Summary
Healthcare workers (HCWs) are proposed as the potential source of transmission of Staphylococcus aureus to hospitalized patients, especially in burn units. This study aimed to investigate S. aureus from burn wound infections and those from the nose of HCWs in terms of antibiotic resistance, the presence of Panton–Valentine leucocidin-encoding gene (pvl) and the arginine catabolic mobile element (ACME), and the ability for biofilm formation. Also, the genetic diversity of isolates was assessed using staphylococcal protein A (spa) typing and staphylococcal cassette chromosome mec (SCCmec) typing. Overall, regarding the studied factors, significant differences were found neither between isolates from patients and HCWs nor between methicillin-resistant and methicillin-susceptible isolates (except for multidrug resistance which was significantly higher in MRSA). The most frequent SCCmec types were type I and III. ACME-arcA was only detected in isolates from patients and similarly the presence of ACME-opp3 was the most prevalent in this group. The presence of common clonal complexes among patient isolates and more importantly between isolates from patients and HCWs is warning. The high prevalence of virulence factors, both in MRSA and MSSA, emphasizes the importance of MSSA in burn centers. Finding no significant difference in the presence of virulence-associated factors between isolates from patients and HCWs demonstrates the need to take HCWs into account as important reservoirs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Burn patients are at a high risk for nosocomial infections. These infections are major challenges in the healthcare setting, so that they have been estimated to be responsible for more than 70% of deaths in burn units [1, 2].
Staphylococcus aureus, particularly methicillin-resistant S. aureus (MRSA), is one of the main causes of healthcare-associated infections and is known as the second most common pathogen for bacteremia in burn patients [1, 3].
In addition to infection, colonization is a matter of concern. It has been reported that colonization of S. aureus during the ICU stay will increase the chance of pneumonia up to 15 times [4]. Healthcare workers (HCWs) are at the forefront of patient care and might contribute to transmission of MRSA either as reservoirs or as vectors. So, despite the emphasis on the control of MRSA exclusively in patients, HCWs have also been accounted for as one of the major sources of MRSA [3, 5].
Several virulence factors in S. aureus have been associated with poor clinical outcomes and even failure of treatment of infections in burn patients. One of these factors is antimicrobial resistance. Most of the resistance associated genes are carried on the staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element which may harbor some antimicrobial resistance genes as well as mecA [6].
Another factor is the arginine catabolic mobile element (ACME), in which two main gene clusters, including the arc genes and the oligopeptide permease operon (opp) genes, have been identified. This novel genetic staphylococcal island is associated with SCCmec and has the potential to increase the virulence or improve the colonization of S. aureus in skin and mucous membranes [6, 7].
The other proposed virulence-associated factor with a cytotoxic effect on human monocytes, macrophages and polymorphonuclear cells, is Panton–Valentine leucocidin (PVL). On the basis of epidemiological and clinical studies, a strong correlation has been found between production of PVL and severe skin/soft tissue infections [6].
The ability for biofilm formation, which enhances the antibiotic resistance, may also predispose the patient to recurrent staphylococcal infections and it is considered as another virulence factor [8].
Due to the importance of S. aureus infections in burn patients, detection of possible transmission routes is of particular significance. To this end, we investigated some potential virulence factors of S. aureus isolated from burn patients as well as those collected from the nose of health personnel working in the same burn center (Yazd, Iran). Additionally, the molecular epidemiology of MRSA isolates in these two groups was analyzed.
Materials and methods
Study population and bacterial identification
This cross-sectional study was approved by the ethical committee at the Kerman University of Medical Sciences (IR.KMU.REC.1397.127) and conducted from April 2018 to May 2019 at Shahid Sadoughi hospital, a 65-bed university burn hospital.
In total, 70 S. aureus isolates from wound infections of burn patients were included in this study. Furthermore, 159 nasal swabs were obtained from the anterior nares of volunteer medical staff including nurses, operating room technicians, and all those who were in contact with burn patients and had not received any antibiotics over at least the past 3 months. All HCWs were provided with a written consent form.
The isolates were identified using standard biochemical procedures and their identities were further confirmed by amplification of the nuc gene [9].
Antimicrobial susceptibility testing
Susceptibility to 10 antimicrobial agents (cefoxitin 30 µg – as a marker for methicillin resistance – ; ciprofloxacin 5 µg; clindamycin 2 µg; erythromycin 15 µg; gentamicin 120 µg; linezolid 30 µg; mupirocin 20 µg; ofloxacin 5 µg; quinupristin/dalfopristin 15 µg; and tetracycline 30 µg [MAST, UK]) was determined by the disk diffusion method according to CLSI criteria. Susceptibility to vancomycin was tested by the agar dilution method (MIC ≤ 2 µg/ml was considered as susceptible) [10]. Multidrug resistance (MDR) was defined as exhibiting resistance to at least four different classes of antibiotics.
Screening of virulence-associated genes
Bacterial DNA was extracted and all isolates were subsequently tested for the presence of the mecA gene as described previously [11]. Additionally, the presence of genes encoding for PVL (pvl) and ACME (arcA and opp3) was investigated by PCR assay [12, 13].
Molecular typing methods
SCCmec typing was performed on all MRSA isolates using multiplex PCR as described by Boye et al. [14]. Amplification of the polymorphic X region of the spa gene was carried out [15] and next to the sequencing of the PCR product (Bioneer Company, Korea), spa types were assigned using http://spaserver.ridom.de.
Biofilm assay
An overnight growth culture in trypticase soy broth (TSB) was adjusted to 0.5-Mc Farland turbidity and then diluted 100-fold in trypticase soy broth supplemented with 1% glucose. Next, the wells of a 96-well microplate were inoculated with 200 μL of the prepared dilution and incubated at 37 °C in a humid atmosphere. After washing, the wells were air-dried and the adherent cells were stained with 200 μL of 0.2% aqueous safranin dye (Merck, Germany) solution. After 40 min, following washing the wells, the dye was dissolved with 200 μL of 95% ethanol solution, and the absorbance of each well was read at 490 nm in a microplate reader (BioTek, Vermont, USA) as described previously. S. epidermidis RP62A was used as positive control. All biofilm assays were carried out in triplicate [8].
Data analysis
IBM SPSS statistics software version 21 (SPSS, Inc.) was used for data analysis. Categorical analysis was carried out using Fisher’s exact test or the Chi-square test, as appropriate. Statistical significance was set at p-value ≤ 0.05.
Results
Totally, 86 S. aureus isolates (from burn patients and HCWs) were included. Nasal carriage of S. aureus was found in 10% of HCWs (16/159). Overall, 45% (39/86) of S. aureus isolates were methicillin resistant, including 43% (30/70) of burn patients and 56% (9/16) of HCWs.
No resistance to linezolid, vancomycin, and quinopristin/dalfopristin was detected. Mupirocin resistance was only detected among MRSA; 19% (3/16) in HCWs and 11% (8/70) in patients. There was no significant difference in MDR phenotype among isolates from burn patients in comparison to HCWs. However, MDR was significantly higher in MRSA compared to MSSA (p = 0.004) (Table 1)
The SCCmec types were assessed in all MRSA isolates. The most frequent types within the two studied groups were related to types I and III.
The ACME-arcA gene was detected in 10 isolates from burn patients of which two isolates were related to MRSA SCCmec types I and III, and the remaining eight isolates were MSSA. The ACME-opp3 was detected predominately in isolates from patients (26%, 18/70), of which 12 isolates (67%) were MRSA. For isolates from HCWs, only two MSSA carried ACME-opp3. Taken together, the most frequent ACME type was ACME-III (18/28; 64%), followed by ACME-II (8/28; 29%) and ACME‑I (2/28; 7%).
The presence of pvl was observed in both HCWs and patients, with a ratio of 75% (12/16) for HCW isolates and 66% (46/70) for isolates from patients.
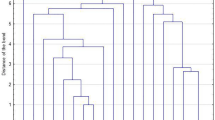
Of 39 MRSA isolates, 25 isolates were spa typed, which represented 8 spa types. One isolate was non-typeable by spa typing. The three most common spa types were t021, t3024, and t2253, representing 80% of spa-typed MRSA from patients. In contrast, spa-typed MRSA from HCWs (n = 5) were grouped into two spa-types, including t224 and t084.
BURP (based upon repeat pattern) analysis [16] clustered four spa types into two groups: A (t021 and t030) and B (t084 and t3024), and the remaining ones were not assigned to any BURP group and were classified as singletons.
The ability for biofilm formation was seen in all studied isolates except for two isolates from patients and two isolates from HCWs. Within both groups, no significant association was found between methicillin resistance, biofilm formation, and the presence of pvl, ACME-arcA, and ACME-opp3 genes. (Table 2).
Discussion
In this study, 70 S. aureus isolates responsible for wound infections in hospitalized burn patients were compared to 16 nasal isolates from HCWs in terms of antibiotic resistance, the presence of virulence-associated gene loci, and genetic diversity.
Using spa typing, a typing method known to provide reliable information on short-term epidemiology, the diversity of MRSA isolates from HCWs was lower than in the patient group. However, 80% of spa-typed MRSA from burn patients were distributed in three specific spa types. So, despite genetic variability which is in accordance with previous studies conducted on the genetic diversity of S. aureus in the hospital settings [17,18,19], the presence of these dominant spa types is warning for intra-hospital spread in this burn center. On the other hand, the placement of MRSA isolates from both groups in a common spa complex implies that some MRSA may have been colonized in the HCWs and are actively circulating in the hospital setting. This issue is also in accordance with several well-documented instances recorded in the literature [5, 20, 21]. However, failure to use pulsed-field gel electrophoresis as the most discriminatory method for genotyping of staphylococcal strains used for hospital outbreak investigations is a limitation of this study.
Based on the results, 87% of MRSA isolates were distributed in SCCmec types I and III, both of which are known as predominant hospital-acquired (HA) types [17]. This corroborates the hypothesis that burn wards facilitate transmission of MRSA from HCWs to patients or vice versa. Additionally, it raises concerns about the transmission of HA-MRSA to the community through HCWs.
Regarding PVL, mainly reported in community-acquired MRSA infections, there are recent reports describing it in HA-MRSA infections, especially in skin or soft tissue infections [22]. This is in agreement with the high prevalence of PVL in our findings and is alarming for the emergence of HA-MRSA infections with increased virulence among burn patients.
In the current study, no significant association was found between methicillin resistance and resistance to other antimicrobial agents. Furthermore, a high rate of antimicrobial resistance was detected among MSSA isolates. Since in most of the studies [23] MRSA strains were the focus of attention, diffusion of MSSA strains that show resistance to commonly prescribed antibiotics limits the options for empiric antimicrobial therapy and strongly reminds us not to ignore them. The prevalence of mupirocin resistance among MRSA in this study did not show a high rate. However, due to relatively high consumption of this antibiotic in the form of topical ointment by patients and particularly hospital staff, continuous epidemiological monitoring is essential to prevent the spread of resistance.
In the current study, high prevalence of biofilm formation was detected. The rate of biofilm formation was higher (but not significantly) in MRSA isolates and in isolates from burn infections. This issue again reinforces the importance of MSSA isolates. Additionally, it strengthen the probability of transmission of virulence associated genes to isolates in HCWs.
In conclusion, the high prevalence of MDR, ability for biofilm formation, and even genes encoding for PVL and ACME—both in MRSA and MSSA—emphasizes the importance of MSSA in burn centers as the same as that of MRSA. Also, the presence of common clonal complexes among burn isolates and, more importantly, between isolates from burn patients and HCWs, is warning about the need for more serious care in controlling the infection in this hospital. Investigation of the S. aureus nasal carriage state in burn patients may provide useful information and is recommended for future studies.
References
Kalligeros M, Shehadeh F, Karageorgos SA, et al. MRSA colonization and acquisition in the burn unit: a systematic review and meta-analysis. Burns. 2019;45:1528–36.
Goudarzi M, Bahramian M, Tabrizi MS, et al. Genetic diversity of methicillin resistant Staphylococcus aureus strains isolated from burn patients in Iran: ST239-SCCmec III/t037 emerges as the major clone. Microb Pathog. 2017;105:1–7.
Price JR, Cole K, Bexley A, et al. Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: a longitudinal cohort study based on whole-genome sequencing. Lancet Infect Dis. 2017;17:207–14.
Paling FP, Wolkewitz M, Bode LG, et al. Staphylococcus aureus colonization at ICU admission as a risk factor for developing S. aureus ICU pneumonia. Clin Microbiol Infect. 2017;23:49.e9.
Pourramezan N, Moghadam SO, Pourmand MR, et al. Methicillin-resistant Staphylococcus aureus tracking spread among health-care workers and hospitalized patients in critical wards at a university hospital, Tehran, Iran. New Microbes New Infect. 2019;27:29–35.
Murray CK, Holmes RL, Ellis MW, et al. Twenty-five year epidemiology of invasive methicillin-resistant Staphylococcus aureus (MRSA) isolates recovered at a burn center. Burns. 2009;35:1112–7.
Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008;16:361–9.
Mahmoudi H, Pourhajibagher M, Chiniforush N, et al. Biofilm formation and antibiotic resistance in methicillin-resistant and methicillin-sensitive Staphylococcus aureus isolated from burns. J Wound Care. 2019;28:66–73.
Lozano C, Gómez-Sanz E, Benito D, et al. Staphylococcus aureus nasal carriage, virulence traits, antibiotic resistance mechanisms, and genetic lineages in healthy humans in Spain, with detection of CC398 and CC97 strains. Int J Med Microbiol. 2011;301:500–5.
Clinical and laboratory standard institute (CLSI). Performance standards for antimicrobial susceptibility testing. 28th informational supplement. Wayne: CLSI; 2015. p. M100-S23.
Zhang K, Sparling J, Chow BL, et al. New quadriplex PCR assay for detection of methicillin and mupirocin resistance and simultaneous discrimination of Staphylococcus aureus from coagulase-negative staphylococci. J Clin Microbiol. 2004;42:4947–55.
Lina G, Piémont Y, Godail-Gamot F, et al. Involvement of panton-valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128.
Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–9.
Boye K, Bartels MD, Andersen IS, et al. A new multiplex PCR for easy screening of methicillin-resistant Staphylococcus aureus SCCmec types I–V. Clin Microbiol Infect. 2007;13:725–7.
Strommenger B, Kettlitz C, Weniger T, et al. Assignment of Staphylococcus isolates to groups by spa typing, SmaI macrorestriction analysis, and multilocus sequence typing. J Clin Microbiol. 2006;44:2533–40.
Mohammadi S, Sekawi Z, Monjezi A, et al. Emergence of SCCmec type III with variable antimicrobial resistance profiles and spa types among methicillin-resistant Staphylococcus aureus isolated from healthcare-and community-acquired infections in the west of Iran. Int J Infect Dis. 2014;25:152–8.
Darban-Sarokhalil D, Khoramrooz SS, Marashifard M, et al. Molecular characterization of Staphylococcus aureus isolates from southwest of Iran using spa and SCCmec typing methods. Microb Pathog. 2016;98:88–92.
Moosavian M, Dehkordi PB, Hashemzadeh M. Characterization of SCCmec, Spa Types and Multidrug Resistant of Methicillin-Resistant Staphylococcus aureus Isolates in Ahvaz, Iran. Infect Drug Resist. 2020;13:1033.
Mirzaii M, Emaneini M, Jabalameli F, et al. Molecular investigation of Staphylococcus aureus isolated from the patients, personnel, air and environment of an ICU in a hospital in Tehran. J Infect Public Health. 2015;8:202–6.
Javidnia S, Talebi M, Saifi M, et al. Clonal dissemination of methicillin-resistant Staphylococcus aureus in patients and the hospital environment. Int J Infect Dis. 2013;17:e691–e5.
El-Ageery SM, Abo-Shadi MA, Elgendy AM, et al. The role of health care workers and environment on transmission of methicillin–resistant Staphylococcus aureus among patients in a medical intensive care unit in a Saudi hospital. J Pure Appl Microbiol. 2011;5:1–8.
Hu Q, Cheng H, Yuan W, et al. Panton-Valentine leukocidin (PVL)-positive health care-associated methicillin-resistant Staphylococcus aureus isolates are associated with skin and soft tissue infections and colonized mainly by infective PVL-encoding bacteriophages. J Clin Microbiol. 2015;53(1):67–72.
Carrel M, Goto M, Schweizer ML, et al. Diffusion of clindamycin-resistant and erythromycin-resistant methicillin-susceptible Staphylococcus aureus (MSSA), potential ST398, in United States Veterans Health Administration Hospitals, 2003–2014. Antimicrob Resist Infect Control. 2017;6:1–8.
Funding
This work was supported by the Kerman University of Medical Sciences (grant No. 585).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
V. Dad, R. Ahmadrajabi, S. Esfahani, and F. Saffari declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dad, V., Ahmadrajabi, R., Esfahani, S. et al. Comparative study of Staphylococcus aureus from burn patients and healthcare workers in a burn center, Yazd, Iran. Wien Med Wochenschr 172, 256–260 (2022). https://doi.org/10.1007/s10354-021-00863-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-021-00863-5