Abstract
Cold-water corals of the Late Pleistocene (21,400–22,500 BP) are recorded from the sea-bottom of two inter-atoll channels (Kardiva Channel at 457-m depth and Malé Vaadhoo Channel at 443-m depth) of the eastern row of the Maldives archipelago. Coral assemblages are composed mainly by Lophelia pertusa and secondarily by Madrepora oculata and Enallopsammia rostrata. These cold-water coral patches are places where the benthic life, mainly sessile, is concentrated, which is clearly absent off-rubble patches. The main epibionts are tube-dwelling polychaetes (mainly Spirorbis and Serpula), bryozoans, siliceous sponges, barnacles, gorgonids, solitary corals, encrusting foraminifera, and microbial mats. The analysis of epibionts assemblages shows different biocoenoses between both studied sites as well as a dependency of the epibiont coverage with regard to the coral genus. Some living benthic organisms such as brachiopods, bivalves, gastropods, barnacles, and ophiuroids find refuge among coral branches. The common record of juvenile specimens of vagile organisms such as small ophiuroids, is probably related to the nursery function of the cold-water corals in spite they are fossils. Environmental requirements of Recent cold-water corals (Lophelia, Madrepora and Enallopsammia) differ of conditions at both sampling sites with sensibly lower oxygenation degree and density of waters than needed for cold-water corals. Therefore, it is proposed that the present-day oxygen and density conditions are the factors which inhibit modern cold-water coral growth in the inter-atoll channels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cold-water coral buildups host communities of associated animals that are distinct from the surrounding background deep-sea fauna and have high species diversity and sometimes a high level of endemism (Henry and Roberts 2007; van Soest et al. 2007; Mastrototaro et al. 2010; Morigi et al. 2012; Schöttner et al. 2012). The biodiversity associated with these ecosystems is comparable to that found in tropical coral reefs (Roberts et al. 2006, 2009). Cold-water coral buildups occur in a variation of settings such as on continental slopes, seamounts, plateaus, ridges, and submerged sides of oceanic islands (Rogers 2004; Davies et al. 2008; Roberts et al. 2009; Wienberg and Titschack 2015).
Cold-water corals are associated with permanent or episodically strong currents, because the currents supply food and disperse eggs sperms and larvae, remove waste products and winnow the sediment thus avoiding the burial of corals (Rogers 2004). However, recent studies have indicated that removal of waste products and winnowing of sediment by currents seems are irrelevant aspects for cold-water coral growth (Larsson and Purser 2011; Purser and Thomsen 2012; Titschack et al. 2015). But the requirement for a strong current influences the distribution and growth form of corals at all scales (Rogers 2004; Davies et al. 2008). Cold-water corals are usually associated to food supply that is commonly driven by the interplay of surface water productivity, the bottom current regime (e.g., internal waves, internal tides, downwelling and cascading) and the topography of the bottom (Frederiksen et al. 1992; Reed 2002; Freiwald et al. 2004; Duineveld et al. 2004, 2007; White et al. 2005; Mienis et al. 2012; Hebbeln et al. 2014).
Cold-water corals were previously not reported from the Maldives or the northern Indian Ocean (Freiwald et al. 2004, 2005; Davies et al. 2008; Roberts et al. 2009). The aim of this study is to present the first record of fossil (upper Pleistocene) cold-water corals from the Maldives. This research is also focused on the analysis of the coral assemblage and the associated epibionts as a proxy for interpreting the paleoenvironmental conditions at the time of coral growth and to compare them with recent conditions. In addition, as a secondary objective, the analysis of epibiont assemblages allows interpreting the behavior of these organisms.
Geological setting
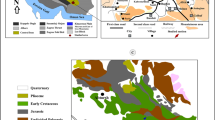
The Maldives archipelago in the central equatorial Indian Ocean is an isolated tropical carbonate platform constituting the central part of the Chagos-Laccadives Ridge, southwest of India (Fig. 1). A north–south-oriented double row of atolls encloses the Inner Sea of the Maldives (Fig. 1). Atolls are separated from each other by inter-atoll channels, which deepen towards the Indian Ocean (Purdy and Bertram 1993). The Inner Sea is a bank-internal basin with water depths of up to 550 m. The Maldives carbonate sedimentary succession is almost 3 km thick; it accumulated since the Eocene, away from any terrigenous input (Aubert and Droxler 1992; Purdy and Bertram 1993; Betzler et al. 2009, 2013).
The archipelago comprises about 1200 smaller atolls. Discontinuous marginal rims formed by smaller atolls (faros) surround lagoons with water depths of up to 50–60 m. The oceanward margins of the Maldives archipelago are generally steeply inclined, with dips of 20°–30° down to 2000 m of water depth. On the Inner Sea side, stepped atoll slopes have the same dip angles, but reach down to water depths of only 550 m, where the gradient rapidly declines (Fürstenau et al. 2010). The Inner Sea is characterized by periplatform ooze deposition (Droxler et al. 1990; Malone et al. 1990), locally accumulated into sediment drift bodies (Betzler et al. 2009, 2013; Lüdmann et al. 2013). Passages separating the atolls formed through the partial demise of larger carbonate banks during the middle Miocene (Betzler et al. 2009, 2013, 2016).
The climate and oceanographic setting of the Maldives is dictated by the seasonally reversing Indian monsoon system (Tomczak and Godfrey 2003). Southwestern winds prevail during northern hemisphere summer (April–November) whereas northeastern winds prevail during winter (December–March). Winds generate ocean currents, which are directed westwards in the winter and eastwards in the summer. Interseasonally, a band of Indian Ocean Equatorial Westerlies establish, enforcing strong, eastward-flowing surface currents showing velocities of up to 1.3 m s−1. Currents reach down to the seafloor (Lüdmann et al. 2013), especially in the inter-atoll passages where submarine dunes and moats occur (Betzler et al. 2009, 2013; Fig. 2a, b).
Multibeam bathymetry. a Eastern Kardiva channel between atolls Gaafaru and Faadhippolhu (see Fig. 1 for location). b Malé Vaadhoo Channel (see Fig. 1 for location). c Backscatter image of the dune field and the submarine blocks of Malé Vaadhoo Channel. d Parasound profile of the dune field at Malé Vaadhoo Channel (Fig. 2b). Red dot shows the position of the cold-water coral accumulations at the top of a flank of the submarine block. VE vertical exaggeration
Materials and methods
The studied samples were recovered during RV SONNE Cruise SO236 in August 2014 (Betzler 2015). The bathymetry of the study area was recorded with the hull-mounted multibeam system Kongsberg EM120 using a nominal sonar frequency of 12 kHz with 191 beams per ping and an angular sector coverage of 120°–140° for most survey lines to suppress low quality signals from the outer range. Equidistant beam spacing was used for all lines. The software package Caris HIPS was used for onboard-processing and editing of the swath echosounder and navigational data. Surfaces with resolutions of 5, 10, and 30 m were created using the CUBE algorithms implemented in CARIS and exported as ESRI ArcGIS grid for visualization with IVS Fledermaus (Interactive Visualization Systems Inc.).
Sub-bottom profiles were recorded using the onboard parametric sediment echosounder Atlas PS70. The system operated with two frequencies (18 and 22 kHz) emitted in a 4° cone from two hull-mounted transducers. Data were stored in the Parasound-native format ps3 and the raw-data format ASD for processing. The ps3 data were converted with the tool ps3segy (Hanno Keil, University of Bremen, Germany) into the standard seismic data format SEG-Y. Processing was performed using the software ReflexW (Sandmeier Software) and comprises automatic gain control (AGC) and amplitude normalization along the profile.
Profiles of conductivity, potential temperature, salinity and dissolved oxygen were derived from calibrated CTD measurements along a W-E transect (Fig. 1c) using a Seabird 911 CTD Sonde mounted on a Seabird Carousel Sampler equipped with 24 10-l Niskin bottles to collect water samples. The different parameters were visualized with the Ocean Data View software package (Schlitzer 2015).
Sea floor sampling was performed with the RV Sonne video grab sampler (five samples) and a box corer (two samples). The video grab sampler also provided a visual record of the sea floor surface that allowed the identification of sedimentary features and cold-water coral distribution prior the sampling. The sediment samples were separated into distinct fractions (>2, >0.25, >0.063, and <0.063 mm) and described accordingly. Composition of coral assemblages was analyzed from 88 specimens from MVC and 112 specimens from KC. In addition, 22 thin-sections (ten from MVC and 12 from KC) were prepared for analyzing preservation of cold-water corals and the presence of microborings.
Sample preparation and measurement as well as correction for 13C/12C ratio and calculation of conventional radiocarbon ages (CRA) were performed by Beta Analytic Inc. (Miami, USA) on selected coral fragments. In order to avoid contamination of samples for dating by younger material, the encrusters as well as the surficial alteration crust on the corals were removed and areas affected by microborings were avoided. Samples were cleaned with ultrasounds in deionized water and were visually inspected for cements, overgrowths and fills. Calibration of conventional radiocarbon ages was done with the software Calib (v.7.0.4., Stuiver and Reimer 1993) using the calibration curve “MARINE13” (Reimer et al. 2013) and a global reservoir correction of 200–500 years to account for the delay in atmosphere–ocean exchange of 14C. Calibrated ages (cal BP) are rounded to the next decade and provided as median of the probability distribution with 2σ error range (95.4% probability).
Epibionts and endobionts (borers and microborers) were analyzed on surface of cold-water corals and thin-sections under stereoscopic microscopy (Olympus SZ60, Universidad de Jaén). Epibiont relationships were studied on cold-water coral surfaces using the overgrowth ability index of Taylor (1979). This index was calculated for each microencruster using Taylor’s formula:
This simple index expresses the number of successful overgrowths as a percentage of the total number of overgrowth interactions. Lateral and frontal overgrowths among microencrusters are not differentiated, because such structures can only be observed in thin-sections. The overgrowth ability index in this paper is treated as an approximation to the true relationships among microencrusters, since overlapping is a complex three-dimensional phenomenon.
Results
Depositional and oceanographical setting
Cold-water corals were found at two stations at the eastern flank of the archipelago. SO236-007 to the south is located at 04°09,07′N, 73°29,28′E, and SO236-017 to the north at 04°51.26′N, 73°28.05′E (Figs. 1, 2).
The southern site is located between Malé Atoll and South Malé Atoll in the eastern part of the Malé Vaadhoo Channel (MVC, Figs. 1, 2b–d). Cold-water corals are recorded at a water depth ranging from 437 to 448 m. Samples were recovered from 443-m depth. The channel floor is covered by some blocks and a several kilometer wide field of submarine dunes at a water depth of 390 m (Fig. 2c, d). The submarine dune sediment consists of bioclast-rich carbonate sands with common skeletal remains >2 mm. The main components are bryozoans, bivalves (mainly pectinids), gastropods, Halimeda plates, pteropods, and benthic foraminifera. The occurrence of Halimeda and the benthic foraminifera Amphistegina indicates sediment export from the shallow-water areas of the faros lining the channel and reworking of the sediment by the bottom currents flowing in the channel. Adjacent to the dune field, there are several blocks, which are up to 40 m high. The block, where the cold-water coral samples were taken, is sediment barren (Fig. 3d) and consists of a moderately bedded and fractured carbonate rock colonized by abundant hydrozoans and echinoids on submarine cliffs (Fig. 3e). At the flanks of the block there are sediment pockets with autochthonous/para-autochthonous cold-water coral rubble (floatstone texture) with a fine-grained wackestone matrix (Fig. 3f) which is rich in bioclasts such as planktic and benthic foraminifera, mollusk fragments, pteropods, echinoid fragments, and sponge spicules. The cold-water corals act as baffles of fine sediment due the surrounding rocky ground, where sediment is winnowed.
Sea-floor images obtained by the TV Grab sampler. The scale length is 20 cm. a Rocky sea floor at the top of the drowned atoll at the eastern Kardiva channel. b Cliff separating two terraces of the drowned atoll at the eastern Kardiva channel. The cliff is nowadays colonized by echinoids, bryozoans, hydrozoans and crustaceans. The bottom of the cliff is in contact with the cold-water coral rubble (right). c Cold-water coral rubble at eastern Kardiva channel. Enallopsammia is dominant in this area and reaches up to 40 cm in size. d Subvertical wall of the large flank of the block at Malé Vaadhoo channel (Fig. 2d). The rock surface is colonized by robust branched bryozoan and with sediment pockets with cold-water coral rubble (upper part of the picture). e Contact between the cold-water coral rubble (left) and the submarine cliff rocky surface encrusted by echinoids, bryozoans and sponges (right). f Cold-water coral rubble with abundant Lophelia floating in a fine grained sediment matrix
The northern site is located at the eastern end of the Kardiva Channel (KC) between Gaafaru Atoll and Kaashidhoo Atoll at a water depth ranging from 453 to 457 m (Figs. 1, 2a, 3a–c). The bottom presents a stepped topography with submarine cliffs. The coral samples were recovered at 457-m depth on the terrace between two submarine cliffs (at the foot of a 9 m submarine cliff and over a 28 m submarine cliff). These submarine cliffs are the wall of a drowned Miocene atoll (Betzler et al. 2009, 2013). The cliff consists of bedded and fractured bare rock and the surface of the rock is locally colonized by echinoids, bryozoans, serpulids, bivalves, sponges, and hydrozoans (Fig. 3b). The cold-water coral rubble is located on a terrace of the drowned atoll flank. The matrix among cold-water coral debris is an unconsolidated carbonate sediment with a poorly sorted floatstone with the debris of the biota associated with cold-water corals floating in a fine-grained wackestone texture with a periplatform ooze matrix. The cold-water corals are autochthonous (autochthonous). The surrounding bottom is a rocky ground where benthic organisms are scarce (Fig. 3a). The fine sediment is retained between cold-water corals meanwhile this sediment is winnowed from surrounding rocky ground.
The surveys with the video grab sampler shows that at both sites there are no living scleractinian corals (Fig. 3c, f). Radiocarbon dating results on the cold-water corals give a conventional radiocarbon age of 21,400–21,840 ± 60 BP for MVC and 22,190–22,540 ± 60 BP for KC (Table 1). The only living macrofauna colonizing the coral rubble is represented by Gorgonia and solitary corals.
The CTD profiles in the Kardiva Channel display strong and stable temperature stratification throughout the Inner Sea with a sharp thermocline at a water depth of 80–90 m (Fig. 4). Average temperatures in the surface mixed layer varied between 28 and 29 °C and plot well within the range of the climatological mean for August. Below the thermocline, at 500 m, temperatures decrease to about 12 °C (Fig. 4). Dissolved oxygen concentrations are around 4.03 ml/l in the upper mixed layer, sharply declining to 1.12–1.34 ml/l below the thermocline. A distinct oxygen minimum with concentrations of about 0.896 ml/l is observed around 500-m water depth. Salinity attains values of around 35.8 psu above the thermocline and averages 35.3 psu below; slightly higher values of 35.35 psu were recorded at the depth where the cold-water corals were found (Fig. 4). The values of neutral density, calculated from the salinity and temperature profiles, are lower than 23 kg/m3 above the thermocline, gradually increasing to 27.25 kg/m3 at the depth of the cold-water coral sampling site (Fig. 4).
Composition of coral assemblages
The coral assemblage is composed by representatives of the Order Scleractinia such as Enallopsammia (Family Dendrophylliidae), Lophelia and Desmophyllum (Family Caryophylliidae), Madrepora (Family Oculinidae) and Stephanophilia (Family Merulinidae) The Order Alcyonacea is represented by the genus Gorgonia (Family Gorgoniidae) (Fig. 5). Coral assemblages from both settings are dominated by Lophelia pertusa (88% at the MVC, 68% at the KC site; Fig. 6) followed by Madrepora oculata (8% at the MVC site and 20% at the KC site) and Enallopsammia rostrata (1% at the MVC site and 8% at the KC site). Desmophyllum and Stephanophyllia are only recorded at the MVC site (Fig. 6).
Preservation of corals
The corals are fragmented and the size of the fragments depends on the taxa. Mean size of Lophelia is 3.8 cm at the MVC site, and 3.2 cm at the KC site. The debris of the second most abundant genus Madrepora is 4.9 cm large in average at the MVC site and 4.2 cm at the KC site. The maximum size of Lophelia fragments is 11 cm the KC site and the maximum size of Madrepora pieces is 34 cm at the MVC site. The larger specimens of corals correspond to genera Enallopsammia (mean size 11.3 cm).
Coral fragments are characterized by distinct colors. At the KC site, Enallopsammia and some Madrepora mostly have a brown surficial phosphatic stain with Fe–Mn oxy-hydroxides (see Freiwald et al. 1997; Freiwald and Wilson 1998). Such specimens are denser and do not have an inner porosity. The upper surface of Enallopsammia specimens are affected by corrasion (sensu Brett and Baird 1986) as well as abundant borings and microborings (Fig. 7a). The white specimens (Lophelia and Madrepora) are comparatively well preserved, without corrasion facets and low incidence of borings. Those are generally located in the theca, in the growth density bands of Lophelia. Growth density bands are poorly developed in Madrepora.
Borings and microborings: a Microborings on an Enallopsammia specimen (KC). b Presence of large sponge chambers on growth density bands of Lophelia (KC) c Presence of large sponge chambers on growth density bands of Madrepora (MVC). d Thin section view of sponge chambers on growth density bands of Madrepora (MVC). e Microborings of fungi on Enallopsammia (KC) in thin section. f Microboring of fungi and boring of sponge on Madrepora in thin section (MVC)
Corals from the MVC site are white, grey-light blue, and brown in color. Corrasion facets and borings are scarcer than in KC samples. Growth density bands are well developed, mainly in Madrepora specimens, where borings of sponges are located in the theca (Fig. 7b–f).
Description of epibiont communities
Malé Vaadhoo Channel
At the MVC site, the main epibionts on the corals are tube-dwelling encrusting serpulids dominated by Spirorbis (small spirally-coiled tubes) and Serpula vermicularis. Filograna, Hydroides, and Placostegus are less abundant (Figs. 8, 9). Other undifferentiated serpulids with a prominent keel have been included in the morphogroup dorsoserpula. The Spirorbis specimens are characterized by a sinistral coiling tube with a smooth porcellanous surface and occasional faint growth rings. These forms are tentatively attributed to Spirorbis corallinae. Less frequent specimens of Spirorbis have sinistral tubes with three prominent longitudinal ridges but preservation impedes species determination. Spirorbis constitutes 79% of the epibionts, while the remaining serpulids constitute 19% (Fig. 8). The proportion of Spirorbis with respect to other epibionts is lower on Lophelia (73%) than on Madrepora (87%). Other epibionts such as bryozoans, bivalves, and barnacles are scarce (Fig. 8). Bryozoans are mainly recorded on Madrepora (2% of the epibionts). Bivalves and barnacles are exclusively recorded on Lophelia (always <1% of the epibionts). Spirorbis exclusively colonizes the external surface of the corals, whereas other serpulids are also located over the coral calice and within the calice between the septa in Lophelia specimens (e.g., Filograna, Fig. 9c). Serpulids located within the calice constitute aggregates of fine tubes. Bioerosion of growth density bands is well developed in Madrepora, where corallites are mainly bioeroded by boring sponge Alectona millari and clionaid sponges (Fig. 7). The boring sponges form networks of cavities which may eventually ramify. Fungal microborings are poorly recorded.
Encrusters from cold-water corals of Malé Vaadhoo Channel. a Colonization of Madrepora by Spirorbis and serpulids. b, c Colonization within the calices of Lophelia by Filograna. d Presence of Spirorbis (blue arrow), serpulids (yellow arrows) and balanids (white arrows) on Lophelia. e Serpulid on calices of Lophelia. f Spirorbis encrusting Madrepora and large serpulid. g Bryozoans encrusting Madrepora surface
Kardiva channel
At the KC site, the assemblage of epibionts, which is mainly represented by encrusters, is more diverse than at the MVC site (Figs. 8, 10). It encompasses serpulids, bryozoans, benthic microbial mats, encrusting foraminifera, sponges, solitary corals, Gorgonia and terebellids, as well as abundant ophiuroids, ostracods, echinoids, gastropods, and brachiopods living between the coral branches (Figs. 10, 11).
Encrusters from cold-water corals of Kardiva Channel. a Spirorbis (spi), bryozoan (bry) and microborings (mb) from Enallopsammia. b Siliceous sponge (sp) encrusting Enallopsammia. c Massive bryozoans encrusting Enallopsammia specimen. d Serpulid (serp), bryozoans (bry) and solitary coral encrusting Lophelia. e Siliceous sponge (sp) within calice of Lophelia. f Calice of Lophelia colonized by siliceous sponge (sp) and bryozoans (yellow arrow). g Living uniserial bryozoans encrusting Lophelia surface. h Massive bryozoan (bry) encrusting calice of Lophelia. i Serpulids, Spirorbis and bryozoans encrusting Madrepora
Encrusters from cold-water corals. a Serpulids and Spirobis colonizing Lophelia specimen from KC. b Living Gorgonia attached to Enallopsammia from KC. c Living microbial biofilm growing on Enallopsammia from KC. d Living massive bryozoans encrusting Lophelia from KC. e Living balanid and solitary coral growing on Lophelia from KC. f Living specimens of solitary stony coral (yellow arrow, probably a Caryophylliidae), brachiopods (white arrows) and bryozoans (blue arrows) attached to Lophelia from MVC. g Living siliceous sponges between Lophelia branches from MVC. h Living indeterminate organism growing on Lophelia from MVC. i Indeterminate agglutinated foraminifera (astrorhizoid) attached to Lophelia from MVC. j Living siliceous sponges growing between Lophelia branches from MVC. k Living balanid attached to Enallopsammia from KC. l Recent uniserial bryozoans colonizing Enallopsammia from KC. m Dendrophryid form Spiculidendron (foraminifera) attached to Enallopsammia downward surface from KC. n Living specimens of uniserial tubular bryozoans growing between branches of Enallopsammia from KC. o Small ophiuroid living on Enallopsammia branches from KC. p Indeterminate sponge living on Enallopsammia from KC
The assemblage of epibionts strongly differs between the corals with the phosphatic crust (mainly Enallopsammia and scarce specimens of Madrepora) and the white corals (Lophelia and Madrepora). The brown corals are covered by a diverse assemblage of living encrusters (Fig. 11) dominated by bryozoans (30% uniserial morphotypes, 14% massive morphotypes) and siliceous sponges (23%). The rest of the assemblage is composed by encrusting agglutinated foraminifera (11%), Gorgonia (5%), ophiuroids (5%), encrusting calcitic foraminifera (4%), serpulids (3%), microbial mats (2%), solitary corals (2%) and barnacles (>1%). Among the sessile foraminifera, the arborescent dendrophryid form Spiculidendron with tubular branches is common and generally attached to the downward-oriented Enallopsammia surfaces. Siliceous sponges and microbial mats are commonly located between the coral branches just in the bifurcation. Moreover, sponges and ostracods are common in the calices of Enallopsammia.
The assemblage of epibionts on white corals is composed mainly of dead organisms and is dominated by serpulids such as Spirorbis (74% on Madrepora and 71% on Lophelia; Fig. 10j, k) and other serpulids (20% on Lophelia and 5% on Madrepora). Uniserial encrusting bryozoans (12% on Madrepora and 3% on Lophelia) and massive encrusting bryozoans (6% on Madrepora and 5% on Lophelia) are very diverse. Less common encrusters are siliceous sponges and terebellids.
In addition to epibionts, an endolith assemblage is recorded composed by clionaid sponges (Entobia ispp.) and fungi (Orthogonum ispp.).
Overgrowth interactions between epibionts
Spirorbis is mainly encrusted by other Spirorbis (63%) and secondarily by other serpulids, sponges, and balanids (n = 45). The main epibionts on the other serpulids are Spirorbis (61%) and other serpulids, microbial mats (30%), and minor bryozoans, sponges, sessile foraminifera and balanids (n = 57). Massive encrusting bryozoans are mainly overgrown by Spirorbis (36%) uniserial bryozoans (21%), and a minority by other serpulids, siliceous sponges, solitary corals and sessile foraminifera (n = 15). Recent microbial mats are only encrusted by agglutinated encrusting foraminifera. Recent sponges related to the coral remains are very small (usually <3 cm diameter) and scarcely encrusted by small serpulids and sessile foraminifera. However, recent sponges overgrow to recent massive bryozoans and recent solitary corals. The analysis of the overgrowth ability index (Taylor 1979) on Upper Pleistocene epibionts (not recent living specimens) shows higher values for sessile foraminifera (100%), Spirorbis (85%) and uniserial bryozoans (83%), meanwhile lower ones correspond to recent solitary corals (33%), large serpulids (29%) and massive bryozoans (12%).
Discussion
Environmental requirements
The controlling parameters of cold-water coral are current dynamics, temperature, water density, vertical and bed-load sediment supply, oxygenation, pH, and the most important, food supply (Freiwald et al. 2004; White et al. 2005; Duineveld et al. 2007; Roberts et al. 2009; Wienberg et al. 2010; Eisele et al. 2011; Fink et al. 2012, 2013; Mienis et al. 2012; Flögel et al. 2014; Hebbeln et al. 2014). Cold-water corals live in aphotic waters by preying mainly on zooplankton that drifts past the coral framework, especially in zones with bottom currents (however, at least for L. pertusa, other food sources have been mentioned; see Duineveld et al. 2007, 2012; Dodds et al. 2009; Tsounis et al. 2010). Moreover, they require a hard substrate on which to attach.
Lophelia pertusa has a nearly cosmopolitan distribution in water with temperatures between 4 and 13.9 °C (Roberts et al. 2006; Freiwald et al. 2009), salinities of 31.7–38.8 psu (Freiwald et al. 2004; Davies et al. 2008) and dissolved oxygen contents lying between 2.7 and 7.2 ml/l (Dodds et al. 2007; Davies et al. 2008, 2010). L. pertusa has been recorded from water depths ranging from 39 to 2775 m (Roberts et al. 2009), and forming buildups between 200- and 1000-m water depth (Davies and Guinotte 2011; Hebbeln et al. 2014).
Madrepora oculata is a secondary framework builder that occurs among colonies of L. pertusa. It is the most widespread cold-water coral taxa extending from 69°N off Norway to 59°S latitude in the Drake Passage (Hourigan et al. 2007) with a depth range from 55 to 1950 m (Zibrowius 1980). Colony branches have distinctive zig-zag morphology.
The Enallopsammia rostrata specimens recovered in the samples form dendroid colonies that usually display an open-spaced growth habit with polyps in branches pointing in the same direction. E. profunda was reported as endemic to the western Atlantic (from Antilles to Massachusetts) at water depths of 145–1750 m (Cairns 1979; Hebbeln et al. 2014), and E. rostrata has been reported from the Atlantic, the Indian, and the western Pacific Ocean (Cairns et al. 1999) in depths from 215 to 2165 m (Cairns 1979).
The two studied cold-water coral occurrences in the Maldives are located at the Indian Ocean facing entrances of the inter-atoll seaways connecting the Inner Sea with the open Indian Ocean. The video surveys and the hydroacoustic data show that these areas are current-swept rocky bottoms as a flow of water is funneled through the straits as indicated by submarine dunes at the Inner Sea facing parts of the channels (Fig. 2; Betzler et al. 2009, 2013; Lüdmann et al. 2013). Therefore, one of the main pre-requisite for deep sea cold-water coral growth, the formation of a current-swept substrate with no or little sedimentation, is fulfilled.
Cold-water corals feed on fresh phytodetritus, zooplankton or on a combination of both (Duineveld et al. 2004, 2007; Kiriakoulakis et al. 2005; Becker et al. 2009; Carlier et al. 2009; Dodds et al. 2009). The locations of the cold-water coral sites in seaways near the edge of the carbonate archipelago are favorable for a constant flow of water providing food to the cold-water corals.
According to Rogers (2004), cold-water corals also tend to live in areas with stable physical conditions and limited annual variations in temperature and salinity. Cold-water corals are often associated with the most salty water mass at a depth that often coincides with the oxygen minimum zone (Freiwald et al. 2002; Rogers 2004) where bacterial activity related to the nutricline is high. Kenyon et al. (2003) suggested that cold-water coral buildups in the North Atlantic are related to the oxygen minimum zone, that usually corresponds with high dissolved inorganic carbon and pCO2, because the oxygen minimum zone arises from remineralization of organic matter and therefore consumption of oxygen and release of CO2 (e.g., Findlay et al. 2014).
The water temperature is a further factor controlling the occurrence of cold-water corals (Roberts et al. 2009). For example L. pertusa is exclusively present at water temperatures between 4 and 13.9 °C (Roberts et al. 2006; Freiwald et al. 2009). However, cold-water corals have been reported from extreme environmental conditions in the Cape Lookout area (NW Atlantic) where is the largest temperature variability (>9 °C in a day) measured in a cold-water coral habitat (Mienis et al. 2014). Water temperature at the depth of cold-water corals at the KC site is approximately 12 °C, and this value is within the normal range of L. pertusa occurrence. Salinity values of around 35.3–35.4 psu at the depth where the cold-water corals were found are within the 31.7–38.8 psu salinity range for dominant L. pertusa, according to Freiwald et al. (2004) and Davies et al. (2008). Therefore, temperature and salinity are within the range of cold-water corals and they are not the reason for explaining the absence of living cold-water corals.
Here, it is proposed the present-day oxygen and density conditions at the sampling sites are the factors which inhibit modern cold-water coral growth. Dissolved oxygen concentrations are around 0.896 ml/l at 500-m depth (Fig. 4), that is a sensibly lower value than the minimum oxygen conditions needed for L. pertusa to survive (>2 ml/l; Dodds et al. 2007; Davies et al. 2008, 2010; Brooke and Ross 2014). Cold-water coral reefs are reported to exclusively occur at certain density ranges, specifically from 27.35 to 27.65 kg/m3 (Dullo et al. 2008), or even 27.38–27.61 kg/m3 (Flögel et al. 2014). This narrow range is closely linked to the aragonite saturation state, which within this density range is easily incorporated to cold-water coral skeletons (Findlay et al. 2014). At the Maldives studied sites, the values of neutral density of around 27.25 kg/m3 at the depth of cold-water coral occurrence are therefore out of the density envelope required for the cold-water coral to live according to Dullo et al. (2008) and Flögel et al. (2014).
Between 21.4 and 22.5 ka BP the global sea level was around 120 m lower than today (Lambeck and Chappell 2001) and therefore the different oxygen and density zones of the water masses with favorable conditions for cold-water corals occurred at the sea floor just at the location of KC and MVC areas where the cold-water coral rubbles are recorded today (these setting during the glacial lowstand of sea level were around 340-m depth for KC and 320-m depth for MVC). According to Tomczak and Godfrey (2003) seasonal and interseasonal currents in the monsoonal controlled area of the Indian Ocean reach water depths of 200 m and more with only slightly reduced velocities, which in the case of the Maldives is reflected by the formation of submarine dunes (Fig. 2) and drift bodies. Therefore, in a context of sea-level lowstand, the Malé Vaadhoo Channel and the Kardiva Channel were probably within favorable oxygen and density values and in a more favorable position with regard to the current regimes supplying the food supply to the cold-water corals. However, changes in other parameters such as changes in sedimentation rate, productivity in shallow waters (affecting to organic matter accumulation in the bottom and oxygenation) among others, related to sea-level lowstand conditions, are not considered as this cannot be resolved with the available data. More studies are needed in the future to elucidate these aspects.
Diversity of corals and epibionts
The benthic assemblage thriving in the Kardiva Channel is more diverse than the benthic assemblage from the Malé Vaadhoo Channel, both with regard to the corals and to the epibionts which are dominated by encrusters. The encrustation density of the corals is also higher in KC than in MVC. In addition, at the KC site the record of living encrusters (bryozoans, sponges, foraminifera, Gorgonia, solitary corals, balanids, dorsoserpula and microbial mats) and other epibionts (brachiopods, ostracods, and ophiuroids) is high. From MVC site, living specimens are less common, and they are restricted to bryozoans, sponges and Gorgonia, and rare cases of brachiopods, solitary corals and Terebella.
One factor which is proposed to control these differences is the sedimentation rate. Longer time of exposure of the coral substrate favors the colonization of bioclasts by epibionts including encrusters and borers and enforces the effect of corrasion (Jensen and Frederiksen 1992; Reolid and Gaillard 2007; Reolid et al. 2007). Especially the development of complex epibiont assemblages calls for a low rate of sedimentation (e.g., Jensen and Frederiksen 1992; Leinfelder et al. 1994). Sessile epibionts recorded at both sites from the Maldives are mainly suspension-feeders and therefore are very sensitive to any influx of fine-grained sediments (Leinfelder et al. 1994; Reolid et al. 2005). Low sedimentation rates and low terrigenous input would be well indicated by the most diverse encruster assemblages and by high abundances of encrusters per surface. Other potential controlling factor on epibionts is the availability of food resources (see Gil et al. 2006; Leite et al. 2016).
Phases of colonization
The corals were colonized, both, during the lifetime and after death. Beuck et al. (2007) and Freiwald and Wilson (1998) indicate that thin soft tissue cover the external surface of juvenile corallites or the distal part of the adult ones, and protect against epibionts, whereas tissue-barren skeletal parts are infested by microborers and encrusters. In a healthy colony, coral is able to encrust repetitively attached epibionts by selectively secreted sclerenchyme layers (Freiwald et al. 1997; Freiwald and Wilson 1998) resulting in a thickening of the skeleton. According to Rosso et al. (2010) and Sanfilippo et al. (2013), serpulids copiously encrust dead colonies of corals and fragments, or tissue-barren branches of living colonies. Freiwald et al. (1997) indicates that skeletal areas which lack a mucus and epithelial tissue protection get rapidly colonized by other sessile invertebrates such as spirorbids and encrusting bryozoans. Therefore a substantial amount of Spirorbis colonization may have occurred on the coral branches during live. Available data, however, do not allow excluding the colonization of death corals and fragments by Spirorbis.
Some bryozoans and serpulids (Filograna) occur within calices of the corals, where they are found shelter between the septa. This is not compatible with the colonization of the polyps during lifetime. Some epibionts from the KC site were alive when sampled coming mainly from brown coral remains. These were siliceous sponges, microbial mats, solitary corals, Gorgonia, bryozoans, brachiopods, ophiurids, and scarce serpulids other than Spirorbis. Living specimens were more common at the KC site and very scarce at the MVC site.
Several corals, especially Enallopsammia from the KC site, were colonized by microborers previously to the encrustation by epibionts. According to Beuck et al. (2007), in adult corallites the lower part of the polyp can be slightly infested due the retraction of the soft tissue that protects against bioeroding organisms. Early postmortem alteration in Lophelia colonies is indicated by the formation of a biofilm (sometimes phosphatic of Fe–Mn oxy-hydroxides) and fungi infestation as reported by Freiwald and Wilson (1998) from Lophelia reefs from Sula Ridge (Mid-Norwegian Shelf). Rapid rates of microbial activity have also been measured from experiments in carbonate substrates in shallow waters, with initial phases of microendolithic organism infestation occurring during the first week, and intense colonization occurring after a few months (Perkins and Tsentas 1976; Kobluk and Risk 1977; Bromley et al. 1990; Chazottes et al. 1995; Wisshak 2006; Wisshak et al. 2010). Diversity and infestation rates of microborers decreases according to depth (light availability) (Vogel et al. 1999; Wisshak et al. 2005a) but hydrographic parameters (such as current velocities) and proximity of cold-water corals favored the diversity and progress of bioerosion (Mortensen et al. 1995; Wisshak et al. 2005a). Endolith assemblage is dominated by boring sponges such as Alectona and clionaids, and fungi. According to Freiwald et al. (1997) and Freiwald and Wilson (1998) fungi and bacteria infestation occurs before clionaid sponges. This assemblage is exclusively of heterotroph organisms and it is regarded as indicative of fossil and Recent open marine aphotic environments (Beuck and Freiwald 2005; Bromley 2005; Wisshak et al. 2005b). Cases of encrustation in previously microbored surfaces, mostly of Enallopsammia, are common, mainly in the brown specimens of the KC site, but epibionts affected by microboring were not observed. Thus, an initial phase of microboring activity could be halted by later colonization by encrusting organisms. Accordingly, the time for settlement was longer for encrusters than for microborers. After colonization and under favorable environmental conditions, encruster growth could be very rapid. For example, the growth rate of serpulids in shallow-marine environments reaches up to 75 mm/year (Simon-Papyn 1965).
Competition for substrate
An interpretation of spatial relationships of the settlement is complicated by the possibility that interacting organisms did not live contemporaneously (Palmer and Palmer 1977; Taylor 1979). As the age of these corals ranges between 21,400 and 22,500 BP, a long exposure time of the coral remains can be assumed and consequently a lower probability of a contemporaneous interaction between epibionts. Some living specimens were recovered with the RV Sonne video grab sampler and box corer and the relationships between these epibionts suggest that active competition for substrate space occur. However, most of the epibionts are empty carcasses with color and fragmentations indicating they were death long time ago. For example, not living specimens of Spirorbis are settled on fragmented tubes of the morphotype dorsoserpula. For these cases, interpretations of spatial and paleoecological relationships among epibionts are hard to perform.
In general, it is assumed that competitively superior microencrusters overgrow the skeletons of less adept competitors, although in some cases, fossilized overgrowths may result from encrustation over an already dead organism (Lescinsky 1997). The lower values of the overgrowth ability index of the massive encrusting bryozoans (13%) can be the result of: (1) a lesser ability of massive encrusting bryozoans than sessile foraminifera, Spirorbis or uniserial bryozoans to compete for the available substrate. Massive encrusting bryozoans would be pioneer organisms colonizing the substrate, including during life of corals. Then, more specialized encrusters would appear; (2) the colonization of massive bryozoans by other organisms occurs when the bryozoans were dead. Colonization of living massive bryozoans by other organisms impeded the metabolic processes and asphyxia the bryozoans. Elsewhere, bryozoans were interpreted as poor space competitors (McKinney and Jackson 1989; Nebelsick 1992).
Preferential colonization
The differential colonization is recorded in the analyzed Pleistocene cold-water coral assemblages. Changes in the composition of encrusters assemblages are identified in both settings depending on the infested coral genus. This selective colonization has been also described in the literature for recent sponges from Mediterranean (Koukouras et al. 1985, 1996). The differences in the proportions of encrusters as well as differences in diversity are stronger in the Kardiva channel.
The preferences of colonization are related to the location of the different encrusters in the coral specimens. Filkorn (1994) indicated that certain species of bryozoa encrusting Madrepora from James Ross Basin (Antarctica) preferentially encrust the upcurrent sides of coral branches. Spirorbis, large serpulids and massive bryozoans among others exclusively grow on the external surface of the corals, but small serpulids (cycloserpulids) are preferently located within the calice between the septa (e.g., in Lophelia from MVC, Fig. 8b, c). Sponges are commonly recorded within the calices of Enallopsammia from KC samples or between the coral branches just in the bifurcation. Because light is not a controlling parameter in the analyzed deposits, this preferential location in protected, less exposed parts responds to a cryptobiontic behavior for larvae fixation. This cryptobiontic distribution might be aimed to avoid predation, with a preferential location of small sessile animals on sheltered parts of available substrates, where larvae are protected. Vagile organisms recorded at the KC site, such as small specimens of ophiurids, are probably juvenile specimens which obtain trophic resources and protection within the coral branches. Thus, these cold-water coral patches are places where the benthic life, mainly sessile, is concentrated, which is clearly scarce off-rubble patches.
Jensen and Frederiksen (1992) indicate the role of random colonization by epibionts on deep-water corals as an additional factor for explaining the distribution of epibionts.
Conclusions
This is the first record of cold-water corals from the Maldives archipelago, where such forms thrived during the last glacial maximum, at least from 21,400 to 22,500 BP. Coral assemblages are composed mainly of Lophelia, followed by Madrepora and Enallopsammia. However, the composition and state of preservation varies between the sampling points in the Kardiva Channel and the Malé Vadhoo Channel. The cold-water corals grew at the eastern mouths of the inter-atoll channels during the Late Pleistocene when these settings presented favorable conditions for developing cold-water coral such as enhanced currents, higher oxygen values, and different density conditions. It is proposed that low oxygen and density conditions are the main restricting factors suppressing cold-water corals in the recent.
The epibiont assemblages are very diverse and abundant, and show different abundances between both studied sites as well as differences with regard to the colonized coral genus. Some living benthic organisms such as brachiopods, bivalves, gastropods, barnacles and ophiurids find refuge among coral branches actually and they are not observed in surrounding rocky ground. However, Spirorbis, the most common encruster reported on Kardiva Channel and the Malé Vaadhoo Channel sites is not recorded as living specimens. Environmental requirements of this group were probably similar to those of cold-water corals.
From our data and observations we deduce a series of general conclusions. (A) although the corals are dead, the coral debris still serves as a cradle for other species; (B) a tentative sequence of colonization is proposed with borers and Spirorbis being the first colonizers followed by other serpulids and bryozoans; (C) epibionts appear to select the coral genus or species to be colonized; (D) cryptobiontic life style of most of the epibionts is not related to photophobic behavior.
References
Aubert O, Droxler AW (1992) General Cenozoic evolution of the Maldives carbonate system (equatorial Indian Ocean). Bull Centre Rech Explor Prod Elf Aquitaine 16:113–136
Becker EL, Cordes EE, Macko SA, Fisher CR (2009) Importance of seep primary production to Lophelia pertusa and associated fauna in the Gulf of Mexico. Deep Sea Res Part I 56:786–800
Betzler C (2015) RV Sonne cruise report/Fahrtbericht SO236, Malé to Colombo, 9 to 29 August 2014. doi:10.2312/cr_so236
Betzler C, IODP Expedition 359 Scientist (2016) The abrupt onset of the modern South Asian monsoon winds. Sci Rep 6:29838. doi:10.1038/srep29838
Betzler C, Hübscher C, Lindhorst S, Reijmer JJG, Römer M, Droxler AW, Fürstenau J, Lüdmann T (2009) Monsoonal-induced partial carbonate platform drowning (Maldives, Indian Ocean). Geology 37:867–870
Betzler C, Lüdmann T, Hübscher C, Fürstenau J (2013) Current and sea-level signals in periplatform ooze (Neogene, Maldives, Indian Ocean). Sediment Geol 290:126–137
Beuck L, Freiwald A (2005) Bioerosion patterns in a deep-water Lophelia pertusa (Scleractinia) thicket on the propeller mound (northern Porcupine Seabight). In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 915–936
Beuck L, Vertino A, Stepina E, Karolczak M, Pfannkuche O (2007) Skeletal response of Lophelia pertusa (Scleractinia) to bioeroding sponge infestation visualised with micro-computed tomography. Facies 53:157–176
Brett CE, Baird GC (1986) Comparative taphonomy: a key to paleoenvironmental interpretation based on fossil preservation. Palaios 1:207–227
Bromley RG (2005) Preliminary study of bioerosion in the deep-water coral Lophelia, Pleistocene, Rhodes, Greece. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 895–914
Bromley RG, Hanken NM, Asgaard U (1990) Shallow marine bioerosion: preliminary results of an experimental study. Bull Geol Soc Den 38:85–99
Brooke S, Ross SW (2014) First observations of the cold-water coral Lophelia pertusa in mid-Atlantic canyons of the USA. Deep Sea Res Part II 104:245–251
Cairns SD (1979) The deep-water Scleractinia of the Caribbean Sea and adjacent waters. Stud Fauna Curaçao Other Caribb Isl 57:341
Cairns SD, Hoeksema BW, van der Land J (1999) List of extant stony corals. Species richness of recent Scleractinia. Atoll Res Bull 459:1–46
Carlier A, Le Guilloux E, Olu-Le Roy K, Sarrazin J, Mastrotorato F, Taviani M, Clavier J (2009) Trophic relationships in a deep Mediterranean cold-water coral bank (Santa Maria di Leuca, Ionian Sea). Mar Ecol Prog Ser 397:125–137
Chazottes V, Le Campion-Alsumard T, Peyrot-Clausade M (1995) Bioerosion rates on coral reefs: interactions between macroborers, microborers and grazers (Moorea, French Polynesia). Palaeogeogr Palaeoclimatol Palaeoecol 113:189–198
Davies AJ, Guinotte JM (2011) Global habitat suitability for framework-forming cold-water corals. PLoS One 6:e18483
Davies AJ, Wisshak M, Orr JC, Roberts JM (2008) Predicting suitable habitat for the cold-water coral Lophelia pertusa (Scleractinia). Deep Sea Res Part I 55:1048–1062
Davies AJ, Duineveld G, van Weering TCE, Mienis F, Quattrini AM, Seim HE, Bane JM, Ross SW (2010) Short-term environmental variability in cold-water coral habitat at Viosca Knoll, Gulf of Mexico. Deep Sea Res Part I 57:199–212
Dodds LA, Roberts JM, Taylor AC, Marubini F (2007) Metabolic tolerance of cold-water coral Lophelia pertusa (Scleractinia) to temperature and dissolved oxygen change. J Exp Mar Biol Ecol 349:205–214
Dodds LA, Black KD, Orr H, Roberts JM (2009) Lipid biomarkers reveal geographical differences in food supply of the cold-water coral Lophelia pertusa (Scleractinia). Mar Ecol Prog Ser 397:113–124
Droxler AW, Haddad GA, Mucciarone DA, Cullen JL (1990) Pliocene-Pleistocene aragonitic cyclic variations in Holes 714A and 716B (the Maldives) compared with Hole 633A (the Bahamas): records of climate-induced CaCO3 preservation at intermediate water depths. Proc Ocean Drill Proj Sci Results 115:539–577
Duineveld GCA, Lavaleye MSS, Berghuis EM (2004) Particle flux and food supply to a seamount cold-water coral community (Galicia Bank, NW Spain). Mar Ecol Prog Ser 277:13–23
Duineveld GCA, Lavaleye MSS, Bergman MJN, De Stigter H, Mienis F (2007) Trophic structure of a cold-water coral mound community (Rockall Bank, NE Atlantic) in relation to the near bottom particle supply and current regime. Bull Mar Sci 81:449–467
Duineveld GCA, Jeffreys RM, Lavaleye MSS, Davies AJ, Bergman MJN, Watmough T, Witbaard R (2012) Spatial and tidal variation in food supply to shallow cold-water coral reefs of the Mingulay Reef complex (Outer Hebrides, Scotland). Mar Ecol Prog Ser 444:97–115
Dullo WC, Flögel S, Rüggeberg A (2008) Cold-water coral growth in relation to the hydrography of the Celtic and Nordic European continental margin. Mar Ecol Prog Ser 371:165–176
Eisele M, Frank N, Wienberg C, Hebbeln D, López Correa M, Douville E, Freiwald A (2011) Productivity controlled cold-water coral growth periods during the last glacial off Mauritania. Mar Geol 280:143–149
Filkorn HF (1994) Fossil scleractinian corals from James Ross Basin, Antarctica. Antarct Res Ser 65:1–95
Findlay HS, Hennige SJ, Wicks LC, Moreno Navas J, Woodward EMS, Roberts JM (2014) Fine-scale nutrient and carbonate system dynamics around cold-water coral reefs in the northeast Atlantic. Sci Rep 4:3671. doi:10.1038/srep03671
Fink HG, Wienberg C, Hebbeln D, McGregor HV, Schmiedl G, Taviani M, Freiwald A (2012) Oxygen control on Holocene cold-water coral development in the eastern Mediterranean Sea. Deep-Sea Res Part I 62:89–96
Fink HG, Wienberg C, De Pol-Holz R, Wintersteller P, Hebbeln D (2013) Cold-water coral growth in the Alboran Sea related to high productivity during the Late Pleistocene and Holocene. Mar Geol 339:71–82
Flögel S, Dullo WC, Pfannkuche O, Kiriakoulakis K, Rüggeberg A (2014) Geochemical and physical constraints for the occurrence of living cold-water corals. Deep-Sea Res II 99:19–26
Frederiksen R, Jensen A, Westerberg H (1992) The distribution of the scleractinian coral Lophelia pertusa around the Feroe Islands and the relation to internal tidal mixing. Sarsia 77:157–171
Freiwald A, Wilson JB (1998) Taphonomy of modern deep, cold-temperate water coral reefs. Hist Biol 13:37–52
Freiwald A, Reitner J, Krutschinna J (1997) Microbial alteration of the deep-water coral Lophelia pertusa: early post-mortem processes. Facies 36:223–226
Freiwald A, Hühnerbach V, Lindberg B, Wilson JB, Campbell J (2002) The Sula reef complex, Norwegian shelf. Facies 47:179–200
Freiwald A, Fosså JH, Grehan A, Koslow T, Roberts J (2004) Cold-water coral reefs. UNEP-WCMC Biodiversity series 22, p 84
Freiwald A, Rogers A, Hall-Spencer J (2005) Global distribution of cold-water corals (version 2). Update of dataset in Freiwald et al. (2004). UNEP World Conservation Monitoring Centre, Cambridge. http://data-unep-wcmc.org/datasets/3
Freiwald A, Beuck L, Rüggeberg A, Taviani M, Hebbeln D, RV Meteor Cruise M70–1 participants (2009) The white coral community in the central Mediterranean Sea revealed by ROV surveys. Oceanography 22:58–74
Fürstenau J, Lindhorst S, Betzler C, Hübscher C (2010) Submerged reef terraces of the Maldives (Indian Ocean). Geo-Mar Lett 30:511–515
Gil M, Armitage AR, Fourqurean JW (2006) Nutrient impacts on epifaunal density and species composition in a subtropical seagrass bed. Hydrobiologia 569:437–447
Hebbeln D, Wienberg C, Wintersteller P, Freiwald A, Becker M, Beuck M, Dullo C, Eberli G, Glogowski S, Matos L, Forster N, Reyes-Bonilla H, Taviani M (2014) Environmental forcing of the Campeche cold-water coral province, southern Gulf of Mexico. Biogeosciences 11:1799–1815
Henry LA, Roberts JM (2007) Biodiversity and ecological composition of macrobenthos on cold-water coral mounds and adjacent off-mound habitat in the bathyal Porcupine Seabight, NE Atlantic. Deep Sea Res I 54:654–672
Hourigan TF, Lumsden E, Dorr G, Bruckner AW, Brooke S, Stone RP (2007) State of deep coral ecosystems of the United States: introduction and national overview, p 64
Jensen A, Frederiksen R (1992) The fauna associated with the bank-forming deepwater coral Lophelia pertusa (Scleractinaria) on the Faroe shelf. Sarsia 77:53–69
Kenyon NH, Akhmetzhanov AM, Wheeler AJ, van Weering TCW, de Haas H, Ivanov MK (2003) Giant carbonate mud mounds in the southern Rockall Trough. Mar Geol 195:5–30
Kiriakoulakis K, Harper E, Wolff GA (2005) Lipids and nitrogen isotopes of two deep-water corals from the North-East Atlantic: initial results and implications for their nutrition. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 715–729
Kobluk DR, Risk MJ (1977) Micritization and carbonate-grain binding by endolithic algae. Am Assoc Pet Geol Bull 61:1069–1082
Koukouras A, Voultsiadou-Koukoura E, Chintiroglou H, Dounas C (1985) Benthic bionomy of the North Aegean sea III. A comparison of the microbenthic animal assemblages associated with seven sponge species. Cah Biol Mar 26:301–319
Koukouras A, Russo A, Voultsiadou-Koukoura E, Arvanitidis C, Stefanidou D (1996) Macrofauna associated with sponge species of different morphology. Mar Ecol 17:569–582
Lambeck K, Chappell J (2001) Sea level change through the last glacial cycle. Science 292:679–686
Larsson AI, Purser A (2011) Sedimentation on the cold-water coral Lophelia pertusa: cleaning efficiency from natural sediments and drill cuttings. Mar Pollut Bull 62:1159–1168
Leinfelder RR, Krautter M, Laternser R, Nose M, Schmid DU, Schweigert G, Werner W, Keupp H, Brugger H, Herrmann R, Rehfeld-Kiefer U, Koch R, Zeiss A, Schweizer V, Christmann H, Menges G, Luterbacher H (1994) The origin of the Jurassic reefs: current research developments and results. Facies 31:1–56
Leite FPP, Pavani L, Yanaka MO (2016) Temporal variation of epi- and endofaunal assemblages associated with the red sponge Tedania ignis on a rocky shore (São Sebastião Channel) SE Brazil. Iheringia Sér Zool 106:e2016007
Lescinsky HL (1997) Epibionts communities: recruitment and competition on North American Carboniferous brachiopods. J Paleontol 71:34–53
Lüdmann T, Kalvelage C, Betzler C, Fürstenau J, Hübscher C (2013) The Maldives, a giant isolated carbonate platform dominated by bottom currents. Mar Pet Geol 43:326–340
Malone MJ, Baker PA, Burns SJ, Swart PK (1990) Minor element and stable isotopic composition of the carbonate fine fraction: site 709, Indian Ocean. Proc Ocean Drill Proj Sci Results 115:661–676
Mastrototaro F, D’Onghia G, Corriero G, Matarrese A, Maiorano P, Panetta P, Gherardi M, Longo C, Rosso A, Sciuto F, Sanfilippo R, Gravili C, Boero F, Taviani M, Tursi A (2010) Biodiversity of the white coral bank off Cape Santa Maria di Leuca (Mediterranean Sea): an update. Deep Sea Res Part II 57:412–430
McKinney F, Jackson L (1989) Bryozoan evolution. Unwin Hyman, Boston, p 238
Mienis F, Duineveld GCA, Davies AJ, Ross SW, Seim H, Bane J, Van Weering TCE (2012) The influence of nearbed hydrodynamic conditions on cold-water corals in the Viosca Knoll area, Gulf of Mexico. Deep Sea Res Part I 60:32–45
Mienis F, Duineveld GCA, Davies AJ, Lavaleye MMS, Ross SW, Seim H, Bane J, Van Haren H, Bergman MJN, De Hass H, Brooke S, Van Weering TCE (2014) Cold-water coral growth under extreme environmental conditions, the Cape lookout area, NW Atlantic. Biogeosciences 11:2543–2560
Morigi C, Sabbatini A, Vitale G, Pancotti I, Gooday AJ, Duineveld GCA, De Stigter HC, Danovaro R, Negri A (2012) Foraminiferal biodiversity associated with cold-water coral carbonate mounds and open slope of SE Rockall Bank (Irish continental margin—NE Atlantic). Deep Sea Res Part I 59:54–71
Mortensen PB, Hovland M, Brattegard T, Farestveit R (1995) Deep water bioherms of the scleractinian coral Lophelia pertusa (L.) at 64ºN on the Norwegian shelf: structure and associated megafauna. Sarsia 80:145–158
Nebelsick JH (1992) Components analysis of sediment composition in early Miocene temperate carbonates from the Austrian paratethys. Palaeogeogr Palaeoclimatol Palaeoecol 91:59–69
Palmer TJ, Palmer CD (1977) Faunal distribution and colonization strategy in a Middle Ordovician hardground community. Lethaia 10:179–199
Perkins RD, Tsentas CI (1976) Microbial infestation of carbonate substrates planted on the St. Croix shelf, West Indies. Bull Geol Soc Am 87:1615–1628
Purdy EG, Bertram GT (1993) Carbonate concepts from the Maldives, Indian Ocean. Am Assoc Pet Geol Stud Geol 34:56
Purser A, Thomsen L (2012) Monitoring strategies for drill cutting discharge in the vicinity of cold-water coral ecosystems. Mar Pollut Bull 64:2309–2316
Reed JK (2002) Comparison of deep-water coral reefs and lithoherms off southeastern USA. Hydrobiologia 471:57–69
Reimer PJ, Bard E, Bayliss A, Beck JW, Blackwell PG, Bronk Ramsey C, Buck CE, Cheng H, Edwards RL, Friedrich M, Grootes PM, Guilderson TP, Haflidason H, Hajdas I, Hatté C, Heaton TJ, Hogg AG, Hughen KA, Kaiser KF, Kromer B, Manning SW, Niu M, Reimer RW, Richards DA, Scott EM, Southon JR, Turney CSM, van der Plicht J (2013) IntCal13 and MARINE13 radiocarbon age calibration curves 0–50,000 years calBP. Radiocarbon. doi:10.2458/azu_js_rc.55.16947
Reolid M, Gaillard C (2007) Microtaphonomy of bioclasts and paleoecology of microencrusters from the Upper Jurassic spongiolithic limestones (External Prebetic, Southern Spain). Facies 53:97–112
Reolid M, Gaillard C, Olóriz F, Rodríguez-Tovar FJ (2005) Microbial encrustation from the Middle Oxfordian-earliest Kimmeridgian lithofacies in the Prebetic Zone (Betic Cordillera, Southern Spain): characterization, distribution and controlling factors. Facies 50:529–543
Reolid M, Gaillard C, Lathuilière B (2007) Microfacies, microtaphonomic traits and foraminiferal assemblages from upper Jurassic oolitic-coral limestones: stratigraphic fluctuations in a shallowing-upward sequence (French Jura, Middle Oxfordian). Facies 53:553–574
Roberts JM, Wheeler AJ, Freiwald A (2006) Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312:543–547
Roberts JM, Wheeler AJ, Freiwald A, Cairns SD (2009) Cold-water corals: the biology and geology of deep-sea coral habitats. Cambridge University Press, New York, p 334
Rogers AR (2004) The biology, ecology and vulnerability of deep-water coral reefs. International Union for Conservation of Nature & Natural Resources, Cambridge, p 11
Rosso A, Vertino A, Di Geronimo I, Sanfilippo R, Sciuto F, Di Geronimo R, Violanti D, Corselli C, Taviani M, Mastrotorato F, Tursi A (2010) Hard and soft-bottom thanatofacies from the Santa Maria di Leuca deep-water coral province, Mediterranean. Deep-Sea Res II 57:360–379
Sanfilippo R, Vertino A, Rosso A, Beuck L, Freiwald A, Taviani M (2013) Serpula aggregates an their role in deep-sea coral communities in the southern Adriatic Sea. Facies 59:663–677
Schlitzer R (2015) Ocean data view. http://odv.awi.de. Accessed 9 Jan 2017
Schöttner S, Wild C, Hoffmann F, Boetius A, Ramette A (2012) Spatial scales of bacterial diversity in cold-water coral reef ecosystems. PLoS One 7:e32093
Simon-Papyn L (1965) Installation expérimentale du benthos sessile des petits substrats durs de l’étage circalittoral en Méditerranée. Recl Trav Stn Mar d’Endoume 39:51–94
Stuiver M, Reimer PJ (1993) Extended 14C database and revised CALIB radiocarbon calibration program. Radiocarbon 35:215–230
Taylor PD (1979) Palaeoecology of the encrusting epifauna of some British Jurassic bivalves. Palaeogeogr Palaeoclimatol Palaeoecol 28:241–262
Titschack J, Baum D, De Pol Holz R, Lopéz Correa M, Forster N, Flögel S, Hebbeln D, Freiwald A (2015) Aggradation and carbonate accumulation of Holocene Norwegian cold-water coral reefs. Sedimentology 62:1873–1898
Tomczak M, Godfrey JS (2003) Regional oceanography: an introduction. Daya Publ House, Delhi, p 390
Tsounis G, Orejas C, Reynaud S, Gili JM, Allemand D, Ferrier-Pagès C (2010) Prey-capture rates in four Mediterranean cold-water corals. Mar Ecol Prog Ser 398:149–155
van Soest RWM, Cleary DFR, de Kluijver MJ, Lavaleye MSS, Maier C, van Duyl FC (2007) Sponge diversity and community composition in Irish bathyal coral reefs. Contrib Zool 76:121–142
Vogel K, Balog SJ, Bundschuh M, Gektidis M, Glaub I, Krutschinna J, Radtke G (1999) Bathymetrical studied in fossil reefs, with microendoliths as paleoecological indicators. Profil 16:181–191
White M, Mohn C, de Stigter H, Mottram G (2005) Deep-water coral development as a function of hydrodynamics and surface productivity around the submarine banks of the Rockall Trough, NE Atlantic. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, The Netherlands, pp 503–514
Wienberg C, Titschack J (2015) Framework-forming scleractinian cold-water corals through space and time: a late quaternary North Atlantic perspective. In: Rossi S (ed) Marine animal forests: the ecology of benthic biodiversity hotspots. Springer, Heidelberg, pp 1–34
Wienberg C, Frank N, Mertens KN, Stuut JB, Marchant M, Fietzke J, Mienis F, Hebbeln D (2010) Glacial cold-water coral growth in the Gulf of Cádiz: implications of increased palaeoproductivity. Earth Planet Sci Lett 298:405–416
Wisshak M (2006) High-latitude bioerosion: the Kosterfjord experiment. Springer, Berlin, p 202
Wisshak M, Gektidis M, Freiwald A, Lundalv T (2005a) Bioerosion along a bathymetric gradient in a cold-temperate setting (Kosterfjord, SW Sweden): an experimental study. Facies 51:93–117
Wisshak M, Freiwald A, Lundalv T, Gektidis M (2005b) The physical niche of the bathyal Lophelia pertusa in a non-bathyal setting: environmental controls and palaeoecological implications. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 979–1001
Wisshak M, Form A, Jakobsen J, Freiwald A (2010) Temperate carbonate cycling and water mass properties from intertidal to bathyal depths (Azores). Biogeosciences 7:2379–2396
Zibrowius H (1980) Les Scléractiniaries de la Méditerranée et de l’Atlantique nord-oriental. Mém Inst Océanogr Monaco 11:247
Acknowledgements
We thank the officers and the crew of the R/V SONNE during Cruise SO236 MALSTROM for efficient assistance, and the Shipboard Scientific Party for substantial support. We thank Michael Kaminski for helping in the determination of dendrophryid foraminifera. The manuscript greatly benefited from comments by reviewers Andre Freiwald and Jürgen Titschack. CB, JR, and SL acknowledge funding by the BMBF (03G0236A). MR research activities were supported by Project RYC-2009-04316 (Ramón y Cajal Program). Eva Vinx is thanked for some close-up photography.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reolid, J., Reolid, M., Betzler, C. et al. Upper Pleistocene cold-water corals from the Inner Sea of the Maldives: taphonomy and environment. Facies 63, 8 (2017). https://doi.org/10.1007/s10347-016-0491-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10347-016-0491-7