Abstract
Purpose
To investigate the additive effects of orthokeratology (OK) and atropine 0.01% ophthalmic solution, both of which are effective procedures to slow axial elongation in children with myopia.
Study design
Prospective randomized clinical trial.
Methods
Japanese children aged 8–12 years with a spherical equivalent refractive error of − 1.00 to − 6.00 diopters were included. A total of 41 participants who had been wearing the OK lenses successfully for 3 months were randomly allocated into two groups to receive either the combination of OK and atropine 0.01% ophthalmic solution (combination group) or monotherapy with OK (monotherapy group). Subjects in the combination group started to use atropine 0.01% ophthalmic solution once nightly from 3 months after the start of OK. Axial length was measured every 3 months using non-contact laser interferometry (IOLMaster), and the axial length measurement at month 3 of OK therapy was used as the baseline value in both groups. The increase in axial length over 1 year was compared between the two groups.
Results
A total of 40 consecutive subjects (20 subjects in the combination group and 20 in the monotherapy group) were followed for 1 year. The increase in axial length over 1 year was 0.09 ± 0.12 mm in the combination group and 0.19 ± 0.15 mm in the monotherapy group (P = 0.0356, unpaired t test).
Conclusion
During the 1-year follow-up, the combination of OK and atropine 0.01% ophthalmic solution was more effective in slowing axial elongation than OK monotherapy in children with myopia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myopia is becoming more prevalent worldwide, especially in East Asia [1,2,3], and increasingly affects younger generations [2, 4, 5]. As myopia tends to progress more rapidly when it develops in younger age groups [6, 7], there are concerns that high myopia will become even more common in the future [8, 9]. Advanced technologies of axial length measurement provide evidence that myopia progression in children is driven largely by axial elongation [6, 10]. The elongation in patients with high myopia causes mechanical stretching of the retina, which may lead to retinal atrophy that increases the risk for macular degeneration, chorioretinal atrophy, glaucoma, retinal detachment, and resulting blindness [2, 11, 12]. Just as good control of blood pressure and glucose levels are important in preventing cerebral and myocardial infarction, controlling axial elongation is important in preventing high myopia. However, there are no known procedures that can stop the progression and prevent pathological high myopia that may lead to blindness.
Nevertheless, recent studies provide evidence of effective measures which slow the progression of myopia. It is reported that treatment with atropine 1% ophthalmic solution reduced the progression of myopia by 77% over a 2-year period [13], and the corresponding percentage with atropine 0.01% ophthalmic solution was 59% [14]. Studies of orthokeratology (OK) report that axial elongation was reduced by 32–56% over a 2-year period [15,16,17,18,19,20], although the percentages differ substantially among studies.
Although atropine 1% ophthalmic solution was the most effective in slowing the progression of myopia [21], it may not be feasible in clinical practice due to adverse drug reactions (ADRs) such as pupillary dilation and loss of accommodation [22]. In addition to ADRs, atropine 1% ophthalmic solution also has been associated with a rebound effect. In the Atropine for the Treatment of Myopia (ATOM) 1 study [13, 23], myopia progression in the atropine 1% group was significantly greater than that in the placebo group following cessation of atropine 1% ophthalmic solution. In the ATOM2 study, atropine 0.01% ophthalmic solution slowed the progression of myopia without causing significant ADRs, and proved suitable for routine clinical use [14]. Moreover, following cessation of treatment atropine 0.01% ophthalmic solution had a more modulated and sustained effect without rebound, compared with higher concentrations of atropine ophthalmic solutions [24, 25]. However, when the investigators compared data in the atropine 0.01% group in the ATOM2 study with those in the placebo group in the ATOM1 study [13, 23], a significant difference between atropine 0.01% ophthalmic solution and placebo was observed for refraction but not for axial length. These findings may be attributed to the difference in the method of axial length measurement: The ATOM1 study [13, 23] used corneal contact A-scan ultrasonography, whereas the ATOM2 study [14, 24, 25] used non-contact laser interferometry (IOLMaster; Carl Zeiss Meditec). Of the two, it is reported that the non-contact IOLMaster is the more accurate method for axial length measurement in children [26, 27]. As atropine ophthalmic solutions cannot correct myopia, additional measures such as spectacles, contact lenses or OK are required to correct myopia in children.
OK is a procedure using specially designed high oxygen-permeable hard contact lenses that flatten the central cornea to reduce refractive errors when worn at night, and, once stabilization of the treatment and full correction have been achieved allows patients to have unaided vision during the day. Recently, reports from around the world describe the efficacy of OK in slowing axial elongation in children [15,16,17,18,19,20]. Although study results vary, the suppressive effect of OK is smaller than that of atropine 1% ophthalmic solution [21, 22]. However, OK has an advantage over atropine ophthalmic solutions as the former can correct myopia and hence, allows spectacle-free vision in the daytime.
Although the mechanisms by which atropine and OK slow the progression of myopia remain uncertain, some hypotheses have been proposed. The most likely mechanism of atropine appears to be blocking of the muscarinic receptors in the retina and sclera that mediate axial elongation [28, 29], while that of OK appears to be improvement of defocus on the peripheral retina [30, 31] with increase in higher-order aberration [32, 33] through corneal epithelial redistribution in which the central cornea is thinned, and the mid-periphery is thickened [34, 35]. Thus, atropine and OK seem to slow the progression of myopia through different mechanisms. Patients with hypertension and those with diabetes mellitus often receive drugs with different mechanisms of action to yield additive effects while reducing the risk of ADRs. In the same way, combining treatments with different mechanisms of action could also be more effective than monotherapies in slowing or preventing myopia progression.
Although atropine 1% ophthalmic solution is not feasible in the clinical setting due to the risk of ADRs and rebound after stopping treatment, using atropine 0.01% ophthalmic solution together with OK may become an optimal treatment option. We thus conducted a prospective clinical study of a combination treatment with OK and atropine 0.01% ophthalmic solution to assess their effect in slowing axial elongation in children with myopia.
Subjects and methods
Participants were Japanese boys and girls 8–12 years of age who wished to undergo OK treatment at Konno Eye Clinic or Omiya Hamada Eye Clinic. The inclusion criteria were cycloplegic spherical equivalent refractive error (SER) of − 1.00 to − 6.00 diopters (D) OU, astigmatism of ≤ 1.50 D OU, anisometropia of ≤ 1.50 D, and best-corrected visual acuity of ≤ 0.00 logarithm of the minimum angle of resolution (logMAR) units OU. Children with ocular disorders such as strabismus and amblyopia, systemic disorders such as cardiac or respiratory illness, low birth weight (≤ 1500 g), or a history of hypersensitivity to atropine were excluded from the study. Children who were already using OK and/or atropine ophthalmic solutions were also excluded. This study was registered on the UMIN Clinical Trials Registry (registration number: UMIN000014362; date of registration: June 24, 2014).
The present study was conducted in accordance with the tenets of the Declaration of Helsinki and was independently reviewed and approved by the ethical committee at Saitama Medical Center, Jichi Medical University. Investigators explained the expected benefits and potential risks associated with treatment to the parents or legal guardians of each child, and written informed consent was obtained. Parents or guardians were also instructed to ensure adequate cleaning and disinfection of OK contact lenses. Explanation to children was done using an easy-to-understand document prior to obtaining informed assent.
As the central corneal thickness stabilizes after the first 1–2 months of OK therapy [34, 36, 37], the axial length measurement at month 3 of OK therapy was used as the baseline value, as in the studies by Hiraoka et al. and Kakita et al. [17, 18]. At baseline, the participants who had been wearing the OK lenses successfully for 3 months after the study entry were randomly allocated by a third person into two groups to receive either the combination of OK and atropine 0.01% ophthalmic solution (combination group) or monotherapy with OK (monotherapy group). Therefore, the subjects in the combination group started to use atropine 0.01% ophthalmic solution OU once nightly at 3 months after the start of OK. A stratified randomization method was used to control for age and SER at enrollment. Specifically, children were divided into four blocks by age (8.0–10.9 years, and 11.0–12.9 years) and SER (− 1.00 to − 3.00 D, and − 3.01 to − 6.00 D), and the children in each block were randomly allocated into the two groups.
The OK lenses used in the present study were four-zone reverse geometry lenses (Breath-O Correct; Universal View Co., Ltd.) made of rigid gas-permeable material constituting of fluoride-containing methacrylate compound and silicone-containing methacrylate compound (Breath-O material; Toray Industries, Inc.) with a nominal oxygen permeability (Dk) of 156 × 10−11 (cm2/s) [mLO2/(mL·mmHg)]. The nominal central thickness of the lens is 0.20 mm, and the diameter 10.6 mm. Children who matched the inclusion criteria in this study were fitted with the lenses using Fitting Master (Universal View Co., Ltd.), a program that calculates and determines the appropriate lens specification based on refractive measurements and topographic maps obtained by refractometer, keratometer, and corneal topography. Subjects in both groups were instructed to wear their OK lenses on both eyes every night for at least 6 consecutive hours.
The atropine 0.01% ophthalmic solution for the combination group was specially prepared for this study by Fuji Yakuhin Co., Ltd., by diluting Nitten ATROPINE Ophthalmic Solution 1% (Nihon Tenganyaku Kenkyusyo Co., Ltd.) with physiological saline at a ratio of 1: 99 in a sterile manner. As the benzalkonium chloride as a preservative was also diluted, parents or guardians of subjects in the combination group were instructed to store the atropine 0.01% ophthalmic solution in a 5 ml polypropylene container under cold conditions. The shelf life for an unopened product was 3 months, and the solution was used within 7–10 days after opening except for cases in which the tip of the container was contaminated. Subjects in the combination group were instructed to instill the atropine 0.01% ophthalmic solution into both eyes once daily at night, at least 5 min before inserting the OK lenses, without their eyelid and eyelashes touching the tip of the container, because the drug reaches a peak concentration in the cornea within 5 min after the ophthalmic solution is topically instilled on an intact eye [38].
Subjects in both groups visited the clinic every 3 months for the measurement of axial length, corneal endothelial cell density, intraocular pressure (IOP), and uncorrected distant and near visual acuity. The adherence in the use of OK lenses and atropine 0.01% ophthalmic solution was also investigated using interview sheets at the time of each visit. Specifically, children and parents or guardians received interview sheets questioning the average weekly use of OK lenses and atropine 0.01% ophthalmic solution since their last visit. Whenever, during the follow-up period measurements showed the uncorrected distant visual acuity changed by more than 0.30 logMAR units, the OK lenses were re-prescribed. Subjects underwent slit-lamp examination to assess the fitting of the OK lenses and the presence/absence of adverse events. Subjects were withdrawn from the study if adverse events occurred that would not allow further use of the OK lenses or atropine 0.01% ophthalmic solution. The axial length was measured using the IOLMaster biometer by examiners who were blinded to the refractive status at enrollment and the group allocation of the participants. During each visit, the axial length was measured five times or more to obtain a mean value for analysis. The corneal endothelial cell density was measured using the Noncon ROBO CA SP-8800 (Konan Medical, Inc.). The IOP was measured using the TONOREF II (Nidek Co., Ltd.). All participants paid a discounted fee for the treatment in this study.
As a primary endpoint of this report, the changes in axial length over 1 year were compared between the combination and monotherapy groups using the unpaired t test, after confirming the normality of the data and the equality of variances of the two groups. Adjustments for multiple comparisons were not performed in the following statistical analyses and thus the results should be considered exploratory. The changes in axial length from enrollment to baseline, over 6 months, and over 1 year stratified by age and SER at enrollment were compared between the two groups using the unpaired t test. The relationships between the change in axial length over 1 year and the age and SER at enrollment were analyzed using the Pearson’s correlation coefficient and linear regression analysis. Multiple linear regression analysis (dependent variable: change in axial length over 1 year, independent variables: age and SER) were performed in order to adjust for potential confounding factors. The effects of adding atropine 0.01% ophthalmic solution to OK therapy were assessed by comparing the changes in corneal endothelial cell density, IOP, and uncorrected distant and near visual acuity between the two groups at the end of 1 year using the unpaired t test. The adherence to the treatment protocol was defined as ≥ 75% expected use of OK lenses, compared between the two groups using the Mann–Whitney U test. All measurements obtained from both eyes of the same participant were averaged and used for statistical analysis. All statistical analyses were performed using the statistical software EZR (Saitama Medical Centre, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing). More precisely, it is a modified version of R Commander designed to add statistical functions frequently used in biostatistics [39]. The data are expressed as the mean ± standard deviation. Differences were considered statistically significant when the P value was < 0.05.
Results
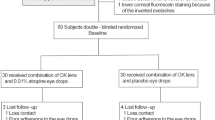
Participants were recruited from June 30, 2014. By November 30, 2016, a total of 43 children had been recruited to undergo fitting with the OK lenses. However, two children failed insertion and removal of the lenses before admittance to the study. A total of 41 OK subjects were randomly allocated into the two groups (20 in the combination group and 21 in the monotherapy group). A total of 40 subjects (20 in the combination group and 20 in the monotherapy group) were followed for 1 year, but one subject in the monotherapy group dropped out after 9 months because of infiltrates in the upper cornea OD (Fig. 1). Table 1 outlines the characteristics at enrollment of subjects in both the combination and monotherapy groups followed for 1 year. The age, gender, SER, axial length, corneal endothelial cell density, IOP, and uncorrected distant visual acuity were well balanced between the two groups, with no statistical differences.
The changes in axial length, corneal endothelial cell density, IOP, and uncorrected distant and near visual acuity over 1 year were compared between the two groups (Table 2). The increase in axial length over 1 year in the combination group was 0.09 ± 0.12 mm and in the monotherapy group it was 0.19 ± 0.15 mm (P = 0.0356, unpaired t test). The difference in axial length increases over 1 year between the two groups was 0.1 mm, and the combination of OK and atropine 0.01% ophthalmic solution was 53% more effective in slowing axial elongation than OK monotherapy. No significant differences between the combination and monotherapy groups were observed in the corneal endothelial cell density, IOP, or uncorrected distant or near visual acuity.
Figure 2 shows the time courses of changes in axial length in the combination group and monotherapy group. During the pre-study period of 3 months after the study entry, the increases in axial length did not differ significantly between the combination (0.0095 ± 0.0691 mm) and the monotherapy group (− 0.0038 ± 0.1072 mm) (P = 0.645, unpaired t test). The increase in axial length over 6 months was significantly smaller in the combination (0.05 ± 0.07 mm) than the monotherapy group (0.11 ± 0.11 mm) (P = 0.0499, unpaired t test). During the 1-year study period, the axial length increased by 0.09 ± 0.12 mm in the combination group and 0.19 ± 0.15 mm in the monotherapy group (P = 0.0356, unpaired t test).
Figure 3 shows the scatter plots of the increase in axial length over 1 year versus age at enrollment in the two groups. No significant correlation between the increase in axial length over 1 year and age at enrollment was observed in either the combination (Pearson’s correlation coefficient; r = − 0.047, P = 0.844) or the monotherapy group (r = − 0.396, P = 0.0837).
Figure 4 shows the scatter plots of the increase in axial length over 1 year versus SER at enrollment in the two groups. A significant positive correlation was observed between the increase in axial length and SER in the monotherapy group (Pearson’s correlation coefficient; r = 0.805, P < 0.001), where the increase in axial length was larger in subjects with lower myopia. This significant correlation was confirmed even after adjustment for age and SER by multiple linear regression analysis (dependent variable: change in axial length over 1 year, independent variables: age; P = 0.299, SER; P < 0.001). However, there was no significant correlation between the two parameters in the combination group (r = 0.306, P = 0.189).
The subjects were stratified by age at enrollment (8.0–10.9 years vs. 11.0–12.9 years) to compare the increases in axial length over 1 year between the combination and monotherapy groups (Fig. 5). In the subgroup of subjects aged 8.0–10.9 years (11 subjects in the combination group and 11 in the monotherapy group), the SERs at enrollment did not differ significantly between the combination (− 2.52 ± 1.04 D) and the monotherapy group (− 2.78 ± 1.67 mm) (P = 0.671, unpaired t-test). In the subgroup of subjects aged 11.0–12.9 years (9 subjects in the combination group and 9 in the monotherapy group), the SERs did not differ significantly between the combination (− 3.17 ± 1.80 D) and the monotherapy group (− 3.17 ± 1.12 D) (P = 1.000, unpaired t-test). In the subgroup of subjects aged 8.0 to 10.9 years, the increases in axial length over 1 year did not differ significantly between the combination (0.10 ± 0.15 mm) and the monotherapy group (0.22 ± 0.18 mm) (P = 0.115, unpaired t test). In the subgroup of subjects aged 11.0–12.9 years, the increases in axial length over 1 year did not differ significantly between the combination (0.09 ± 0.08 mm) and monotherapy group (0.16 ± 0.11 mm) (P = 0.154, unpaired t test). The increases in axial length over 1 year in both subgroups were similar and tended to be smaller in the combination than the monotherapy group, although they did not reach significance.
The subjects were stratified by SER at enrollment (− 1.00 to − 3.00 vs. − 3.01 to − 6.00 D) to compare the increases in axial length over 1 year between the combination and monotherapy groups (Fig. 6). In the subgroup of subjects with a SER of − 1.00 to − 3.00 D (12 subjects in the combination group and 11 in the monotherapy group), the increase in axial length over 1 year was significantly smaller in the combination (0.11 ± 0.10 mm) than in the monotherapy group (0.28 ± 0.12 mm) (P < 0.001, unpaired t test), with a difference of 0.17 mm between the groups. In the subgroup of subjects with a SER of − 3.01 to − 6.00 D (8 subjects in the combination group and 9 in the monotherapy group), the increases in axial length over 1 year did not differ significantly between the combination (0.08 ± 0.15 mm) and the monotherapy group (0.07 ± 0.08 mm) (P = 0.900, unpaired t test). In the subgroup of subjects with a SER of − 1.00 to − 3.00 D, the ages at enrollment did not differ significantly between the combination (10.71 ± 1.27 years) and the monotherapy group (9.83 ± 1.95 years) (P = 0.207, unpaired t test), although the age in the monotherapy group tended to be younger than that in the combination group. In the subgroup of subjects with a SER of − 3.01 to − 6.00 D, the ages did not differ significantly between the combination (11.12 ± 1.57 years) and the monotherapy group (11.09 ± 1.57 years) (P = 0.975, unpaired t test).
Comparison of the increases in axial length over 1 year between the orthokeratology and atropine 0.01% combination group and the orthokeratology monotherapy group stratified by spherical equivalent refractive error at enrollment [− 1.00 to − 3.00 diopters (D) vs. − 3.01 to − 6.00 D]. Error bars represent the standard deviation
The adherence rates to the treatment protocol during the 1-year period, defined as ≥ 75% expected use of OK lenses, were 95% for the combination, and 100% for the monotherapy group. No significant difference between the two groups was observed in the adherence to the use of OK lenses (P = 0.342, Mann–Whitney U test). The adherence rate to the treatment protocol, defined as ≥ 75% expected use of atropine 0.01% ophthalmic solution, was 80% in the combination group during the 1-year period. During the 1-year study period of OK therapy in both groups, none of the subjects were re-prescribed with new OK lenses due to a decrease in the uncorrected distant visual acuity of over 0.3 logMAR units.
One subject in the monotherapy group suffered from infiltrates in the upper cornea OD after 9 months. She was withdrawn from the study and did not complete the follow-up evaluation at the end of 1 year. She discontinued OK therapy and switched to spectacles. The corneal infiltrates resolved after topical antimicrobial therapy. One subject in the combination group had mild superficial punctate keratopathy (SPK) OU after 6 months and was observed since she had no subjective symptoms. However, the SPK worsened and subjective symptoms appeared at the end of 1 year, at which point she switched from OK to spectacles. The SPK resolved 1 month after she had started to use spectacles. No subjects in the combination group dropped out because of photophobia, impairment of near visual acuity, allergic reaction, microbial infection, or systemic adverse effects caused by the atropine 0.01% ophthalmic solution.
Discussion
To our knowledge, this is the first study evaluating the additive effects of OK and atropine 0.01% ophthalmic solution in slowing axial elongation in children with myopia. Axial elongation over 6 months and 1 year was significantly suppressed by combining OK and atropine 0.01% ophthalmic solution compared to OK monotherapy, although axial elongation over the first 3 months of only OK lenses wear in both groups did not differ significantly between the two groups (Fig. 2).
Table 3 compares the axial elongation over 1 year in this and other studies. Chia et al. report that the axial length in children receiving atropine 0.5, 0.1, and 0.01% ophthalmic solutions increased by 0.11, 0.13, and 0.24 mm, respectively [14]. The increase in axial length over 1 year in children in our study receiving OK and atropine 0.01% ophthalmic solution was smaller than that in children receiving atropine 0.5% ophthalmic solution, although simple comparison might not be appropriate because of differences between the two studies in the ethnicity and age of the subject population and methodology. In the study by Hiraoka et al., which was similar to our study in terms of the ethnic background (Japanese) and age (8–12 years) of the subject population, as well as the method (IOLMaster) of axial length measurement, the axial length increased by 0.19 mm in children receiving OK and 0.38 mm in those using single focus spectacles [17]. In our study, the result in children receiving OK monotherapy, 0.19 mm, was consistent with the study by Hiraoka et al., and the combination of OK and atropine 0.01% ophthalmic solution was 53% more effective in slowing axial elongation than OK monotherapy.
Adherence rate to therapy, defined as ≥ 75% expected use of atropine 0.01% ophthalmic solution, was 80% in the combination group during the 1-year period. The adherence to the use of atropine 0.01% ophthalmic solution in the combination group in this study was worse compared to the adherence rate of 98.8% to the use of atropine 0.01% ophthalmic solution during the 2-year period in the ATOM2 study [14]. This finding might have resulted from the need to perform two procedures before sleeping. A timing device to remind the subject to apply atropine 0.01% ophthalmic solution was required.
In the present study, a strong positive correlation was observed between the increase in axial length over 1 year and SER at enrollment in the monotherapy group, showing a larger increase in subjects with lower myopia. This significant correlation was confirmed even after adjustment for age by multiple linear regression analysis. There was no significant correlation between the two parameters in subjects receiving OK and atropine 0.01% ophthalmic solution (Fig. 4). When the subjects were stratified by SER at enrollment to compare the increases in axial length over 1 year, the difference in axial length increases between the two groups was larger in subjects with a SER of − 1.00 to − 3.00 D than in all subjects with a SER of − 1.00 to − 6.00 D, while no difference between the two groups was observed in subjects with a SER of − 3.01 to − 6.00 D (Fig. 6). The ages in both subgroups of subjects with a SER of − 1.00 to − 3.00 and − 3.01 to − 6.00 D did not differ significantly between the two groups, although the age of subjects with a SER of − 1.00 to − 3.00 D in the monotherapy group tended to be younger than that in the combination group. These findings suggest that the suppressive effect of OK monotherapy on axial elongation was affected by the SER rather than the age of the children. In their meta-analysis, Li et al. report that OK slows axial elongation more effectively in children with higher myopia than with lower myopia [40]. Therefore, it was considered that the addition of atropine 0.01% ophthalmic solution to OK therapy was more effective in slowing axial elongation in children with lower myopia who responded poorly to OK therapy compared to those with higher myopia, while OK monotherapy was as effective as the combination of OK and atropine 0.01% in children with higher myopia.
Although the mechanisms by which atropine and OK slow the progression of myopia remain uncertain, the most credible mechanism of OK appears to be improvement of defocus on the peripheral retina [30, 31] with increases in the higher-order aberration [32, 33] through corneal epithelial redistribution in which the central cornea is thinned, and the mid-periphery is thickened [34, 35]. According to this hypothesis, when the amount of myopia correction by OK therapy becomes larger, the defocus on the peripheral retina is further improved with change from hyperopic toward myopic defocus. Therefore, the defocus on the peripheral retina in OK monotherapy subjects with a SER of − 3.01 to − 6.00 D at enrollment might have been sufficiently improved, but not in those with a SER of − 1.00 to − 3.00 D. The most credible theory of mechanism of atropine appears to be blocking of the muscarinic receptors in the retina and sclera that mediate axial elongation [28, 29]. It seems reasonable that adding atropine 0.01% ophthalmic solution to OK therapy was more effective through this mechanism in subjects with a SER of − 1.00 to − 3.00 D at enrollment, where the defocus on the peripheral retina was insufficiently improved by OK monotherapy. However, we did not measure the peripheral refraction and higher-order aberration in this study.
Recently, it has been reported that violet light (VL, 360–400 nm wavelength), abundant in the outdoor environment, reduced the progression of myopia [41,42,43,44]. In the ATOM2 study, pupil diameter in the atropine 0.01% group increased by about 1 mm after starting instillation [14]. Increasing VL exposure of the eyes can make combined OK and atropine 0.01% ophthalmic solution more effective in slowing axial elongation than OK monotherapy. It was considered that, before the start of OK many subjects with higher myopia had used non-VL transmitting spectacles or contact lenses regularly. Therefore, in subjects with higher myopia VL exposure of the eyes could have been suddenly increased after the start of OK, where the suppressive effect of myopia progression was greater. However, in this study we did not take into consideration the measures used to correct myopia before the start of OK, and the pupil diameters of subjects.
This report has some limitations. The number of subjects was small and observation period was relatively short. A greater number of subjects should be compared for a longer period of time to confirm the real effectiveness of this therapy. We will continue to enroll additional subjects and follow them for a longer period of time. Secondly, we did not measure the peripheral refraction and higher-order aberration in this study. The measurement of these values will be required to clarify the mechanism of the additive effects of OK and atropine 0.01% ophthalmic solution. A study should be conducted to clarify this mechanism. Thirdly, we did not take into consideration the environmental factors such as near work time and outdoor activity time, the measures used to correct myopia before the start of OK, and the pupil diameters of subjects. The suppressive effect of myopia progression by VL exposure should be also taken into consideration in future studies.
Our conclusions are: during the 1-year follow-up, the combination of OK and atropine 0.01% ophthalmic solution was more effective in slowing axial elongation than OK monotherapy in children with myopia. The additive effects of OK and atropine 0.01% ophthalmic solution in slowing axial elongation was greater in children with lower myopia, while OK alone was as effective as combining OK and atropine 0.01% ophthalmic solution in children with higher myopia. The combination of OK and atropine 0.01% ophthalmic solution, two treatments that complement each other, could be an effective procedure to slow the progression of myopia.
References
Dolgin E. The myopia boom. Nature. 2015;519:276–8.
Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739–48.
Vitale S, Sperduto RD, Ferris FL. Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127:1632–9.
Lin LL, Shih YF, Hsiao CK, Chen CJ. Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann Acad Med Singap. 2004;33:27–33.
Matsumura H, Hirai H. Prevalence of myopia and refractive changes in students from 3 to 17 years of age. Surv Ophthalmol. 1999;44:S109–15.
Saw SM, Chua WH, Gazzard G, Koh D, Tan DTH, Stone RA. Eye growth changes in myopic children in Singapore. Br J Ophthalmol. 2005;89:1489–94.
Hyman L, Gwiazda J, Hussein M, Norton TT, Wang Y, Marsh-Tootle W, et al. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch Ophthalmol. 2005;123:977–87.
Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123:1036–42.
Chua SY, Sabanayagam C, Cheung YB, Chia A, Valenzuela RK, Tan D, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt. 2016;36:388–94.
Kimura S, Hasebe S, Miyata M, Hamasaki I, Ohtsuki H. Axial length measurement using partial coherence interferometry in myopic children: repeatability of the measurement and comparison with refractive components. Jpn J Ophthalmol. 2007;51:105–10.
Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012;31:622–60.
Asakuma T, Yasuda M, Ninomiya T, Noda Y, Arakawa S, Hashimoto S, et al. Prevalence and risk factors for myopic retinopathy in a Japanese population The Hisayama Study. Ophthalmology. 2012;119:1760–5.
Chua WH, Balakrishnan V, Chan YH, Tong L, Ling Y, Quah BL, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285–91.
Chia A, Chua WH, Cheung YB, Wong WL, Lingham A, Fong A, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology. 2012;119:347–54.
Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) Study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53:7077–85.
Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain: refractive and biometric changes. Invest Ophthalmol Vis Sci. 2012;53:5060–5.
Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci. 2012;53:3913–9.
Kakita T, Hiraoka T, Oshika T. Influence of overnight orthokeratology on axial elongation in childhood myopia. Invest Ophthalmol Vis Sci. 2011;52:2170–4.
Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol. 2009;93:1181–5.
Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005;30:71–80.
Huang JH, Wen DZ, Wang QM, McAlinden C, Flitcroft I, Chen HS, et al. Efficacy comparison of 16 interventions for myopia control in children a network meta-analysis. Ophthalmology. 2016;123:697–708.
Walline JJ. Myopia control: a review. Eye Contact Lens. 2016;42:3–8.
Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572–9.
Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2 myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123:391–9.
Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014;157:451–7.
Chan B, Cho P, Cheung SW. Repeatability and agreement of two A-scan ultrasonic biometers and IOLMaster in non-orthokeratology subjects and post-orthokeratology children. Clin Exp Optom. 2006;89:160–8.
Carkeet A, Saw SM, Gazzard G, Tang W, Tan DTH. Repeatability of IOLMaster biometry in children. Optom Vis Sci. 2004;81:829–34.
Gallego P, Martinez-Garcia C, Perez-Merino P, Ibares-Frias L, Mayo-Iscar A, Merayo-Lloves J. Scleral changes induced by atropine in chicks as an experimental model of myopia. Ophthalmic Physiol Opt. 2012;32:478–84.
McBrien NA, Arumugam B, Gentle A, Chow A, Sahebjada S. The M4 muscarinic antagonist MT-3 inhibits myopia in chick: evidence for site of action. Ophthalmic Physiol Opt. 2011;31:529–39.
Kang PL, Swarbrick H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses. Optom Vis Sci. 2011;88:476–82.
Smith EL, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49:2386–92.
Hiraoka T, Kakita T, Okamoto F, Oshika T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology. 2015;122:93–100.
Mathur A, Atchison DA. Effect of orthokeratology on peripheral aberrations of the eye. Optom Vis Sci. 2009;86:476–84.
Alharbi A, Swarbrick HA. The effects of overnight orthokeratology lens wear on corneal thickness. Invest Ophthalmol Vis Sci. 2003;44:2518–23.
Swarbrick HA, Wong G, O’Leary DJ. Corneal response to orthokeratology. Optom Vis Sci. 1998;75:791–9.
Soni PS, Nguyen TT, Bonanno JA. Overnight orthokeratology: refractive and corneal recovery after discontinuation of reverse-geometry lenses. Eye Contact Lens. 2004;30:254–62 (discussion 63-4).
Nichols JJ, Marsich MM, Nguyen M, Barr JT, Bullimore MA. Overnight orthokeratology. Optom Vis Sci. 2000;77:252–9.
Sieg JW, Robinson JR. Mechanistic studies on transcorneal permeation of pilocarpine. J Pharm Sci. 1976;65:1816–22.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Li SM, Kang MT, Wu SS, Liu LR, Li H, Chen Z, et al. Efficacy, safety and acceptability of orthokeratology on slowing axial elongation in myopic children by meta-analysis. Curr Eye Res. 2016;41:600–8.
Williams KM, Bentham GC, Young IS, McGinty A, McKay GJ, Hogg R, et al. Association between myopia, ultraviolet B radiation exposure, serum vitamin D concentrations, and genetic polymorphisms in vitamin D metabolic pathways in a Multicountry European Study. JAMA Ophthalmol. 2017;135:47–53.
Torii H, Kurihara T, Seko Y, Negishi K, Ohnuma K, Inaba T, et al. Violet light exposure can be a preventive strategy against myopia progression. EBioMedicine. 2017;15:210–9.
Torii H, Ohnuma K, Kurihara T, Tsubota K, Negishi K. Violet light transmission is related to myopia progression in adult high myopia. Sci Rep. 2017;7:14523.
Xiong S, Sankaridurg P, Naduvilath T, Zang J, Zou H, Zhu J, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. 2017;95:551–66.
Acknowledgements
The authors thank Shigeto Shimmura, MD (Keio University School of Medicine) for English editing, and J-SENSATION (Jichi Medical University) for their editing support. This study was supported by JSPS KAKENHI Grant Number JP26462646 from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
N. Kinoshita, None; Y. Konno, None; N. Hamada, None; Y. Kanda, None; M. S. -Tomita, None; A. Kakehashi, None.
Additional information
Corresponding author: Nozomi Kinoshita
About this article
Cite this article
Kinoshita, N., Konno, Y., Hamada, N. et al. Additive effects of orthokeratology and atropine 0.01% ophthalmic solution in slowing axial elongation in children with myopia: first year results. Jpn J Ophthalmol 62, 544–553 (2018). https://doi.org/10.1007/s10384-018-0608-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-018-0608-3