Abstract
Aim
Lymphatic filariasis is an important public health problem that causes economic loss and poverty in many endemic regions of India. This study explores the influence of socioeconomic factors on filariasis prevalence in the Chittoor district of Andhra Pradesh.
Subjects and methods
To understand the influence of socioeconomic variables on lymphatic filariasis, a pilot-scale epidemiological and socioeconomic study was conducted in 30 villages of Chittoor district, Andhra Pradesh, India, from 2004 to 2007. Data were analyzed statistically by frequency distribution, multivariate logistic regression and principal component analysis (PCA).
Results
A total of 5133 blood samples were collected and screened for microfilaria; 77 were found to be positive (1.52 %). The multivariate analysis showed that variables such as age (OR = 2.4, 95 % CI: 1.47–4.01), income [Indian rupees (INR): <1000: OR = 4.2, 95 % CI: 1.48–11.76; INR: 1000–3000: OR = 3.84, 95 % CI:1.92–7.68], drainage system (OR = 3.5, 95 % CI: 1.62–7.5), mosquito avoidance (OR = 1.41, 95 % CI: 0.69–2.87) and participation in mass drug administration (MDA) programs (OR = 1.33, 95 % CI:0.74–2.38) were risk factors for filariasis. The socioeconomic index derived from the PCA was categorized into low (1.7 %), medium (1.7 %) and high (1.3 %) in relation to the percentage of parasite prevalence.
Conclusion
This study reveals that filariasis is largely associated with various socioeconomic factors. Hence, health officials should focus on improving the quality of life to minimize the filarial incidence in the endemic villages by considering the socioeconomic index as a marker for targeting low and medium socioeconomic level groups for disease control programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lymphatic filariasis (LF) is one of the neglected tropical diseases caused by three dominant species of parasites (viz., Wuchereria bancrofti, Brugia malayi and Brugia timori) and mostly transmitted by Culex and Anopheles mosquito species (Bockarie and Molyneux 2009). LF is most prevalent in Africa, South Asia, Southeast Asia and parts of South America, accounting for over 127 million people from 81 countries infected with this parasite. Around 1.3 billion people are at risk of infection and 45 million suffer from the consequences of chronic disease manifestations (WHO 2009; Michael and Bundy 1997). In India, filariasis is an important public health problem in 18 states, and the Union Territories alone contribute 40 % of filarial cases globally (Ottesen et al. 2008). India’s National Health Policy on LF was started in 2002. The main objective of this program is to eliminate LF transmission and prevent disability due to LF by the year 2015 by providing an annual single dose of diethyl carbamazine citrate (DEC). Under this program, in Andhra Pradesh, 16 districts were included in a mass drug administration (MDA) program, which covered 54 million people (NVBDCP 2004). The Government of India also launched a nationwide MDA program in 2004 in many areas identified as endemic. In 2004, only 202 districts were covered, with a coverage rate of 72.6 %; from 2007 onwards, MDA coverage was increased to 250 districts in different states of India (Upadhyayula et al. 2012b).
The human development index (HDI) and LF endemicity were analyzed in 161 countries; of these, 94 % of the countries with the lowest HDIs were found endemic for lymphatic filariasis. Based on the per capita incomes of 175 countries, it was observed that a high LF endemicity of 73 % was observed in low-income countries, 33 % in middle-income countries and 5 % in high-income countries (Durrheim et al. 2004). Hence, it is assumed that LF mostly prevails in low socioeconomic conditions. However, the association between socioeconomic conditions and LF is poorly understood and has not been quantified (Ahorlu et al. 1999; Upadhyayula et al. 2012a).
LF has a wide range of clinical symptoms such as fever, chronic symptoms of hydroceles, lymphoedema, elephantiasis of the limbs, enlarged breasts and kidney damage, causing great morbidity and disability of the infected persons (WHO 1992). Apart from these clinical manifestations, LF infection has many other significant effects such as causing disabilities and poor mental health, leading to social stigma, discrimination and inability to work, placing a socioeconomic burden on the affected person, their family and the country as a whole (Mishra 2009; Okon et al. 2010; Litt et al. 2012). Most neglected diseases grow in a society because of lack of proper knowledge about transmission and awareness of the disease, poor quality of habitats, and negligence in targeting the pathogens and vectors (Mwakitalu et al. 2013). Factors such as population migrations (Ramaiah 2013), presence of mosquito habitats, high vector density and availability of infected mosquitoes support the transmission of filariasis (Boyd et al. 2010). Similarly, other associated factors such as not implementing personal protection measures (such as mats, repellents, liquid vaporizers, bed nets and mosquito coils) increase the human-vector contact (Charu Kohli et al. 2014), as do noncompliance with mass drug administration (MDA) programs, lack of proper MDA distribution centers, non-consumption of DEC drugs (Mukhopadhyay et al. 2008) and lack of awareness about MDA programs (Litt et al. 2012; Ghosh et al. 2013). Apart from these above factors, the transmission of filariasis is also strongly influenced by various socioeconomic and demographic factors that play an important role in the disease transmission (Cano et al. 2014; Upadhyayula et al. 2012a). Hence, to understand the influence of various socioeconomic factors on LF, disease surveillance studies were undertaken in villages in the Chittoor district of Andhra Pradesh, India, and a socioeconomic index was also developed as a marker to help health officials monitor the prevalence of filariasis among the different socioeconomic groups for immediate remedies.
Methods
Study site
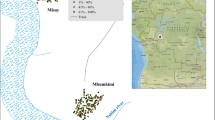
The survey was conducted in selected villages of the Chittoor district of Andhra Pradesh, India (Fig. 1). The Chittoor district (12°37′-14°8′ N & 78°3′-79°55′ E) lies in the Southern part of Andhra Pradesh along the banks of the Ponnai River. It is bounded on the north by the Anantapur and Cuddapah districts, on the East by the Nellore and Chengai-Anna district of Tamilnadu, on the South by the North Arcot Ambedkar and Dharmapuri districts of Tamilnadu, and on the west by the Kolar District of Karnataka state. Around 30 % of the land in Chittoor is covered by forests. The topography of these selected villages is generally undulating, and the altitude varies between a low of 117 mt and high of 431 mt. The temperature in the western part of the district is relatively lower than in the eastern part of the Chittoor district. This is because of the higher altitude of the western compared to the eastern parts. During the study period, the summer temperatures peak at 46 °C in the eastern parts, whereas in the western parts it ranges from 36° to 39 °C. Similarly, the winter temperatures in the western parts are relatively low, ranging between 13 °C and 16 °C, and in the eastern parts from 17 °C to 19 °C. According to the 2011 census, the total population of the Chittoor district is 4.173 million, of which 2.09 million are male and 2.083 million female. About 29.50 % of the population lives in urban sectors, and 70.50 % of the population lives in rural areas. The population data concerning castes in the Chittoor district show that around 18.8 % and 3.8 % of the population belong to scheduled castes and scheduled tribes, respectively. The average literacy rate in the Chittoor district is 71.53 %, of which 79.83 % males and 63.28 % females are literate. Concerning monthly income (Indian rupees, INR), the basic wage in the Chittoor district is INR/Rs: 2635. Agriculture is the main source of income for the populace residing in rural areas; the majority of the population in this district depends on this activity directly or indirectly to earn their daily income. For about 1.933 million (46.3 %) of the population, the main occupation is as laborers, of which 31.3 % are engaged in agricultural labor and the rest work in various industries and other sectors.
Study design
Chittoor district is considered to be highly endemic for LF. Thirty villages were identified by health authorities and marked as filarial endemic. Both epidemiological and socioeconomic surveys were conducted from 2004 to 2007. The houses in the villages were chosen randomly for the study. Residents of selected households were interviewed using a structured questionnaire, and blood samples were collected at night (because the microfilariae that cause lymphatic filariasis circulate in the peripheral blood at night, and the process of LF is called nocturnal periodicity) by the finger prick method. Similarly, the global position system (GPS) data of each village were collected during the study period.
Ethics statement
The study received ethical clearance from the Ethics Committee of the CSIR-Indian Institute of Chemical Technology, affiliated with the Ministry of Science and Technology, Government of India. The survey was supervised overall by the concerned health officials of Chittoor district, Government of Andhra Pradesh. In addition, all the respondents were informed and gave written consent before beginning the epidemiological survey in the presence of the health officials. Similarly, written consent of the parents/guardians was also obtained for the minors who participated in this survey. All participants in the survey/questionnaire element of the study also provided written consent.
Collection of socioeconomic data
A short questionnaire was designed to collect socioeconomic data to help assess the risk factors for filariasis. The socioeconomic information was collected from respondents who were registered to participate in the epidemiological study. Information on family characteristics with a possible influence on filariasis infection, such as age, gender, occupation, education, income (INR: Indian rupees), house structure, breeding habitats of mosquitoes, drainage system, mosquito avoidance measures, participation in mass drug administration (MDA) programs and awareness of filariasis, was collected by interviewing each head of the family if he/she was available or from another member of the household answering the questionnaire. The questionnaire was composed according to local requirements and appropriateness.
Serological survey
Households of residents who provided socioeconomic data and were selected for interviews were visited at night from 8–11 p.m. Using the finger prick method, 20-μl blood samples were collected on clean, prenumbered slides. Approximately 170 blood smears were collected from each village. The samples were dehemaglobinized, fixed and stained with JSB (Jaswant-Singh-Bhattacherji) stain, then checked under the microscope for microfilaria (MF) of Wuchereria bancrofti.
Development of the socioeconomic index
Principal component analysis (PCA) was done using SPSS, version 15.0, to estimate the socioeconomic index from a combination of household and asset variables (Filmer and Pritchett 2001). The household socioeconomic position is defined as the complex of social and economic factors that influence the position of the individuals and groups within the structure of the society (Lynch and Kaplan 2000). Socioeconomic and disease-related information included occupation, age group, education level, monthly income (INR), house structure, drainage system, mosquito breeding habitats and participation in an MDA program. Data on these variables were collected from the head of the family using a structured questionnaire.
The socioeconomic index was derived after performing the PCA by including all socioeconomic variables. Information on these variables was used to generate eigenvectors (weights) by PCA, using the varimax rotation method. The higher the eigenvector of a variable, the stronger is their association with high socioeconomic status (WHO 2002). The PCA analysis showed that the first three factors [occupation, education and income (INR)] had 61 % cumulative variance (eigene value >1) (Table 2). These weights were used to create a socioeconomic index for the study area. Based on the weights, the data were grouped according to socioeconomic status into three categories, low, medium and high status (Table 4).
Statistical analysis
SPSS version 15.0 was used for statistical analysis. The frequency distribution of different socioeconomic variables was calculated, and the occurrence of filariasis was compared with these variables by chi-square test. The socioeconomic index was derived by PCA. The odds ratio with 95 % CI was calculated for all independent variables (socioeconomic factors) and dependent variables (filariasis prevalence) using the multivariate logistic regression method, and the level of significance was considered 0.05.
Results
Filarial infection was assessed for each respondent by examining the microfilaria (MF) from the collected blood sample using light microscopy. During the survey 5133 blood samples were collected from 931 households, of which 77 (1.52 %) serum samples were found to be positive for microfilaria (MF). Among the (30) surveyed villages, high microfilaria (MF) rates were reported from Thumendula Palem (7 %), Kanipaka Patnam (4 %), Ramapuram (3 %) and Chittapara (3 %). The MF rates of Penumuru (2 %), Pymagam (1.5 %), Mittapalli, Mahasamudram and Chinnakam Palli (1 %) villages were reported, respectively. The remaining villages in the district (Anoop Palli, Chinnareddy Palle, Ekambara Kuppam, Nangamangalam and Poola Kandiga) reported an MF rate <1 %. In some of the villages no MF was recorded. Table 1 summarizes the epidemiological data on filariasis and its prevalence related to various demographic and socioeconomic factors in the Chittoor district of Andhra Pradesh, India. This study showed that higher numbers of filarial cases were mostly found among the middle and high age groups compared to the younger age groups (P < 0.001). More filariasis cases were found among male (52.6 %) than female (47.4 %) respondents; however, the difference was not significant (P = 0.525). When considering the socioeconomic factors, the disease prevalence was highly associated with occupation (P = 0.006), education (P = 0.021), income (INR) (P = <0.001), breeding habitats of mosquitoes (P = <0.001), drainage system (<0.001), mosquito avoidance (P = <0.001) and filariasis awareness (P = 0.002) (Table 1). Although more filariasis cases were found among respondents who lived in tiled houses than in huts and RCCs, the occurrence of LF was not associated with the type of housing structure (P = 0.615).
Socioeconomic index
All socioeconomic variables with relevant contributions were used to generate a combined socioeconomic index using PCA. To asses the number of factors, eigenvalues and scree plots were used (Fig. 2). The first principal component factor showed an eigenvalue of 2.402 with 26.686 % variance, factor 2: 1.86 (20.687 % variance) and factor 3: 1.219 (13.545 % variance) (Table 2). The three factors contribute nearly 61 % of the total variance of the variables included in the PCA. In factor 1, four socioeconomic variables [occupation, education, income (INR) and house structure] were strongly associated with filariasis. However, factor 2 (mosquito avoidance and filariasis awareness) and factor 3 (drainage and participation in an MDA program) (Table 3) were moderately associated with the occurrence of filariasis. Based on the percent rank derived from these values, the socioeconomic status of households was classified into three groups, i.e., low, medium and high, respectively. The socioeconomic status/index shows that there was not much difference in disease prevalence among those with low (1.7), medium (1.7) and high (1.3) socioeconomic status. The disease prevalence among these socioeconomic groups did not show any statistical significance (P = 0.613) (Table 4).
Socioeconomic risk factors for lymphatic filariasis
The socioeconomic factors and prevalence of filariasis were analyzed by bivariate and multivariate logistic regression analysis to determine the association between parasitic infections and the possible role of specific risk factors. In the bivariate analysis, being in the higher age group (≥26 years) was found to be a significant risk factor for filariasis (OR: 2.4, 95 % CI: 1.5–3.9, P = 0.001). Having an income (INR) of rupees <1000 (OR: 2.3, 95 % CI: 1.0–5.1, P = 0.040) or 1000–3000 (OR: 3, 95 % CI: 1.7–5.2, P = 0.001) was reported to be a significant risk factor for filariasis. Breeding habitats such as cesspools (OR: 1.8, 95 % CI: 1.1–3.1, P = 0.025), open drainage (OR: 2, 95 % CI: 1.1–3.7, P = 0.020) and kutcha drainage systems (OR: 3.9, 95 % CI: 2.5–6.1, P = 0.001) were also significant risk factors for filariasis. Similarly, not participating in an MDA program was a risk factor for filariasis (OR: 1.9, 95 % CI: 1.2–3.1, P = 0.006) (Table 5).
To understand the most specific conditions within these socioeconomic factors, multivariate analysis (AOR: adjusted odds ratio) can provide detailed insight into specific conditions or factors that can influence the higher occurrence of filariasis (Table 6). The likelihood (AOR) of individuals who were ≥26 years age old to develop filariasis was 2.4 times higher than that of individuals ≤25 years old. People with incomes of INR <1000 and 1000–3000 had a 4.2 (95 % CI: 1.5–11.8) and 3.8 (95 % CI: 1.9–7.7) times higher risk of filariasis infection, than the INR >3000 group respectively. In addition, residents living near a kutcha drainage system had a 3.5 times greater risk (95 % CI: 1.6–7.5) of filariasis infection than those living near a pucca drainage system. These risk factors were found to be statistically significant (Table 6).
Discussion
A national health policy statute of the Government of India aimed to eliminate filariasis by 2015, according to the National Vector-Borne Disease Control Program (NVBDCP). The Goverment of India has shown that the population covered by an MDA program improved from 73 % in 2004 to 83 % in 2013. LF is reported to be mainly regulated by the type of habitat, sanitation, socioeconomic conditions, and demographic and eco-climatic factors (WHO 2000; Testi and Ivaldi 2009; Ottesen and Molyneux 2006; Ahorlu et al. 1999). Previous studies assessing the association of socioeconomic factors and LF were conducted on small populations (Molyneux 2003). Hence, to understand how various socioeconomic factors influence LF, a detailed study was undertaken in Chittoor district of Andhra Pradesh, and, considering these factors, a socioeconomic index was developed for application in the monitoring of the LF load in endemic areas.
The study results reveal that Chittoor district is highly endemic for filariasis even after four rounds (till 2007) of MDA programs. From 2004 to 2007, the MDA coverage in all districts of Andhra Pradesh was 84.78 %, 84.33 %, 89.66 % and 89.13 %, respectively. Table 1 shows that MF prevalence was higher among males (52.6 %) than females (47.4 %). The higher number of infections in males may be due to more time of exposure to bites, and they have a high probability of attracting more mosquitoes because of the higher relative body heat, more carbon dioxide output and greater surface area (Murty et al. 2010). The males are mainly agricultural workers or laborers, providing a higher chance of human-mosquito contact. It was also observed that the MF rate increased relatively with increasing age. The highest MF rate was observed among those between 41 and 60 years old (39.7 %) followed by the 26–40-year-old age group (32.1 %). This study result is in agreement with our earlier reports on the distribution of MF prevalence among different age groups (Murty et al. 2010; Upadhyayula et al. 2012a). If transmission is interrupted in these age groups by improving socioeconomic conditions and involving them in MDA programs, the disease rate can be reduced substantially.
Income (INR) was found to be a significant risk factor for the occurrence of MF (Table 6). The incidence of MF among the various income groups suggests that most of the respondents were from the INR 1000-3000 group (66.7 %) followed by the INR >3000 group (20.5 %), whereas there were fewer respondents (12.8 %) from the low-income group (INR <1000). Many studies have shown that low-income groups/communities have increased risks of various infectious diseases (Thrane et al. 2005; Azab et al. 2014; Aguiar-Santos et al. 2013). The present study also suggests that more cases were reported from the middle and lower income (INR) groups. People who are infected with filariasis in low-income households had less opportunity to obtain effective treatment from distant clinics, coupled with their living and working conditions, which made hygiene and compliance with treatment regimes more difficult. They were also less protected from social stigma (Perera et al. 2007).
In the study areas, more cases were observed among the agricultural community, which may be due to the nature of jobs in agricultural fields where there is a higher possibility of proliferation of mosquitoes and high probability of human-mosquito contact, which can lead to transmission of microfilaria (Erlanger et al. 2005). The housing structure is an important variable that has a direct influence on the transmission dynamics of vector-borne diseases (Webb 1985; Schofield and White 1984). The present study showed that living in house structures such as tiled houses and huts was a greater risk factor for filariasis than living in an RCC house. Baruah and Rai (2000) also observed that there is a high correlation between the type of house structure and the density of C. quinquefasciatus and transmission of filariasis in Varanasi, India. Higher densities of indoor resting mosquitoes were found in poorly constructed houses, leading to greater potential for filariasis transmission among the population.
Bancroftian filariasis is prevalent in both urban and rural areas in India, and the parasite is mainly transmitted by C. quinquefasciatus (Babu et al. 2009). This study showed that there is a high association between MF prevalence and the type of drainage system (P = 0.001). More cases were observed among respondents who live near a kutcha drainage system compared to those near better drainage systems in the study area (OR: 3.491, 95 % CI: 1.627–7.491, P < 0.001). In the study areas, the drainage systems were sedimented with solid wastes; the sewage disposal system has become transformed into rudimentary cesspits and ditches. This drainage system condition significantly favors the proliferation of C. qninquefaciatus, which leads to transmission of the parasite. In addition, there were several other mosquito -breeding habitats such as cesspools, cesspits and open drainage that have become ideal breeding grounds for this vector to transmit MF.
To control the disease, the most important factor is the practice of personal protection measures against mosquitoes, which have a direct impact on the disease prevalence. In the study villages, most of the population is not implementing personal protection measures (79.9 %). Very few (20.1 %) use mosquito avoidance methods such as bed nets, liquid vaporizers, mosquitocidal coils, etc. These studies also suggested that there is a significant association between not using a mosquito net and the presence of microfilaremia (Bonfim et al. 2009). Apart from this, it is estimated that many respondents did not participate in a regular MDA program (51.1 %), which might have influenced disease transmission in different socioeconomic groups of respondents. A study in Orissa found that 83 % of the population had received the drugs, but only 49.5 % consumed them (Babu and Mishra 2008). Similarly, in the present study, although 48.9 % participated in MDA programs, we do not have a clear figure to estimate how many people actually consumed the given drugs.
Considering the goal of elimination of filariasis as the primary objective, it would be better to understand the existing relationship between the epidemiology of the disease and socioeconomic status of the inhabitants living in the endemic areas. Based on the multivariate analysis among the various socioeconomic factors, variables such as gender, income (INR), house structure, drainage system and mosquito avoidance measures and non-participation in MDA programs are risk factors for the occurrence of disease (Table 6).
Similarly, PCA analysis was used to determine the relationship between socioeconomic inequality and the prevalence of filariasis disease. The results showed that the same percentage of microfilaremia cases were observed among the low (1.7 %), medium (1.7 %) and high (1.3 %) income groups, respectively. Although various socioeconomic parameters regulate the possible occurrence of MF, from this study it was inferred that all of the socioeconomic factors play a major role among the various strata of respondents, which may be because the study area was categorized as endemic for LF. This index will help health officials to pinpoint the exact socioeconomic population for controlling lymphatic filariasis. Apart from this, by increasing the awareness of disease transmission and vector management in targeted socioeconomic groups, effective control can be achieved.
A study from Orissa showed that only 42 % of people had knowledge about filariasis (Rath et al. 2006). Most of lower income (INR) people do not know that filariasis is transmitted by mosquitoes (Babu and Kar 2004). It is well known that filariasis disease is caused by poverty, and it perpetuates the poverty cycle. It is evident that filariasis is closely associated with the economic situation and infrastructure of endemic communities (Haddix and Kestler 2000). This study also showed that most of the population (76.4 %) was unaware of filariasis and its chronic manifestations. These results may be used in the development of community-based health education campaigns and disease awareness programs and encourage the respondents to participate in MDA programs. Implementing protective measures against mosquito bites and improving the socioeconomic situation will help reduce the intensity of lymphatic filariasis.
References
Aguiar-Santos AM, Medeiros Z, Bonfim C, Rocha AC, Brandão E, Miranda T, Oliveira P, Sarinho ES (2013) Epidemiological assessment of neglected diseases in children: lymphatic filariasis and soil-transmitted helminthiasis. J Pediatr (Rio J) 89:250–255
Ahorlu CK, Dunyo SK, Koram KA, Nkrumah FK, Aagaard-Hansen J, Simonsen PE (1999) Lymphatic filariasis related perceptions and practices on the coast of Ghana: implications for prevention and control. Acta Trop 73:251–261
Azab SF, Sherief LM, Saleh SH, Elsaeed WF, Elshafie MA, Abdelsalam SM (2014) Impact of the socioeconomic status on the severity and outcome of community-acquired pneumonia among Egyptian children: a cohort study. Infect Dis Poverty 3:14
Babu BV, Kar SK (2004) Coverage, compliance and some operational issues of mass drug administration during the programme to eliminate lymphatic filariasis in Orissa, India. Trop Med Int Health 9:702–709
Babu BV, Mishra S (2008) Mass drug administration under the programme to eliminate lymphatic filariasis in Orissa, India: a mixed-methods study to identify factors associated with compliance and non-compliance. Trans R Soc Trop Med Hyg 102:1207–1213
Babu BV, Mishra S, Nayak AN (2009) Marriage, sex, and hydrocele: an ethnographic study on the effect of filarial hydrocele on conjugal life and marriage ability from Orissa, India. PLoS Negl Trop Dis 3:e414
Baruah K, Rai RN (2000) The impact of housing structures on filarial infection. Jpn J Infect Dis 53:107–110
Bockarie MJ, Molyneux DH (2009) The end of lymphatic filariasis? BMJ 338:b1686
Bonfim C, Netto MJ, Pedroza D, Portugal JL, Medeiros Z (2009) A socioenvironmental composite index as a tool for identifying urban areas at risk of lymphatic filariasis. Trop Med Int Health 14:877–884
Boyd A, Won KY, McClintock SK, Donovan CV, Laney SJ et al (2010) A community-based study of factors associated with continuing transmission of lymphatic filariasis in Leogane, Haiti. PLoS Negl Trop Dis 4
Cano J, Rebollo MP, Golding N, Pullan RL, Crellen T, Soler A, Kelly-Hope LA, Lindsay S, Hay SI, Bockarie MJ, Brooker SJ (2014) The global distribution and transmission limits of lymphatic filariasis: past and present. Parasit Vector 7:466. doi:10.1186/s13071-014-0466-x
Charu Kohli, Rajesh Kumar, Meena GS, Singh MM, Jyotiranjan Sahoo, Ingle GK (2014) Usage and perceived side effects of personal protective measures against mosquitoes among current users in Delhi. J Parasitol Res 628090
Durrheim DN, Wynd S, Liese B, Gyapong JO (2004) Editorial: Lymphatic filariasis endemicity—an indicator of poverty? Trop Med Int Health 9:843–845
Erlanger TE, Keiser J, Caldas De Castro M, Bos R, Singer BH, Tanner M, Utzinger J (2005) Effect of water resource development and management on lymphatic filariasis, and estimates of populations at risk. Am J Trop Med Hyg 73:523–533
Filmer D, Pritchett LH (2001) Estimating wealth effect without expenditure data or tears: an application to educational enrolments in States of India. Demography 38:115–132
Ghosh S, Samanta A, Kole S (2013) Mass drug administration for elimination of lymphatic filariasis: recent experiences from a district of West Bengal, India. Trop Parasitol 3:67–71
Haddix AC, Kestler A (2000) Lymphatic filariasis: economic aspects of the disease and programmes for its elimination. Trans R Soc Trop Med Hyg 94:592–593
Litt E, Baker MC, Molyneux D (2012) Neglected tropical diseases and mental health: a perspective on comorbidity. Trends Parasitol 28:195–201
Lynch J, Kaplan G (2000) Socioeconomic position. In: Berkman L, Kawachi I (eds) Social epidemiology. Oxford University Press, Oxford, pp 13–35
Michael E, Bundy DAP (1997) Global mapping of lymphatic filariasis. Parasitol Today 13:472–476
Mishra A, Bhadoriya RS (2009) An epidemiological study of filariasis in a village of District Datia, MP. Indian J Community Med 34:202–205
Molyneux DH (2003) Lymphatic filariasis (elephantiasis) elimination: a public health success and development opportunity. Filaria Journal 2:13
Mukhopadhyay AK, Patnaik SK, Satya Babu P, Rao KNMB (2008) Knowledge on lymphatic filariasis and mass drug administration (MDA) programme in filaria endemic districts of Andhra Pradesh, India. J Vector Borne Dis 45:73–75
Murty US, Rao MS, Sriram K, Rao KM (2010) Assessment of microfilaria prevalence in Karimnagar and Chittoor Districts of Andhra Pradesh, India. Asian Pacific J Trop Med 3:647–650
Mwakitalu ME, Malecela MN, Pedersen EM, Mosha FW, Simonsen PE (2013) Urban lymphatic filariasis in the metropolis of Dar es Salaam, Tanzania. Parasit Vector 6:286
NVBDCP (2004) Operational guidelines on elimination of lymphatic filariasis. Directorate of National Vector Borne Disease Control Programme, New Delhi p10
Okon OE, Iboh CI, Opara KN (2010) Bancroftian filariasis among the Mbembe people of Cross River state, Nigeria. J Vector Borne Dis 47:91–96
Ottesen EA, Molyneux D (2006) Lymphatic filariasis: treatment, control and elimination. Adv Parasitol 61:395–441
Ottesen EA, Hooper PJ, Bradley M, Biswas G (2008) The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Negl Trop Dis 2:e317
Perera M, Whitehead M, Molyneux D, Weerasooriya M, Gunatilleke G (2007) Neglected patients with a neglected disease? A qualitative study of lymphatic filariasis. PLoS Negl Trop Dis 1:e128
Ramaiah KD (2013) Population migration: implications for lymphatic filariasis elimination programmes. PLoS Negl Trop Dis 7:e2079
Rath K, Nath N, Shaloumy M, Swain BK, Suchismita M, Babu BV (2006) Knowledge and perceptions about lymphatic filariasis: a study during the programme to eliminate lymphatic filariasis in an urban community of Orissa, India. Trop Biomed 23:156–162
Schofield CJ, White GB (1984) Housing design and domestic vectors of disease. Trans R Soc Trop Med Hyg 78:285–292
Testi A, Ivaldi W (2009) Material versus social deprivation and health: a case study of an urban area. Eur J Health Econ 10:323–328
Thrane N, Sondergaard C, Schonheyder HC, Sørensen HT (2005) Socioeconomic factors and risk of hospitalization with infectious diseases in 0- to 2-year-old Danish children. Eur J Epidemiol 20:467–474
Upadhyayula SM, Mutheneni SR, Kadiri MR, Kumaraswamy S, Nagalla B (2012a) A cohort study of lymphatic filariasis on socio economic conditions in Andhra Pradesh, India. PLoS ONE 7:e33779
Upadhyayula SM, Mutheneni SR, Kumaraswamy S, Kadiri MR, Pabbisetty SK, Yellepeddi VS (2012b) Filaria monitoring visualization system: a geographical information system–based application to manage lymphatic filariasis in Andhra Pradesh India. Vector-Borne Zoonot Dis 12:418–427
Webb DJ (1985) Low-cost housing and parasite vectors. Parasitol Today 1:65–66
WHO (2000) Eliminate filariasis: attack poverty. proceedings of the first meeting of the global alliance to eliminate lymphatic filariasis. WHO, Geneva
WHO (2002) Lymphatic filariasis: the disease and its control. fifth report of the WHO expert committee on filariasis. WHO, Geneva
WHO (2009) Global programme to eliminate lymphatic filariasis. Wkly Epidemiol Rec 84:437–444
WHO (World Health Organisation) (1992) Lymphatic filariasis: the disease and its control. fifth report of the WHO Expert Committee on filariasis. World Health Organ Tech Rep Ser 821:1–71
Acknowledgments
We thank the Director, Council of Scientific and Industrial Research, Indian Institute of Chemical Technology, for her encouragement and support. Thanks go to the Ministry of Communication and Information Technology (MC&IT), Government of India, for funding the project.
Conflict of interest
The authors declare that they have no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mutheneni, S.R., Upadhyayula, S.M., Kumaraswamy, S. et al. Impact of socioeconomic factors on the prevalence of lymphatic filariasis in Andhra Pradesh, India. J Public Health 23, 231–240 (2015). https://doi.org/10.1007/s10389-015-0673-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-015-0673-8