Abstract
Purpose
Vernal keratoconjunctivitis (VKC) is a severe and recurrent allergic conjunctivitis, the mechanism of which is not well understood. In this study, we investigated the role of oncostatin M (OSM) in the pathogenesis of VKC, with a focus on tissue remodeling.
Study design
Clinical and experimental.
Patients and methods
The OSM concentrations in tear fluid samples obtained from VKC patients and healthy controls were measured using ELISA, and the expression of OSM mRNA and protein in giant papillae resected from VKC patients was investigated using RT-PCR and immunohistochemistry, respectively. In cultured human conjunctival epithelial cells (HconEpiCs), expression of OSM receptor β (OSMRβ) was detected using immunocytochemical and FACS analyses. Finally, we investigated whether recombinant OSM activated STAT1 and STAT3 to induce the expression of various genes related to tissue remodeling in HconEpiCs, by using Western blot analysis, microarray analysis, and RT-PCR.
Results
The OSM concentration was higher in the tear fluid of VKC patients than in that of the healthy controls, and strong expression of OSM mRNA was found in the giant papillae. We also detected T cells expressing OSM in the giant papillae. In addition, HconEpiCs showed surface expression of OSMRβ. Recombinant human OSM strongly activated both STAT1 and STAT3 in HconEpiCs and induced various tissue remodeling-related genes, including MMP-1, MMP-3, IL-24, IL-20, serpinB3, S100A7, tenascin C, and SOCS3.
Conclusion
Our results suggest that OSM is one of the key molecules involved in remodeling of giant papillae in VKC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vernal keratoconjunctivitis (VKC) is characterized by chronic allergic inflammation of the conjunctiva associated with tissue remodeling, including the formation of giant papillae and fibrosis. Histopathologic examination has revealed that the conjunctiva is infiltrated by eosinophils, T cells, B cells, mast cells, macrophages, basophils, plasma cells, and dendritic cells [1]. These various cells produce many kinds of cytokines and chemokines, but which cytokines have important roles in tissue remodeling is not well understood. In this study, we focused on oncostatin M (OSM), a member of the IL-6 family. Human OSM signaling is mediated via 2 receptors: the type I receptor is a heterodimer of leukemia inhibitory factor receptor and gp130, whilst the type II receptor is a heterodimer of OSM receptor beta (OSMRβ) and gp130 [2]. Gp130 is a receptor ubiquitously expressed by human cells. When inflammation occurs, OSM is released by monocytes/macrophages, dendritic cells, T cells, and neutrophils, after which it binds to the above-mentioned receptors. It has been reported that OSM signaling is mainly mediated via the JAK/STAT pathway [2,3,4,5]. OSM is involved in various physiologic and pathologic processes, including hematopoiesis, mesenchymal stem cell differentiation, liver regeneration, cardiac remodeling, nociception, inflammation, and metabolism [1]. There have been previous reports of increased OSM levels in sinus tissue from patients with allergic rhinitis, in psoriatic skin, and in sputum from asthma patients [6,7,8]. Moreover, intratracheal administration of adenoviral OSM to mice was shown to induce a type 2 immune response [4]. OSM also displays profibrotic activity in various tissue and disease models [9, 10]. For example, OSM stimulates the accumulation of extracellular matrix (ECM) in a transgenic mouse model of connective tissue disease [11]. In addition, overexpression of OSM induces matrix deposition, activation of STAT3, and dysregulation of SMAD1 in the lungs of fibrosis-resistant BALB/c mice [12]. OSM may be involved in fibrotic diseases of the lung, liver, heart, vessels, kidney, pancreas, and skin, suggesting that it is a proinflammatory cytokine with a crucial role in the pathogenesis of various fibrotic diseases. Moreover, OSM has been reported to be a potent mediator of pulmonary inflammation and ECM accumulation. However, involvement of OSM in inflammation and fibrosis may vary, depending on the disease pathology and cellular microenvironment. For example, OSM inhibits TGF-β1-induced expression of extracellular matrix proteins in human proximal renal tubule cells [13]. Abe and associates demonstrated that OSM directly inhibits TGF-β1-mediated activation of cardiac fibroblasts via extracellular signal-regulated kinase (ERK)1/2-dependent phosphorylation of the SMAD linker region [14]. In addition, Huguier and associates revealed that OSM inhibits TGF-β1-induced secretion of extracellular matrix components by normal and pathologic skin fibroblasts [15]. Therefore, OSM may not only induce fibrosis, but also inhibit tissue fibrosis mediated via TGF-β1. In this study, we discovered that OSM was highly expressed in both the tear fluid and the giant papillae of VKC patients and also showed that OSM potentially activated tissue remodeling in human conjunctiva via JAK/STAT signaling, suggesting that OSM plays an important role in recurrent and severe allergic conjunctivitis such as VKC.
Patients and methods
Human samples
Tear fluid samples were obtained from 7 VKC patients and 6 controls (healthy volunteers) by using Microcaps (Drummond Scientific Company). Giant papillae were resected for therapeutic purposes from 4 VKC patients and control conjunctival tissue was biopsied from 8 conjunctivochalasis patients during resection surgery after obtaining their written informed consent, as described previously [16]. Because control specimens were obtained during the conjunctivochalasis surgery, these samples were bulbar conjunctival samples. Thus, a fundamental anatomic difference between the palpebral and bulbar conjunctiva underlies the methodology of this evaluation. Giant papillae are extremely rarely found in the bulbar conjunctiva. All procedures were approved by the ethics committees of Juntendo University School of Medicine and Kyoto Prefectural University of Medicine, and the study was conducted in accordance with the tenets of the Declaration of Helsinki.
This study was approved as an observational study, not a specified clinical trial, by the institutional review board in August 2018. The study period was 2 years (2018–2020).
Culture of human conjunctival epithelial cells
Human conjunctival epithelial cells (HConEpiCs) [17,18,19] were generously gifted from Dr Satoshi Kawasaki (Department of Ophthalmology, Osaka University, Japan.) Frozen cells were warmed in a 37 °C water bath, and the thawed cell pellet was washed in 5 mL medium and centrifuged at 1200 rpm for 3 min at room temperature. Then HConEpiCs were gently resuspended in approximately 10 mL of Defined Keratinocyte-SFM medium (Thermo Fisher Scientific) with growth supplements that eliminate the requirement for bovine pituitary extract. The medium was changed every 2–3 days. After the cells reached 75–90% confluence, subculture was performed.
Measurement of OSM in tear fluid by use of enzyme-linked immunosorbent assay (ELISA)
The tear samples were diluted at 1:50 using double distilled water (DDW) and subjected to measurement of the OSM concentration by use of an ELISA kit (Abcam) according to the manufacturer’s instructions. The detection limit of OSM was about 15.6 pg/mL, on the basis of our standard curves.
Quantitative real-time PCR
For the human tissues of the giant papillae, total RNA was extracted using a NucleoSpin II RNA isolation kit (Macherey–Nagel), and cDNAs were prepared from 750 ng of total RNA using random primers and the RevaTra-Ace reverse transcriptase (both from Toyobo) according to the manufacturer’s protocol. HConEpiCs in the third passage were prepared for quantitative PCR incubated in Defined Keratinocyte-SFM medium without growth supplements for 24 h before the subsequent experiments. The cells were stimulated for 12 h with 20 ng/mL or 100 ng/mL of recombinant human OSM (Sigma Aldrich), and then RNA was extracted using an RNeasy Mini kit (Qiagen) according to the manufacturer’s protocol. cDNA was synthesized from 750 ng of total RNA using PrimeScript RT Master Mix (Takara). Quantitative PCR was then performed using a SYBR Premix Ex Taq II (TliRNaseH Plus) with an Applied Biosystems 7900HT thermocycler and analyzed using Sequence Detection System 2.4 (Applied Biosystems). The primers used are shown in Table 1. GAPDH was used as the reference gene.
Immunohistochemistry
Giant papillae specimens were processed for immunohistochemistry as previously described [20]. The slides were blocked with PBS containing 10% BSA for 1 h at room temperature (RT). Double immunostaining was carried out on pairs of rabbit anti-OSM polyclonal antibody (Proteintech Japan) and mouse anti-CD3 monoclonal antibody (Abcam Cambridge). The pair of primary antibodies was applied to the slides simultaneously overnight at 4 °C and then washed with PBS. Next, the slides were incubated with donkey Alexa 488-conjugated anti-rabbit IgG antibody, and donkey Alexa 594-conjugated anti-mouse IgG antibody (Invitrogen Japan) was used as the secondary antibody. The slides were mounted with SlowFade gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole) counterstain. The stained slides were examined with a confocal fluorescence microscope (Keyence).
Immunocytochemistry
The preparation of cell cultures for immunocytochemistry was performed using Chamber Slide System/4 well Chamber Slide (Thermo Fisher). After 24 h of incubation, the cells were rinsed with PBS, fixed with 4% PFA for 15 min, and blocked with 2% BSA for 30 min. Then, the cells were incubated with primary antibodies, mouse anti-human OSMRβ antibody (clone D-10, 1:100 dilution; Santa Cruz Biotechnology) or mouse IgG1 kappa isotype control (eBioscience), for 2 h at room temperature. After 3 washes with PBS, the cells were incubated with an Alexa Fluor 594-conjugated secondary antibody (1:1000) (Thermo Fisher Scientific) for 30 min at room temperature, after which they were examined with a fluorescence microscope (Keyence).
Flowcytometry
For flow cytometry, the cells were stained with a mouse anti-human OSMRβ antibody (D-10, 1:50; Santa Cruz Biotechnology) or isotype control for 30 min and then with a PE-conjugated secondary antibody for 15 min. The stained cells were analyzed using a FACSCalibur (BD Biosciences).
Western blot analysis for phosphorylation of STAT1 and STAT3
HConEpiCs in the third passage were incubated in Defined Keratinocyte-SFM medium without growth supplements for 24 h. Then, the cells were treated with 100 ng/mL, 500 ng/mL recombinant human IL-31 (Peprotech), or 100 ng/mL recombinant human OSM (Sigma Aldrich) for 10 min before the subsequent experiments. Total cell extracts from cultured HConEpiCs were obtained by lysis in RIPA buffer (50 mM Tris–HCL, pH 7.5, 5 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 1% nonidet P-40, and 150 mM NaCl) containing a serine protease inhibitor (phenylmethylsulfonyl fluoride; Roche, Molecular Biochemicals). After centrifugation to remove the cell debris, the supernatants were subjected to sodium dodecyl sulfate–polyacrylamide (SDS-PAGE) gel electrophoresis using NuPAGE 4–12% Bis–Tris Gels (Life Technologies) and Laemmli buffer (4% SDS, 10% 2-mercaptoethanol, 20% glycerol, 0.004% bromophenol blue, and 0.125 M Tris–HCl). The proteins were subsequently electrotransferred to an Immobilon-P Transfer Membrane (Millipore,). The membrane was blocked in 2% ECL Prime Blocking Solution (Amersham Pharmacia Biotech)/TBS with 0.1% Tween-20 (TBS-T) for 1 h at room temperature, which was followed by incubation with the primary antibodies overnight at 4℃. The working concentrations of the primary antibodies in PBS-T with 5% skim milk were 1:1000 dilution for rabbit anti-phospho-STAT1 (Tyr701), rabbit anti-phospho-STAT3 (Tyr705), rabbit anti-STAT1, and mouse anti-STAT3 (Cell Signaling Technology) or 1:5000 dilution for GAPDH (Cell Signaling Technology). After 5 washes in TBS-T, the blots were incubated with the secondary antibodies (horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G) at 1:2000 dilution. An ECL detection system (Amersham Pharmacia Biotech) was used to visualize the target proteins on the blots according to the manufacturer’s instructions. The exposure time ranged from 5 s to 1 min, depending on the protein. A digital imaging system, Amersham Imager 600 (GE Healthcare), was used to record and analyze the images of the membranes.
DNA microarray analysis
HConEpiCs in the third passage were incubated in Defined Keratinocyte-SFM medium without growth supplements for 24 h before the subsequent experiments. The cells were also stimulated for 12 h with 100 ng/mL of recombinant human OSM (Sigma Aldrich), and then RNA was extracted using a RNeasy Mini kit (Qiagen) according to the manufacturer’s protocol. Gene expression microarray analysis using the Human Gene 2.0 ST Array (Affymetrix, Thermo Fisher Scientific) containing 53,617 probes characterized as human genes was consigned to Filgen Inc. Predictive enrichment analysis was performed on sets of genes exhibiting an expression change (fold change > 2, < 0.5). Pathway analysis was carried out by using the “KEGG pathway function.” Data are presented as heatmap and MA plots generated using MeV (http://mev.tm4.org) and Prism 8 software (GraphPad).
Statistical analysis
All results are expressed as means and SDs. The data were analyzed using GraphPad Prism software. Data comparisons between 2 groups were performed using the 2-tailed t test, and data comparisons between multiple groups were performed using 1-way ANOVA with a Bonferroni correction. Probability values below 0.05 were considered significant.
Results
High OSM expression in tear fluid and giant papillae of VKC patients
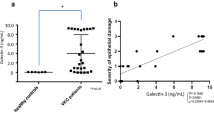
We first performed an ELISA to measure the OSM concentration in tear fluids obtained from VKC patients. In total, 1500–4000 pg/mL of OSM was detected in the tear fluid samples of all 7 VKC patients, whilst the ter fluid OSM concentration in all 6 healthy controls was under the detection limit (Fig. 1a).
Expression of oncostatin M (OSM) in the tear fluid and giant papillae of vernal keratoconjunctivitis (VKC) patients. a The concentrations of OSM in the tear fluid of 7 VKC patients and 6 healthy controls were measured using enzyme-linked immunosorbent assay (detection limit = 15.6 pg/mL). The error bars indicate means ± standard errors of the mean (SEM) *P < .05. b The expression of OSM at the mRNA level in 4 giant papillae from VKC patients and 7 conjunctival specimens from conjunctivochalasis patients during resection surgery was investigated by use of quantitative RT-PCR. The error bars indicate means ± standard deviations (SDs). ****P < .0001, *P < .05
OSM mRNA expression in the giant papillae of VKC patients was also assessed by use of quantitative RT-PCR. We found that OSM mRNA expression was significantly increased in the giant papillae of the VKC patients as compared with those of the controls (Fig. 1b).
T cells expressing OSM under the epithelium
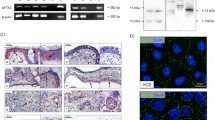
To determine which cell type was making OSM in the giant papillae, we tried double immunostaining with specific antibodies for OSM and a T cell marker (CD3). We observed many T cells expressing OSM under the epithelium in the giant papillae (Fig. 2b). However, in the substantia propria in the giant papillae, T cells expressing OSM were not observed (Fig. 2a). These results suggest that T cells under the epithelium may be one of the sources of OSM.
T cells expressing OSM in giant papillae. a Double-immunostained with anti-OSM and anti-CD3 antibodies. Many CD3-positive T cells (red) and several OSM-positive cells (green) were observed in the substantia propria. However, T cells expressing OSM were not observed. b Many T cells expressing OSM (yellow) were observed under the epithelium. Arrow: T cells expressing OSM
OSM-specific receptors were expressed in human conjunctival epithelial cells
To assess if OSM overexpression in tear fluid or giant papillae tissue affects the biologic activities of human conjunctiva, we investigated expression of OSMRβ in HConEpiCs, which is an OSM-specific receptor. Flow cytometric analysis revealed strong OSMRβ immunoreactivity (Fig. 3a), and the immunocytochemical results showed positive staining of the cell membranes of HConEpiCs with OSMRβ antibody (Fig. 3b).
Expression of oncostatin M-specific receptor in cultured human conjunctival epithelial cells. a Flow cytometry analysis of the surface expression of oncostatin M-specific receptor (OSMRβ). The expression of OSM receptor beta (OSMRβ) in cultured human conjunctival epithelial cells (HconEpiCs) was represented by use of flow cytometry. Rightward shifts of the histogram (OSMRβ monoclonal antibody) from the filled histogram (isotype control antibody) indicate the OSMRβ expression on the cell surface. b Immunofluorescence signals were shown on the cell surface of HconEpiCs. DAPI 4,6-diamice-2-phenylindole. Scale bars: 20 μm
OSM activated the JAK/STAT pathway in human conjunctival cells
Binding of OSM to its receptor subunits is known to induce the activation of the JAK/STAT signaling pathway [1]. Phosphorylated STATs translocate to the nucleus and induce gene expression binding to regulatory elements in the promoter of OSM-responsive genes. To investigate whether OSM activates the JAK/STAT signaling pathway in HConEpiCs, we treated the cells with 100 ng/mL of recombinant human OSM for 10 min and assessed the total and phosphorylated STAT1 and STAT3 expressions by means of Western blot analysis. We also treated the cells with IL-31, which belongs to the same IL-6 family as OSM as a positive control, whilst OSMRβ (alias, IL-31 receptor subunit beta) associates with IL-31RA to form the IL-31 receptor. Although the phosphorylation of STAT1 and STAT3 were not seen in the control, treatment of OSM and IL-31, especially OSM, markedly increased the level of both phospho-STAT1 and phospho-STAT-3 (Fig. 4). Therefore, it was revealed that OSM can activate the JAK/STAT pathway via OSMRβ in HConEpiCs.
Oncostatin M induced STAT activation in cultured human conjunctival epithelial cells. Activation of STAT1 and STAT3 were measured by use of Western blot analysis. Cultured human conjunctival epithelial cells (HconEpiCs) were treated with 100 ng/mL of human recombinant oncostatin M (OSM) and 100 ng/mL or 500 ng/mL of human recombinant interleukin (IL)-31 for 10 min. The concentrations of total and phosphorylated STAT1 and STAT3 were visualized by means of Western blot analysis
Global gene expression analysis in OSM-treated human conjunctival epithelial cells
To investigate how OSM affects human conjunctival diseases such as VKC, global gene expression in OSM-treated and -nontreated HConEpiCs was analyzed by microarray. The HConEpiCs were incubated with 100 ng/mL OSM for 12 h, after which RNA was extracted for microarray analysis. One hundred sixty genes (495 probes) in the OSM-treated HConEpiCs were upregulated twofold or more (log2 FC > 1), and 171 genes (453 probes) were downregulated 0.5-fold or less (log2 FC < – 1) (Fig. 5a). The most upregulated genes in the OSM-treated HConEpiCs were matrix metalloproteinases 1 and 3 (MMP-1, MMP-3), whose expression ratios were 14.1 and 13.9 fold-changes, respectively (Fig. 5b). Among the top 20 genes that were upregulated in the OSM-treated HConEpiCs, several were related to tissue remodeling or fibrosis, such as SOCS3, SERPINβ3, IL-20, IL-24, and TNC (Fig. 5b). KEGG pathway analysis showed that genes associated with the JAK/STAT signaling pathway, TNF signaling pathway, NF-kappa B signaling pathway, ECM-receptor interaction, or focal adhesion are enriched in the upregulated genes (Fig. 5c). We also confirmed the expression change of these genes by real-time qPCR with 0, 20, or 100 ng/mL of OSM. The expressions of MMP-1, MMP-3, SOCS3, SERPINβ3, and IL-20 were suppressed in the OSM-treated HConEpiCs in a dose-dependent manner (Fig. 5d).
Gene expression changes in human conjunctival epithelial cells after oncostatin M treatment. After cultured human conjunctival epithelial cells (HconEpiCs) were treated with 100 ng/mL of human oncositatin M (OSM) recombinant or vehicle for 12 h, the comprehensive gene expressions were analyzed by use of microarray. a MA plot visualized the gene expression change between the control vehicle and the OSM-treated samples in terms of log2 fold change on the Y-axis and the log of the mean of the expression counts of the normal and OSM-treated samples on the X-axis. b Top 20 genes that were upregulated or downregulated in HconEpiCs with OSM as compared with the control were shown in the heat map (upregulated genes: 3.74 < fold change < 14.1; downregulated genes: 0.07 < fold change < 0.30). c Pathway analysis was performed on sets of genes exhibiting an expression change (fold change > 2, < 0.5). d The expression levels of MMP1, MMP3, Serpine B3, IL-20, SOCS3, and TNC were analyzed by use of qPCR after 12-h incubation with 20 ng/mL or 100 ng/mL human OSM recombinant. MMP1 matrix metalloproteinase 1, MMP3 matrix metalloproteinase 3, serpine B3 serpin family B member 3, IL-20 interleukin 20, SOCS3 suppressor of cytokine signaling 3, TNC tenascin C. The error bars indicate means ± standard deviations (SDs). *P < .05, **P < .01, ***P < .001, ****P < .0001
Discussion
This study showed that the OSM concentration in tear fluid samples from VKC patients was higher than that from healthy controls and that OSM mRNA was strongly expressed in the giant papillae. We also tried to identify the cell type responsible for OSM production in the giant papillae of VKC patients. Several reports demonstrated that neutrophils, dendritic cells, macrophages, mast cells, or T cells produced OSM [3, 21,22,23]. Some dendritic cells and macrophages activated in vitro express OSM. For example, OSM production was increased in human dendritic cells or macrophages by lipopolysaccharides from Staphylococcus aureus [22]. In the circulation, however, neutrophils are the predominant cells that express OSM. Neutrophils store OSM in granules that could be readily mobilized [24, 25]. Pothoven and associates revealed that neutrophils are a major source of OSM in the nasal polyps of chronic rhinosinusitis patients [21]. On the other hand, Boniface and associates demonstrated that T cells infiltrating inflammatory skin such as atopic dermatitis and psoriasis are important sources of OSM [3]. In this study, we observed many T cells expressing OSM under the epithelium. However, T cells expressing OSM were not observed in the substantia propria. The mechanism by which only T cells under the epithelium express OSM is unclear. The epithelium may produce some kinds of cytokines to induce OSM in T cells.
Next, we demonstrated OSMR-β expression in HconEpiCs by use of FACS and immunocytochemical analyses. We found that various cytokines (TGF-β, TNF-α, IL-4, and IL-13, which cause allergic inflammation) did not enhance the expression of OSMR-β (data not shown). On the other hand, recombinant OSM strongly promoted STAT1 and STAT3 phosphorylation in HconEpiCs. Furthermore, microarray analysis and q-PCR showed that recombinant OSM strongly enhanced the expression of MMP-1, MMP-3, IL-24, serpinβ3, IL-20, SOCS3, S100A7, and tenascin C in HconEpiCs.
The matrix metalloproteinases (MMPs) are a family of 24 zinc-dependent proteases involved in diverse physiologic processes such as tissue remodeling, embryonic development, wound healing, angiogenesis, and inflammation [26]. MMPs have been demonstrated to have a role in ECM degradation and remodeling [26]. The ECM acts as a reservoir of biologically active molecules and as a storage site for growth factors, cytokines, and proteases. Recently, Ryan and associates reported that bioactive OSM binds to ECM molecules such as type I collagen, laminin, and fibronectin. Therefore, MMPs may be important for the development of chronic inflammation and fibrosis mediated via OSM [27]. Leonardi and associates found significantly increased MMP-1 and MMP-3 activity in VKC tear fluid as compared with that in the tear fluid of control participants [28]. Thus, increased levels and/or activity of MMP-1 and MMP -3 may be involved in OSM production in the giant papillae.
Serpinβ3 belongs to a family of serine peptidase inhibitors with antiprotease activity, most of which are serine and cysteine proteases [29]. It is well known that house dust mites are one of the major allergens in VKC. House dust mite allergens include several kinds of proteases, such as serine and cysteine proteases [30]. Exogenous proteases derived from allergens disrupt the epithelial barrier and have been implicated in the pathogenesis of allergic disorders, such as atopic dermatitis, asthma, and allergic rhinosinusitis [30]. Upregulation of serpinβ3 by OSM may inhibit house dust mite allergen protease activity, resulting in maintenance of the epithelial barrier and attenuation of allergic inflammation in VKC. On the other hand, OSM has been reported to play a role in epithelial barrier dysfunction associated with mucosal diseases [31]. Previous studies have shown that OSM induces significant loss of barrier function and disorganization of tight junctions [32]. Therefore, the role of OSM in the epithelial barrier function is controversial.
The IL-20 subfamily comprises IL-19, IL-20, IL-22, IL-24, and IL-26. These cytokines are mainly produced by immune cells, such as myeloid cells and lymphocytes [33]. However, epithelial cells (the main target cells of the IL-20 subfamily) can also secrete IL-19, IL-20, and IL-24 in response to stimulation by other cytokines. For example, IL-1, IL-8, IL-17A, and TNF have been found to induce IL-20 production by keratinocytes [34]. In this study, OSM strongly induced the expression of IL-20 and IL-24 mRNA. Members of the IL-20 cytokine subfamily represent a key link between the immune system and epithelial tissues and are essential for promoting innate epithelial immunity and wound healing.
S100A7 (psoriasin) was originally identified as a molecule highly expressed in psoriatic lesions [35]. It belongs to the S100 family of calcium-binding proteins that are involved in several cellular processes, including proliferation, differentiation, invasion, and metastasis [36]. Elevated expression of psoriasin has been detected in some epithelial carcinomas, including squamous cell carcinoma of the skin and bladder [37, 38]. Recently, Vegfors and associates showed that S100A7 enhances the expression of angiogenic factors such as vascular endothelial growth factor (VEGF) in keratinocytes and acts on dermal endothelial cells to promote angiogenesis [39]. S100A7 induced angiogenesis by endothelial cells in a VEGF-independent manner, suggesting that S100A7 is itself an angiogenic factor. Therefore, upregulation of psoriasin by OSM may contribute to epithelial cell hyperplasia and promote angiogenesis in giant papillae.
Tenascin-C is usually under tight spatial and temporal regulation, showing prominent expression during embryogenesis but being undetectable in most healthy adult tissues, whilst transient re-expression occurs during wound healing and dynamic tissue remodeling [40]. Persistent expression of tenascin-C is associated with a variety of chronic pathologic conditions. Tenascin-C interacts with many matrix proteins such as periostin, fibronectin, and perlecan and is well known to drive chronic organ fibrosis [41]. In humans, both idiopathic and systemic sclerosis-associated forms of pulmonary fibrosis are accompanied by elevation of tenascin-C [41]. Alterations of tenascin-C expression or function are also linked with fibrosis in animal models. In our previous study, we demonstrated strong extracellular expression of tenascin-C in the substantia propria of giant papillae from VKC patients [42]. Therefore, OSM may stimulate conjunctival epithelial cells to produce tenascin-C, resulting in tissue remodeling and fibrosis in giant papillae.
In a similar fashion to other IL-6 type cytokines, OSM is negatively regulated by SOCS, particularly SOCS3. In this study, microarray analysis and q-PCR showed that OSM strongly induced SOCS3 expression, which may be evidence that SOCS3 acts as a potent feedback inhibitor of OSM.
In conclusion, a high concentration of OSM was detected in the tear fluid of VKC patients. In HconEpiCs, OSM induced the expression of genes related to tissue remodeling via the OSMβ/gp130 receptor through STAT1/STAT3 activation. These findings suggest that OSM potentially acts as a key molecule for tissue remodeling in VKC. Further analysis of OSM will shed light on its potential role as a target for the future treatment of VKC.
References
Tanaka M, Miyajima A. Oncostatin M: a multifunctional cytokine. Rev Physiol Biochem Pharmacol. 2003;149:39–52.
Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18:773–89.
Boniface K, Diveu C, Morel F, Pedretti N, Froger J, Ravon E, et al. Oncostatin M secreted by skin infiltrating T lymphocytes is a potent keratinocyte activator involved in skin inflammation. J Immunol. 2007;178:4615–22.
Fritz DK, Kerr C, Fattouh R, Llop-Guevara A, Khan WI, Jordana M, et al. A mouse model of airway disease: oncostatin M-induced pulmonary eosinophilia, goblet cell hyperplasia, and airway hyperresponsiveness are STAT6 dependent, and interstitial pulmonary fibrosis is STAT6 independent. J Immunol. 2011;186:1107–18.
Nagahama KY, Togo S, Holz O, Magnussen H, Liu X, Seyama K, et al. Oncostatin M modulates fibroblast function via signal transducers and activators of transcription proteins-3. Am J Respir Cell Mol Biol. 2013;49:582–91.
Kang HJ, Kang JS, Lee SH, Hwang SJ, Chae SW, Woo JS, et al. Upregulation of oncostatin M in allergic rhinitis. Laryngoscope. 2005;115:2213–6.
Mozaffarian A, Brewer AW, Trueblood ES, Luzina IG, Todd NW, Atamas SP, et al. Mechanisms of oncostatin M-induced pulmonary inflammation and fibrosis. J Immunol. 2008;181:7243–53.
Johnson RB. Use of NSAIDs in long-distance runners: a risk factor for sudden death? South Med J. 1989;82:95.
Scaffidi AK, Mutsaers SE, Moodley YP, McAnulty RJ, Laurent GJ, Thompson PJ, et al. Oncostatin M stimulates proliferation, induces collagen production and inhibits apoptosis of human lung fibroblasts. Br J Pharmacol. 2002;136:793–801.
Duncan MR, Hasan A, Berman B. Oncostatin M stimulates collagen and glycosaminoglycan production by cultured normal dermal fibroblasts: insensitivity of sclerodermal and keloidal fibroblasts. J Invest Dermatol. 1995;104:128–33.
Bamber B, Reife RA, Haugen HS, Clegg CH. Oncostatin M stimulates excessive extracellular matrix accumulation in a transgenic mouse model of connective tissue disease. J Mol Med (Berl). 1998;76:61–9.
Wong S, Botelho FM, Rodrigues RM, Richards CD. Oncostatin M overexpression induces matrix deposition, STAT3 activation, and SMAD1 Dysregulation in lungs of fibrosis-resistant BALB/c mice. Lab Invest. 2014;94:1003–16.
Sarkozi R, Hauser C, Noppert SJ, Kronbichler A, Pirklbauer M, Haller VM, et al. Oncostatin M is a novel inhibitor of TGF-beta1-induced matricellular protein expression. Am J Physiol Renal Physiol. 2011;301:F1014–25.
Abe H, Takeda N, Isagawa T, Semba H, Nishimura S, Morioka MS, et al. Macrophage hypoxia signaling regulates cardiac fibrosis via Oncostatin M. Nat Commun. 2019;10:2824.
Huguier V, Giot JP, Simonneau M, Levillain P, Charreau S, Garcia M, et al. Oncostatin M exerts a protective effect against excessive scarring by counteracting the inductive effect of TGFbeta1 on fibrosis markers. Sci Rep. 2019;9:2113.
Iwamoto S, Asada Y, Ebihara N, Hori K, Okayama Y, Kashiwakura J, et al. Interaction between conjunctival epithelial cells and mast cells induces CCL2 expression and piecemeal degranulation in mast cells. Invest Ophthalmol Vis Sci. 2013;54:2465–73.
Tanioka H, Kawasaki S, Yamasaki K, Ang LP, Koizumi N, Nakamura T, et al. Establishment of a cultivated human conjunctival epithelium as an alternative tissue source for autologous corneal epithelial transplantation. Invest Ophthalmol Vis Sci. 2006;47:3820–7.
Ang LP, Tanioka H, Kawasaki S, Ang LP, Yamasaki K, Do TP, et al. Cultivated human conjunctival epithelial transplantation for total limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 2010;51:758–64.
Kinoshita S, Kawasaki S, Kitazawa K, Shinomiya K. Establishment of a human conjunctival epithelial cell line lacking the functional TACSTD2 gene (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2012;110:166–77.
Matsuda A, Ebihara N, Yokoi N, Kawasaki S, Tanioka H, Inatomi T, et al. Functional role of thymic stromal lymphopoietin in chronic allergic keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2010;51:151–5.
Pothoven KL, Norton JE, Suh LA, Carter RG, Harris KE, Biyasheva A, et al. Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. J Allergy Clin Immunol. 2017;139:1966–78.
Suda T, Chida K, Todate A, Ide K, Asada K, Nakamura Y, et al. Oncostatin M production by human dendritic cells in response to bacterial products. Cytokine. 2002;17:335–40.
Salamon P, Shoham NG, Puxeddu I, Paitan Y, Levi-Schaffer F, Mekori YA. Human mast cells release oncostatin M on contact with activated T cells: possible biologic relevance. J Allergy Clin Immunol. 2008;121:448–55.
Grenier A, Dehoux M, Boutten A, Arce-Vicioso M, Durand G, Gougerot-Pocidalo MA, et al. Oncostatin M production and regulation by human polymorphonuclear neutrophils. Blood. 1999;93:1413–21.
Uriarte SM, Powell DW, Luerman GC, Merchant ML, Cummins TD, Jog NR, et al. Comparison of proteins expressed on secretory vesicle membranes and plasma membranes of human neutrophils. J Immunol. 2008;180:5575–81.
Young D, Das N, Anowai A, Dufour A. Matrix metalloproteases as influencers of the cells’ social media. Int J Mol Sci. 2019;20:3847.
Ryan RE, Martin B, Mellor L, Jacob RB, Tawara K, McDougal OM, et al. Oncostatin M binds to extracellular matrix in a bioactive conformation: implications for inflammation and metastasis. Cytokine. 2015;72:71–85.
Leonardi A, Brun P, Abatangelo G, Plebani M, Secchi AG. Tear levels and activity of matrix metalloproteinase (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2003;44:3052–8.
Turato C, Pontisso P. SERPINB3 (serpin peptidase inhibitor, clade B (ovalbumin), member 3). Atlas Genet Cytogenet Oncol Haematol. 2015;19:202–9.
Reithofer M, Jahn-Schmid B. Allergens with protease activity from house dust mites. Int J Mol Sci. 2017;18:1368.
Pothoven KL, Norton JE, Hulse KE, Suh LA, Carter RG, Rocci E, et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J Allergy Clin Immunol. 2015;136(737–46):e4.
Pothoven KL, Schleimer RP. The barrier hypothesis and Oncostatin M: restoration of epithelial barrier function as a novel therapeutic strategy for the treatment of type 2 inflammatory disease. Tissue Barriers. 2017;5:e1341367.
Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines: from host defence to tissue homeostasis. Nat Rev Immunol. 2014;14:783–95.
Tohyama M, Hanakawa Y, Shirakata Y, Dai X, Yang L, Hirakawa S, et al. IL-17 and IL-22 mediate IL-20 subfamily cytokine production in cultured keratinocytes via increased IL-22 receptor expression. Eur J Immunol. 2009;39:2779–88.
Madsen P, Rasmussen HH, Leffers H, Honore B, Dejgaard K, Olsen E, et al. Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly up-regulated in psoriatic skin. J Invest Dermatol. 1991;97:701–12.
Hoffmann HJ, Olsen E, Etzerodt M, Madsen P, Thogersen HC, Kruse T, et al. Psoriasin binds calcium and is upregulated by calcium to levels that resemble those observed in normal skin. J Invest Dermatol. 1994;103:370–5.
Alowami S, Qing G, Emberley E, Snell L, Watson PH. Psoriasin (S100A7) expression is altered during skin tumorigenesis. BMC Dermatol. 2003;3:1.
Ostergaard M, Wolf H, Orntoft TF, Celis JE. Psoriasin (S100A7): a putative urinary marker for the follow-up of patients with bladder squamous cell carcinomas. Electrophoresis. 1999;20:349–54.
Vegfors J, Ekman AK, Stoll SW, Bivik Eding C, Enerback C. Psoriasin (S100A7) promotes stress-induced angiogenesis. Br J Dermatol. 2016;175:1263–73.
Giblin SP, Midwood KS. Tenascin-C: form versus function. Cell Adh Migr. 2015;9:48–82.
Bhattacharyya S, Wang W, Morales-Nebreda L, Feng G, Wu M, Zhou X, et al. Tenascin-C drives persistence of organ fibrosis. Nat Commun. 2016;7:11703.
Ohtomo K, Ebihara N, Matsuda A, Tokura T, Funaki T, Murakami A. Role of TGF-beta in tissue eosinophilia associated with vernal keratoconjunctivitis. Exp Eye Res. 2010;91:748–54.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
K. Mashimo, None; A. Usui-Ouchi, None; Y. Ito, None; R. Wakasa-Arai, None; N. Yokoi, None; S. Kawasaki, None; A. Murakami, None; A. Matsuda, None; N. Ebihara, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Nobuyuki Ebihara
About this article
Cite this article
Mashimo, K., Usui-Ouchi, A., Ito, Y. et al. Role of oncostatin M in the pathogenesis of vernal keratoconjunctivitis: focus on tissue remodeling. Jpn J Ophthalmol 65, 144–153 (2021). https://doi.org/10.1007/s10384-020-00791-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00791-8