Abstract
Purpose
Our purpose was to evaluate the anatomical and functional changes in retinae of rabbit eyes following monocular intravitreal injection of sodium iodate (SI).
Methods
Twenty albino rabbits were divided into four groups and underwent monocular intravitreal injection with four different doses of SI (0.1, 0.2, 0.4, and 0.8 mg). Before and for 28 days after injection, the eyes were examined using fundus photography, optical coherence tomography (OCT), and electroretinography (ERG). At postinjection days 2, 7, and 28, the eyes were enucleated and underwent histological examination.
Results
On fundus examination, no distinct retinal changes were seen in any group except the 0.8-mg group, which showed chorioretinal vascular attenuation. In 0.1 and 0.2-mg groups, no significant anatomical changes were found except transient hyperreflective dots over the vitreoretinal interface on OCT. In 0.4 and 0.8-mg groups, disruption of the ellipsoid zone and diffuse retinal swelling were observed in the early period on OCT. In the 0.4-mg group, the outer retina was significantly destroyed at day 28, whereas the inner retina was relatively preserved. In the 0.8-mg group, the entire retina was destroyed irreversibly. The b-wave of ERG was reduced immediately in all groups, which recovered fully (0.1- and 0.2-mg groups), partially (0.4-mg group), or never (0.8-mg group). No structural or functional abnormalities were found in the fellow control eyes.
Conclusions
Retinal degeneration following intravitreal injection of SI appears to be dose dependent; retinal damage is reversible at low doses but irreversible at high doses. At a certain dose, the outer retina may be preferably ablated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Experimental animal models for retinal degeneration are required to investigate diseases involving retinal pigment epithelium (RPE) and photoreceptors [1]. Transgenic animal models have been developed to meet this need [2, 3], and a laser-induced model has also been promoted [4]. Most commonly, several retinotoxic agents, including iodoacetate, nitroprusside, and N-methyl-N-nitrosourea, have been used to induce retinal degeneration [5–7].
Among these, sodium iodate (SI) has been widely adopted to induce outer retinal degeneration in various animals [8, 9]. Systemic injection of SI is reported to damage the RPE and photoreceptors selectively [10, 11] and has been used in many studies on retinal physiology, stem-cell therapy, and retinal prostheses [12–15]. However, intravenous administration always induces bilateral retinal degeneration, leaving no healthy eye for use as a normal control. Systemic toxicity may also occur after intravenous injections [16]. To settle these issues, some investigators tried monocular intravitreal injection of SI [17], but the sequential retinal changes after injection and dose–response relationship are yet to be explored in detail.
In this study, we investigated the anatomical and functional changes of rabbit retinae over time after monocular intravitreal injection of SI using several different doses. Rabbits, which have an eyeball size similar to that of humans, were chosen to facilitate successive studies. Elucidating the process of retinal degeneration would provide a better understanding of the mechanism of SI-induced retinal toxicity and the repair course after toxic retinal damages. To our knowledge, this is the first comprehensive study of retinal degeneration after intravitreal injection of SI in animals.
Materials and methods
Animals
Adult female New Zealand white rabbits weighing 2.5–3.5 kg were used for the study. The animals were 2 months of age and were reared in a temperature- and light-controlled environment that provided 12 h of light and 12 h of dark. Rabbits with any ocular pathology under pre-experimental examination were excluded; 20 rabbits were studied. The study design and experimental protocols were approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital. All experimental procedures involving animals adhered to the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research.
Intravitreal injection of SI
Sterile solutions of SI were freshly prepared at four concentrations (2, 4, 8, and 16 mg/ml) using solid SI powder (S4007, Sigma-Aldrich, St. Louis, MO, USA) dissolved in 0.9 % normal saline. Rabbits were anesthetized using a mixture of tiletamine hydrochloride/zolazepam hydrochloride (Zoletil®, Virbac, Carros, France; 15 mg/kg) and xylazine hydrochloride (Rompun®, Bayer Corp., Shawnee Mission, KA, USA; 0.2 ml/kg). Topical proparacaine hydrochloride (Alcaine®, Alcon laboratories, Inc., Fort Worth, TX, USA) and a diluted povidone–iodine solution were applied to the eye before injection.
Twenty rabbits were randomly allocated to four groups (N = 5 for each group) and injected with a volume of 0.05 ml corresponding to the doses of 0.1, 0.2, 0.4, or 0.8 mg assigned to each group. The injections were administered intravitreally to the right eye using a 30-gauge needle syringe inserted 2 mm posterior to the limbus. No injections were administered to the left eyes. The injected right eyes were treated with a daily application of topical fluoroquinolone solution for up to 3 days after injection. All eyes were evaluated anatomically and functionally before (day 0), and after injection on days 1, 2, 4, 7, 14, 21, and 28.
Fundus photography and optical coherence tomography (OCT)
To observe apparent anatomical retinal changes, fundi were examined using a SuperQuad® 160 lens (Volk Optical, Inc., Mentor, OH, USA) positioned over the cornea after applying topical phenylephrine hydrochloride and tropicamide drops (Mydrin®-P, Santen, Osaka, Japan) to achieve maximal pupillary dilation. Color fundus photographs were obtained from all eyes using a digital camera (D60, Nikon Corp., Tokyo, Japan). Then, in vivo swept-source (SS) OCT was performed using a deep-range imaging (DRI) OCT-1 Atlantis® system (Topcon, Tokyo, Japan) and following a 9-mm high-resolution volume scan protocol. The SS-OCT system used a light source with a wavelength–sweeping laser centered at 1050 nm and had an axial resolution of 8 µm [18]. The area of visual streak below the optic disc, 3-mm ventral to the inferior edge of the optic nerve head, was scanned in cross section without a lens.
Electroretinography
All eyes were evaluated using electroretinography (ERG) during each follow-up examination. Before ERG, rabbits were adapted to the dark for at least 30 min and were anesthetized by intramuscular injections using a mixture of tiletamine hydrochloride/zolazepam hydrochloride and xylazine hydrochloride, as described above. The pupils were maximally dilated before ERG recording, and the cornea was anesthetized with topical proparacaine hydrochloride drops. ERGs were recorded using a hand-held multispecies ERG unit (HMsERG, OcuScience, Henderson, NV, USA). A contact lens electrode with a built-in light-emitting diode (EW-202/LS-C, Mayo Corp., Inazawa, Japan) was used on a drop of 2 % methyl cellulose over the cornea. A reference electrode was placed on the occipital area subcutaneously halfway between the base of the ear and the eye, and the ground electrode was placed in the ear. ERG signals were filtered between a low-pass 300-Hz and high-pass 0.3-Hz filter [19].
ERGs were performed using the scotopic 3.0 protocol for maximal combined rod and cone response with a flash intensity of 1000 cd/m2 and a duration of 3 ms, according to the International Society for Clinical Electrophysiology of Vision standard protocol [19]. All examinations were monocular, with the companion eye occluded, carried out at room temperature, and with no evident tachypnea observed. Five responses were measured in each eye with an interval of >10 s. Each measurement was averaged with four responses with a stimulus interval of 10 s. Analysis was based on amplitude measurements of the a-wave (from baseline to negative trough) and b-wave (from a-wave trough to the positive peak of the b-wave). ERG changes were considered significant if the difference in a-wave or b-wave amplitude was >30 % of baseline [20].
Histological examination
On days 2, 7, and 28, one rabbit from each group was euthanized using a lethal dose of potassium chloride (35 mg/kg) injected intravenously into the marginal auricular vein. Just after death, both eyes were enucleated and immersed in a mixture of 10 % neutral-buffered formalin and 2.5 % glutaraldehyde for 4 days. After fixation, the globes were cut into two pieces bisecting the visual streak perpendicularly. One of the bisected pieces was dehydrated using a series of 95 % ethanol baths and embedded in paraffin. Transretinal sections 5-μm-thick were obtained by microtome sectioning on poly-l-lysine-coated microscopic slides and stained with hematoxylin and eosin (H&E). The slides were examined to detect pathological changes in the retina using a light microscope (BX-50, Olympus Corp., Tokyo, Japan) and photographed with a digital video camera (DP-71 CCD, Olympus). Consistency of retinal layers, loss of cellular elements, apoptotic or necrotic degeneration, and density of stained nuclei within each layer were assessed [20]. To determine ultramicroscopic pathologic changes, the other bisected piece was immersed in a 2.5 % glutaraldehyde solution and processed with osmium tetroxide for transmission electron microscopy (TEM). Consistency of intracellular organelles such as mitochondria, endoplasmic reticulum, or nucleoli was carefully examined.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD). Data were analyzed using the Mann–Whitney U test, Wilcoxon signed-rank test, or repeated measure Kruskal–Wallis test. All statistical tests were two-tailed, and significance was defined as P < 0.05. Statistical analyses were performed using SPSS 20.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
Anatomical changes on fundus examination
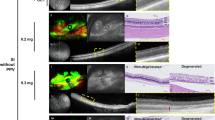
Color-fundus photographs of the eyes are presented in Fig. 1. In low-dose groups (0.1 and 0.2 mg), no apparent retinal changes were observed during the follow-up examinations (Fig. 1a, b). On the other hand, there was transient vitreous haze at day 7 in the 0.4-mg group, which cleared thereafter (Fig. 1c). In the 0.8-mg group, attenuation of the choroidal and retinal vasculature was observed after day 7, as well as atrophy of the retina and choroid (Fig. 1d). Chorioretinal atrophy and vascular attenuation progressed during the follow-up period, leading to retinal detachment in one eye at day 21. The anterior segment showed no distinct abnormal findings in all dose groups.
Sequential fundus photographs at days 0, 7, 14, and 28 of rabbits that received intravitreal injection of sodium iodate (SI). a–d Fundus images of the eyes injected with 0.1, 0.2, 0.4, and 0.8 mg of SI, respectively. e Fundus images of the noninjected eye of the rabbit that received intravitreal injection of 0.8 mg SI
In fellow control eyes, no abnormalities were found in fundi of all dose groups throughout the follow-up period, even in the group receiving the highest dose (0.8 mg) (Fig. 1e). All rabbits showed normal behavior and activity without any significant weight loss.
Anatomical changes on OCT
OCT images over time are presented in Fig. 2. In the low-dose groups (0.1 and 0.2 mg), hyperreflective dots were observed over the vitreoretinal surface and in the posterior vitreous cavity on OCT examination at day 7; three of four (75 %) living rabbits at day 7 showed hyperreflective dots. In the 0.1-mg group, sparse hyperreflective dots appeared at day 7, but no other anatomical abnormality was seen on OCT during the follow-up period. At day 28, OCT images returned to normal, being the same as the image at day 0, in all rabbits (Fig. 2a). In the 0.2-mg group, multiple hyperreflective dots appeared at day 4, accompanied by mild blurring of the ellipsoid zone, which appeared in two of the four (50 %) living rabbits. These changes recovered over time, achieving normal retinal structures at day 7, except for a few remnant hyperreflective dots (Fig. 2b).
Sequential retinal optical coherence tomography (OCT) images at days 0, 1, 2, 4, 7, 14, and 28 of rabbits that received intravitreal injection of sodium iodate (SI). a–d OCT images of eyes injected with 0.1, 0.2, 0.4, and 0.8 mg of SI, respectively. e OCT images of the noninjected eye of the rabbit that received intravitreal injection of 0.8 mg SI. Arrows on OCT show hyperreflective dots; arrowheads indicate blurring of the ellipsoid zone on OCT
In the 0.4-mg group, disruption of the ellipsoid zone was observed at day 1 in all rabbits (Fig. 2c). Entire retinal layers showed an increased signal reflectance and mild swelling, with swelling of the retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL) being more prominent. At day 2, the internal limiting membrane (ILM) was split from the RNFL in part of the retina in three of five (60 %) living rabbits. Seven days after intravitreal injection, the outer nuclear layer (ONL) was significantly reduced. By contrast, the inner nuclear layer (INL) was readily identified. After 2 weeks, the inner plexiform layer (IPL) and INL were slightly thinned, but the outer plexiform layer (OPL) was significantly reduced and the ONL almost gone. This outer retinal thinning and the relative preservation of the inner retina remained until day 28 in all living rabbits. At day 28, the increased signal reflectance on OCT returned to normal and hyperreflective dots disappeared.
In the 0.8-mg group, significant swelling of the inner retinal layer was found at postinjection day 1 among all rabbits (Fig. 2d). The swollen inner retina eventually split the next day. After splitting away of the inner retina, destruction of the outer retina was continuous, and it became almost completely atrophied by day 7 in three of the four (75 %) living rabbits. No recovery was observed by day 28, when the retinal layer was hardly visible on OCT in all living rabbits, and the underneath choroid was also thinned. By contrast, the fellow eye showed no abnormalities during the follow-up period (Fig. 2e).
Functional changes on ERG
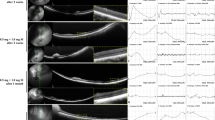
Changes in ERG waveform in each group are demonstrated in Fig. 3. Even in the lowest dose group (0.1 mg), an instantaneous change was observed in the ERG waveform beginning 1 day after injection (Fig. 3a). The prompt change was loss of b-wave in all dose groups and loss of a-wave in the ≥0.2 mg dose groups. These changes recovered completely in the low-dose groups (0.1 and 0.2 mg), but recovery was only partial in the 0.4-mg group. In the 0.8-mg group, ERG was extinguished from the day after injection and never recovered (Fig. 3d).
Changes over time in a- and b-wave amplitude of ERG in each group are suggested in Figs. 4 and 5. The a-wave amplitude was reduced in all dose groups in the early period (Fig. 4) and was reversible in the 0.1- and 0.2-mg groups but irreversible in the 0.8-mg group until day 28.
The a-wave amplitude of electroretinography (ERG) in rabbits before and after intravitreal injection of sodium iodate (SI). a–d Change of a-wave amplitude over time after injection with 0.1, 0.2, 0.4, and 0.8 mg doses of SI, respectively. Dot-dash-dot lines indicate injected eyes; solid lines represent noninjected fellow eyes
The b-wave amplitude of electroretinography (ERG) in rabbits before and after intravitreal injection of sodium iodate (SI). a–d The change of b-wave amplitude over time after injection with 0.1, 0.2, 0.4, and 0.8 mg doses of SI, respectively. Dot-dash-dot lines indicate injected eyes; solid lines represent noninjected fellow eyes
Amplitude of the b-wave was decreased at day 1 in all groups (Fig. 5), being reversible in low-dose groups (0.1 and 0.2 mg). In the 0.4-mg group, mean b-wave amplitude at baseline was 178.7 ± 37.1 µV, but the magnitude was significantly decreased to one fifth (35.5 ± 16.2 µV) at day 28 (P = 0.005). In comparison, the a-wave and b-wave were extinguished completely and were never restored in the 0.8-mg group.
Histological changes on light microscopy
Histological examination using H&E staining under light microscopy is presented in Fig. 6. There were no remarkable abnormalities in the RPE or neurosensory retina in eyes injected with SI at doses of 0.1 or 0.2 mg (Fig. 6a, b). All retinal layers showed normal cellularity and intact structures.
In the 0.4 mg group, there was photoreceptor destruction at day 7 showing only scanty photoreceptor nuclei (Fig. 6c). The ONL and OPL thicknesses were significantly decreased. After 1 month, photoreceptor layers, including the ONL and OPL, had hardly recovered, but the inner retinal layers, including the INL, IPL, and GCL, were quite preserved. In the highest dose group (0.8 mg), the retinal specimen at day 2 showed the split of ILM and destruction of the inner retina (Fig. 6d). At day 7, the entire retina was destroyed almost completely, which persisted during follow-up examinations.
Histologic changes on electron microscopy
TEM images at days 2 and 28 of each group are shown in Fig. 7. RPE cells were not significantly influenced by 0.1 or 0.2 mg of SI at day 2 (Fig. 7a, b). In eyes injected with 0.4 mg of SI, mitochondrial swelling was found in the cytoplasm of the RPE cells (Fig. 7c). In the 0.8-mg group, disruption of nuclear membrane, shrinkage of nuclei, loss of endoplasmic reticulum, as well as intracellular organelles and multiple inclusion bodies, were observed in RPE cells (Fig. 7d).
Transmission electron microscopy images of rabbits that received intravitreal injection of sodium iodate (SI). a–d Retinal pigment epithelium (RPE) at day 2 of eyes injected with 0.1, 0.2, 0.4, and 0.8-mg dose of SI, respectively (e–h) RPE at day 28 in eyes injected with 0.1, 0.2, 0.4, and 0.8 mg dose of SI, respectively
At day 28, electron microscopic examination showed no remarkable changes in the RPE and photoreceptors of eyes injected with 0.1 mg of SI (Fig. 7e). In the 0.2-mg group, photoreceptors did not show significant changes, but some RPE cells showed intracellular vacuoles at day 28 (Fig. 7f). In the 0.4-mg group, photoreceptors were very scarce, and RPE cells were observed to be partly covering and in between the INL and choroid. The nucleolus was less prominent compared with baseline, and the number of cytoplasmic pigmentary bodies increased (Fig. 7g). In eyes injected with 0.8 mg of SI, the retina was entirely destroyed at day 7, and only deformed RPE cells with multiple cytoplasmic vacuoles were sparsely left. At day 28, RPE cells were slightly increased in number, had a lengthy nucleus, and composed a thin layer over the choroid (Fig. 7h).
Discussion
Here we report results of a novel method of introducing retinal degeneration using intravitreal injection of SI, retinal changes observed over time for several different doses of SI. Both anatomical and functional changes occurred in a dose-dependent manner: retinal degeneration was reversible on low and irreversible on high doses; no abnormalities were seen in noninjected fellow eyes.
Structural changes after intravitreal injection of SI appeared consistently on OCT and histologic examinations. Anatomical changes were very subtle when low doses were injected but prominent following high doses. Typical initial findings were retinal swelling and disruption of the ellipsoid zone, which were observed immediately from day 1 in high-dose groups. Electron microscopic findings of RPE cells were also marked in high-dose groups. Previous studies reported that retinal degeneration, such as breakage of the RPE barrier, can appear within 24 h of intravenous injection of SI [10, 21, 22]. The early findings on electron microscopy included defragmentation of RPE cell nuclei, mitochondrial swelling, loss of smooth endoplasmic reticulum and cytoplasmic organelles, disappearance of basal infoldings, and intracellular granular debris [12, 23, 24]. When a high dose of SI is injected intravenously, these changes occur as early as 7–10 h after injection [23]. Similar changes were observed in the 0.8-mg group of this study and at day 2 were very similar to those at 24 h after intravenous injection of 50 mg/kg SI [23].
Notably, the significant changes in the inner retina during the early period in this study, such as pronounced swelling of RNFL and split of ILM from RNFL, are not reported in previous studies using intravenous delivery [11, 14, 24]. Main structural changes during the first few days after intravenous injection were necrotic destruction of the outer retina but not that of the inner retina [8, 25]. The intense inner retinal reaction in the study presentedhere might be attributable to the administration route, because intravitreally injected SI meets the inner retina before meeting the outer retina. Conversely, intravenous injection would deliver chemical agents to inner and outer retinas simultaneously through retinal vessels or to the outer retina in greater abundance because of choriocapillaris blood flow is more plentiful than that of retinal vessels [23].
In this study, retinal destruction occurring within the first week became stable thereafter. Previous studies report recovery of RPE cells 1–2 weeks after SI injection [22, 26, 27]. Necrotic RPEs are removed by macrophages after vigorous destruction, and then the spared RPEs regenerate [28]. Korte el al. reported recovery of RPE barrier breakdown 1 week after injection [29], and it is also reported that RPE cells regenerate or are replaced by a heterogeneous cell layer 1 week after intravenous injection of SI [30]. Two weeks following SI injection, regenerated flat-shaped RPE cells with short microvilli were observed over Bruch’s membrane in rats [31]. Regenerating RPE showed changes in cell shape, polarity, and intercellular adhesion [29] and are suggested to support surviving photoreceptors and help their recovery [32]. It is suggested that the regenerative process in the retina involves the expression of multiple genes involved in retinal neurogenesis, cell differentiation, and re-establishment of morphologic structure [33]. The final retinal status 1 month after injection of high-dose SI included retinal thinning, reduction of RPE and photoreceptors, retinal vascular attenuation, and atrophy of the choriocapillaris, which were similar to those seen after intravenous injection [11, 29, 34]. To sum up, changes over time after intravitreal injection in this study were consistent with those after intravenous administration [26].
On the other hand, fundus changes visible to the naked eye were not distinct in most groups except the highest dose (0.8 mg) group. While there was depigmentation of RPE and RPE and choriocapillaris atrophy in pigmented rabbits after the injection of SI [9, 14], visible fundus changes in albino rabbits are suggested to be indistinct [9], probably because of the absence of melanin pigments. However, we identified the attenuation of chorioretinal vasculature and retinal detachment in eyes of the 0.8-mg group. A close fundus examination would enable detection of subtle changes caused by SI toxicity.
Functional changes occurred instantaneously in all investigated doses, although structural changes were not apparent in low-dose groups. The early change was an abrupt vanishment of b-wave, which occurred immediately after injection, as previously reported [34, 35]. Whereas histologic findings at day 2 and OCT findings at days 1 and 2 appeared to be normal in low-dose groups, the ERG waveform dramatically diminished, showing the negative ERG pattern beginning the day after injection. The transient negative ERG was previously reported in studies using intravenous injection of SI and was attributed to third-order neuronal responses [36]. In the ≥0.2 mg dose groups, the loss of a-wave—which might be considered as the dysfunction of photoreceptors—was also observed. It is possible that hidden microscopic cellular changes may have existed. However, ERG was helpful in detecting more subtle functional changes after toxic retinal damage.
One of the interesting findings in this study is that although the outer and inner retinae were both ablated after intravitreal injection of SI, the inner retina was relatively preserved at a certain dose. After the 0.4-mg SI injection, the inner retina was not fully destroyed by day 7, and the damage did not worsen after that period. The ERG also showed a remaining response in this group, and response recovered slightly at day 28. The intact structure of the ganglion and bipolar cell layers resembled the histological finding in retinitis pigmentosa (RP) [37], a finding that may imply the usefulness of this model in RP research. The precise mechanism of damage to RPE cells is yet to be determined [35], but the degeneration process is hypothesized as being necrosis combined with kariolysis and melanin clumping [11, 23]. The mechanisms suggested for RPE cell damage by SI include inhibition of intracellular enzymes, such as triose phosphate dehydrogenase or lactate dehydrogenase [38]; destruction of zonula occludens; and alterations in the strength of adhesion [35, 39], breakdown of RPE cell basal membrane [40]; and denaturation of some retinal protein involved in metabolic cycles [23]. The increased ability of melanin to convert glycine to glyoxylate, a cytotoxic compound, might also contribute [41]. Of note, co-administration of cysteine reduced retinal damage caused by SI, presumably by interfering with the initial uptake, binding, or toxicity of SI within the eye [42].
On the other hand, the effect of SI on photoreceptors is considered to be secondary to RPE cell damage [11, 38, 43]. After breakdown of the blood–retinal barrier supported by RPE, photoreceptors are exposed to choroidal circulation directly and could be harmed continuously [11]. Destruction of photoreceptors is considered a process of apoptosis [11]; however, recent experiments propose that neuronal cells are also sensitive to SI toxicity and that intravenous administration of SI has a direct effect on photoreceptors [34, 44]. Further experiments are required to elucidate the mechanism of SI toxicity in greater detail.
In this study, retinal architecture and function were fully preserved in noninjected contralateral eyes, as seen in previous studies [17]. Recently, intravitreal injection of N-methyl-N-nitrosourea was used to induce monocular retinal degeneration [7]. Intravenous injection of verteporfin followed by photodynamic therapy, or intravitreal injection of sodium nitroprusside, has been suggested as a monocular photoreceptor degeneration model for rabbits [4, 6]. The benefits of a monocular model for retinal degeneration include use of the companion eye as a control, which provides a more controlled comparison, being within the same individual. It also reduces the number of animals necessary for the experiment and is more compatible with current ethical considerations for animal experiments. Our study suggests a new way of establishing monocular retinal degeneration using SI. The use of chemicals is also better from an economic perspective than expensive genetically engineered models. Achieving monocular degeneration within a short period is another advantage of this model.
There may be some shortcomings in the intravitreal administration of drugs. One possible disadvantage may be the unequal distribution of SI in the vitreous cavity due to uneven liquefaction of vitreous gel, which may reduce reproducibility of the SI effect on the retina. Nevertheless, reproducibility in this study was quite high. The high solubility of SI and the abundant content of water in the vitreous gel might explain the result. On the other hand, the margin between the effective and lethal dose was greater compared with that for intravenous injection [9]. The ratio of concentration resulting in entire retinal destruction to the ratio of concentration inducing minimal anatomical changes was within 8, which was higher than in the intravenous injection of SI [38]. Limitations of the study include the small number of subjects and individual differences in the responses to SI toxicity. In addition, if we had used pigmented rabbits, we could possibly have evaluated RPE damage more distinctly on fundus examination or fluorescein angiography. Nevertheless, a merit of this study was the use of OCT to evaluate structural changes over time as in vivo images. We determined that in vivo OCT images showed good correlation with histologic findings. Because most research on the retinal toxicity of SI was performed before the development of OCT [22, 23, 45], those researchers could not measure serial anatomical changes in the same individual. Recently, OCT enabled in vivo evaluation of the retina after SI administration in mice and rats, without needing to sacrifice the animals [10, 34, 46], but OCT data after SI injection in rabbits are not widely available [14], especially in albino rabbits.
This study is the first to present OCT data after SI injection in albino rabbit eyes. Based on this study, future studies can use in vivo OCT images withouth the need to sacrifice the animals for histological examination.
In conclusion, retinal degeneration over time following intravitreal injection of SI was dependent on injection dose. Anatomical and functional retinal changes were reversible at low and irreversible at high doses. In addition, we identified the possibility of novel monocular retinal degeneration, especially outer retinal degeneration, using intravitreal injection of SI. The outer retina was preferentially damaged after injecting a certain dose. This method could thus be useful for investigating outer retinal diseases, such as RP or age-related macular degeneration.
References
Chader GJ. Animal models in research on retinal degenerations: past progress and future hope. Vis Res. 2002;42:393–9.
Kondo M, Sakai T, Komeima K, Kurimoto Y, Ueno S, Nishizawa Y, et al. Generation of a transgenic rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci. 2009;50:1371–7.
Li T, Snyder WK, Olsson JE, Dryja TP. Transgenic mice carrying the dominant rhodopsin mutation P347S: evidence for defective vectorial transport of rhodopsin to the outer segments. Proc Natl Acad Sci USA. 1996;93:14176–81.
Nishida K, Kamei M, Kondo M, Sakaguchi H, Suzuki M, Fujikado T, et al. Efficacy of suprachoroidal-transretinal stimulation in a rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci. 2010;51:2263–8.
Yamauchi Y, Agawa T, Tsukahara R, Kimura K, Yamakawa N, Miura M, et al. Correlation between high-resolution optical coherence tomography (OCT) images and histopathology in an iodoacetic acid-induced model of retinal degeneration in rabbits. Br J Ophthalmol. 2011;95:1157–60.
Isago H, Sugano E, Murayama N, Tamai M, Tomita H. Establishment of monocular-limited photoreceptor degeneration models in rabbits. BMC Ophthalmol. 2013;13:19.
Rosch S, Johnen S, Mataruga A, Muller F, Pfarrer C, Walter P. Selective photoreceptor degeneration by intravitreal injection of N-methyl-N-nitrosourea. Invest Ophthalmol Vis Sci. 2014;55:1711–23.
Kiuchi K, Yoshizawa K, Shikata N, Moriguchi K, Tsubura A. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Curr Eye Res. 2002;25:373–9.
Sorsby A. Experimental pigmentary degeneration of the retina by sodium iodate. Br J Ophthalmol. 1941;25:58–62.
Yang Y, Ng TK, Ye C, Yip YW, Law K, Chan SO, et al. Assessing sodium iodate-induced outer retinal changes in rats using confocal scanning laser ophthalmoscopy and optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55:1696–705.
Machalinska A, Lubinski W, Klos P, Kawa M, Baumert B, Penkala K, et al. Sodium iodate selectively injuries the posterior pole of the retina in a dose-dependent manner: morphological and electrophysiological study. Neurochem Res. 2010;35:1819–27.
Kitano S, Hori S, Nagataki S. Transport of fluorescein in the rabbit eye after treatment with sodium iodate. Exp Eye Res. 1988;46:863–70.
Amirpour N, Karamali F, Rabiee F, Rezaei L, Esfandiari E, Razavi S, et al. Differentiation of human embryonic stem cell-derived retinal progenitors into retinal cells by Sonic hedgehog and/or retinal pigmented epithelium and transplantation into the subretinal space of sodium iodate-injected rabbits. Stem Cells Dev. 2012;21:42–53.
Wang K, Li XX, Jiang YR, Dong JQ. Influential factors of thresholds for electrically evoked potentials elicited by intraorbital electrical stimulation of the optic nerve in rabbit eyes. Vis Res. 2007;47:3012–24.
Siu T, Morley J. Implantation of episcleral electrodes via anterior orbitotomy for stimulation of the retina with induced photoreceptor degeneration: an in vivo feasibility study on a conceptual visual prosthesis. Acta Neurochir (Wien). 2008;150:477–85 (discussion 485).
Murray MM. The effects of administration of sodium iodate to man and animals. Bull World Health Organ. 1953;9:211–6.
Siu TL, Morley JW. Influence of callosal transfer on visual cortical evoked response and the implication in the development of a visual prosthesis. Graefes Arch Clin Exp Ophthalmol. 2007;245:1797–803.
Matsuo Y, Sakamoto T, Yamashita T, Tomita M, Shirasawa M, Terasaki H. Comparisons of choroidal thickness of normal eyes obtained by two different spectral-domain OCT instruments and one swept-source OCT instrument. Invest Ophthalmol Vis Sci. 2013;54:7630–6.
Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol. 2009;118:69–77.
Dolz-Marco R, Gallego-Pinazo R, Pinazo-Duran MD, Pons-Vazquez S, Domingo-Pedro JC, Diaz-Llopis M. Intravitreal docosahexaenoic acid in a rabbit model: preclinical safety assessment. PLoS One. 2014;9:e96872.
Sorsby A, Reading HW. Experimental degeneration of the retina. XI. The effect of sodium iodate on retinal -SH levels. Vision Res. 1964;4:511–4.
Ringvold A, Olsen EG, Flage T. Transient breakdown of the retinal pigment epithelium diffusion barrier after sodium iodate: a fluorescein angiographic and morphological study in the rabbit. Exp Eye Res. 1981;33:361–9.
Grignolo A, Orzalesi N, Calabria GA. Studies on the fine structure and the rhodopsin cycle of the rabbit retina in experimental degeneration induced by sodium iodate. Exp Eye Res. 1966;5:86–97.
Redfern WS, Storey S, Tse K, Hussain Q, Maung KP, Valentin JP, et al. Evaluation of a convenient method of assessing rodent visual function in safety pharmacology studies: effects of sodium iodate on visual acuity and retinal morphology in albino and pigmented rats and mice. J Pharmacol Toxicol Methods. 2011;63:102–14.
Korte GE, Wanderman MC. Distribution of Na+K(+)-ATPase in regenerating retinal pigment epithelium in the rabbit. A study by electron microscopic cytochemistry. Exp Eye Res. 1993;56:219–29.
Korte GE, Rappa E, Andracchi S. Localization of alkaline phosphatase on basolateral plasma membrane of normal and regenerating retinal pigment epithelium. A cytochemical study in rabbits. Invest Ophthalmol Vis Sci. 1991;32:3187–97.
Obata R, Yanagi Y, Tamaki Y, Hozumi K, Mutoh M, Tanaka Y. Retinal degeneration is delayed by tissue factor pathway inhibitor-2 in RCS rats and a sodium-iodate-induced model in rabbits. Eye (Lond). 2005;19:464–8.
Korte GE, Mrowiec E, Landzberg KS, Youssri A. Reorganization of actin microfilaments and microtubules in regenerating retinal pigment epithelium. Exp Eye Res. 1995;61:189–203.
Korte GE, Reppucci V, Henkind P. RPE destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci. 1984;25:1135–45.
Flage T, Ringvold A. The retinal pigment epithelium diffusion barrier in the rabbit eye after sodium iodate injection. A light and electron microscopic study using horseradish peroxidase as a tracer. Exp Eye Res. 1982;34:933–40.
Ogata N, Kanai K, Ohkuma H, Uyama M. Pathologic response of the regenerated retinal pigment epithelium (RPE)—affected by sodium iodate (NaIO3). Nihon Ganka Gakkai Zasshi. 1989;93:466–74.
Mizota A, Adachi-Usami E. Functional recovery of retina after sodium iodate injection in mice. Vis Res. 1997;37:1859–65.
Machalinska A, Kawa MP, Pius-Sadowska E, Roginska D, Klos P, Baumert B, et al. Endogenous regeneration of damaged retinal pigment epithelium following low dose sodium iodate administration: an insight into the role of glial cells in retinal repair. Exp Eye Res. 2013;112:68–78.
Wang J, Iacovelli J, Spencer C, Saint-Geniez M. Direct effect of sodium iodate on neurosensory retina. Invest Ophthalmol Vis Sci. 2014;55:1941–53.
Franco LM, Zulliger R, Wolf-Schnurrbusch UE, Katagiri Y, Kaplan HJ, Wolf S, et al. Decreased visual function after patchy loss of retinal pigment epithelium induced by low-dose sodium iodate. Invest Ophthalmol Vis Sci. 2009;50:4004–10.
Tanaka M, Machida S, Ohtaka K, Tazawa Y, Nitta J. Third-order neuronal responses contribute to shaping the negative electroretinogram in sodium iodate-treated rats. Curr Eye Res. 2005;30:443–53.
Stone JL, Barlow WE, Humayun MS, de Juan E, Milam AH Jr. Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol. 1992;110:1634–9.
Enzmann V, Row BW, Yamauchi Y, Kheirandish L, Gozal D, Kaplan HJ, et al. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp Eye Res. 2006;82:441–8.
Ashburn FS Jr, Pilkerton AR, Rao NA, Marak GE. The effects of iodate and iodoacetate on the retinal adhesion. Invest Ophthalmol Vis Sci. 1980;19:1427–32.
Konda BR, Pararajasegaram G, Wu GS, Stanforth D, Rao NA. Role of retinal pigment epithelium in the development of experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 1994;35:40–7.
Baich A, Ziegler M. The effect of sodium iodate and melanin on the formation of glyoxylate. Pigment Cell Res. 1992;5:394–5.
Heike M, Marmor MF. l-cystein protects the pigment epithelium from acute sodium iodate toxicity. Doc Ophthalmol. 1990;75:15–22.
Noell WK. Experimentally induced toxic effects on structure and function of visual cells and pigment epithelium. Am J Ophthalmol. 1953;36:103–16.
Tao Z, Dai J, He J, Li C, Li Y, Yin ZQ. The influence of NaIO(3)-induced retinal degeneration on intra-retinal layer and the changes of expression profile/morphology of DA-ACs and mRGCS. Mol Neurobiol. 2013;47:241–60.
Negi A, Marmor MF. The resorption of subretinal fluid after diffuse damage to the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1983;24:1475–9.
Machalinska A, Lejkowska R, Duchnik M, Kawa M, Roginska D, Wiszniewska B, et al. Dose-dependent retinal changes following sodium iodate administration: application of spectral-domain optical coherence tomography for monitoring of retinal injury and endogenous regeneration. Curr Eye Res. 2014;39:1033–41.
Acknowledgments
This study was supported by the Public Welfare and Safety Program 2012-0006566 by Ministry of Education and Science Technology of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
B.-J. Cho, None; J.-M. Seo, None; H. G. Yu, None; H. Chung, None.
About this article
Cite this article
Cho, BJ., Seo, JM., Yu, H.G. et al. Monocular retinal degeneration induced by intravitreal injection of sodium iodate in rabbit eyes. Jpn J Ophthalmol 60, 226–237 (2016). https://doi.org/10.1007/s10384-016-0429-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-016-0429-1