Abstract
Purpose
We introduce selective internal limiting membrane (ILM) peeling, a guideline procedure to determine whether to remove the ILM during vitrectomy for rhegmatogenous retinal detachment (RRD).
Study design
Retrospective case series
Methods
Patients who underwent pars plana vitrectomy for RRD and were followed up for 12 months or longer were included. When vitreous cortex remnants (VCRs) were detected with triamcinolone acetonide, the ILM was removed; otherwise, the ILM was preserved (“selective ILM peeling”). The factors associated with the presence of VCRs and incidence of secondary epiretinal membrane (ERM) were analyzed.
Results
VCRs were detected in 87 of 133 eyes (65.4%) in which the ILM was removed. Younger age, better preoperative visual acuity, and vitreous hemorrhage were negatively correlated with the presence of VCRs. No ERM occurred in the eyes after ILM peeling. Among the eyes with ILM preservation, subclinical ERM was noticed in 4 eyes (8.7%), and 1 eye (2.1%) required additional surgery owing to ERM. ERM occurred more commonly in eyes with the ILM preserved (P = .004). However, no differences in the rate of additional surgeries were found between the 2 groups.
Conclusion
Selective ILM peeling offers an alternative option to reduce the burden of ILM peeling or additional surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Secondary epiretinal membrane (ERM) often occurs after vitrectomy for rhegmatogenous retinal detachment (RRD). Fast-progressing secondary ERM after vitrectomy for RRD, or macular pucker [1], may cause metamorphopsia or disturb visual recovery. Macular pucker is a known type of proliferative vitreoretinopathy (PVR), of which the main pathologic component is thought to be retinal pigment epithelium dispersed through retinal breaks that settles on the macular surface [2] and subsequently transdifferentiates into myofibroblasts [3].
The outermost lamella of the vitreous cortex, posterior to the liquefied vitreous, may remain on the macular surface when posterior vitreous detachment (PVD) occurs, a condition known as vitreous cortex remnants (VCRs) [4]. VCRs can act as a scaffold for myofibroblast proliferation in the process of ERM formation [5]. VCRs can be detected intraoperatively with biostaining with triamcinolone acetonide (TA) [6, 7].
The internal limiting membrane (ILM) is the outermost layer of the retina, and removal of the ILM around the macular area is a common procedure in macular surgery. ILM removal is the best way to prevent secondary ERM formation, as it can guarantee complete removal of the VCRs and the ILM itself, which act as a scaffold for ERM formation [8]. A series of studies showed that ILM removal during vitrectomy for RRD repair significantly lowers the incidence of secondary ERM formation [9,10,11]. However, many surgeons think that ILM removal is not essential unless preoperative ERM is distinct, given that ILM removal in a detached retina is difficult and time-consuming, especially for inexperienced surgeons [12]. Additionally, ILM removal may cause structural deformity of the macula [13], and severe ERM requiring additional surgery does not commonly occur even if the ILM is not removed [14, 15]. Whether prophylactic ILM removal improves visual outcomes after RRD repair remains to be established [9,10,11]. Thus, the necessity of ILM removal is still a matter of debate.
We expected that if ILM removal were performed only in eyes with intraoperatively detected VCRs, it would reduce the burden of the procedure, as well as that of the secondary surgery related to postoperative ERMs. We refer to this procedure as “selective ILM peeling.” Here, we attempted to determine the factors related to VCRs among RRD patients and analyzed the incidence of secondary ERM after selective ILM peeling.
Methods
Ethical approval and patient selection
This retrospective, interventional, comparative study adhered to all the tenets of the Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Board of Pusan National University Hospital (approval no: 2112-003-109) before the study was started.
Patients who underwent pars plana vitrectomy for RRD sometime between January 2017 and July 2020 and who were followed up for 12 months or more were included. Eyes with proliferative diabetic retinopathy, tractional retinal detachment, diabetic macular edema, retinal detachment (RD) with macular hole, uveitis, retinal vascular occlusions, or age-related macular degeneration were excluded. Myopic eyes with vision-threatening macular disease such as lacquer cracks, myopic choroidal neovascularization, Fuchs spot, myopic retinoschisis, macular hole, and macular atrophy were also excluded. Those with a history of vitrectomy or penetrating trauma were also excluded. Eyes with PVR of more than grade B, PVR involving the macula, or ERM noted preoperatively and intraoperatively were excluded. The first eye was included if the patient had undergone vitrectomy for RRD in both eyes. Eyes requiring additional surgery owing to redetachment were excluded.
All the patients were diagnosed with RRD and underwent comprehensive ophthalmologic examinations, including best-corrected visual acuity (BCVA) assessment, slit-lamp examination, tonometry, fundus examination, fundus photography (Optos Ultra-widefield Retinal Imaging; Optos), and optical coherence tomography (OCT; Triton, Topcon Medical Systems) at baseline and at 1, 3, 6, and 12 months postoperatively. BCVA was measured with a Snellen chart and converted to logarithm of the minimal angle of resolution (logMAR) units for statistical analysis.
Demographic data, duration of symptoms, comorbidities (diabetes, hypertension), ocular characteristics (BCVA, axial length, previous cataract surgery, previous scleral buckling, previous retinal photocoagulation, vitreous hemorrhage, or choroidal detachment), RD status (extent of RD, foveal status, presence of giant retinal tear, size, location, and number of retinal breaks, and presence of lattice degenerations), and the surgical method (concurrent scleral buckling, concurrent cataract surgery, ILM peeling, 360° barrier laser photocoagulation, or intravitreal tamponade with air or silicone oil) were retrieved from the medical records.
Surgical method
Vitrectomy was performed in all eyes with the Constellation Vitrectomy System (Alcon Laboratories) and a noncontact viewing system (Resight 700; Carl Zeiss Meditec). Cataract surgery was performed at the surgeon’s discretion through a 2.8-mm superior clear corneal incision.
We determined the presence of VCRs under a wide-field lens. After core vitrectomy, 0.2 mL of TA (40 mg/mL; Dong Kwang Pharmaceutical) was applied to the vitreous cavity. It was then washed out by means of gentle flushing with a backflush needle. When a TA particle adhered to the macula, we defined it as the presence of VCRs. If the TA was just sedimented on the ILM, it was readily washed out from the macula. When some of the TA remained on the surface of the macula, it was considered an indication of the presence of VCRs and the ILM was removed within a 4 disc-diameter of the foveal center and stained with 0.025% Brilliant Blue G solution (Sigma-Aldrich); these eyes were assigned to the VCR group (ILM peeled). If TA did not remain on the macula after washing out, the ILM was preserved; these eyes were assigned to the no-VCR group (ILM preserved) [6] (Fig. 1). Peripheral vitreous was removed and the vitreous base was shaved as much as possible. Perfluorocarbon liquid (PFCL) was routinely applied to drain the subretinal fluid. Fluid-air exchange was performed. Prophylactic 360° barrier photocoagulation was done at the surgeon’s discretion. An air tamponade was routinely used as a temporary tamponade; a long-acting gas was not used, in line with our previous reports [16]. Silicone oil (1300 centistokes) was applied as the tamponade at the surgeon’s discretion, on the basis of the risk assessment for postoperative PVR. All surgeries were performed by a single surgeon (S.W.P.).
Method to detect vitreous cortex remnants (VCRs) on the macula during vitrectomy. a and b After core vitrectomy, 0.2 mL of triamcinolone acetonide (TA) is applied into the vitreous cavity. c and d The TA is then washed out by use of a backflush needle. e When some of the TA remains on the macular surface, it is considered to be VCR. f When the TA seems not to remain on the macula, it is considered as no VCR
Outcome measures and statistical analysis
To find factors associated with VCRs, univariate logistic regression was performed between the presence of VCRs and variables including age, preoperative BCVA, duration of symptoms, age, sex assigned at birth, comorbidities, axial length, lens status, previous retinal photocoagulation, vitreous hemorrhage, choroidal detachment, area of RD, whether to invade the foveal center, size of retinal tears, number of retinal tears, and presence of lattice degeneration. Variables with probability values below .20 in the univariate analysis were entered into the multivariate logistic regression analysis to calculate the odds ratios (ORs) for significant parameters. IBM SPSS software (version 22.0; IBM) was used for all statistical analyses. In all analyses, significance was set at P <.50.
The incidence of postoperative ERM was analyzed until 12 months after the vitrectomy. The ERM was diagnosed through fundus photography and OCT. It was graded according to the Gass classification [17] as follows: grade 0 = early, translucent form of the ERM without distortion of the inner retina; grade 1 = intermediate, translucent form of the ERM with mild distortion of the inner retina; grade 2 = late opaque form of the ERM with severe distortion of the inner retina, which was suspected of causing metamorphopsia and decreased vision and ultimately needed secondary vitrectomy for ERM removal. The incidences of ERMs for each grade were compared between the VCR group and the no-VCR group at 12 months after vitrectomy. The central macular thickness (CMT) in the 2 groups was compared at postoperative 12 months.
Descriptive statistics, ie, means and standard deviations (SDs), were calculated for the continuous variables. The Kolmogorov-Smirnov test and Shapiro-Wilk test were performed to test for normal distribution of the variables. The Levene test was used to evaluate variations. Differences in means and distributions of variables between the groups were analyzed by use of the independent t test and the Mann-Whitney U test for continuous variables and the Fisher exact test for nonnumeric variables. The changes in BCVA before and after the surgery were evaluated by use of the Wilcoxon signed rank test.
Results
From a total of 175 eyes that underwent primary vitrectomy for RRD, 42 (24%) were excluded: 21 eyes had either preoperative ERM or advanced PVR (>grade B), 14 were not followed up until 12 months, 4 were second eyes of bilateral RRD cases, and 3 eyes required additional surgery owing to redetachment. Thus, 133 eyes of 133 patients were included. The mean age of the 133 patients was 54.6 ± 12.9 years (range: 12–80 years) and 80 patients (60.2%) were male. Vitreous cortex remnants were noticed during vitrectomy in 87 eyes (65.4%) of the VCR group in which the ILM was removed. The ILM was preserved in the other 46 eyes (34.6%), which were assigned to the no-VCR group in which the ILM was preserved.
During vitrectomy for RRD, scleral buckling was concurrently performed in 27 eyes (16.3%) and combined cataract surgery was done in 114 eyes (68.7%) (20 eyes underwent concurrent buckling, cataract surgery, or vitrectomy). Prophylactic 360° barrier photocoagulation was performed in 125 eyes (75.3%) during vitrectomy. Tamponade was done with air in 143 eyes (86.1%) and with silicone oil (1000 centistokes) in 21 eyes (12.7%).
The mean preoperative and postoperative BCVAs were better in the no-VCR group than in the VCR group (preoperative BCVA: 1.045 ± 0.820 vs 0.772 ± 0.845 [P = .043]; postoperative BCVA: 0.311 ± 0.473 vs 0.228 ± 0.698 [P = .016]). The patients in the VCR group (57.41 ± 10.79 years, range: 25–80 years) were older than those in the no-VCR group (49.26 ± 14.86 years, range: 12–74 years) (P = .002).
The results of the univariate and multivariate logistic regression analyses for the detection of VCRs are listed in Table 1. A 1-year increase in age was related to an approximately 5% increase in the OR (OR = 1.05, 95% CI = 1.02–1.09, P = .001). Axial length >26.0 mm (OR = 0.31, 95% CI = 0.14–0.68, P = .003), previous scleral buckling (OR = 0.27, 95% CI = 0.07–0.97, P = .045) and vitreous hemorrhage (OR = 0.27, 95% CI = 0.09–0.79, P = .017) were negatively correlated with VCR detection. An increase in BCVA of 1.0 in logMAR (ie, worse BCVA) was related to an approximately 1.5-fold increase in the incidence of VCRs, although the significance was borderline in the univariate analysis (OR = 1.52, 95% CI = 0.96–2.41, P = .075). The ORs of the significant variables in the multivariate logistic analysis are listed in Table 1. An increase in BCVA of 1.0 in logMAR (ie, worse BCVA) was related to a 2-fold increase in the incidence of VCRs (OR = 2.00, 95% CI = 1.17–3.41, P = .011). A 1-year increase in age was related to a 6% increase in the odds of VCRs (OR = 1.06, 95% CI = 1.03–1.09, P <.001). The presence of vitreous hemorrhage with RRD was associated with a reduction in the odds of VCR detection of approximately 83% (OR = 0.17, 95% CI = 0.05–0.59, P = .005).
VCRs were noticed during vitrectomy in 87 eyes (65.4%), in which the ILM was removed. The ILM was preserved in the other 46 eyes (34.6%) in which VCRs were not detected. The mean postoperative CMT (μm) was 275.13 ± 41.58 in the VCR group and 272.65 ± 31.62 in the no-VCR group; the difference was not significant (P = .725). Among 46 eyes, grade 0–2 ERMs were noticed in 3 eyes (6.5%), 1 eye (0.75%), and 1 eye (0.75%) in the no-VCR group. The eye with grade-2 ERM in the no-VCR group underwent additional vitrectomy. On the other hand, among 87 eyes, none showed postoperative ERM in the VCR group until 12 months. No significant difference was found between the 2 groups in terms of the incidence of grade-2 ERM (requiring additional surgery) (0% vs 0.75%, P = .346), but a significant difference in the incidence of ERMs was found (0% vs 10.9%, P = .004)
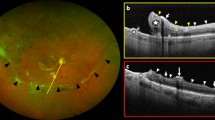
Figure 2 illustrates a case of secondary ERM in a 57-year-old woman who needed additional surgery. Figure 3 shows the OCT findings of 5 eyes with postoperative ERM.
a Wide fundus photograph of the right eye of a 57-year-old woman with rhegmatogenous retinal detachment (RRD) caused by multiple atrophic holes in the lattice degeneration at the superotemporal retina. b Optical coherence tomography (OCT) of the right eye with RRD shows a normal foveal contour and attachment. c Wide fundus photography at postoperative 3 months shows the reattached retina, but with macular pucker. d OCT shows a thick epiretinal membrane (ERM) with thickening and deformation of the sensory retina at 3 months postoperatively. The best-corrected visual acuity (BCVA) was 20/100. e Wide fundus photography at 9 months after vitrectomy for peeling of the ERM and internal limiting membrane. f OCT with no ERM at 9 months after ERM peeling when the BCVA was 20/20
Discussion
In the present study of 133 eyes, whether to remove the ILM was decided on the basis of the intraoperative detection of VCRs, guided by a TA bio-stain. We named this method “selective ILM peeling.” The ILM was removed in 87 eyes (65.4%) in which VCR was detected, whereas the ILM was preserved in the other 46 eyes (34.6%). Older age and lower preoperative visual acuity were associated with VCRs, and vitreous hemorrhage was negatively correlated with VCRs. Secondary ERM did not occur within 12 months in the ILM-peeled eyes. On the other hand, in the ILM-preserved eyes (no-VCR), the incidence of ERM was 11.0% (5 eyes) under the guidance of selective ILM peeling, and the incidence of severe ERM requiring an additional surgery was 2.2% (only 1 eye). When compared with the ILM-peeled eyes, ERM occurred more frequently (P = .004), but no significant difference was found in the incidence of severe ERM requiring an additional surgery (P = .346).
In previous studies, the incidence of postoperative ERMs after vitrectomy for RRD that needed a secondary vitrectomy varied widely, from 2.8% to 33.3% [18,19,20]. In 2009, Aras and colleagues [9] proposed ILM peeling for RRD to prevent secondary ERM; since then, many studies have proven the effectiveness of this approach. A meta-analysis of 9 studies showed that the incidence of secondary ERM was 3% in ILM-peeled eyes, which was lower than the rate of 29% in the other eyes [21]. In the present study, the incidence of secondary ERM was 11.0% in eyes in which the ILM was preserved under the guidance of selective ILM peeling, thus lower than the incidence of 29% in the meta-analysis. This implies that selective ILM peeling is a reasonable guidance to reduce the incidence of secondary ERM.
That VCRs are highly associated with postoperative ERM is not disputed; however, how to detect or manage VCRs remains a matter of debate. Kato and colleagues [22] showed that the method used to detect VCRs affected the detection rate: the incidence of VCRs was 15.4% with a wide-field lens, but 41.5% with a floating lens during vitrectomy for RRD. Cho and colleagues [23] classified VCRs into fragmented and membranous types (41.03% vs 23.81%). We determined the presence of VCRs under a wide-field lens and differentiated sedimented TA from TA adhered to VCRs by flushing it with a backflush needle. TA entangled with the cortical vitreous moved but did not go away during the washout. The best way for detecting or defining VCRs has yet to be established. More studies are needed to derive a standard detection method.
In the present study, VCRs were found in 65.4% of the patients, which is comparable to the rate of 41.5–75% reported previously [20, 23,24,25]. Unlike in the present study, in which the ILM was removed in cases with VCR detection, Cho and colleagues [23] attempted to remove the VCRs without ILM peeling in such cases and reported postoperative ERM in 35% of eyes with VCRs and in 15% of eyes without VCRs [23]. Although the incidence of postoperative ERM in the eyes without VCRs in their study was similar to that in ours (15% vs 11%), there was a considerable difference in that of the eyes with VCRs (35% vs 0%). An indirect comparison of these results indicated that ILM removal is better, as compared with VCR removal, in terms of the incidence of secondary ERMs. However, large prospective studies are required for further clarification.
In this study, the mean preoperative and postoperative BCVAs of the no-VCR group were better than those of the VCR group. We do not think the VCRs themselves influence preoperative visual acuity, given that VCRs are translucent and not thick. We rather theorize that VCRs were likely formed in eyes with worse preoperative visual acuity. Visual acuity can be affected by multiple factors such as stage of macular detachment, age, duration of retinal detachment, or vitreous hemorrhage, thus making it hard to analyze which one was a major factor in the formation of VCRs. We think poor postoperative visual acuity resulted from a poor preoperative visual acuity. In the multivariate analysis, older age and worse preoperative visual acuity were positively correlated with VCR detection. A 1-year increase in age was associated with a 6% greater risk of VCRs (OR = 1.06, 95% CI = 1.03–1.09, P <.001), which may be related to the higher prevalence of vitreoschisis in ageing eyes [26]. Vitreous hemorrhage had a negative correlation with the detection of VCRs (OR = 0.17, 95% CI = 0.05–0.59, P = .005). We explain this correlation as follows: First, eyes without VCRs may have stronger vitreous traction than do eyes with VCRs, thus making vitreous hemorrhage more likely. Second, the vitreous hemorrhage that occurs at the time of PVD development contains iron, which makes hyaluronic acid depolymerized [27, 28], which can facilitate degradation of VCRs on the macula. To the best of our knowledge, a study by Assi and colleagues [29] has been the only one to analyze the factors associated with VCRs. Their findings regarding ageing were similar to our findings; however, they did not analyze vitreous hemorrhage or preoperative visual acuity.
ILM peeling did not have a beneficial effect on anatomic and functional outcomes in macula-off RRD with preoperative PVR in a large-sized, multicenter study performed in Japan [30]. Our study differs in that we evaluated the incidence of postoperative ERM and excluded every patient who already had ERMs or advanced PVR before surgery. Some other studies did not exclude such patients [14, 18, 19, 31]. In addition, our study differs from those of previous studies [20, 23, 24] in terms of the method for detecting VCRs. Our study has limitations in that it was retrospective and recruited only a small number of patients from a single hospital. Further prospective studies including more patients with longer follow-up are warranted to validate this study’s results.
In conclusion, VCRs were detected in 65.4% of eyes during vitrectomy for RRD. Younger age, better preoperative visual acuity, and vitreous hemorrhage were negatively related to VCRs. ILM peeling was done only in eyes with VCRs, using selective ILM peeling. Only 1 eye (0.8%) required secondary surgery for secondary ERM. Selective ILM peeling could be an alternative option to reduce the burden of ILM peeling or additional surgery.
References
Lobes LA Jr, Burton TC. The incidence of macular pucker after retinal detachment surgery. Am J Ophthalmol. 1978;85:72–7.
Roth AM, Foos RY. Surface wrinkling retinopathy in eyes enucleated at autopsy. Trans Am Acad Ophthalmol Otolaryngol. 1971;75:1047–58.
Guenther SR, Schumann RG, Hagenau F, Wolf A, Priglinger SG, Vogt D. Comparison of surgically excised premacular membranes in eyes with macular pucker and proliferative vitreoretinopathy. Curr Eye Res. 2019;44:341–9.
Wakabayashi T, Mahmoudzadeh R, Salabati M, Garg SJ, Ho AC, Spirn MJ. Utility of removal of vitreous cortex remnants during vitrectomy for primary rhegmatogenous retinal detachment repair. Curr Eye Res. 2022;47:1444–9.
van Overdam KA, Busch EM, Verdijk RM, Pennekamp CWA. The role of vitreous cortex remnants in proliferative vitreoretinopathy formation demonstrated by histopathology: a case report. Am J Ophthalmol Case Rep. 2021;24: 101219.
Lee JE, Byon IS, Park SW. Internal limiting membrane surgery. Singapore: Springer; 2021.
Sakamoto T, Ishibashi T. Visualizing vitreous in vitrectomy by triamcinolone. Graefes Arch Clin Exp Ophthalmol. 2009;247:1153–63.
Kwok A, Lai TY, Yuen KS. Epiretinal membrane surgery with or without internal limiting membrane peeling. Clin Exp Ophthalmol. 2005;33:379–85.
Aras C, Arici C, Akar S, Muftuoglu G, Yolar M, Arvas S, et al. Peeling of internal limiting membrane during vitrectomy for complicated retinal detachment prevents epimacular membrane formation. Graefes Arch Clin Exp Ophthalmol. 2009;247:619–23.
Nam KY, Kim JY. Effect of internal limiting membrane peeling on the development of epiretinal membrane after pars plana vitrectomy for primary rhegmatogenous retinal detachment. Retina. 2015;35:880–5.
Rao RC, Blinder KJ, Smith BT, Shah GK. Internal limiting membrane peeling for primary rhegmatogenous retinal detachment repair. Ophthalmology. 2013;120:1102-3.e1-2.
Forlini M, Date P, Ferrari LM, Lorusso M, Lecce G, Verdina T, et al. Comparative analysis of retinal reattachment surgery with or without internal limiting membrane peeling to prevent postoperative macular pucker. Retina. 2018;38:1770–6.
Hisatomi T, Tachibana T, Notomi S, Koyanagi Y, Murakami Y, Takeda A, et al. Internal limiting membrane peeling-dependent retinal structural changes after vitrectomy in rhegmatogenous retinal detachment. Retina. 2018;38:471–9.
Heo MS, Kim HW, Lee JE, Lee SJ, Yun IH. The clinical features of macular pucker formation after pars plana vitrectomy for primary rhegmatogenous retinal detachment repair. Korean J Ophthalmol. 2012;26:355–61.
Szigiato A-A, Antaki F, Javidi S, Touma S, Duval R, Cordahi G, et al. Risk factors for epiretinal membrane formation and peeling following pars plana vitrectomy for primary rhegmatogenous retinal detachment, an OCT guided analysis. Int J Retina Vitreous. 2022;8:70.
Pak KY, Lee SJ, Kwon HJ, Park SW, Byon IS, Lee JE. Exclusive use of air as gas tamponade in rhegmatogenous retinal detachment. J Ophthalmol. 2017;2017:1341948.
Gass JDM. Stereoscopic atlas of macular diseases: diagnosis and treatment. 3rd ed. USA: Mosby; 1997.
Ishida Y, Iwama Y, Nakashima H, Ikeda T, Emi K. Risk factors, onset, and progression of epiretinal membrane after 25-gauge pars plana vitrectomy for rhegmatogenous retinal detachment. Ophthalmol Retina. 2020;4:284–8.
Katira RC, Zamani M, Berinstein DM, Garfinkel RA. Incidence and characteristics of macular pucker formation after primary retinal detachment repair by pars plana vitrectomy alone. Retina. 2008;28:744–8.
Kishi S, Demaria C, Shimizu K. Vitreous cortex remnants at the fovea after spontaneous vitreous detachment. Int Ophthalmol. 1986;9:253–60.
Fallico M, Russo A, Longo A, Pulvirenti A, Avitabile T, Bonfiglio V, et al. Internal limiting membrane peeling versus no peeling during primary vitrectomy for rhegmatogenous retinal detachment: a systematic review and meta-analysis. PLoS ONE. 2018;13: e0201010.
Kato Y, Inoue M, Hirakata A. Effect of foveal vitreous cortex removal to prevent epiretinal membrane after vitrectomy for rhegmatogenous retinal detachment. Ophthalmol Retina. 2021;5:420–8.
Cho EH, Ku HC, Il W, Lee EK. Residual vitreous cortex at the fovea during vitrectomy for primary rhegmatogenous retinal detachment repair. Retina. 2018;38:1549–55.
Chen TY, Yang CM, Liu KR. Intravitreal triamcinolone staining observation of residual undetached cortical vitreous after posterior vitreous detachment. Eye (Lond). 2006;20:423–7.
Sonoda KH, Sakamoto T, Enaida H, Miyazaki M, Noda Y, Nakamura T, et al. Residual vitreous cortex after surgical posterior vitreous separation visualized by intravitreous triamcinolone acetonide. Ophthalmology. 2004;111:226–30.
van Overdam KA, van Etten PG, Accou G, Wubbels RJ, van Meurs JC, Verhoekx JSN. Prevalence of vitreoschisis-induced vitreous cortex remnants over the peripheral retinal surface in eyes undergoing vitrectomy for primary rhegmatogenous retinal detachment. Acta Ophthalmol. 2024;102:99–106.
Jena S, Tripathy K. Vitreous hemorrhage. StatPearls Publishing; 2022.
Squire C, McEwen WK. The effect of iron compounds on rabbit vitreous. Am J Ophthalmol. 1958;46:356–8.
Assi A, Khoueir Z. Prevalence of vitreous cortex remnants in eyes with primary rhegmatogenous retinal detachment undergoing vitrectomy. Retina. 2021;41:1403–6.
Obata S, Sawada O, Kakinoki M, Matsumoto R, Saishin Y, Ohji M. Effects of internal limiting membrane peeling on anatomical and functional outcomes in macula-off rhegmatogenous retinal detachment complicated by proliferative vitreoretinopathy: Japan-retinal detachment registry. Jpn J Ophthalmol. 2023;67:417–23.
Martinez-Castillo V, Boixadera A, Distefano L, Zapata M, Garcia-Arumi J. Epiretinal membrane after pars plana vitrectomy for primary pseudophakic or aphakic rhegmatogenous retinal detachment: incidence and outcomes. Retina. 2012;32:1350–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E. Kim, None; Y. Choi, None; I. Byon, Grants or contracts (Novartis), Consulting fees (Bayer, Novartis), Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Alcon, Samil, Taejun); J. E. Lee, Consulting fees (Bayer, Novartis, Samsung Bioepis), Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Bayer, Novartis, Abbvie); S. W. Park, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Sung Who Park
About this article
Cite this article
Kim, E., Choi, Y., Byon, I. et al. Selective internal limiting membrane peeling for prevention of secondary epiretinal membrane after vitrectomy for rhegmatogenous retinal detachment. Jpn J Ophthalmol 68, 216–224 (2024). https://doi.org/10.1007/s10384-024-01056-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-024-01056-4