Abstract
The participation of microorganisms in construction and destruction of sedimentary structures is widely recognized, and so is the importance of studying such geological processes in modern systems, where the conditions, participating forces, and the results can be observed and recorded. This information is important for understanding and interpreting corresponding processes if their effects were preserved as part of the fossil record. The present contribution refers to topics discussed during the 9th International Bioerosion Workshop in Rome on Oct. 23–27, 2017, dedicated to the evaluation of microbial traces as paleoecological and paleobathymetric indicators. The paper reviews the habitats, methods of collection, and preparation of samples, followed by observation of extracted microbial euendoliths. This approach is complemented by producing images of three-dimensional display of inhabited microborings in their original positions using resin-casting and double embedding of the microbially invaded substrates. This contribution stresses the value of recognizing the microboring organisms’ identities as a key aspect of the interpretation of their traces. It discusses different and complementary ways of how to achieve such parallel assessments. It reports on the importance of photo-documentation and morphometric evaluation of microbial populations, while avoiding possible artefacts caused by the methods used. The study also briefly summarizes the distribution patterns of microboring organisms and their boring and etching traces along depth profiles. Problems arising in the naming of complex traces and the relation to biological nomenclature are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The contribution of microorganisms to modern and ancient sedimentary processes is generally recognized (Seckbach and Oren 2010; Riding 2011; Reitner et al. 2011). The microbiota play significant roles in sediment stabilization and construction as well as in sediment and rock destruction by bioerosion (Seilacher 2007; Tribollet 2008; Tribollet et al. 2011b; Wisshak 2012), by contributing to karstification of coastal limestone, by generating fine sedimentary particles (Schneider and Torunski 1983), as well as by promoting mineral recycling in the process (Golubic et al. 1979b; Berner 1999; Archer 2010). Microorganisms colonize the surfaces of hard substrates, such as rocks, sediment particles, and bioclasts as biofilms (Krumbein et al. 2003; di Donato et al. 2016). Some adhere to external rock surfaces as epiliths, while others colonize the interior of rocks as endoliths contributing to form a complex lithobiontic ecological niche (Golubic et al. 1981). The microorganisms that actively penetrate carbonate substrates and reside partially or completely inside the cavities of their own making exhibit specific rock-boring behavior and are called euendoliths (endoliths sensu Bachmann 1915) to distinguish them from microbial chasmo-endoliths, which occupy rock fissures (endoliths sensu Diels 1914) and cryptoendoliths, which colonize pre-existing spaces within porous rocks (endoliths sensu Friedmann 1971). Only euendoliths produce microborings as specific traces of their activity, whereas chasmo- and cryptoendoliths adhere to the internal substrate surfaces and, like epiliths, are able to affect the substrate indirectly by their metabolic products. They may leave etching textures, as a special trace category, which are less specific than microborings, a distinction noticed early in reference to bio-erosion by algae: “les algues cariant et perforant le roche” (Frémy 1945).
The microborings conform closely to the outlines of the organism that produce them, leaving a specific trace (Campbell and Hoffman 1979), but they also reflect the mineral properties of the substrate along the surfaces of contact (Golubic 1969; Golubic et al. 1975, fig. 12.6). The illustrations of the present contribution are referred to in the text consistently with capital letters (Fig. x) and those cited from published sources with small letters (fig. x). Because the microbial traces are engraved into hard and permanent substrates, such as limestone or calcareous skeletons of foraminifera, corals, mollusks and brachiopods, they produce “instant” fossils (Campbell and Hoffman 1979; Radtke and Golubic 2011). They can be studied at the level of microbial populations and trace assemblages. Direct fossil to modern comparisons allow for determination of taxonomic affinity. Golubic et al. (1979a) recognized that as much as 600 million years of trace fossils could now be directly compared to extant taxa.
Microbial euendoliths have a geologic antiquity approaching that of stromatolites. The oldest known are cyanobacterial euendoliths penetrating lithified stromatolites in the over 1600-My-old Paleoproterozoic Dahongyu Formation of China (Zhang and Golubic 1987). Cyanobacterial euendoliths in ooid sands were well established and diversified during the Neoproterozoic (Campbell 1982a; Knoll et al. 1986), long before the evolution of metazoans, whose skeletons they regularly penetrated throughout the Phanerozoic (e.g., Hessland 1949; Vogel et al. 2000; Glaub et al. 2007; Vogel and Brett 2009 and the bibliography therein).
Geological significance of microboring organisms has been recognized regarding several problems, including the role of microboring organisms in the formation of micritic envelopes (Bathurst 1966; Hook et al. 1984), in biokarstification of coastal limestone (Schneider and Torunski 1983; see Tribollet et al. 2011a, fig. 2h), the initiation and persistent participation of microbial euendoliths in bioerosion (Rioult and Dangeard 1967; Wisshak 2012), and the use of fossil traces of phototrophic euendoliths as indicators of depositional depths in ancient oceans (Swinchatt 1969).
The microboring habit evolved in light-dependent, phototrophic cyanobacteria and microscopic green and red algae, but also in light-independent organotrophic microorganisms such as bacteria, protists and fungi. Light-dependent microorganisms occur in the upper, illuminated part of the ocean, whereas the light-independent euendoliths can occur at any depth. The value of microboring traces as paleo-bathymetric indicators that was recognized early (Swinchatt 1969; Golubic 1972; Budd and Perkins 1980), depends on the ability to distinguish between traces of phototrophic vs. organotrophic microorganisms studied in modern oceans (Campbell 1982b). Owing to rather tight-fitting tunnels made by some microborers, their traces are often quite similar to the body outlines of microboring organisms in the present and in the past (Campbell and Hoffman 1979). The distinction between traces of phototrophic and organotrophic euendoliths is necessary for any paleobathymetric or paleoecological application, but due to convergent evolution in morphology of microorganisms and their traces (Golubic et al. 2016), it is not always easy. With this biological distinction achieved, the depth distribution of euendoliths and their traces in modern settings can be applied to their fossil counterparts and serve as paleobathymetric indicators.
Regarding the organisms that live inside rocks in cavities of their own making, the question “why do they bore” intrigues. A reasonable suggestion “to escape grazers”, was rebuked after the fossil microborers were discovered a billion years earlier than their grazers evolved (Zhang and Golubic 1987). Also, the endolithic habitat offered little shelter in view of the efficiency of the grazing tools of mollusks, echinoderms, and fishes able to remove layers of rock together with the endoliths (Schneider and Torunski 1983; Tribollet et al. 2011b). Once the euendoliths mastered the chemistry of carbonate dissolution (see Garcia-Pichel et al. 2010; Guida and Garcia-Pichel 2016; Couradeau et al. 2017), microbial euendoliths made the interior of limestones, dolomites, and carbonate skeletons their regular habitats, forming an internal biofilm within these substrates (Golubic and Schneider 2003).
Bioerosion is an integrated process initiated by microorganisms as primary actors in microbiocorrosion (Tribollet et al. 2011a), followed by various assemblages of grazing animals (Schneider 1976; Tribollet and Golubic 2005; Tribollet et al. 2011b). Common grazers of epilithic and endolithic microorganisms like gastropods, chitons, sea urchins, and parrot fish, also remove a thin layer of the rock, thereby significantly enhancing the bioerosion and contributing to the production, suspension, and deposition of fine grain sediments (Schneider and Torunski 1983). By pursuing microboring organisms for food, grazing animals constitute a major landscape-forming force along carbonate coasts, with formation of biokarst and coastal bioerosional notches (Neumann 1966; Radtke et al. 1996; Couradeau et al. 2017). Microbial bioerosion also affects the rocks in terrestrial habitats that are exposed to freshwater (Ercegović 1925; Schneider and Le Campion-Alsumard 1999) including weather-exposed ancient marble statues and monuments (Macedo et al. 2009; Golubic et al. 2015).
This contribution was a part of the 9th International Bioerosion Workshop held in Rome, Italy, Oct. 23–27, 2017. It is focused on multidisciplinary approaches in the study of marine microboring organisms, their relation to the substrate, and formation of traces they leave behind. It underlines the importance of evaluating both the microbial endoliths and their traces. The depth-distribution of microbial euendoliths and their traces are reviewed.
From materials and methods to results
Microboring organisms inhabit marine coastal waters from the supratidal spray levels down to the abyssal depths (Campbell 1982a, b; Le Campion-Alsumard et al. 1982; Golubic et al. 1984), documented as deep as 4000 m (Campbell 1982b). Phototrophic euendoliths inhabit the upper, illuminated parts of the ocean, where they are often arranged in zones of distinct microbial composition (Ercegović 1932; Le Campion-Alsumard 1969; Radtke and Golubic 2011). The zones in supratidal (wave spray) and intertidal ranges are narrow and sharply outlined, whereas those in the subtidal ranges are wider and less uniform (Golubic et al. 1975, fig. 12.2). Organotrophic euendoliths follow the distribution of organic nutrients. They are expected to dominate with the increasing depth but may actually occur at any depth. The aphotic depths of the sea are populated exclusively by such light-independent, mainly organotrophic euendoliths. There are no known chemolithotrophic euendoliths, but the microenvironments they create may support chemolithotrophic colonizers. This presentation addresses collection, preparation, and observation of endoliths and traces in marine systems. The same approach would be applicable to bioerosion in freshwater and subaerial habitats as well. The materials and methods described, discussed, and recommended here both contributed to and were derived from this work.
Collecting and preserving endolith samples
As reviewed in our Introduction, microborings occur in solid limestone, dolomite, and phosphate rocks as well as in animal skeletons of similar mineral composition, including shoaling ooids and sand-size shell fragments. Each of these substrates requires a different sample collection procedure. The multidisciplinary approach recommended in the present contribution requires that each sample be subdivided so that subsamples can be exposed to different and complementary methods of preparation and analyses. This is a key feature of our method.
Rock fragments are best removed by hammer and chisel, so as to include intact bioeroded surfaces. Obtaining samples by core drilling should be used with caution, because the vibration may shatter delicate biokarst features. The preparation of petrographic thin-sections is the next preparation step that retains information on the relationship between endoliths and substrate. Sand samples and ooids are collected by scooping them from the sediment surface by diving, or from undisturbed box-core sediment samplers. Samples collected from submerged habitats and those that remain wet during tides are best wet-preserved in 3% formaldehyde solution in environmental water. The procedure preserves the specimens close to their natural color; 70% ethanol is also commonly used for preservation, which is practiced to preserve DNA, but may dissolve some of the pigments. For nucleic acid preservation, DNase suppressants, e.g., 4% solution of guanidine thiocyanate in sea water (Abed et al. 2003), is recommended. The possibility of freezing and freeze-drying of samples is usually limited while in the field. Air-exposed samples from the supratidal ranges that are naturally subject to desiccation during low tides preserve well dry.
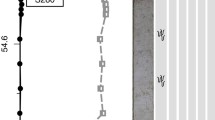
The zonation of lithobionts on the rocky limestone coast of Croatia (Fig. 1) is largely controlled by water supply and water retention. The physico-chemical conditions are increasingly erratic in the upper supratidal or wave-spray zone, both in frequency and chemical composition. Below that level, the zonation is subject to rhythmic yet regular changes by tidal oscillation. Zonation is typically more uniform and optimized in the subtidal zone. The width of this horizontal zone varies, depending on wave exposure (Ercegović 1934). The sampling of coastal endoliths is typically performed as a vertical transect across the zones expressed by color lines (Le Campion-Alsumard 1969; Palinska et al. 2017).
Extraction and observation of microbial euendoliths
Microbial euendoliths are surrounded by the carbonate substrate they penetrate. In translucent shells and shell fragments, they can be observed by transmitted light microscopy. Solid and opaque carbonate needs to be dissolved to extract the microbial endoliths. Larger borings, including those of boring worms and sponges, can be observed in intact substrates by X-rays and by micro-computing tomography (Schönberg and Shields 2008; Färber et al. 2016; Wisshak et al. 2017), but the microbial borings that are orders of magnitude smaller require special preparation, including extraction by acids. A traditional combination of fixation and carbonate dissolution is achieved by using Perenyi solution (nitric and chromic acid in ethanol). Dissolution by EDTA (ethylenediamine-tetraacetic acid) is used in preference to Perenyi if DNA extraction is planned. For dissolution of carbonate in formaldehyde-fixed samples a 3% HCl solution is commonly used. Following carbonate removal, the organisms may be mounted on microscope slides, observed by light microscopy and photo-documented.
All extraction procedures remove the carbonate support of endoliths so they collapse and lose their original orientation in relation to the substrate. Complementary preparation of petrographic thin-sections, cut perpendicular to and including the bioeroded surface, may help to restore information about their original positions and orientation. This often requires staining. Different water-soluble pigments (e.g., methylene or tolouidin blue) are used to emphasize cellular outlines of the organisms (Tribollet et al. 2011a, fig. 1a). Similar effect and better resolution is achieved by methods recommended for the transmission electron microscopy (TEM) of tissues, using fixation in 2.5% glutaraldehyde solution in 0.1 M buffer, followed by staining with 1.5% osmium tetroxide (e.g., Stirling et al. 2013). A large proportion of euendoliths contain their own pigments as parts of the light harvesting system and photosynthesis, which can be detected and analyzed using fluorescence techniques (e.g., Miyashita et al. 2003; Baker and Oxborough 2004). A large number of fluorescent and other compound-specific pigments are for use with confocal fluorescence microscopy to enhance the perception of different cellular structures (Macedo et al. 2009, fig. 11). Extracellular pigments that protect the organisms from excessive solar irradiation including UV have been evaluated using Raman spectrum analysis (Storme et al. 2015).
Epilithic microorganisms and biofilms
In shallow-marine environments, the euendoliths are often associated with epilithic biofilm cover and may be obscured by it. Some euendoliths are also partially epilithic, and most of them maintain regular contact with the substrate surface. The structure and composition of the biofilm and other epilithic overgrowth can be studied by SEM of critical-point-dried samples or by low-vacuum “natural” SEM analysis. Epilithic overgrowth cannot be removed mechanically without damaging delicate biokarst structures (e.g., Tribollet et al. 2011b, fig. 20), or disrupting shallow endolithic traces otherwise often positioned tightly under the substrate surface or contaminating the sample with extraneous DNA. Instead, the epilithic cover should be removed chemically using strong oxidizing agents that do not affect the substrate (e.g., hydrogen peroxide or sodium hypochlorite). Such agents were successfully applied in removing the organic periostracum layer that protects bivalve shells, exposing resin-cast microborings within it (Hook and Golubic 1990, 1992). With the epilithic coating removed, the shell’s surface and the effects of bioerosion can be observed by incident light microscopy and, in more detail, by scanning electron microscopy (SEM) of gold–palladium-coated surfaces (e.g., Radtke et al. 1996, fig. 13). Biofilm coating is absent on shoaling ooids, on shells and shell fragments in sand samples, and on fossils, so that the effect of bioerosion on substrate surface can be observed directly and evaluated (e.g., Chazottes et al. 1995).
Bioerosion assessment
Microbial endoliths often enter new substrates as germinating spores through small perforations but expand in the interior of the substrate. On the basis of figures taken from the study of fractured tests of the foraminifer Oolina (Golubic et al. 1984, fig. 2c), we have now measured the volume of the carbonate excavated from the interior of the test underneath individual entry holes (observed on the face of a fracture), and compared it with the volume estimated from the size of the opening (observed on the surface of the test). We found that the excavation in the interior may be ten to a hundred times greater than predicted on the basis of the dimensions of entry holes alone. This information is relevant in estimating and measuring of the extent of modern and fossil microbial bioerosion to avoid underestimating the worldwide effect of microbial bioerosion.
Resin-casting of microborings
Microbial euendoliths and their borings can be preserved and observed in their original orientation in serial two-dimensional microscopy and via three-dimensional display by SEM, if embedded and cast in polymerizing resins (e.g., Epon, Araldite or Spurrs’ Low Viscosity Medium) and exposed by partial or complete dissolution and removal of the surrounded carbonate matrix (Golubic et al. 1970, 1975). The procedure varies depending on the state of preservation of the organisms as well as their traces. The formaldehyde-preserved samples containing fragments of carbonate substrates with microborings are washed in distilled water and gradually dehydrated by moving the sample through a series of baths involving increasing acetone concentrations. Dehydration with ethanol is possible but requires an additional bath in propylene oxide to avoid hygroscopy. This stage is followed by a similar gradual stepwise transfer from acetone through a mixture of acetone with a complete polymerizing resin to pure resin including polymerizer, softener, and hardener. The resin for embedding-casting should be selected to have an extended polymerization time at room temperature to allow sufficient time for penetration into finest pores and fissures in the rock. The viscosity of the polymerizing resin is lowered by acetone, which mixes well with the resin in all proportions, just as acetone does with water in the process of gradual dehydration of the specimens. Vacuum is applied at the end stage of resin infiltration, which causes the remaining acetone to evaporate and bubble out of the resin mix, which flushes out residual air.
This is especially important in the preparation of dry samples harboring modern or fossil microborings because the large proportion of the latter typically remained empty (air filled). Dry samples can be exposed to acetone gradually by placing them in a shallow pool of acetone, taking advantage of the capillarity action. This drives the air out of the porous samples prior to resin infiltration, which then follows as described above. In fossil borings, the resin casts incorporate most of the precipitates that may have accumulated in the course of diagenesis. This procedure is used, with some modification, for the study of fossil microborings (Golubic et al. 1983).
Alternative treatment involves removing the content of microborings prior to the embedding-casting procedure (Wisshak 2006, p. 39, fig. 4; 2012, fig. 2). The euendoliths residing in their borings can be removed by strong oxidizing agents, as described above for the removal of the external biofilm and periostracum. The precipitates and fills that often occur in fossil microborings are loosened by surfactants and treated in an ultrasonic bath, washed and then dried. Wisshak (2012) recommends applying a vacuum during the embedment process to enhance infiltration of liquid resin into cleaned microborings. He performs this by using a low-viscosity epoxy resin, e.g., Ciba-Geigy Araldite BY 158 resin + Aradur 21 hardener, possibly also including the use of Keystone oil blue died resin to increase the contrast of outlines of the borings when viewed in petrographic thin-sections (Wisshak 2012, fig. 1A, B). The procedure depends on research objectives. It may not to work on preserved partly or completely carbonized organic residues.
The resin solidifies by polymerizing at the temperature and time as specified by the resin manufacturer. A solid block ready for oriented sectioning results. A hardened resin-block harboring an embedded rock fragment or bioclast with microborings is subsequently cut into differently oriented sections to be followed by partial or complete removal of the carbonate matrix. At least two complementary sections are recommended, which could be obtained from the same resin block: a “vertical” section, cut perpendicular to the substrate surface followed by partial carbonate removal and a “horizontal” section combined with complete carbonate removal.
The “vertical” section reveals the borings in side view against the background of the remaining carbonate matrix. When viewed by SEM, the preparation shows the borings as they progressed from the rock surface to the proliferation depth of particular microborers (Fig. 2a, insert, Fig. 3a arrow). The section offers the opportunity to compare the surface imprints on the casts of the borings together with the adjacent mineral texture on the mineral matrix. A separate section can be used for double embedding (Fig. 3b, arrow; Fig. 4).
Traces of cyanobacteria dominating the upper intertidal ranges. a Resin-replicated interior surface view of Fascichnus traces of the microboring cyanobacterium Hyella caespitosa; Insert: The same traces in vertical section. The arrow points to the rock surface. b Detail of a; note bush-like traces diverging into the rock
Traces of eukaryotic algae dominating the subtidal ranges. a Traces of chlorophytes close to the transition between tidal range and permanent see-level. Note the rock surface marked by an arrow. b Chlorophytes Phaeophyla sp. and Eugomontia sacculata double-embedded in situ. c Traces of green alga Ostreobium in a coral skeleton (removed by acid). d Conchocelichnus seilacheri, resin-cast endolithic trace of the red alga Pyropia sp. inside coral skeleton
Double-embedded sections with coastal euendoliths and their traces in their original positions at the supratidal (a–d), intertidal (e–h) and upper subtidal (i) levels. a Podocapsa pedicellatum in boring position (oblique section) with envelopes and borehole outlines marked by gold–palladium coating from previous SEM survey. Scale bar in a is for all images. b Scytonema endolithicum. c Hormathonema violaceo-nigrum (on top) and Solentia foveolarum (below). d Entophysalis granulosa and Hyella balani. e Hyella caespitosa. f Section close to sea level including epilith-endolith transition. Black coating from SEM survey marks the contact between microborers and the substrate. g Kyrtuthrix dalmatica, note intercalary heterocysts. h Mastigocoleus testarum, note terminal heterocysts on short side branches. i Green algae with large resting spores dominate euendoliths and epiliths below the sea level
A “horizontal” section cut parallel to the substrate surface and followed by a complete dissolution and removal of carbonate opens a view from the interior of the bored substrate toward the surface. When metal coated and observed by SEM, the view presents the horizontal distribution of microborings. The resin-replicas of borings are shown emerging from a plane representing the interior view of the shell surface (Fig. 5a, c), or from the domed interior surface of a micro-bored foraminiferal test (Fig. 5b). The area covered by those “interior landscapes” depends on the magnification used. Such views may show the interactions among microboring traces, produced by a single variable euendolithic taxon or by several taxa competing for the same space (Fig. 5a). Such interactions may show species-specific avoidance and/or anastomosing of microboring tunnels, the relations between vegetative (tubules) and reproductive (swellings) structures (Fig. 5b, c), and between microborings in the interior of the substrates and traces that are etched into the substrate surface (Fig. 5d). The complete removal of carbonate matrix often removes the original support to microboring traces, displacing especially the distribution of finer microborings, producing artefacts (see below). Resin replication of microborings in complex coral skeletons can be confusing, showing the replicated microborings crisscrossing the complex space networks left behind after the dissolution of the coral’s skeleton (Fig. 3c). Yet, the outlines of microboring replicas (Fig. 3d) include morphological detail that permits recognition of the microborer’s biological identity. To make such identifications, the paleontologist must have great familiarity with modern microbial taxa. It actually calls for expertise in different relevant disciplines or a highly collaborative group of professionals representing these fields.
Deep-sea traces and etching textures. a Microscopic interior landscape of boring replicas belonging to ichnogenera Orthogonum and Saccomorpha. b Irregular microborings in the deep-sea foraminifer Laticarinina with traces close to Saccomorpha guttulata. c Ichnocoenosis assembly of deep-sea traces: a fragment of an Orthogonum tube (upper right), two interconnected sporangial swellings of Saccomorpha with hyphal tunnels, and two Scolecia isp. adhering to the interior substrate surface. d Scolecia-type integration of traces into substrate surface as grooves (1 and 2), also shown as resin-cast trace textures (3 and 4)
The study of assemblages of microborings benefits greatly from the use of multiple parallel sample preparations—a multidisciplinary approach using techniques favored by microbiologists as well as those favored by micropaleontologists. For example, some subsamples permit microscopic identification of the microboring organisms following simple acid extraction, whereas other subsamples are used to replicate their borings. In this manner, we obtained enough information to compare with type descriptions and have learned that the dense assemblage of borings in Fig. 2 was the product of the cyanobacterium Hyella caespitosa that inhabited and dominated the upper intertidal zone shown on top of the lower brown band in Fig. 1.
Double-embedded sections
When applied to modern settings, the resin casting-embedding often incorporates the resident microbial euendoliths. This yields the possibility to compare their external shape with the shape of their traces (Campbell and Hoffman 1979). This is best achieved by the use of the double-embedding procedure (Figs. 3b, 4), developed as a means of interpretation of resin-casts microborings (see Golubic et al. 1975, figs. 12.4A, 12.6A, Inserts). Preparation departs from the embedding-casting procedure at the state of samples embedded and cured in a solid block and involves sectioning, carbonate matrix removal, and re-embedding. Sections are cut from the solid block of polymerized resin aiming to cut perpendicular to the bioeroded surface of the embedded sample. The sections are mounted onto glass (using the same resin) and the carbonate is removed completely by slow dissolution. Sections are carefully washed, dried, and embedded again in a layer of the polymerizing resin (complete mixture), and subsequently cured at elevated temperature. The second resin mix replaces the carbonate matrix making such double-embedded sections transparent. These sections can be trimmed and polished to a thickness of 50 µm (standard in petrography), observed, and photo-documented by light microscopy (Fig. 4). The contrast is achieved naturally by optical properties of the extracellular polymeric substance (EPS) of the cyanobacterial sheaths, and by natural intra- and extracellular pigments (Fig. 3b). Some sections may be produced by embedding the preparations cast and observed by SEM. In that case, the outlines of the boring replicas may be marked by a gold–palladium coating (Fig. 4a, d). When stained by osmium tetroxide, such preparations can be further exposed to ultrafine sectioning and prepared for TEM.
The filaments interspersed in the coral skeleton in Fig. 3c were identified to belong to the chlorophyte Ostreobium quekettii, while the other coral inhabitant in Fig. 3d are borings made by the euendolithic developmental Conchocelis stage of the rhodophyte Pyropia (former Porphyra). The -identification euendolithic microorganisms was made by microscopy of specimens extracted from subsamples. A technique of double embedding in polymerizing resin has been developed for observing both the microboring organisms and their traces in their original position and orientation. Using the double-embedding preparation technique, we could identify cyanobacteria in position (Fig. 4) and compare epilithic biofilm with endolithic microboring organisms and traces.
Photomicrographic documentation
The examination of endolithic microboring systems usually starts at low magnifications in order to obtain an overview, followed by zooming into ever higher magnifications while searching for significant details. It is recommended to make thereafter another zoom out for oversight of the detail’s surrounding context, and to publish such photo-documents in pairs of images at two different magnifications (Figs. 2a–b, 5d 1–2, 3–4). The perception of three-dimensional display of borings may be derived from evaluating the “vertical” and “horizontal” views and by cybernetic 3D reconstructions, replacing the earlier publication of stereo-pairs observed with special glasses. However, the stereo vision may be simply enhanced by obliquely angled photomicrographs by inclining the specimen while under SEM, thereby providing a different perspective of roughly 7° (Fig. 5a, c). Capturing an oblique view is also important to show the contacts between borings and the substrate surface (Fig. 5c, center). The specific problem with resin-casting of boreholes and grooves in the substrate refers to “positive to negative translation” (Fig. 5d 1–3, 2–4). Images of this kind illustrate the bioerosion traces that are integrated in the substrate surface, i.e., depressions or channels on the surface of substrates as opposed to tunnels penetrating the interior of the substrates (see also serrate Scolecia traces in Fig. 5c).
Morphometric evaluation
The nomenclatural rules for traces are largely the same as for animals, as they are both governed by the International Code of Zoological Nomenclature (ICZN 1999). What are different are the practices of the ichnotaxonomy versus taxonomy. For example, the ichnotaxobasis has been debated to center on shape and substrate, while the measurement of traces is recommended if it supports the expression of proportions (Bertling et al. 2006, 2007) The ichnotaxobase accepted for all trace fossils is their form, i.e., general geometry and detailed morphology, as it reflects the producer geometry, shape, and behavior (Bertling 2007), while the identity of the trace maker is considered irrelevant for the ichnotaxonomical treatment.
Using fossil traces in stratigraphy and paleoecology depends on some level of identification of their biological sources. In our case, this involves distinguishing between light-dependent and light-independent microboring organisms. For a similar reason, morphometric evaluation of size distribution, variability of size proportions, and other demographic methods—obtained by the multidisciplinary approach—help in recognizing the boundaries of natural populations of microbial euendoliths or their traces. The measurements are performed on the basis of in-scale projections and photographic images. Such evaluations of trace assemblages may provide insights in the process of microbial diversification (see Radtke and Golubic 2005, 2011). Accordingly, size measurements that help identifying physiological properties of organisms are considered valid in the study of microborings for paleobathymetric purposes. The problem of prokaryotic speciation is a subject intensely discussed (e.g., Cohan 2002; Gevers et al. 2005; Marin et al. 2017). It still remains unresolved but is not within scope of this contribution.
Artefacts
In science and technology, it is an accepted fact that methods produce artefacts, which need to be acknowledged, if possible removed, or at least explained. The artefacts of greatest concern are those potentially introduced during the preparation of the specimens for study, including: (1) cleaning and storage, (2) resin-casting of microbial borings, and (3) in failure to remove or identify residual organic or inorganic matter.
Biofilms
Mineral surfaces in the sea are invariably coated by biofilms, adding organic and mineral deposits, which may cover and obscure the effect of bioerosion by euendoliths. Many euendoliths, after penetrating the carbonate substrates, continue to bore parallel to, and immediately below the surface, making periodic contact with the surface and making it fragile. For this reason, the mechanical removal of epilithic overgrowth is to be avoided, because it may cause a collapse of the roof of horizontal tunnels, making them appear to be trenches in the substrate. Such effects must be considered artefacts to be distinguished from microbially caused surface trenches and grooves, which constitute specific engravings of particular bioeroding microorganisms (Fig. 5c, d). Subaerial biocorrosion associated with differential water exposure and retention is known to produce biokarst (Schneider and Torunski 1983). If subjected to water level changes, these structures may become biofilm-covered. Mechanical removal of biofilm may destroy such traces. It is important to recognize that the finest and most delicate traces are also the most vulnerable (Tribollet et al. 2011a, fig. 2h).
Artefacts with endolith extraction
Distinguishing between epiliths hat tightly adhere to the substrate, and the endoliths is not easy, because the process of extraction by carbonate dissolution deprives the endoliths of their support and they can lose their orientation to the substrate surface. There are historic examples of such confusions between epiliths and endoliths. The euendolithic Herpyzonema intermedium was described as epilithic but “tightly adhering to the rock” (Weber van Bosse 1913, p. 36). Similar uncertainty occurred with the description of upper supratidal epi-endoliths Podocapsa pedicellatum and Brachynema litorale (Ercegović 1931).
As explained above, the extraction by acids removes the carbonate support and disorients the extracted endoliths, which is the most common artefact. In order to minimize the degree of disorientation, we recommend using very slow and gradual dissolution accompanied by visually guided micromanipulation under dissecting microscope. In cases where euendoliths form interconnected mat-like layers, they remain coherent after carbonate is removed. Parts of such layers can be flipped over and followed by microscopic observation, so as to distinguish the side that originally faced the interior of the rock from the side that faced the water column.
Resin-related artefacts
Other possible artefacts deal with resin penetration, polymerization, and hardening. The dimensions of microendolithic traces that can be replicated by the casting-embedding procedure range over five orders of magnitude, from submicron to mm-size scale. Very fine tunnels produced by some microboring organisms are easily replicated but are subject to displacement. They may be dislodged and suspended in the liquid during carbonate dissolution or distorted during subsequent drying of the specimens. Narrow resin-cast tunnels, e.g., the hyphal tunnels that connect sporangial swellings of Saccomorpha frequently sag, leaning on the substrate surface, although they may have originally been suspended deeper inside it. Very fine and long tunnels, identified as traces of the ichnogenus Scolecia, may form “spaghetti-like” accumulations leaning on the replicated substrate surface. Although the outlines and size of such tunnels may be accurate and well represented in the cast, their position and interrelations have to be recognized as artefacts in such situations. Other artefacts originate when the sporangial swellings rested on very narrow connections with the substrate surface and their resin replicas become detached and displaced in the process of mounting of the specimens for SEM—a problem that the finest, most delicate traces are especially susceptible to.
Insoluble residue
The preparation of samples, including chemical and ultrasonic cleaning treatment as discussed above, usually precedes the resin infiltration and casting and subsequent chemical carbonate removal steps. These procedures might well remove most of the extraneous organic and inorganic matter, but in the process can expose structures that are insoluble in hydrochloric acid such as the organic fibers supporting the skeletal carbonate. In order to separate preserved organic fibers from resin-replicated microborings, it is recommended again to subdivide the sample and exposed one subsample to strong oxidizing agents (e.g., hydrogen peroxide or Na-hypochlorite or both). Our experience shows that the finest resin-replicated microborings are not adversely affected by the agents listed above.
Bioerosion along depth profiles
To assess the importance of a finding of specific microorganisms in a given environmental setting, one needs to distinguish between their growth and survival (Golubic 1980). The endolithic niche in the ocean is shared by prokaryotic and eukaryotic microborers. As shown in most studies, the prokaryotes dominate in supratidal and intertidal levels, joined by eukaryotic green and red algae at permanently submerged subtidal levels. Fungi appear to occur throughout the profile, often parasitizing on endolithic phototrophs (e.g., Priess et al. 2000) or forming lichen associations. The combined morphotype/genotype studies of coastal profile (Palinska et al. 2017) found fairly high diversity at the intertidal level, indicating that stable patterns of change such as diurnal wetting and drying as in the intertidal may provide for sufficiently extended time for physiological functions as well as for growth by such microbiota that are also optimized to surviving repeated wetting and drying events. Intertidal zone is apparently a less extreme environment than generally assumed.
From the sea level upward (Fig. 1), it is the water supply and water retention (e.g., drainage) as well as solar irradiation that provide the principal selective pressures. Some organisms have developed compensatory ways of slowing desiccation. For example, dense and uniform populations of cyanobacteria (e.g., Fig. 2) are able to store some water or retard evaporation loss by their extracellular polysaccharide (EPS) envelopes (Richert et al. 2005) during air exposure at low tide and to respond to excessive radiation by production of UV-protecting pigments (Storme et al. 2015). Timing of their physiological functions and growth is subject to tidal rhythmicity. Above the tidal range, the microorganisms are exposed to more extreme conditions of water shortage and salinity fluctuations, depending on wave spray and rain (Ercegović 1934). Their presence is granted by relatively short periods of active growth and metabolic functioning and extended periods in a latent state of survival. The distribution of intertidal and supratidal microbial euendoliths and their microboring traces are composed almost exclusively by cyanobacteria (Ercegović 1932; Le Campion-Alsumard 1969) and fungi (Golubic et al. 2005). The polysaccharide sheaths of the cyanobacteria (Richert et al. 2005) and the thick cell walls of fungi are able to slow water loss and also imbibe it quickly when rewetting occurs. A schematic presentation of euendolith depth distribution profile (Golubic et al. 1975) shows that the zones characterized by microbial populations above the sea level are narrow, becoming wider and less uniform with depth. The coastal profile, starting with the upper part of the range, is illustrated here by the in situ positions of several euendoliths species using a series of double embedding sections (Fig. 4).
Upper ranges of the wave-wetted supratidal are settled by colonies of single-celled droplet-shaped euendoliths (Fig. 4a) that penetrate carbonate with their wider sides, with a few short series of cells. The penetration is mediated by wide EPS envelopes and results in hemispheric depressions outlined by an SEM metal-coating. Ercegović (1931) described two cyanobacteria from the upper supratidal levels, Podocapsa pedicellatum and Brachynema litorale, that both showed polar differentiation in cell division and envelope production, but he considered them to be epilithic. Ercegović later added the description of Epilithia adriatica (Ercegović 1932) also from the uppermost portion of the lithophyte belt exposed to wave impact. He noted for this form that the parts he described as apical were oriented toward the rock. Ercegović’s original drawings show close similarity between these three forms, suggesting that they may represent growth stages of a single taxon, revised independently as Ecegovicia by De Toni (1936), honoring the above author. The deep-boring filamentous heterocystous cyanobacterium Scytonema endolithicum (Fig. 4b) occurs commonly at the supratidal wave spray levels (Golubic et al. 1999, p. 69, Pl. III. fig. D) but were not observed in lower supratidal and intertidal ranges.
We found the growth orientation to be toward the rock as well as euendolithic rock penetration of this and other coccoid cyanobacteria (Fig. 4c–e). This observation is supported by the distribution of UV-protecting extracellular pigments and by the position of the reproductive cell clusters closer to the water column. The smaller coccoid cells forming dense epilithic-endolithic colonies and containing the extracellular UV-protecting pigments gloeocapsin (blue) and scytonemin (yellow–brown) characterize the pleurocapsalean genus Hormathonema with species H. violaceo-nigrum and H. luteobrunneum (Ercegović 1929). Their colonies extend along the rock surface above the deeper boring endoliths (Fig. 4b–e). Small depressions in the rock represent microenvironments with locally retarded water drainage, usually occupied by Solentia foveolarum (Fig. 4c), which together with Hormathonema violaceo-nigrum provides the rock with a macroscopically perceived bluish coloration (Le Campion-Alsumard et al. 1995; Palinska et al. 2017, fig. 2B). The relationship between Hormathonema and Solentia has been revised by unifying H. paulocellulare into Solentia (Le Campion-Alsumard and Golubic 1985a, figs. 5 and 6).
Rock surfaces with more efficient drainage are brown in color from the pigment scytonemin extracellular to compact spherical groups of Entophysalis granulosa and Hyella balani (Fig. 4d), which is organized in sparely branched serial filaments (Le Campion-Alsumard and Golubic 1985b, fig. 18). H. caespitosa with distinctly serial cell organization (Fig. 4e) dominates the middle level of the intertidal range, but has a significantly wider distribution, as it has been observed in the subtidal down to a depth of 100 m in clear ocean waters off the Florida coast (Lukas 1978). The question of how to identify a biological species is beyond the scope of this contribution. The gene sequence analyses have only recently started to evaluate morphological variability of euendoliths in terms of their phylogenetic origins (Brandes et al. 2015; Palinska et al. 2017; Couradeau et al. 2017). The present approach is ecological, using the traditional distinction of forms while illustrating the relationship between epilithic and endolithic microbial constituents (Figs. 3a vs. 3b, 4f vs. i). The lower intertidal ranges are dominated by filamentous, heterocystous cyanobacteria, Kyrtuthrix dalmatica (Fig. 4g) and Mastigocoleus testarum (Fig. 4h). The substrates exposed to microbial bioerosion in the intertidal and supratidal ranges represent mostly limestone and dolomite rocks, including local sedimentary environments in rock pools and microbioeroded skeletons of corals and mollusks. Cyanobacterial euendoliths are present in the shallow subtidal range (see Palinska et al. 2017, fig. 9), but yield the local dominance to euendolithic green and red algae (Figs. 3a, b, 4f, i).
The frequency of encountering inhabited borings is particularly high in the intertidal and supratidal wave spray ranges. These ranges are dominated by cyanobacteria that are able to persist through times having conditions unfavorable for their growth. The uniformity of euendolithic populations there is also very high (Fig. 2a, b). The seasonality in the life cycle of eukaryotic microborers is more expressed in permanently submersed habitats (Golubic and Radtke 2008; Radtke et al. 1996). Organisms that spend only parts of their life cycle inside carbonate substrates are more common among eukaryotes (Radtke et al. 1997; Tribollet et al. 2017). In shallow subtidal ranges, the substrates exposed to microbial bioerosion are also more varied. In addition to hard rocks, there are skeletons of a variety of invertebrates and their accumulated post-mortem remains, including sand-sized shell fragments. On the coasts of tropical seas there are, in addition, microbialite-type accretions exposed to tidal currents (Reid et al. 2011), shoaling ooids, and coral reefs. The shoaling ooid grains represent an environment closest to the subtidal-intertidal boundary (Radtke and Golubic 2011). The distribution of euendolithic populations is less regular and more substrate-specific, thus requiring an increase in the number and variety of samples to determine the diversity of the shallow subtidal ranges.
The relative contribution of prokaryotic vs. eukaryotic microborers to the euendolithic population in the shallow subtidal is increasingly difficult to discern. SEM images of oriented, partially exposed borings (Fig. 3a) enable recognition of chlorophyte borings characterized by rhythmic alternation in diameter to be distinguished from the more consistently cylindrical borings of cyanobacteria. Double-embedded samples (Fig. 3b) add an insight to condition above the substrate surface marked by white arrows and help establish the relations between epi- and endolithic colonization, adding the advantage of comparing both microbial euendoliths and their borings in situ. Coral skeletons are colonized by the green siphonal alga Ostreobium quekettii soon after their larvae attach to the hard ground (Massé et al. 2018) and they continue to penetrate the coral skeleton (Fig. 3c) keeping up with coral growth (Le Campion-Alsumard et al. 1995, 1996) and possibly contribute to coral’s health (del Campo et al. 2017). The Ostreobium networks in coral skeletons are parasitized by euendolithic fungi (Priess et al. 2000), which also attack the coral coelenterate (Bentis et al. 2000).
Other common eukaryotes that penetrate coral skeletons are Conchocelis stages in the development of bangialean rhodophytes (Fig. 3d) known to range back over 400 My, as the fossil Palaeochonchocelis starmachii penetrated crinoid ossicles in Silurian strata of Poland (Campbell et al. 1979). Modern Conchocelis stages were studied as penetrating the skeletons of stylasterid corals in Indonesian coral reefs (Pica et al. 2016). These morphologically characteristic traces were formally described as Conchocelichnus seilacheri (Radtke et al. 2016).
Depth distribution information and the lower limit of the occurrence of phototrophic microboring organism have long been sought as potential paleo-depth indicators (Swinchatt 1969). Geologists and ecologists seek to determine the extent of the photic zone as the energy base for oceanic primary production. Previous estimates, based on primary production in plankton, have largely underestimated the distribution of benthic oligo-photic microorganisms (Vogel et al. 1995; Englebert et al. 2017), since Ostreobium quekettii was found to grow inside shells of benthic brachiopods at depths of 140–220 m in clear Mediterranean waters (Fredj and Falconetti 1977), at 200 m depth off the coast of Florida (Lukas 1978; Le Campion-Alsumard et al. 1982), and at 300 m depth in carbonate rocks collected by a submarine in clear waters off the Bahamas (Vogel et al. 2000; Glaub et al. 2007), while providing macroscopic green coloration to the limestone cliffs at a depth of 150 m. The Conchocelis stages of the rhodophyte Porphyra were recorded at 78-m depth in the North Sea (Clokie et al. 1981), but was not observed in clear oligotrophic waters. The deepest occurrence of phototrophic microorganisms is reported at 370-m depth in clear waters off the Florida coast for the cyanobacterium Leptolyngbya (Plectonema) terebrans, followed distantly at this location by Hyella caespitosa at 100-m depth (Lukas 1978). The light-limitation appears to be an important determining factor in distribution of phototrophic euendoliths with increasing depth. However, it is the efficiency in light harvesting that determines the limits separately for each species.
The study of microboring traces extends into the aphotic deep sea in order to refine the distinction between light-dependent and light-independent microboring organisms. This study, currently in progress, is important because the light-independent microboring organisms may occur at any depth, but the aphotic depths exclude morphologically similar light-dependent forms (Golubic et al. 2016). The SEM images of microbial bioerosion in the deep sea present an internal landscape with a combination of simple tubular traces classified under the ichnogenus Orthogonum as well as complex traces, comprised of sporangia-containing cavities interconnected by hyphal tunnels classified as Saccomorpha, juxtaposed against an interior view of the bored shell surface that forms the background of the photo (Fig. 5a). Both the borings and the bored substrate are more complex in the case of borings in deep-sea foraminifera (Fig. 5b), including gradual transitions between tubular and sack-shaped parts of the borings classified as Saccomorpha guttulata (Wisshak et al. 2018).
Continuing studies of deep-sea microborings in the aphotic depth called attention to shallow traces, identified as etching patterns (Fig. 5c, d) in the substrate surface. The deep-sea endolithic landscape containing microboring and etching is presented in Fig. 5c, with a simple Saccomorpha bag emerging from a hyphal tunnel and producing another such tunnel by a spore that germinated within the sporangial bag. A large Orthogonum tube is in the background, upper right. Two densely wound serrated traces appear to adhere to the shell’s surface. They were classified as ichnospecies of Scolecia. The nature of Scolecia traces is analyzed in Fig. 5d, employing an external SEM view (Fig. 5d, 1–2) showing that they represent grooves rather than tunnels, produced by an epilithic dweller that loosens and removes the crystallite structure of the shell, thus leaving a serrate outline. When replicated by polymerizing resin (Fig. 5d, 3–4), the image appears more like those of Scolecia shown in Fig. 5c.
Naming and ichnotaxonomy of complex traces: tubes vs. sacs
Recognizing and comparing microbial euendoliths on the basis of their traces is important if we want to use them as paleobathymetric or any kind of paleoecological indicator. The recognition and application of traces requires that they be described and named as ichnotaxa on the basis of fossil appearances, including determination of the boundaries of these units. The taxonomic procedures for fossil traces are similar to those traditionally employed in the classification of extant and fossil organisms, but the names given to traces compete with those of biotaxa for homonymy (Wisshak et al. 2005).
The naming and grouping of the traces causes little problem when they are simple and uniform; it becomes more difficult to provide meaningful names for highly variable and complex traces (see Miller 2007 for definition) as, for example, in traces that are spreading by tubes, but also form bags, sometimes containing cells, but more often containing sporangia as parts of the reproduction of the trace producer. Some descriptions emphasize the tunnels, e.g., Orthogonum (Fig. 5a), while treating the swellings and other irregularities as secondary (e.g., Rhopalia), while others emphasize the conspicuous bags and swellings, while treating the tubular interconnections as a secondary property. In the case of Saccomorpha (Fig. 5a, c), the bags are known to have a reproductive function and contain sporangia with spores. Their size is age-dependent until they reach full size, as the spores inside mature, to be released through a tunnel to the substrate surface, recognized as and thus called a “bottleneck.” Note that the term “spore” is in wide use for eukaryotic organisms. In cyanobacteria, such reproductive structures are termed “beocytes”, resulting from multiple fission (Komárek 2016). Some spores are not released but germinate while still in the sporangium and produce a hypha, which departs from the swelling and continues to bore (Fig. 5c, right). The relationship between tubes and sacs may vary seasonally within the same ichnotaxon, and from one ichnotaxon to another. However, the distinction between the tubes and bags as separate morphological elements is not always clear, as the diameter may change gradually (Fig. 5b) as in Saccomorpha guttulata (Wisshak et al. 2018). Other microboring organisms maintain periodic contacts with the substrate surface, including fine hair-like tubules that extend into the surrounding water column (Fig. 4i), possibly for exchange of nutrients and metabolic products. A detailed view shows several members of such complex microboring assemblage (Fig. 3b).
Ichnotaxonomy is not safe from taxonomic lumping vs. splitting, which has historically plagued biological classifications as well. While some researchers perceived the morphological variability as intraspecific, including it in the description of an ichnotaxon, others preferred to treat the morphological variants as separate ichnotaxa. The information as to whether a variant is genetically coded or is instead a modification introduced by environmental conditions is neither required nor recommended in the description of traces. However, it is generally accepted that a trace should be morphologically distinct and substrate-specific while expressing a behavior of its producer. Substrate-specificity is sometimes though not usually a feature of the taxonomic descriptions of living species.
Conclusions
A multidisciplinary approach in the study of microbioerosion in modern environments is necessary to support the use of microboring traces as paleoecological indicators. The combination of different methods applied in parallel to subsamples helps to complement their strengths and to limit the “typical” artefacts that stem from the techniques of preparation and study. Introduction of double-embedding preparation method enables in situ comparison of the microboring organism and its trace and offers insights into the relations between epiliths and endoliths (external and internal microbial biofilms) and the mineral surfaces they affect—identified as characteristic ichnotaxon-specific etching textures and microborings. The depth-related decrease in light available to phototrophs is an important determinant for their distribution, but the depth limits they reach also depend on their species-specific efficiency in harvesting light. Thus, the species designations of such bathymetric indicators need to be worked out and related to their traces. The distribution of light-dependent and light-independent euendoliths requires additional research that should include the genotypic diversity of extant organisms or fossilized biological remains with preserved nucleic acids.
References
Abed RMM, Golubic S, Garcia-Pichel F, Camoin GF, Sprachta S (2003) Characterization of microbialite-forming Cyanobacteria in a tropical lagoon: Tikehau Atoll, Tuamotu, French Polynesia. J Phycol 39:862–873
Archer D (2010) The global carbon cycle. Princeton University Press, Princeton (ISBN 9781400837076)
Bachmann E (1915) Kalklösende Algen. Ber Dtsch Bot Ges 33:45–57
Baker NR, Oxborough K (2004) Chlorophyll fluorescence as a probe of photosynthetic productivity. Chapter 3. In: Papaqeorgiou GC (ed) Chlorophyll a fluorescence a signature of photosynthesis. Springer, Berlin, pp 66–79
Bathurst RGC (1966) Boring algae, micrite envelopes and lithification of molluscan biosparites. Geol J 5:15–32
Bentis CJ, Kaufman L, Golubic S (2000) Endolithic fungi in reef-building corals (Order: Scleractinia) are common, cosmopolitan, and potentially pathogenic. Biol Bull 198:254–260
Berner RA (1999) A new look at the long-term carbon cycle. GSA Today 9(11):1–6
Bertling M (2007) What’s in a name? Nomenclature, systematics, ichnotaxonomy. In: Miller W III (ed) Trace Fossils. Elsevier, Amsterdam, pp 81–91
Bertling M, Braddy SJ, Bromley RG, Demathieu R, Genise J, Mikulas JK, Nielsen JK, Nielsen KSS, Rindsberg K, Schlirf M, Uchman A (2006) Names for trace fossils: a uniform approach. Lethaia 39:265–286
Brandes M, Albach DC, Vogt JC, Mayland-Quellhors E, Mendieta-Leiva G, Golubic S, Palinska KA (2015) Supratidal extremophiles—cyanobacterial diversity in the rock pools of the Croatian Adria. Microbial Ecol 70(4):876–888. https://doi.org/10.1007/s00248-015-0637-0
Budd DA, Perkins RD (1980) Bathymetric zonation and paleoecological significance of microborings in Puerto Rican shelf and slope sediments. J Sediment Res 50:881–904
Campbell SE (1982a) Precambrian endoliths discovered. Nat Lond 299:429–431
Campbell SE (1982b) The modern distribution and geological history of calcium carbonate boring microorganisms. In: Westbroek P, de Jong EW (eds) Biomineralization and biological metal accumulation. D. Reidel Pub., Co., Dordrecht, pp 99–104
Campbell SE, Hoffman EJ (1979) Endoliths and their microborings: how close is the fit? In: 2nd international symposium on fossil algae. Abstract with Program
Campbell SE, Kazmierczak J, Golubic S (1979) Palaeoconchocelis starmachii gen. n., sp. n., an endolithic rhodophyte (Bangiaceae) from the Silurian of Poland. Acta Palaeontol Pol 24:405–408
Chazottes V, Le Campion-Alsumard T, Peyrot-Clausade M (1995) Bioerosion rates on coral reefs: interaction between macroborers, microborers and grazers (Moorea, French Polynesia). Palaeol Palaeol Palaeol 113:189–198
Clokie JJP, Scoffin TP, Boney AD (1981) Depth maxima of Conchocelis and Phymatolithon rugulosum on the N.W. Shelf and Rockall Plateau. Mar Ecol Prog Ser 4:131–133
Cohan FM (2002) What are bacterial species. Ann Rev Microbiol 56:457–487
Couradeau E, Roush D, Guida BS, Garcia-Pichel F (2017) Diversity and mineral substrate preference in endolithic microbial communities from marine intertidal outcrops (Isla de Mona, Puerto Rico). Biogeosciences 14:311–324
de Toni G (1936) Notarelle di nomenclatura algological, vol VIII. Terzo elenco di Missoficee omonime, Brescia, p 5
del Campo J, Pombert J-F, Slapeta J, Larkum A, Keeling PJ (2017) The ‘other’ coral symbiont: Ostreobium diversity and distribution. ISME J 11:296–299
di Donato P, Poli A, Taurisano V, Abbamondi GR, Nicolaus B, Tommonaro G (2016) Recent advances in the study of marine microbial biofilm: from the involvement of quorum sensing in its production up to biotechnological application of the polysaccharide fractions. Mar Sci Eng 4(2):1–14. https://doi.org/10.3390/jmse4020034
Diels L (1914) Die Algenvegetation der Südtiroler Dolomitenriffe. Ber Dtsch Bot Ges 32:507–531
Englebert N, Bongaerts P, Muir PR, Hay KB, Pichon M, Hoegh-Guldberg O (2017) Lower mesophotic coral communities (60–125 m depth) of the Northern Great Barrier Reef and Coral Sea. PLoS One. https://doi.org/10.1371/journal.pone.0170336
Ercegović A (1925) Litofitska vegetacija vapnenaca i dolomita u Hrvatskoj. (La végétation lithophytes sur les calcaires et les Dolomites en Croatie). Acta Bot Inst Bot Univ Zagreb. 1:64–114
Ercegović A (1929) Sur quelques nouveaux types des Cyanophycées lithophytes de la côte adriatique. Arch f Protistenk 66(1):164–174
Ercegović A (1931) Podocapsa et Brachynema deux genres nouveaux chamésiphonales de la côte adriatique de Dalmatie. Acta Botanica Croatica 6(1):33–37
Ercegović A (1932) Ekološke I sociološke studije o litofitskim cijanoficejama sa jugoslavenske obale Jadrana. Rad JAZU 244:129–220 (Pl. 1-7. (Études écologieques et sociologiques des cyanophycées lithophytes de la côte yougoslave del’Adriatique. Bull Internat Acad Yougosl Sci Arts Class Sci Math Nat 26:33–36)
Ercegović A (1934) Wellengang und Lithophytenzone an der Adriatischen Küste. Acta Adriatica Split 3:1–20
Färber C, Titschack J, Schönberg CHL, Ehrig K, Boos K, Baum D, Illerhaus B, Asgaard U, Bromley RG, Freiwald A, Wisshak M (2016) Long-term macrobioerosion in the Mediterranean Sea assessed by micro-computed tomography. Biogeosciences 13:3461–3474. https://doi.org/10.5194/bg-13-3461-2016
Fredj G, Falconetti C (1977) Sur la présence d’Algues filamenteuses perforantes dans le test des Gryphys vitreus (Born) (Brachiopodes, Térébratulidés) de la limite inférieure du plateau continental méditerranéen. C R Acad Sci Paris 284(Ser D):1167–1170
Frémy P (1945) Contribution a la physiologie des Thallophytes marines perforant et cariant les roches calcaires et les coquilles. Ann Inst Oceanogr 22:107–144
Friedmann EI (1971) Light and scanning electron microscopy of the endolithic desert algal habitat. Phycologia 10:411–428
Garcia-Pichel F, Ramirez-Reinat E, Gao Q (2010) Microbial excavation of solid carbonates powered by P-type ATPase-mediated transcellular Ca2+ transport. PNAS 107:21749–21754
Gevers D, Cohan FM, Lawrence JG, Spratt BG, Coenye T et al (2005) Opinion: re-evaluating prokaryotic species. Nat Rev Microbiol 3:733–739
Glaub I, Golubic S, Gektidis M, Radtke G, Vogel K (2007) Microborings and microbial endoliths: geological implications. In: Miller W III (ed) Trace fossils: concepts, problems, prospects. Elsevier, Amsterdam, pp 368–390
Golubic S (1969) Distribution, taxonomy and boring patterns of marine endolithic algae. Am Zool 9:747–751
Golubic S (1972) Scanning electron microscopy of recent boring cyanophyta and its possible paleontological application. In: Desikachary TV (ed) Taxonomy and biology of blue-green algae. University of Madras, India, pp 167–170
Golubic S (1980) Halophily and halotolerance in cyanophytes. Orig Life 10:169–183
Golubic S, Radtke G (2008) The trace Rhopalia clavigera isp. n. reflects the development of its maker Eugomontia sacculata Kornmann 1960. In: Wisshak M, Tapanila L (eds) Current developments in bioerosion. Springer, Berlin, pp 96–108
Golubic S, Schneider J (2003) Microbial endoliths as internal biofilms. In: Krumbein WE, Dornieden T, Volkmann M (eds) Fossil and recent biofilms. Kluwer Academic Publishers, Dordrecht, pp 249–263
Golubic S, Brent G, Le Campion T (1970) Scanning electron microscopy of endolithic algae and fungi using a multipurpose casting-embedding technique. Lethaia 3:203–217. https://doi.org/10.1111/j.1502-3931.1970.tb01858.x
Golubic S, Perkins RD, Lukas KJ (1975) Boring microorganisms and microborings in carbonate substrates. In: Frey RW (ed) The study of trace fossils. Springer, Berlin, pp 229–259
Golubic S, Hoffman EJ, Campbell SE (1979a) Study of fossil microbial borings—new approach. In: American Association of Petroleum Geology (AAPG-SEPA) Annual Meeting. Abstracts with programs p 94
Golubic S, Krumbein WE, Schneider J (1979b) The carbon cycle. In: Trudinger PA, Swaine DJ (eds) Biogeochemical cycling of mineral-forming elements. Elsevier, Amsterdam, pp 29–45
Golubic S, Friedmnn I, Schneider J (1981) The lithobiontic ecological niche, with special reference to microorganisms. J Sediment Res 51:475–478. https://doi.org/10.1306/212F7CB6-2B24-11D7-8648000102C1865D
Golubic S, Campbell SE, Spaeth C (1983) Kunsharzausgüsse fossiler Mikroben-Bohrgänge. Der Präparator, Bochum 29:197–200
Golubic S, Campbell SE, Drobne K, Cameron B, Balsam WL, Cimerman F, Dubois L (1984) Microbial endoliths: a benthic overprint in the sedimentary record, and a paleobathymetric cross-reference with foraminifera. J Paleontol 58:351–361
Golubic S, Le Campion-Alsumard T, Campbell SE (1999) Diversity of marine cyanobacteria. In: Charpy L, Larkum AWD (eds) Marine cyanobacteria, Bulletin de l’ Institut Océanographique, Monaco, special issue 19:53–76
Golubic S, Radtke G, Le Campion-Alsumard T (2005) Endolithic fungi in marine ecosystems. Trends Microbiol 13(5):229–235. https://doi.org/10.1016/j.tim.2005.03.007
Golubic S, Pietrini AM, Ricci S (2015) Euendolithic activity of the cyanobacterium Chroococcus lithophilus Erc. in biodeterioration of the Pyramid of Caius Cestius, Rome, Italy. Int Biodeterior Biodegradation 100:7–16
Golubic S, Campbell SE, Lee S-J, Radtke G (2016) Depth distribution and convergent evolution of microboring organisms. Pal Z 90(2):315–326. https://doi.org/10.1007/s12542-016-0308-6
Guida BS, Garcia-Pichel F (2016) Extreme cellular adaptations and cell differentiation required by a cyanobacterium for carbonate excavation. PNAS 113:5712–5717
Hessland I (1949) Investigation of the Lower Ordovician of the Siljan District, Sweden II. Lower Ordovician penetrative and enveloping algae from the Siljan District. Bull Geol Inst Univ Uppsala 33:409–428
Hook JE, Golubic S (1990) Mussel Periostracum from deep sea redox communities as a microbial habitat—2: the pit borers. PSZNI Marine Ecol 11:239–254
Hook JE, Golubic S (1992) Mussel Periostracum from deep-sea redox communities as a microbial habitat 3: secondary inhabitants. PSZNI Marine Ecol 13:119–131
Hook JE, Golubic S, Milliman JD (1984) Micritic cement in microborings is not necessarily a shallow-water indicator. J Sediment Res 54:425–431
ICZN (1999) International Code on zoological nomenclature, XXIX. Natural History Museum, London
Knoll AH, Golubic S, Green J, Swett K (1986) Organically preserved microbial endoliths from the Late Proterozoic of East Greenland. Nat Lond 321:856–857
Komárek J (2016) A polyphasic approach for the taxonomy of cyanobacteria: principles and applications. Eur J Phycol 51:346–353. https://doi.org/10.1080/09670262.2016.1163738
Krumbein WE, Paterson DM, Zavarzin GA (eds) (2003) Fossil and recent biofilms, a natural history of life on earth. Kluwer Academic Publishers, Dordrecht
Le Campion-Alsumard T (1969) Contribution à l’étude des Cyanophycées lithophytes des étages supralittoral et medio-littoral (Région de Marseille). Thétis, Marseille 1:119–172
Le Campion-Alsumard T, Golubic S (1985a) Ecological and taxonomic relationships between euendolithic cyanophytes Hormathonema and Solentia. Arch Hydrobiol Suppl 71 Algol Stud 38(39):115–118
Le Campion-Alsumard T, Golubic S (1985b) Hyella caespitosa Bornet et Flahault and Hyella balani Lehman (Pleurocapsales, Cyanophyta): a comparative study. Arch Hydrobiol Suppl 71 Algol Stud 38(39):119–148
Le Campion-Alsumard T, Campbell SE, Golubic S (1982) Endoliths and the depth of the photic zone, discussion. J Sediment Res 52:1333–1338
Le Campion-Alsumard T, Golubic S, Hutchings P (1995) Microbial endoliths in skeletons of live and dead corals: Porites lobata (Moorea, French Polynesia). Mar Ecol Prog Ser 117:149–157. https://doi.org/10.3354/meps117149
Le Campion-Alsumard T, Golubic S, Pantazidou A (1996) On the euendolithic genus Solentia Ercegović (Cyanophyta/Cyanobacteria). Algol Stud 83:107–127
Lukas KJ (1978) Depth distribution and form among common microboring algae from the Florida continental shelf. Geological Society of America annual meeting, abstracts with programs, p 448
Macedo MF, Miller AZ, Dionisio A, Saiz-Jimenez C (2009) Biodiversity of cyanobacteria and green algae on monuments in the Mediterranean Basin: an overview. Microbiology 155:3476–3490
Marin J, Battistuzzi FU, Bown AC, Hedges SB (2017) The timetree of prokaryotes: new insight into their evolution and speciation. Mol Biol Evol 34(2):437–446
Massé A, Domart-Coulon I, Golubic S, Duché D (2018) Tribollet A (2018) Early skeletal colonization of the coral holobiont by the microboring Ulvophyceae Ostreobium sp. Scientific Reports 8:2293. https://doi.org/10.1038/s41598-018-20196-5
Miller W III (2007) Complex trace fossils. In: Miller W III (ed) Trace fossils: concepts, problems, prospects. Elsevier, Amsterdam, pp 458–465
Miyashita H, Ikemoto H, Kurano N, Miyachi S, Chihara M (2003) Acaryochloris Marina gen. et sp. nov. (Cyanobacteria), an oxygenic photosynthetic prokaryote containing Chl D as a major pigment. J. Phycol 39(6):1247–1253
Neumann AC (1966) Biological erosion of limestone coasts. In: Fairbridge RW (ed) Encyclopedia of geomorphology. Reinhold Book. Corp, New York, pp 75–81
Palinska KA, Abed RMM, Vogt J, Radtke G, Golubic S (2017) Diversity of microbial endoliths on east Adriatic limestone coast: morphological vs molecular diversity. Geomicrobiol J 34(10):903–915
Pica D, Tribollet A, Golubic S, Bo M, Di Camillo CG, Bavestrello G, Puce S (2016) Microboring organisms in living stylasterid corals (Cnidaria, Hydrozoa). Mar Biol Res 12:573–582. https://doi.org/10.1080/17451000.2016.1169298
Priess K, Le Campion-Alsumard T, Golubic S, Gadel F, Thomassin BA (2000) Fungi in corals: black bands and density-banding of Porites lutea and P. lobata skeleton. Mar Biol 136:19–27
Radtke G, Golubic S (2005) Microborings in mollusk shells, Bay of Safaga, Egypt: morphometry and ichnology. Facies 51:125–141
Radtke G, Golubic S (2011) Microbial euendolithic assemblages and microborings in intertidal and shallow marine habitats: insight in cyanobacterial speciation. In: Reitner J, Queric V, Arp G (eds) Advances in stromatolite geobiology. Lecture Notes in Earth Sciences, 131. Springer, Berlin, pp 213–244
Radtke G, Le Campion-Alsumard T, Golubic S (1996) Microbial assemblages of the bioerosional “notch” along tropical limestone coasts. Algol Studies 83:469–482
Radtke G, Gektidis M, Golubic S, Hofmann K, Kiene WE, Le Campion-Alsumard T (1997) The identity of an endolithic alga: Ostreobium brabantium Weber-van Bosse is recognized as carbonate-penetrating rhizoids of Acetabularia (Chlorophyta, Dasycladales). Cour Forsch Inst Senckenberg 201:341–347
Radtke G, Campbell SE, Golubic S (2016) Conchocelichnus seilacheri igen. et isp. nov., a complex microboring trace of Bangialean rhodophytes. Ichnos 23(3–4):228–236. https://doi.org/10.1080/10420940.2016.1199428
Reid RP, Foster JS, Radtke G, Golubic S (2011) Modern marine stromatolites of Little Darby Island, Exuma Archipelago, Bahamas: Environmental setting, accretion mechanisms and role of endoliths. In: Reitner N, Queric V, Arp G (eds) Advances in Stromatolite geobiology—lecture notes in earth sciences 131. Springer, Berlin, pp 77–89,
Reitner N, Queric V, Arp G (eds) (2011) Advances in stromatolite geobiology. Lecture Notes in Earth Sciences, 131, Springer, Berlin (ISBN 9783642104152)
Richert L, Golubic S, Le Guédès R, Ratiskol J, Payri C, Guezennec J (2005) Characterization of exopolysaccharides produced by cyanobacteria isolated from Polynesian microbial mats. Curr Microbiol 5:379–384. https://doi.org/10.1007/s00284-005-0069-z
Riding R (2011) Calcified cyanobacteria. In: Reitner J, Thiel V (eds) Encyclopedia of Geobiology. Encyclopedia of earth science series. Springer, Berlin, pp 211–223
Rioult M, Dangeard L (1967) Importance des cryptogames perforantes marines en géologie. Botaniste 50:389–413
Schneider J (1976) Biological and inorganic factors in the destruction of limestone coasts. Contrib Sedimentol 6:1–112
Schneider J, Le Campion-Alsumard T (1999) Construction and destruction of carbonates by marine and freshwater cyanobacteria. Eur J Phycol 34:417–426
Schneider J, Torunski H (1983) Biokarst on limestone coasts, morphogenesis and sediment production. Mar Ecol 4:45–63
Schönberg CHL, Shields G (2008) Micro-computing tomography for studies on Entobia: transparent substrates versus modern technology. In: Wisshak M, Tapanila L (eds) Current developments in bioerosion. Springer, Berlin, pp 146–164
Seckbach J, Oren A (eds) (2010) Microbial mats: modern and ancient microorganisms in stratified systems, cellular origin, life in extreme habitats and astrobiology 14. Springer, Berlin, p 606
Seilacher A (2007) Trace fossil analysis. Springer, Berlin, p 226
Stirling J, Curry A, Eyden B (eds) (2013) Diagnostic electron microscopy: a practical guide to interpretation and technique. Wiley, New York, p 492 (ISBN: 978-1-119-97399-7)
Storme J-Y, Golubic S, Wilmotte A, Kleinteich J, Velasquez D, Javaux EJ (2015) Raman characterization of the UV-protective pigment gloeocapsin and its role in the survival of cyanobacteria. Astrobiology 15(10):843–857. https://doi.org/10.1089/ast.2015.1292
Swinchatt JP (1969) Algal boring: a possible depth indicator in carbonate rocks and sediments. Geol Soc Am Bull Boulder CO 80:1391–1396
Tribollet A (2008) The boring microflora in modern coral reef ecosystems. In: Wisshak M, Tapanila L (eds) Current developments in bioerosion. Springer, Berlin, pp 67–94. https://doi.org/10.1007/978-3-540-77598-0
Tribollet A, Golubic S (2005) Cross-shelf differences in the pattern and pace of bioerosion of experimental carbonate substrates exposed for 3 years on the northern Great Barrier Reef, Australia. Coral Reefs 24:422–434. https://doi.org/10.1007/s00338-005-0003-7
Tribollet A, Golubic S, Radtke G, Reitner J (2011a) On Microbiocorrosion. In: Reitner J, Queric NV, Arp G (eds) Advances in stromatolite geobiology, lecture notes in earth sciences. Springer, Berlin, pp 265–276
Tribollet A, Radtke G, Golubic S (2011b) Bioerosion. In: Reitner J, Thiel V (eds) Encyclopedia of geobiology, encyclopedia of earth sciences series. Springer, Berlin, pp 213–244
Tribollet A, Pica D, Puce S, Campbell SE, Radtke G, Golubic S (2017) Euendolithic Conchocelis stage (Bangiales, Rhodophyta) in the skeletons of stylasterid reef corals. Marine Biodiversity 48(4):1855–1862. https://doi.org/10.1007/s12526-017-0684-5
Vogel K, Brett CE (2009) Record of microendoliths in different facies of the Upper Ordovician in the Cincinnati Arch region USA: the early history of light-related microendolith zonation. Palaeol Palaeol Palaeol 81:1–24
Vogel K, Bundschuh M, Glaub I, Hofmann K, Radtke G, Schmidt H (1995) Hard substrate ichnocoenoses and their relations to light intensity and marine bathymetry. N Jb Geol Paläont Abh 195:49–61. https://doi.org/10.1080/00241160025100053
Vogel K, Gektidis M, Golubic S, Kiene WE, Radtke G (2000) Experimental studies on microbial bioerosion at Lee Stocking Island, Bahamas and One Tree Island, Great Barrier Reef, Australia: implications for paleoecological reconstructions. Lethaia 33:190–204
Weber van Bosse A (1913) Liste des Algues de Siboga. EJ Brill Publisher, Leiden
Wisshak M (2006) High-latitude bioerosion: the Kosterfjord experiment. Lecture Notes in Earth Sciences, vol 109. Springer, Berlin. https://doi.org/10.1007/978-3-540-36849-6
Wisshak M (2012) Microbioerosion. In: Knaust D, Bromley R (eds) Trace fossils as indicators of sedimentary environments. Developments in sedimentology 64. Elsevier, Amsterdam, pp 213–243
Wisshak M, Gektidis M, Freiwald A, Lundälv T (2005) Bioerosion along a bathymetric gradient in a cold-temperate setting (Kosterfjord, SW Sweden): an experimental study. Facies 51:93–117
Wisshak M, Titschack J, Kahl W-A, Girod P (2017) Classical and new bioerosion trace fossils in Cretaceous belemnite guards characterised via micro-CT. Fossil Record 20:173–199. https://doi.org/10.5194/fr-20-173-20
Wisshak M, Meyer N, Radtke G, Golubic S (2018) Saccomorpha guttulata: a new marine fungal microbioerosion trace fossil from cool- to cold-water settings. PalZ 92(3):525–533
Zhang Y, Golubic S (1987) Endolithic microfossils (cyanophyta) from early Proterozoic stromatolites, Hebei, China. Acta Micropaleont Sin 4:1–12
Acknowledgements
This work is in memory of our collaborator Professor Dr. Jürgen Schneider of the University of Göttingen and his work on environmental protection. Sediment samples with pteropod shell hash were obtained courtesy of Dr. T. Sleeter, Bermuda Biological Station and Dr. R. Janssen, Senckenberg, Frankfurt, which provided the valuable deep-sea samples for this study. EM application was generously supported by E. Seling and P. Price at MCZ Harvard University. Drs. Aline Tribollet, Annick Wilmotte, and Max Wisshak are thanked for critical comments and discussions. The research was supported by NSF Grant EAR-8306179 to S. Golubic and S. E. Campbell, and a Boston University Grant to J. E. Hook.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of a Topical Collection in Facies on Bioerosion: An interdisciplinary approach, guest edited by Ricci, Uchman, and Wisshak.
Rights and permissions
About this article
Cite this article
Golubic, S., Schneider, J., Le Campion-Alsumard, T. et al. Approaching microbial bioerosion. Facies 65, 25 (2019). https://doi.org/10.1007/s10347-019-0568-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10347-019-0568-1