was cut from each block and was microradiographed using transverse microradiography (TMR) as previously described (Amaechi et al. 1998). The microradiographs were analyzed with TMR software version 3.0.0.11 (Inspektor Research Systems, The Netherlands) to quantify the parameters of integrated mineral loss (ΔZ, vol % μm) (Amaechi et al. 2010).
Intra-oral appliance fabrication
The two lesion-bearing enamel blocks from each tooth, assigned to one study subject, were mounted on the buccal site within the acrylic portion of a removable lower jaw intra-oral appliance. Each block was retained within the acrylic using fluoride-free intermediate restorative material. The blocks were not covered with polyester gauze to prevent plaque accumulation, but were rather mounted slightly recessed below the edge of the acrylic to prevent contact of the block surface with the oral mucosa. The appliances were sterilized with gamma irradiation (Amaechi et al. 1999).
Study procedure
The 45 enrolled subjects, each with an assigned intra-oral appliance bearing two enamel blocks, were randomly assigned to one of the three treatment groups (15 subjects/group; n = 30 samples/group): (T1) an aqueous NaF rinse with functionalized β-tricalcium phosphate (225 ppm F + 40 ppm fTCP) prepared as described previously (Karlinsey et al. 2009; Mathews et al. 2012); (T2) a commercially available aqueous NaF rinse with 225 ppmF (ACT®), and (T3) no mouthrinse (saliva). A qualified dentist, who was different from the laboratory assistant who processed and analyzed the samples to produce the final data, fitted the appliance. All subjects were seen at the clinic in the mornings but were asked to commence the use of their mouthrinse in the night. This was to allow enough time for formation of a salivary pellicle on the enamel blocks. Also the mouthrinses were specially prepared and coded by the manufacturing company, and the codes were not released until data collection and analysis had been completed. This procedure permitted blinding of the clinician, laboratory assistant and the study coordinator providing instructions to patients. The treatment, which last for 14 days, was preceded by a 7-day washout period to balance for residual effects of the participant’s previously used home product. During the washout period, all subjects used fluoride-free toothpaste for their usual oral hygiene procedure without wearing any appliance. During the treatment, the participants were supplied with 1100 ppm F toothpaste (Crest©, 1100 ppm NaF, Procter & Gamble Co., Cincinnati, OH, USA), a toothbrush, their assigned mouthrinse, and written instructions and a schedule. The appliances were worn for 24 h except during meals. When outside the mouth, appliances were stored in a sealed moist plastic bag at room temperature until reinsertion. After meals, 15 min elapsed before re-insertion.
The participants were asked to first brush their teeth with the 1100 ppm F toothpaste (Crest©) for 2 min, rinse with 15 ml of water for 10s, and then rinse with 15 ml of the assigned treatment mouthrinse depending on the protocol. This was done twice daily (first thing in the morning and last thing before bed). Subjects were instructed to avoid eating or drinking for 30 min after the product usage. Compliance was monitored by (1) providing each subject with a diary to record the number of treatment events performed each day and the time it was done and (2) weighing the product before dispensing and after the study. Therefore, subjects were instructed to return the remaining product at the completion of the study. All subjects were asked to maintain their normal dietary habits. The use of any other oral hygiene product was prohibited. These measures were used to ensure uniformity in the use of oral hygiene products, which may otherwise interfere with the de-/remineralization cycle during the study period. Subjects were instructed to avoid brushing directly on the enamel slab. At the end of the 14-day period, the appliance was removed.
Post-treatment processing
Following the study, the enamel slabs were removed from their respective appliances, and the post-exposure surface microhardness (SMHp) was measured as previously described. After SMH measurements, an enamel slice (about 100 μm thick) was cut from each block and processed for TMR measurement of the post-treatment mineral loss. Although the pre-test slices had been microradiographed and analyzed for selection of the appropriate lesions, they were microradiographed again together with the post-test slices to enable both sets to be microradiographed and analyzed under the same conditions. In doing so, both the pre-test (ΔZi, vol% μm) and post-treatment (ΔZp, vol% μm) integrated mineral loss was obtained. Then the relative percent change in SMH (%SMHC) and the percent change mineral loss (%ML) were calculated as follows (Amaechi et al. 1998):
Statistical analysis
Data were analyzed statistically using the SPSS statistical software (IBM Statistics 19.0), with a 0.05 significance level. The assumptions of normal distributions were checked for all the variables. The mean and standard error of the mean (SD) values of SMH and TMR parameters were calculated for the pre- and post-test treatment data. The pre- and post-test parameters within each group were compared using the paired t-test at the 95 % confidence level. Intergroup comparisons were performed using one-way analysis of variance (ANOVA, p < 0.05) followed by post-hoc multistep comparisons (Tukey HSD).
Results
All participants completed the study satisfactorily. No adverse events were reported in the study. Three samples were lost from the appliances during the experimental period, one from each group. ANOVA did not show significant differences in SMHe among the groups, indicating that the groups are comparable in pre-treatment data. Intragroup comparison (paired t-test) of the pre-treatment and post-treatment SMH indicated a significant rehardening of the eroded lesion with exposure to T1 (p < 0.001) and T2 (p < 0.01) but not with T3 (Table 1). Intergroup comparison, using the percentage change in SMH, demonstrated a statistically significant difference in the amount of rehardening observed among the groups (ANOVA) with T1 being significantly different from T3 (p < 0.05; Tukey HSD) but not from T2, and T2 not being different from T3.
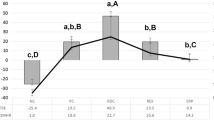
ANOVA did not show any statistically significant difference (p > 0.05) in pre-treatment mineral loss among the groups (Table 2). Following 14-day treatment, intragroup comparison with the t-test showed a statistically significant (t-test, p < 0.001) reduction in ΔZ (i.e., gain in mineral) only in the T1 group, but neither in T2 nor T3. Intergroup comparison (Tukey HSD) indicated that the percentage mineral gain was significantly (p < 0.01) higher in T1 compared with T2 and T3. The T2 and T3 did not differ from each other in percentage mineral gain (p > 0.05).
Discussion
Previous studies have shown that fluoride and other anticaries compounds involving calcium and phosphate ions may have an important role in the prevention and control of dental erosion (Amaechi et al. 2012; Ghosh et al. 2008; Karlinsey and Pfarrer 2012; Magio et al. 2010; Mathews et al. 2012). A number of studies have shown that, unlike dental caries, a high fluoride concentration is required to arrest or remineralize eroded lesion (Ganss et al. 2001; Magalhaes et al. 2011). However, considering the implication of using high-concentration fluoride, especially in children among whom erosion is also common (Jaeggi and Lussi 2006), the present study investigated the possibility of combining low-level fluoride (225 ppm) with calcium technology (fTCP) to achieve the remineralization of eroded lesions.
Within the limits of the present study, the combination of fTCP with 225-ppm fluoride in a mouthrinse caused a significant rehardening of the eroded lesion as indicated by the high percentage gain in surface microhardness of the lesions following exposure to the mouthrinse. The significant reduction in the amount of mineral loss, an indication of mineral gain, is evidence of remineralization of the eroded lesions through exposure to the mouthrinse. This observation is not surprising considering that the base of the crater formed by erosion is made of a softened layer that is incompletely demineralized and as such retains a framework of partly dissolved crystals that could form a substrate for crystal growth, thus rendering the layer amenable to remineralization (Amaechi et al. 1998), and the remineralization of eroded lesions has long been demonstrated in a number of studies (Amaechi and Higham 2001; Amaechi et al. 1998; Amaechi et al. 2010; Ganss et al. 2001; Karlinsey et al. 2010a, b; Magio et al. 2010; Reynolds et al. 2003). On the same basis, it was not surprising that significant remineralization was observed with the 225 ppm fluoride alone, although less than that observed when it was combined with fTCP. This low remineralization produced by the standard fluoride mouthrinse has been reported by other studies (Karlinsey et al. 2010a, b; Karlinsey and Pfarrer 2012; Mathews et al. 2012). It has also been reported that erosive lesions tend to require higher levels of fluoride in order to promote a substantial antierosive response (Imfeld 1996; Karlinsey and Pfarrer 2012). However, the results of the present study and other previous studies that used 450 ppm F (Amaechi et al. 2010; Karlinsey et al. 2009; Karlinsey et al. 2010a, b; Karlinsey and Pfarrer 2012) suggest that combining low fluoride with fTCP will serve as an alternative to high-concentration fluoride in promoting eroded lesion remineralization. However, the lack of significant remineralization of the eroded lesion observed with exposure to saliva alone was contrary to the report of previous studies (Amaechi and Higham 2001; Attin et al. 2001; Attin et al. 2004; Jaeggi and Lussi 1999). As saliva contains dissolved calcium and phosphate ions, and indeed is supersaturated with respect to hydroxyapatite, it is theoretically possible that it could support remineralization of erosive lesions between acid challenges, which would restore the mechanical integrity of the softened layer. A number of studies have explored this possibility and shown that some reduction of erosive tissue loss occurs after various periods of in situ exposure to saliva of acid-challenged tooth surfaces (Amaechi and Higham 2001; Attin et al. 2001; Attin et al. 2004; Jaeggi and Lussi 1999). The results have led to recommendations that tooth brushing should be avoided for about 60 min after consumption of erosive products (Attin et al. 2001; Jaeggi and Lussi 1999). However, the inability of saliva alone to cause significant remineralization was reported in studies by Lippert et al. (2004) who attributed it to the poor capacity of saliva to supply bioavailable calcium and phosphate ions to promote significant remineralization (Karlinsey et al. 2008; Reynolds et al. 2003; Vogel et al. 2006). Furthermore, based on the nature of tooth erosion, the precipitation of substantial amounts of calcium phosphate from saliva may be frustrated by the presence of some salivary proteins (Karlinsey et al. 2010a, b). Studies also confirmed that the softened enamel is not capable of complete rehardening by exposure only to saliva (Mathews et al. 2012; Poggio et al. 2010).
Unlike caries, eroded lesions require an agent applied primarily to re-harden the softened layer at the base of the crater to render it more resistant to subsequent acid attack (Fejerskov et al. 1994; Magalhaes et al. 2011). This has been reported to be achievable using either highly concentrated fluoride agents (Amaechi et al. 2012; Magalhaes et al. 2011) or fluoride compounds of tin or stannous ions (Ghosh et al. 2008; Magalhaes et al. 2011; Mathews et al. 2012). The result of the present study has shown that combining fluoride with fTCP can also accomplish this. Functionalizing TCP enables it to coexist with fluoride during storage in an aqueous environment without a premature reaction to calcium fluoride, thus ensuring the full bioavailability of the individual ions, calcium, phosphate and fluoride before and during intraoral application (Karlinsey et al. 2010a, b; Karlinsey and Pfarrer 2012). The results presented in this study accord with and corroborate the findings of previous studies that demonstrated the benefit of the addition of functionalized β-tricalcium phosphate (fTCP) to a fluoride rinse (Amaechi et al 2010; Karlinsey et al. 2009; Karlinsey et al. 2010a, b; Karlinsey and Pfarrer 2012; Mathews et al. 2012).
The results of this in situ study demonstrated that the combination of low fluoride plus fTCP produced a statistically significant rehardening of the surface of eroded enamel lesions compared to either fluoride or saliva alone. These results support future clinical evaluations of the promising combination of different concentrations of fluoride plus fTCP.
References
Amaechi BT, Higham SM (2001) In vitro remineralisation of eroded enamel lesions by saliva. J Dent 29:371–376
Amaechi BT, Hingham SM, Edgar VW (1998) Use of transverse microradiography to quantify mineral loss by erosion in bovine enamel. Caries Res 18:17–24
Amaechi BT, Higham SM, Edgar WM (1999) The use of gamma irradiation for the sterilization of enamel for intra-oral cariogenicity tests. J Oral Rehabil 26:809–813
Amaechi BT, Karthikeyan R, Poornima KM, Najibfard K, Mackey AC, Karlinsey RL (2010) Remineralization of eroded enamel by a NaF rinse containing a novel calcium phosphate agent in an in situ model: a pilot study. Clin Cosmet Investig Dent 2:93–100
Amaechi BT, Ramalingam K, Mensinkai PK, Chedjieu I (2012) In situ remineralization of early caries by a new high-fluoride dentifrice. Gen Dent 60(4):186–192
Attin T, Knöfel S, Buchalla W, Tütüncü R (2001) In situ evaluation of different remineralization periods to decrease brushing abrasion of demineralized enamel. Caries Res 35:216–222
Attin T, Siegel S, Buchalla W, Lennon ÀM, Hannig C, Becker K (2004) Brushing abrasion of softened and remineralised dentin: an in situ study. Caries Res 38:62–66
Cochrane NJ, Cai F, Huq NL, Burrow MF, Reynolds EC (2010) New approaches to enhance remineralisation of tooth enamel. J Dent Res 89:1187–1197
Fejerskov O, Larsen MJ, Richards A, Baelum V (1994) Dental tissue effects of fluoride. Adv Dent Res 8:15–31
Ganss C, Klimek J, Schäffer U, Spall T (2001) Effectiveness of two fluoridation measures on erosion progression in human enamel and dentine in vitro. Caries Res 35:325–330
Ghosh SK, Nandi SK, Kundu B, Datta S, De DK, Roy SK, Basu D (2008) In vivo response of porous hydroxyapatite and beta-tricalcium phosphate prepared by aqueous solution combustion method and comparison with bioglass scaffolds. J Biomed Mater Res 86:217–227
Imfeld T (1996) Dental erosion: definition, classification and links. Eur J Oral Sci 104:207–214
Jaeggi T, Lussi A (1999) Toothbrush abrasion of erosively altered enamel after intraoral exposure to saliva: An in situ study. Caries Res 33:455–461
Jaeggi T, Lussi A (2006) Prevalence, incidence and distribution of erosion. In: Lussi A (ed) Dental Erosion: from diagnosis to therapy. Monography in Oral Science. Karger, Basel, pp 44–65
Karlinsey RL, Pfarrer AM (2012) Fluoride plus functionalized β-TCP: a promising combination for robust remineralization. Adv Dent Res 24:48–52
Karlinsey RL, Yi K, Duhn CW (2006) Nucleation and growth of apatite by a self-assembled polycrystalline bioceramic. Bioinspir Biomim 1:12–19
Karlinsey RL, Mackey AC, Walker ER, Frederick KE (2008) Spectroscopic evaluation of native, milled, and functionalized β-TCP seeding into dental enamel lesions. J Mater Sci 44:5013–5016
Karlinsey RL, Mackey AC, Walker ER, Frederick KE, Fowler CX (2009) In vitro evaluation of eroded enamel treated with fluoride and a prospective tricalcium phosphate agent. J Dent Oral Hyg 1:52–58
Karlinsey RL, Mackey AC, Walke ER, Frederick DE (2010a) Surfactant-modified β-TCP: structure, properties, and in vitro remineralisation of subsurface enamel lesions. J Mater Sci Mater Med 21:2009–2020
Karlinsey RL, Mackey AC, Walker ER, Frederick KE (2010b) Preparation, characterization and in vitro efficacy of an acid-modified β-TCP material for dental hard-tissue remineralisation. Acta Biomater 6:969–978
Lippert F, Parker DM, Jandt KD (2004) In vitro demineralization/remineralisation cycles at human tooth enamel surfaces investigated by AFM and nanoindentation. J Colloid Interface Sci 280:442–448
Lussi A (2006) Erosive tooth wear—a multifactorial condition of growing concern and increasing knowledge. In: Lussi A (ed) Dental erosion. Karger, Basel, pp 1–8
Magalhaes AC, Wiegand A, Rios D, Buzalaf MA, Lussi A (2011) Fluoride in dental erosion. Monogr Oral Sci 22:158–170
Magio B, Guibert RG, Mason SC, Karwal R, Rees GD, Kelly S, Zero DT (2010) Evaluation of mouthrinse and dentifrice regimens in an in situ erosion remineralisation model. J Dent 38:37–44
Mathews MS, Amaechi BT, Ramalingam K, Ccahuana-Vasquez RA, Chedjieu IP, Mackey AC, Karlinsey RL (2012) In situ remineralisation of eroded enamel lesions by NaF rinses. Arch Oral Biol 57(5):525–530
Poggio C, Lombardini M, Colombo M, Bianchi S (2010) Impact of two toothpastes on repairing enamel eroson produced by a soft drink: an AFM in vitro study. J Dent 38:868–874
Reynolds EC, Cai F, Shen P, Walker GD (2003) Retention in plaque and remineralisation of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum. J Dent Res 82:206–211
Srinivasan N, Kavitha M, Longanathan SC (2010) Comparison on the remineralisation potential of CPP-ACP and CPP-ACP with 900ppm fluoride on eroded human enamel: an in situ study. Arch Oral Biol 55:541–544
Vogel GL, Shim D, Schumacher GE, Carey CM, Chow LC, Takagi S (2006) Salivary fluoride from fluoride dentifrices of rinses after use of a calcium pre-rinse or calcium dentifrice. Caries Res 40:449–454
Voronets J, Jaeggi T, Buergin W, Lussi A (2008) Controlled toothbrush abrasion of softened human enamel. Caries Res 42:286–290
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The present study had its own financing. The authors would like to thank the Coordination of Training of Higher Education Graduates (CAPES) for the scholarship (BEX# 6636-10).
Conflict of interest
The authors declare no conflict of interest in this work with a personal or financial relationship that might have introduced any bias or affected our judgment.
Ethical approval
The Local Institutional Ethics Committee granted ethical approval for the study. The study followed the guidelines of Good Clinical Practice and was conducted in full accordance with the ethical standards of the Institutional Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All participants signed the informed written consent.
Rights and permissions
About this article
Cite this article
de Oliveira, A.F.B., Mathews, S.M., Ramalingam, K. et al. The effectiveness of an NaF rinse containing fTCP on eroded enamel remineralization. J Public Health 24, 147–152 (2016). https://doi.org/10.1007/s10389-016-0709-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-016-0709-8