Abstract
The western capercaillie (Tetrao urogallus) is a species of conservation concern that has been experiencing a severe decline in many regions of Europe. The poor breeding success rather than mortality of full-grown birds has been suggested to be responsible for its decline, been the nest loss a potential driver of the low productivity (number of chicks per female and year) of capercaillie. Working in a capercaillie population in the Pyrenees, we aimed to provide further insights into nest predation by monitoring 82 artificial nests (58 of them with camera traps), and estimating the population density of predators using camera traps and applying random encounter model and camera trap distance sampling. Pine/stone martens -Martes foina, Martes martes- were the species with higher nest predation rates (23%) despite being the less abundant (0.49 ind/km2), followed by foxes -Vulpes vulpes- (11%) and a population density of 3.37 ind/km2, and wild boar -Sus scrofa- (4%) and 6.95 ind·km− 2; predators could not be identified at 27% of the events. After fitting statistical models on the nest predation data, we observed that the martens’ encounter rate (i.e. number of martens detected per camera and day) and the altitude had a positive significant effect on the artificial nest predation probability. While caution is recommended in artificial nest experiments, our results are valuable highlighting the importance of martens and red foxes when addressing nest predation to promote the breeding success and population recovery of western capercaillie populations.
Resumen
El urogallo (Tetrao urogallus) es una especie amenazada y que ha experimentado un grave declive en muchas regiones de Europa. Se ha sugerido que la causa de su declive es el escaso éxito reproductor, en lugar de la mortalidad de individuos adultos; siendo la pérdida de nidos un posible factor causante de la baja productividad (número de pollos por hembra y año) del urogallo. Trabajando en una población de urogallos en los Pirineos, nuestro objetivo fue aportar más datos sobre la depredación de nidos mediante el seguimiento de 82 nidos artificiales (58 de ellos monitorizados con cámaras trampa), y la estimación de la densidad de población de depredadores utilizando cámaras trampa y aplicando el modelo de encuentro aleatorio y el muestreo a distancia con cámaras trampa. Las martas y garduñas -Martes foina, Martes martes- fueron las especies con mayores tasas de depredación de nidos (23% a pesar de ser las menos abundantes (0,49 ind/km2), seguidas de los zorros -Vulpes vulpes- (11%) y una densidad poblacional de 3,37 ind/km2, y los jabalíes -Sus scrofa- (4%) y 6,95 ind-km-2; en el 27% restante de los nidos predados no se identificó al depredador. Tras ajustar los modelos lineares generalizados a los datos de depredación de nidos, observamos que la tasa de encuentro de martas (es decir, el número de martas detectadas por cámara y día) y la altitud tenían un efecto positivo significativo sobre la probabilidad de depredación de nidos. Aunque se recomienda precaución en la interpretación de los resultados en experimentos con nidos artificiales, nuestros resultados son valiosos y destacan la importancia de las martas y los zorros a la hora de abordar la depredación de nidos para promover el éxito reproductor y la recuperación poblacional de las poblaciones de urogallo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The western capercaillie Tetrao urogallus is a Eurasian species that inhabits the extensive boreal coniferous forests of northern Europe and Asia, as well as the large mountain ranges of southern Europe (including the Alps, Balkans, the Carpathians, the Jura Mountains, the Pyrenees, and the Cantabrian Mountains) (Leclercq and Ménoni 2018). While the species status is “Least concern” according to the IUCN, the global population trend is decreasing, with a severe decline in many regions -particularly in southern and central Europe, and Scotland-, and even extinction in others (IUCN 2016; Jahren et al. 2016). In this respect, recent genetic studies suggested the existence of two evolutionarily significant units; individuals from the Iberian unit on the one hand (southwestern limit of the species’ global range), and all the other populations from the rest of Europe and Asia forming another evolutionary significant unit (Duriez et al. 2007; Escoda et al. 2023).
The southwestern population of capercaillie are distributed across two isolated areas: the Cantabrian mountains (Spain) and the Pyrenees (Andorra, Spain and France), and it was isolated after the last quaternary glaciation (Robles et al. 2005). Both populations represent the southwestern edge of the species distribution range, which makes these populations especially sensitive to climate change (Moss et al. 2001). Other populations in south and western Europe have become extinct, for instance in Ireland, Scotland (but later reintroduced) or Belgium (Gil 2011; Klaus and Bergmann 1994; Saniga 2012). The Cantabrian population is catalogued as “Critically endangered” (Ministerio para la Transición Ecológica 2018). This population suffered a reduction of more than 80% in the distribution range in the last 50 years, and the last census estimated a population size of around 200 individuals (Jiménez et al. 2022). The southern slope of the Pyrenean population has been recently catalogued as “Endangered” (Ministerio para la Transición Ecológica y el Reto Demográfico 2023). A decline of close to 40% in the number of males counted on leks was reported between 1980 and 2005 (Gil et al. 2020; Robles et al. 2022). In both populations, low productivity has been reported (Gil et al. 2020; Jiménez et al. 2022). Productivity (or recruitment rate) is usually measured as the mean number of chicks per hen from transects carried out with trained dogs in August (Gil et al. 2020). In the Cantabrian population, the average productivity is 0.37 chicks per female (range: 0–1.87) (Jiménez et al. 2022; Ministerio para la Transición Ecológica 2018). In the Pyrenean population, the average productivity reported is 0.95 chicks per female (range: 0–2.13) (Gil et al. 2020). Previous studies have suggested that several factors linked to forest structure (Kortmann et al. 2018), climate change (Moss et al. 2001), predation(Jahren et al. 2016; Moreno-Opo et al. 2015) and ground cover of bilberry (Baines et al. 1994) can affect capercaillie productivity. It has been also concluded that poor breeding success rather than mortality of full-grown birds was responsible for the decline of capercaillie (Jahren et al. 2016; Moss et al. 2000). In the southwestern populations, productivity does not compensate for adult mortality and the populations are expected to further decline unless productivity rates increase. Thus, a better understanding of capercaillie productivity is a priority aspect to preserve its populations.
Nest predation (or nest loss) is an important factor in the breeding success of birds, particularly for species whose nests are easily accessible (Martin 1993). A recent review showed that nests built in trees were safer than those closer to the ground (Matysioková & Remes, 2024). Artificial nest experiments to evaluate predation risk have been frequently used as a standard tool for assessing nest predation, especially for capercaillie and other tetraonids (Oja et al. 2018; Summers et al. 2004, 2009). Artificial nest advantages include sufficient sample sizes, the possibility to pre-plan complex comparative experiments, and little disturbance to nesting birds. Previous artificial nest studies have shown that mid-sized carnivores and corvids have been identified as a significant threat to many grouse populations and the driving force of some regional declines of the capercaillie (Matysek et al. 2021; Storaas 1988; Summers et al. 2004). Thus, reliable data on predators’ abundance is desired to better understand the implications of carnivores in capercaillie productivity, particularly as regards nest predation (Summers et al. 2004).
In this context, this study aimed to provide further insights into the nest predation rates, a possible driver of the low productivity observed in capercaillie in the southwestern population. Our objective was not to directly extrapolate predation rates on artificial nests to those expected in real ones, but just to compare relative predation rates among predators, and to better understand the drivers that determined the process of nest predation. Specifically, we considered three specific objectives: (i) to identify species which predated the artificial nest and its rates, (ii) to identify the main factors that determine the probability of artificial nest predation; and (iii) to estimate predators population density. We expected martens and foxes as the main predators, with higher predation rates by the more abundant species.
Methods
Study area
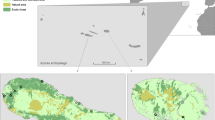
The study was conducted in the Alt Pirineu Natural Park, a protected area in northern Spain (Pyrenees, Lat: 42° 36’ N, Long: 1° 16’ W). Specifically, we monitored an area of 1,000 hectares with a northwest orientation and with capercaillie presence (Fig. 1). The area was characterized by a homogeneous forest of Pinus uncinata and Pinus sylvestris, with some Quercus forest at the lowest altitudes. We covered an altitudinal range from 940 to 2,292 m.a.s.l.
Map showing the capercaillie Tetrao urogallus distribution range according to the International Union for Conservation of Nature (IUCN) and the location (rectangle) of the study area (panel A). Map showing the distribution model of capercaillie in Catalonia, indicating adequate (light red), good (red) and optimal (dark grey) zones (from www.sig.gencat.cat, panel B) Map showing the location of the artificial nests (red squares) and those cameras (black crosses) used to estimate mammal population density with altitude as a base layer (panel C)
Field methods
Nest predation
From March 27th to June 26th 2023, 82 artificial nests were monitored (58 of them with a camera trap) for 30 days. Nests were deployed at two different times, from 27th to 30th March (first period), and from 2nd to 4th May (second period). Artificial nests consisted of two chicken eggs placed into a small depression on the ground, one of the eggs was gypsum-filled (Oja et al. 2018). Each gypsum-filled egg has embedded strings tied to a tree so predators cannot easily remove the egg (increasing the chance of the cameras being activated). At the bottom of each nest, we placed 30 g of dried grass. This grass was cut from the field and stored in a cage for a month in a chicken coop, thus the grass acquired the smell of hen. A nest was judged to have been lost to predators when one or more of the hen eggs were damaged or removed. The locations of artificial nests were chosen randomly using a geographic information system. At these locations, we then defined a buffer to 15-meter radius to place the nest in an area with dense vegetation or scrubland (e.g. under Rhododendron ferrugineum plants), according to the capercaillie preferences in the area (Grané 2020). We did not place any nests on roads or wildlife trails. Camera traps (Browning Recon Force Edge - BTC-7E) were set at 2–4 m from the nest and pointed downwards to maximize the probability of camera activation (Palencia et al. 2022). Cameras were set to record a burst of 8 pictures each time it was activated, with a time gap of 1 min between consecutive activations. To reduce the effect of human presence on the nest predation rate, we (i) put in place the nest between 9:00 and 18:00 to limit disturbance to nocturnal mammals (such time may increase predation by birds, but we expected the influence of birds to be minor compared with that of mammals), (ii) placed the nest and the camera in less than 10 min, (iii) did not mark the nest with any artificial mark to make easier to recover it, (iv) used latex gloves and dedicated field clothes (boots, trousers and jacket), and (v) did not revisit the nest until 30 days after. Finally, on each nest, we defined a buffer of 2 m radius in which we measured the ‘resistance to passage’ and ‘the scrubland coverage’. These variables were categorized into three levels: low, medium and high. Regarding the resistance to passage, we considered three characteristics: slope, fallen trees and scrubland. Thus, a nest surrounded by a high number of fallen trees, in an area with a high slope, and with a high coverage of scrubland was categorized as a ‘high’ resistance. On the contrary, a nest located in an area without fallen trees, plain terrain and with low coverage of scrubland was categorized as ‘low’ resistance. Regarding the scrubland coverage, we measured scrubland with a height over 30 cm, and we considered ‘low’, when the coverage in the buffer was lower than 33%, ‘medium’ when the coverage ranged from 33 to 66%, and ‘high’ when the scrubland coverage was higher than 66%.
Predators’ monitoring: density estimation
To monitor predators’ abundance, 28 camera traps (Tetrao Spromise S308) were randomly deployed simultaneously with nests (i.e. from March 27th to June 26th ). Cameras were placed heading toward the north, 50 cm above ground, and with the sensor angled parallel to the slope. Cameras were set to be operative all day, to record a burst of 10 consecutive pictures at each activation, with the minimum time lapse between consecutive activations. Nocturnal pictures were illuminated with infrared flash (low glow). Cameras were checked once to check the status of the batteries and memory cards.
Data processing
The pictures were filtered to discard false activations using Megadetector (Beery et al. 2019), and subsequently processed in the open software TrapTagger (https://wildeyeconservation.org/trap-tagger-about/) to classify the pictures by species. We grouped pine marten (Martes martes) and stone marten (Martes foina) in the same category “martens” due to the difficulties in accurately identifying the species from camera trap pictures illuminated with infrared flash.
Nest predation
We recorded the species that predated the nest, and the encounter rate (number of independent events per camera and day) of all the species recorded by the camera. An independent event was considered a detection (group of pictures) of a species spaced more than 10 min from the next detection of the same species (see Appendix S1). Exceptionally, for the species that predated a given nest, we selected the data recorded before predation to estimate the encounter rate. We considered this criterion because we observed a tendency in the predator to revisit the nest after predating it, which would result in an inflated encounter capture if we considered the entire monitoring period.
Predators’ monitoring: density estimation
The random encounter model (REM) and camera-trap distance sampling (CTDS) were considered the reference methods to estimate population density (Howe et al. 2017; Rowcliffe et al. 2008). The REM was applied for abundant species, while CTDS was applied for rare -low abundant- species (Palencia et al. 2021). Both methods require the locations of the animals in the field of view of the camera to subsequently derive the needed parameters to estimate population density. To estimate the locations of the animals in the field of detection, we applied a photogrammetry approach (Appendix S2).
Analysis
Nest predation
Factors that determine the probability of nest predation
Generalized linear models (GLM) with a binomial distribution and the logit link function were used to evaluate which factors determine the probability of nest predation. First, we fitted a model to assess the effect of environmental and habitat-related predictors including the data on all the nests monitored (n = 82). The response variable was whether the nest was predated (1’s) or not (0’s). As continuous predictors, we included: the slope, the altitude, the distance to water streams, and, the percentage of scrubland, riparian, deciduous, and evergreen forest in the 10 m radii buffer. As factors: the orientation (eight levels: N, NE, E, SE, S, SW, W and NW), the scrubland coverage (three levels: low, medium and high), the resistance to passage (three levels: low, medium and high), and whether the nest was monitored with a camera trap or not (two levels: yes or no). The slope, the altitude and the orientation were obtained from a Digital Terrain Model. The rest of the environmental and habitat-related variables were obtained from the land cover map provided by Center for Ecological Research and Forestry Applications (CREAF 2009), from which we defined a 10 m radii buffer and measured the percentage of riparian, deciduous, and evergreen forest, and scrubland to explore the effect of small-scale habitat characteristics. We applied a backward stepwise procedure based on Akaike’s Information Criteria to select the most parsimonious model (Akaike 1974). See a more detailed explanation of this model in Appendix S3.
Then, using the predictors maintained in the environmental and habitat-related best model (namely: altitude, slope, distance to water streams, and percentage of scrubland in a 10 m radius buffer), we subsequently explored the effect of predator species (martens, red foxes and wild boar) on the probability of nest predation using the data from those artificial nests monitored with camera traps (n = 58). Thus, to the above-mentioned environmental and habitat-related variables, we also included as predictors the encounter rate and presence of the predators. In total, we tested fifteen different models (including the null model). We fitted twelve single-species models on nest predation both for the species presence (as a factor) and the species encounter rate -six models- (e.g. model-1: predation ~ speciesa presence; model-2; predation ~ speciesa encounter rate), and also more complex models including environmental and habitat-related variables -six models- (e.g. model-3: predation ~ environmental/habitat-related + speciesa presence; model-4; predation ~ environmental/habitat-related + speciesa encounter rate). We also fitted two models including the presence and the encounter rate of the three species. The best model was selected based on AIC (Akaike 1974). In all the models described above, we excluded those predictors with a variance-inflation factor value > 3 (Zuur et al. 2010). On the best model selected, we applied the variation partitioning procedure to elucidate the relative weight of the predictors. This quantitative method allows specifying how much of the variation in the model is explained by each predictor (or group of related predictors) (Hortal et al. 2008). Briefly, this approach is based on fitting independent models for each factor (e.g. predation ~ predators), and also partial models for each pair of factors (e.g. predation ~ predators + environmental-habitat), to finally estimate the variation attributable to each factor independently of the other others as well their shared effects. All the above-mentioned models were validated according to the exploration of residuals and following Zuur et al. (2010).
Predators’ monitoring: density estimation
Both for CTDS and REM, the required parameters to apply the methods (namely: encounter rate, distances between animals and cameras, and activity level for CTDS; and encounter rate, day range and detection zone for REM were estimated from the camera trap pictures (Palencia, Fernández-López et al., 2021, Rowcliffe et al. 2011, 2014).
When applying CTDS, we modelled the decrease in the probability of detection as the distance between animals and the cameras increased. We fitted distance sampling models including the half-normal, hazard rate and uniform key functions, with a combination of expansion terms (Howe et al. 2017). The best model was selected using QIAC and following a two-step method (i.e. model selection within key functions -step 1-, and model selection among key functions -step 2-, Howe et al. 2019).
When applying REM, we modelled the encounters (detection) between animals and cameras, by accounting for the daily distance travelled by the target population (i.e. day range), and the camera trap-effective detection zone (i.e. the sector defined by radius and angle in which the camera effectively detects and individual of the target species). For further details on the application of CTDS and REM, please see (Howe et al. 2017; Palencia et al. 2022; Rowcliffe et al. 2008).
Results
No camera was stolen and a total of 184,959 pictures containing animals were recorded during a total sampling period of 4,026 camera trap nights. Capercaillie was detected in four cameras.
Nest predation
A total of 53 nests (64.63%) were predated (37 of them were monitored with cameras, and the remaining 16 were not monitored with cameras). The camera traps evidenced predation by martens (n = 19), red fox – Vulpes vulpes - (n = 9), and wild boar – Sus scrofa - (n = 3). We did not identify the predator in the remaining 22 nests due to not being monitored with cameras (n = 16) and malfunction issues (mainly empty batteries and full cards when the nests, n = 6). We also observed multiple examples of predators detected in a nest, but not predating it (martens: 9 nests, red fox: 12 nests, and wild boar: 23 nests, Appendix S4). We did not record any nest predated by the other carnivores present in the area, including badger (Meles meles), genet (Genetta genetta), brown bear (Ursus arctos) and weasel (Mustela nivalis), not by corvids. No nest was trampled by wild not domestic ungulates. The density of nests in a 25 m radius buffer around each nest was 5.2·10− 4 nests/m2; at a larger scale (500 m radius buffer), the density of nests was 9.4·10− 6 nests/m2. We did not observe an effect of the density of nests, not the period in which the nests were deployed on the probability of nest predation (see Appendix S4).
Statistical analysis
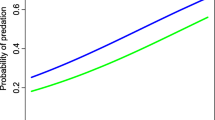
On the binomials GLMs fitted to explore the effect of environmental and habitat-related predictors in combination with the predator’s predictors, the best model included the marten’s encounter rate, the altitude, the slope, the distance to the water streams and the scrubland percentage (Table 1). We observed a strong positive effect of altitude and martens’ encounter rate on the probability of nest predation (Fig. 2). In this model, we discarded three nests due to camera malfunction issues, and two more due to the Cook’s distance > 1 (Zuur et al. 2010). The R2 of this model was 0.59. Regarding the variation partitioning analysis, independently of other variables, the encounter rate of martens explained 0.30 of the deviance, and the environmental and habitat-related variables explained 0.21 of the deviance; while 0.08 of the deviance was explained by the intersection of the two groups of variables.
Predators’ monitoring: density estimation
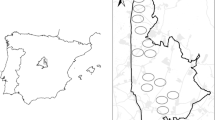
Martens and foxes were recorded in 25 cameras, and wild boar in 27 out of 28 cameras (Fig. 3.). Naïve occupancy for all the predators was higher than 0.9.
Applying REM, we estimated the population density of wild boar (6.95 ind·km− 2 ± 1.33, standard error -SE-), and red fox (3.37 ind·km− 2 ± 1.12 SE). Applying CTDS, we estimated the population density of pine and stone martens (0.49 ind·km− 2 ± 0.15 SE). REM and CTDS parameters for each species can be found in Appendix S5.
Spatial variation in encounter rates (number of events per camera and day) for martens (upper-left panel), red fox (upper-right panel) and wild boar (bottom-left panel). Circle size is proportional to the encounter rate. Bottom-right panel show the species that predated the nest (silhouettes), nests predated but predator not identified (red circles), and nests not predated (green circles)
Discussion
We have shown that martens, red foxes and wild boars were the species recorded predating ground artificial nests. While martens were the predators with less abundance in the study area (0.49 ind·km− 2), they were the species with the higher percentage of predation (23.17%). Martens encounter rate was also the main covariate explaining the probability of nest predation.
Martens, foxes and wild boars were abundant and widespread across the study area (Fig. 3), with a naïve occupancy higher than 0.9 for all of them. The confirmed presence of predators in most of the cameras made it useless to fit occupancy models, because accounting for imperfect detection (i.e. a species present but not detected in a camera) resulted in an occupancy estimate of 1, indicating predators occupying the entire study area (Goldstein et al. 2024). On the other hand, population densities were estimated at the study area level, so the unique variable that reflects the intensity at which predators used a habitat patch was the encounter rate. Additionally, the fact that not all encounters of predators with a nest resulted in a predation event reinforced the utility of this metric to model the predation process, (Appendix S4). The main limitations of the utility of encounter rate emerged when comparing different species, years and camera brands (Palencia et al. 2022a; Sollmann et al. 2013), but here we compared encounter rates among sampling points at the species level, we included data from only one year, and the same camera model was used in all the nests. Thus, we included predators’ encounter rate as a predictor in the models to account not only for environmental and habitat-related variables, but also for predators’ intensity of use (O’Brien et al., 2003; Ibáñez-Álamo et al. 2015; Shannon et al. 2023). Marten’s encounter rate was the predictor with the strongest effect and highest proportion of the deviance explained − 0.30- in the probability of nest predation (Table 1; Fig. 2). For any habitat patch in which martens’ encounter rate was higher than 0.04, the probability of nest predation was higher than 0.5 (Fig. 2). In our study area, the median and mean martens encounter rates were 0.04 and 0.07 marten’s detections/camera·day respectively in random cameras, supporting the martens as the main predator of nests. These results could be explained by the different foraging patterns of martens and red foxes. It has been described red foxes used mostly voles and carrion remains of ungulates, whereas martens used cached eggs, small birds and food remains (Lanszki et al. 2007; Selva et al. 2005; Tobajas et al. 2021; Willebrand et al. 2017). Previous studies have also reported that martens are the main predator of capercaillie nests, -and artificial nests simulating capercaillie ones- (Grané 2020; Summers et al. 2004). These results suggest that martens could be more specialized in searching and exploiting ground nests, and are also more efficient in detecting the nests. Regarding environmental and habitat-related variables such as altitude, slope, distance to water streams and percentage of scrubland in the 10 m buffer, all together explained a smaller proportion of the deviance − 0.21-. We observed a significant and positive effect of altitude on the predation rate (Table 2). This result can be explained by considering the low availability of resources at higher altitudes (Lee et al. 2021), forcing carnivores to exploit all available resources. In no case, the higher probability of nest predation at higher altitudes could be explained by a higher encounter rate of martens, since these variables were not correlated (Pearson’s correlation: r = 0.13, p-value = 0.35) likely explained because we grouped pine martens and stone martens in the same category, the latter being more frequent at lower altitudes.
The predation rates reported here, as well as the species recorded predating the artificial nests were similar to those previously published in the context of capercaillie conservation. Predation rates on real capercaillie and hazel grouse (Bonasa bonasia) nests in Slovakia ranged from 57 to 82%, being pine and stone marten, and red fox the main predators (22%), followed by wild boar (9%) (Saniga 2002). Similarly, an artificial nest experiment carried out in the context of capercaillie and black grouse (Tetrao tetrix) conservation reported nest predation rates ranging from 42 to 94%, with pine martens and red fox the main predators (Summers et al. 2004). More relevant, a nest predation rate of 42% was reported from 45 real capercaillie nests monitored in the southern slope of the Pyrenees from 2009 to 2015, being again martens and red foxes as the predators (Tobajas et al. 2021). Interestingly, the Tobajas et al. (2021) also reported a positive significant trend in nest predation rate over the years. Using artificial nests, we found higher predation rates (65%). The higher predation rates observed could be expected due to the increase in nest predation rate over the years, but also for the intrinsic characteristics of artificial nests (Moore and Robinson 2004; Pärt and Wretenberg 2002). Higher predation rates are expected when using artificial nests because: (i) some real nests are predated before being detected by humans; (ii) real nests are defended by the hens; and (iii) human effects (such as scent, or corvids detecting humans deploying the nest) could increase predation rates. For instance, from the 45 real nests monitored by Tobajas et al. 2021, 10 of them (22%), were opportunistically found by people searching for mushrooms and found during the summer census. These 10 nests would never have been detected if they had been predated in advance, leading to a possible underestimation of the real predation rate in capercaillie nests. Monitoring of real nests is valuable and strongly recommended, but the low number of nests, a consequence of the low abundance of hens, enhances the utility of artificial experiments to better understand nest predation and, lastly implement conservation measures to increase capercaillie productivity.
Regarding the predators’ abundance, to the best of our knowledge this is the first study that assessed nests predation rates by accounting also for predators’ population density. This is especially interesting because this allows us to discuss that martens were the species with a higher impact on nest predation (23.17%) despite they occurred at lower densities (0.49 ind·km− 2) compared to red fox (3.37 ind·km− 2 and 10.98% predation rate) and wild boar (6.95 ind·km− 2 and 3.66% predation rate). This could be explained by the foraging strategy of martens, with a clear preference for eggs (Willebrand et al. 2017). We would like to mention that all the cameras were deployed at ground level, and thus, we missed martens’ activity at canopy level which would result in an underestimation of population density. Data on the proportion of the activity period that martens spent on trees are scarce. From snow tracking during winter, one study reported 26% and 38% of the activity at the canopy level for stone marten and pine marten respectively (Goszczyński et al. 2007). Assuming similar values in our population, the abundance of martens will be still lower than that of foxes. Additionally, the marten’s density reported here was similar to the ones reported in other areas (Burgos et al. 2023; Manzo et al. 2012). Red fox population density was higher than in other populations (Jimenez et al. 2019; Palencia et al. 2021), but more relevant, we found a prevalence of ca. 20% of foxes with sarcoptic mange (Sarcoptes scabei) compatible lesions (Barroso and Palencia 2023). The outbreak of sarcoptic mange in the Scandinavian red fox population during the late 1970s and 1980s increased capercaillie and black grouse abundance (Lindström et al. 1994; Smedshaug et al. 1999). A long-term monitoring program could be useful to address if the capercaillie population in this area will also benefit from the sarcoptic mange outbreak. The absence of apex predators and the high abundance of ungulates could be contributing to the high abundance of martens and foxes (Jahren et al., Jiménez et al. 2019; 2016; Martin and Joron 2003; Tobajas et al. 2021). A recent study reported that the cumulative density of all wild ungulates in the study area was ca. 30 ind·km− 2 (Palencia and Barroso 2024), likely providing offal and carcasses for carnivores during winter bottlenecks, contributing to maintaining its high abundance (Selås and Vik 2006; Tobajas et al. 2021). In addition to the high availability of resources, a reduction in winter severity due to climate change may also benefit mesopredators (Baines et al. 2016; Bartoń and Zalewski 2007).
We would also like to emphasize the relevance of those species present in the study area that were not observed predating a nest. Previous studies have reported that corvids (e.g. Garrulus glandarius or Corvus corone), brown bears and badgers predated real capercaillie nests, or artificial nests simulating capercaillie ones (Baines et al. 2004; Bamber et al., 2024; Saniga 2002). However, we did not observe any bird, badger or bear predating a nest. Other carnivores present in the study area (genet and weasel) were not observed predating nests. Previous studies have also reported that red deer, fallow deer and roe deer predated nests, likely representing an unusual habit to obtain minerals (Krüger et al. 2018; Vazquez et al. 2023); but we did not observe any nest predated by these species. Regarding wild boar, we detected wild boar in 26 nests frequently reacting to the eggs, but without consuming them (Appendix S6). The predation rate of 3% observed for wild boar matched with previous studies (Fig. 3). For instance, Saniga (2002) concluded that wild boar is responsible for 9% of the nest predation cases for the ground-nesters capercaillie and hazel grouse in Slovakia; and no nest of capercaillie was predated by wild boar in the southern slope of the Pyrenees (Tobajas et al. 2021). Thus, the direct impact of wild boar on capercaillie nests could be much lower compared to that of other ground-nesting birds (Carpio et al. 2014, 2016; Mori et al. 2021).
Conservation implications
Considering all the above, conservation actions targeted to reduce nest predation should be focused on martens and red foxes. Previous studies carried out in our study area showed that the breeding success of capercaillie was enhanced in areas where martens and red foxes were removed (Moreno-Opo et al. 2015). In other areas, capercaillie productivity also increased when predators were controlled (Baines et al. 2004; Summers et al. 2004). A recent review also concluded that predator control was associated with a positive effect on reproductive success, adult abundance, and survival in grouse populations (Kämmerle and Storch 2019). However, predator control is very time-consuming, logistically and financially constrained and not socially accepted due to ethical issues (Moreno-Opo et al. 2015). Reducing predator’s impact can be addressed by other perspectives different to a removal/lethal approach. In this respect, the recovery of apex predators to revert capercaillie trend has been already suggested (Gil et al. 2020; Moreno-Opo et al. 2015). Restoration of an apex predator has been shown to reduce mesopredator abundance, with evidence of resonating positive impacts on lower trophic levels (Jiménez et al. 2019). In the context of capercaillie conservation, the recovery of Eurasian lynx (Lynx lynx) was accompanied by a reduction in red fox abundance (Elmhagen et al. 2010). Diversionary feeding (deliberate provisioning of food to explore the propensity of individuals to exploit the most easily accessed resource) has also been suggested as a promising conservation action to reduce the pressure of carnivores in capercaillie (Bamber et al. 2024; Finne et al. 2019). Similarly, another non-lethal tool is the use of conditioned food aversion, which occurs when an animal associates the taste of a certain food with negative symptoms such as nausea or vomiting, and they then avoid consuming it (Garcia et al. 1974). Conditioned food aversion has already been tested in the context of capercaillie conservation, with a reduction of nest predation in foxes, but a limited effect on martens (Tobajas et al. 2023). On the other hand, effective management of ungulate carrion, especially during winter, could be also recommended (Selås and Vik 2006; Tobajas et al. 2021). Similarly, predator densities are often higher in the vicinity of tourist facilities and ski slopes due to the supply of discarded food and/or improved predator movement (Storch and Leidenberger 2003).
In conclusion, our results evidenced the relevance of martens in the artificial nest predation rates despite being the less abundant predator. We reported that martens’ encounter rate explained nearly one-third of the variation in nest predation. Despite using artificial nests, our results are similar to those observed in real capercaillie nests monitored in the Pyrenees, both in terms of the percentage of nests predated and the species which predated. Monitoring real nests and chicks is strongly recommended to disentangle the low productivity rates observed in western capercaillie.
Data availability
The datasets generated during and/or analyzed during the current study will be available in supplementary materials after paper acceptance.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19(6):716–723. https://doi.org/10.1109/TAC.1974.1100705
Arlettaz R, Patthey P, Baltic M, Leu T, Schaub M, Palme R, Jenni-Eiermann S (2007) Spreading free-riding snow sports represent a novel serious threat for wildlife. Proc Royal Soc B: Biol Sci 274(1614):1219–1224. https://doi.org/10.1098/rspb.2006.0434
Baines D, Sage RB, Baines MM (1994) The implications of red deer grazing to Ground Vegetation and Invertebrate communities of Scottish native pinewoods. J Appl Ecol 31(4):776. https://doi.org/10.2307/2404167
Baines D, Moss R, Dugan D (2004) Capercaillie Breeding Success in Relation to Forest Habitat and Predator Abundance. In Source: Journal of Applied Ecology (Vol. 41, Issue 1)
Baines D, Aebischer NJ, Macleod A (2016) Increased mammalian predators and climate ch1ange predict declines in breeding success and density of Capercaillie tetrao urogallus, an old stand specialist, in fragmented Scottish forests. Biodivers Conserv 25(11):2171–2186. https://doi.org/10.1007/s10531-016-1185-8
Bamber JA, Kortland K, Sutherland C, Payo-Payo A, Lambin X (2024) Evaluating diversionary feeding as a method to resolve conservation conflicts in a recovering ecosystem. J Appl Ecol
Barroso P, Palencia P (2023) Camera traps reveal a high prevalence of sarcoptic mange in red foxes from northern Spain. Res Vet Sci 105098. https://doi.org/10.1016/j.rvsc.2023.105098
Bartoń KA, Zalewski A (2007) Winter severity limits red fox populations in Eurasia. Glob Ecol Biogeogr 16(3):281–289. https://doi.org/10.1111/j.1466-8238.2007.00299.x
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting Linear mixed-effects models using lme4. J Stat Softw 67(1). https://doi.org/10.18637/jss.v067.i01
Beery S, Morris D, Yang S (2019) Efficient Pipeline for Camera Trap Image Review
Burgos T, Salesa J, Fedriani JM, Escribano-Ávila G, Jiménez J, Krofel M, Virgós E (2023) Top-down and bottom-up effects modulate species co-existence in a context of top predator restoration. Sci Rep 13(1):4170
Carpio AJ, Guerrero-Casado J, Tortosa FS, Vicente J (2014) Predation of simulated red-legged partridge nests in big game estates from South Central Spain. Eur J Wildl Res 60(2):391–394. https://doi.org/10.1007/s10344-013-0786-8
Carpio AJ, Hillström L, Tortosa FS (2016) Effects of wild boar predation on nests of wading birds in various Swedish habitats. Eur J Wildl Res 62(4):423–430. https://doi.org/10.1007/s10344-016-1016-y
Core Team R (2013) R: A language and environment for statistical computing
CREAF (2009) Mapa de cobertes del sòl de Catalunya [WWW Document]. URL http://www.creaf.uab.es/mcsc/ (accessed 8.3.23)
Duriez O, Sachet JM, Ménoni E, Pidancier N, Miquel C, Taberlet P (2007) Phylogeography of the capercaillie in Eurasia: what is the conservation status in the Pyrenees and cantabrian mounts? Conserv Genet 8(3):513–526. https://doi.org/10.1007/s10592-006-9165-2
Elmhagen B, Ludwig G, Rushton SP, Helle P, Lindén H (2010) Top predators, mesopredators and their prey: interference ecosystems along bioclimatic productivity: gradients. J Anim Ecol 79(4):785–794. https://doi.org/10.1111/j.1365-2656.2010.01678.x
Escoda L, Piqué J, Paule L, Foulché K, Menoni E, Castresana J (2023) Genomic analysis of geographical structure and diversity in the capercaillie (Tetrao urogallus). Conserv Genet. https://doi.org/10.1007/s10592-023-01567-6
Finne MH, Kristiansen P, Rolstad J, Wegge P (2019) Diversionary feeding of red fox in spring increased productivity of forest grouse in southeast. Nor Wildl Biology 2019(1):1–12
Garcia J, Hankins WG, Rusiniak KW (1974) Behavioral regulation of the Milieu Interne in Man and Rat. Science 185(4154):824–831. https://doi.org/10.1126/science.185.4154.824
Gil JA (2011) El urogallo pirenaico en Aragón. www.quebrantahuesos.org
Gil JA, Gómez-Serrano MÁ, López-López P (2020) Population decline of the Capercaillie Tetrao urogallus aquitanicus in the Central Pyrenees. Ardeola 67(2):285–306. https://doi.org/10.13157/arla.67.2.2020.ra4
Goldstein BR, Jensen AJ, Kays R, Cove MV, McShea WJ, Rooney B, Pacifici K (2024) Guidelines for estimating occupancy from autocorrelated camera trap detections. Methods in Ecology and Evolution
Goszczyński J, Posłuszny M, Pilot M, Gralak B (2007) Patterns of winter locomotion and foraging in two sympatric marten species: Martes martes and Martes foina. Can J Zool 85(2):239–249. https://doi.org/10.1139/Z06-212
Grané A (2020) Estudi i caracterització del microhàbitat reproductiu del gall fer (Tetrao urogallus) Al Parc Natural De l’Alt Pirineu. University of Girona
Hortal J, Rodríguez J, Nieto-Díaz M, Lobo JM (2008) Regional and environmental effects on the species richness of mammal assemblages. J Biogeogr 35(7):1202–1214. https://doi.org/10.1111/j.1365-2699.2007.01850.x
Howe EJ, Buckland ST, Després-Einspenner M-L, Kühl HS (2017) Distance sampling with camera traps. Methods Ecol Evol 8(11):1558–1565. https://doi.org/10.1111/2041-210X.12790
Howe EJ, Buckland ST, Després-Einspenner M, Kühl HS (2019) Model selection with overdispersed distance sampling data. Methods Ecol Evol 10(1):38–47. https://doi.org/10.1111/2041-210X.13082
Ibáñez-Álamo JD, Magrath RD, Oteyza JC, Chalfoun AD, Haff TM, Schmidt KA, Martin TE (2015) Nest predation research: recent findings and future perspectives. J Ornithol 156:247–262
IUCN (2016) Tetrao urogallus. The IUCN Red List of Threatened Species 2016
Jahren T, Storaas T, Willebrand T, Moa F, P., Hagen BR (2016) Declining reproductive output in capercaillie and black grouse-16 countries and 80 years. Anim Biology 66(3–4):363–400. https://doi.org/10.1163/15707563-00002514
Jimenez J, Chandler R, Tobajas J, Descalzo E, Mateo R, Ferreras P (2019) Generalized spatial mark–resight models with incomplete identification: an application to red fox density estimates. Ecol Evol 9(8):4739–4748. https://doi.org/10.1002/ece3.5077
Jiménez J, Nuñez-Arjona JC, Mougeot F, Ferreras P, González LM, García-Domínguez F, Muñoz-Igualada J, Palacios MJ, Pla S, Rueda C, Villaespesa F, Nájera F, Palomares F, López-Bao JV (2019) Restoring apex predators can reduce mesopredator abundances. Biol Conserv 238:108234. https://doi.org/10.1016/j.biocon.2019.108234
Jiménez J, Godinho R, Pinto D, Lopes S, Castro D, Cubero D, Osorio MA, Piqué J, Moreno-Opo R, Quiros P, González-Nuevo D, Hernandez-Palacios O, Kéry M (2022) The cantabrian capercaillie: a population on the edge. Sci Total Environ 821:153523. https://doi.org/10.1016/j.scitotenv.2022.153523
Kämmerle J-L, Storch I (2019) Predation, predator control and grouse populations: a review. Wildl Biology 2019(1). https://doi.org/10.2981/wlb.00464
Klaus S, Bergmann H (1994) Distribution, status and limiting factors of capercaillie (Tetrao urogallus) in Central Europe, particularly in Germany, including an evaluation of reintroductions. Gibier Faune Sauvage 11:57–80
Kortmann M, Heurich M, Latifi H, Rösner S, Seidl R, Müller J, Thorn S (2018) Forest structure following natural disturbances and early succession provides habitat for two avian flagship species, capercaillie (Tetrao urogallus) and hazel grouse (Tetrastes bonasia). Biol Conserv 226:81–91. https://doi.org/10.1016/j.biocon.2018.07.014
Krüger H, Väänänen VM, Holopainen S, Nummi P (2018) The new faces of nest predation in agricultural landscapes—a wildlife camera survey with artificial nests. Eur J Wildl Res 64(6). https://doi.org/10.1007/s10344-018-1233-7
Lanszki J, Zalewski A, Horváth G (2007) Comparison of red fox Vulpes vulpes and pine marten Martes martes food habits in a deciduous forest in Hungary. Wildl Biology 13(3):258–271. https://doi.org/10.2981/0909-6396(2007)13[258:CORFVV]2.0.CO;2
Leclercq B, Ménoni E (2018) Le grand tétras. Biotope Éditions, Mèze
Lee MA, Burger G, Green ER, Kooij PW (2021) Relationships between resource availability and elevation vary between metrics creating gradients of nutritional complexity. Oecologia 195(1):213–223. https://doi.org/10.1007/s00442-020-04824-4
Lindström ER, Andrén H, Angelstam P, Cederlund G, Hörnfeldt B, Jäderberg L, Lemnell A, Martinsson B, Sköld K, Swenson JE (1994) Disease Reveals the Predator: Sarcoptic Mange, Red Fox Predation, and Prey Populations. In Source: Ecology (Vol. 75, Issue 4)
Manzo E, Bartolommei P, Rowcliffe JM, Cozzolino R (2012) Estimation of population density of European pine marten in central Italy using camera trapping. Acta Theriol 57:165–172
Martin TE (1993) Nest predation and nest sites. Bioscience 43(8):523–532
Martin JL, Joron M (2003) Nest predation in forest birds: influence of predator type and predator’s habitat quality. Oikos 102(3):641–653. https://doi.org/10.1034/j.1600-0706.2003.12040.x
Matysek M, Gwiazda R, Figarski T, Zięba F, Klimecki M, Mateja R, Krzan P, Piątek G (2021) What habitat parameters are important for the survival of ground nests in mountain forests? Recommendations for protection of Hazel Grouse Tetrastes bonasia based on an experiment with artificial nests. Bird Study 68(2):258–266. https://doi.org/10.1080/00063657.2022.2026875
Matysioková B, Remeš V (2024) Nest predation decreases with increasing nest height in forest songbirds: a comparative study. J Ornithol 165(1):257–261
Ministerio para la Transición Ecológica (2018) Orden TEC/1078/2018, de 28 de septiembre, por la que se declara la situación crítica de Cistus heterophyllus carthaginensis, Lanius minor, Margaritifera auricularia, Marmaronetta angustirostris, ustela lutreola, Pinna nobilis y Tetrao urogallus cantabricus en España, y se declaran de interés general las obras y proyectos encaminados a la recuperación de dichos taxoneshttp://www.boe.es
Ministerio para la Transición Ecológica y el Reto Demográfico (2023) Orden TED/339/2023, de 30 de marzo, por la que se modifica el anexo del Real Decreto 139/2011, de 4 de febrero, para el desarrollo del Listado de Especies Silvestres en Régimen de Protección Especial y del Catálogo Español de Especies Amenazadas, y el anexo del Real Decreto 630/2013, de 2 de agosto, por el que se regula el Catálogo Español de Especies Exóticas Invasorashttps://www.boe.es
Moore RP, Robinson WD (2004) Artificial bird nests, external validity, and bias in ecological field studies. In Ecology (Vol. 85, Issue 6, pp. 1562–1567). Ecological Society of America. https://doi.org/10.1890/03-0088
Moreno-Opo R, Afonso I, Jiménez J, Fernández-Olalla M, Canut J, García-Ferré D, Piqué J, García F, Roig J, Muñoz-Igualada J, González LM, López-Bao JV (2015) Is it necessary managing carnivores to reverse the decline of endangered prey species? Insights from a removal experiment of mesocarnivores to benefit demographic parameters of the pyrenean capercaillie. PLoS ONE 10(10). https://doi.org/10.1371/journal.pone.0139837
Mori E, Lazzeri L, Ferretti F, Gordigiani L, Rubolini D (2021) The wild boar Sus scrofa as a threat to ground-nesting bird species: an artificial nest experiment. J Zool 314(4):311–320. https://doi.org/10.1111/jzo.12887
Moss R, Picozzi N, Summers RW, Baines D (2000) Capercaillie Tetrao urogallus in Scotland - Demography of a declining population. Ibis 142(2):259–267. https://doi.org/10.1111/j.1474-919x.2000.tb04865.x
Moss R, Oswald J, Baines D (2001) Climate Change and Breeding Success: Decline of the Capercaillie in Scotland. In Journal of Animal Ecology (Vol. 70, Issue 1)
O’Brien TG, Kinnaird MF, Wibisono HT (2003), May Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Animal Conservation Forum> (Vol. 6, No. 2, pp. 131–139). Cambridge University Press
Oja R, Pass E, Soe E, Ligi K, Anijalg P, Laurimaa L, Saarma U, Lõhmus A, Valdmann H (2018) Increased nest predation near protected capercaillie leks: a caveat against small reserves. Eur J Wildl Res 64(1). https://doi.org/10.1007/s10344-018-1165-2
Palencia P, Barroso P (2024) How many sequences should I track when applying the random encounter model? Journal of Zoology, accepted, https://doi.org/10.1111/jzo.13204
Palencia P, Rowcliffe JM, Vicente J, Acevedo P (2021) Assessing the camera trap methodologies used to estimate density of unmarked populations. J Appl Ecol 58(8):1583–1592. https://doi.org/10.1111/1365-2664.13913
Palencia P, Vicente J, Soriguer RC, Acevedo P (2022) Towards a best-practices guide for camera trapping: assessing differences among camera trap models and settings under field conditions. J Zool 316(3):197–208. https://doi.org/10.1111/jzo.12945
Palencia P, Barroso P, Vicente J, Hofmeester TR, Ferreres J, Acevedo P (2022a) Random encounter model is a reliable method for estimating population density of multiple species using camera traps. Remote Sens Ecol Conserv 8(5):670–682. https://doi.org/10.1002/rse2.269
Palencia P, Vada R, Zanet S, Calvini M, De Giovanni A, Gola G, Ferroglio E (2023) Not just pictures: utility of Camera Trapping in the Context of African Swine Fever and Wild Boar Management. Transbound Emerg Dis 2023:1–9. https://doi.org/10.1155/2023/7820538
Pärt T, Wretenberg J (2002) Do artificial nests reveal relative nest predation risk for real nests? J Avian Biol 33(1):39–46. https://doi.org/10.1034/j.1600-048X.2002.330107.x
Patthey P, Wirthner S, Signorell N, Arlettaz R (2008) Impact of outdoor winter sports on the abundance of a key indicator species of alpine ecosystems. J Appl Ecol 45(6):1704–1711. https://doi.org/10.1111/j.1365-2664.2008.01547.x
Robles L, Ballesteros F, Canut J (2005) El urogallo en España, Andorra Y Pirineos franceses. SEO/Birdlife)
Robles L, Martínez J, Obeso R, Gil JA, García-Ferré D, Robles C, Gil JR, García-Ferré Y (2022) J. A. Urogallo común: III Atlas de las aves en época de reproducción en España. SEO/BirdLife. https://atlasaves.seo.org/ave/urogallo-comun/
Rowcliffe JM, Field J, Turvey ST, Carbone C (2008) Estimating animal density using camera traps without the need for individual recognition. J Appl Ecol 45(4):1228–1236. https://doi.org/10.1111/j.1365-2664.2008.01473.x
Rowcliffe JM, Carbone C, Jansen PA, Kays R, Kranstauber B (2011) Quantifying the sensitivity of camera traps: an adapted distance sampling approach. Methods Ecol Evol 2(5):464–476
Rowcliffe JM, Kays R, Kranstauber B, Carbone C, Jansen PA (2014) Quantifying levels of animal activity using camera trap data. Methods Ecol Evol 5(11):1170–1179
Saniga M (2002) Nest loss and chick mortality in capercaillie (Tetrao urogallus) and hazel grouse (Bonasa bonasia) in West Carpathians (Vol. 51, Issue 3)
Saniga M (2012) Population dynamics of Capercaillie Tetrao urogallus on leks in Central Slovakia in the period 1981–2012. Grouse News 44:5–9
Selås V, Vik JO (2006) Possible impact of snow depth and ungulate carcasses on red fox (Vulpes vulpes) populations in Norway, 1897–1976. J Zool 269(3):299–308. https://doi.org/10.1111/j.1469-7998.2006.00048.x
Selva N, Jȩdrzejewska B, Jȩdrzejewski W, Wajrak A (2005) Factors affecting carcass use by a guild of scavengers in European temperate woodland. Can J Zool 83(12):1590–1601. https://doi.org/10.1139/z05-158
Shannon G, Valle S, Shuttleworth CM (2023) Capturing red squirrels (Sciurus vulgaris) on camera: a cost-effective approach for monitoring relative abundance and habitat preference. Ecol Evol 13(10). https://doi.org/10.1002/ece3.10536
Smedshaug CA, Selås V, Lund SE, Sonerud GA (1999) The effect of a natural reduction of red fox Vulpes vulpes on small game hunting bags in Norway. Wildl Biology 5(3):157–166. https://doi.org/10.2981/wlb.1999.020
Sollmann R, Mohamed A, Samejima H, Wilting A (2013) Risky business or simple solution – relative abundance indices from camera-trapping. Biol Conserv 159:405–412. https://doi.org/10.1016/j.biocon.2012.12.025
Storaas T (1988) A Comparison of Losses in Artificial and Naturally Occurring Capercaillie Nests. In Source: The Journal of Wildlife Management (Vol. 52, Issue 1). https://about.jstor.org/terms
Storch I, Leidenberger C (2003) Tourism, mountain huts and distribution of corvids in the bavarian alps, Germany. Wildl Biology 9(4):301–308. https://doi.org/10.2981/wlb.2003.018
Summers RW, Green RE, Proctor R, Dugan D, Lambie D, Moncrieff R, Moss R, Baines D (2004) An experimental study of the effects of predation on the breeding productivity of capercaillie and black grouse. J Appl Ecol 41(3):513–525. https://doi.org/10.1111/j.0021-8901.2004.00891.x
Summers RW, Willi J, Selvidge J (2009) Capercaillie Tetrao urogallus nest loss and attendance at Abernethy Forest, Scotland. Wildl Biology 15(3):319–327. https://doi.org/10.2981/08-036
Tobajas J, Oliva-Vidal P, Piqué J, Afonso-Jordana I, García-Ferré D, Moreno-Opo R, Margalida A (2021) Scavenging patterns of generalist predators in forested areas: the potential implications of increase in carrion availability on a threatened capercaillie population. Anim Conserv acv12735. https://doi.org/10.1111/acv.12735
Tobajas J, Roig J, Afonso I (2023) Reducing nest predation of the endangered Western Capercaillie using Conditioned Food Aversion. 15th INTERNATIONAL GROUSE SYMPOSIUM, 80–80
Vazquez MS, Gonzalez DV, Amico GC (2023) Herbivores but not vegans: deer as nest predators. Austral Ecol. https://doi.org/10.1111/aec.13374
Wickham H (2016) Elegant Graphics for Data Analysis. Springer-, New York
Wickham H, François R, Henry L, Müller K, Vaughan D (2023) A Grammar of Data Manipulation. R package version 1.1.0
Willebrand T, Willebrand S, Jahren T, Marcström V (2017) Snow tracking reveals different foraging patterns of red foxes and pine martens. Mammal Res 62(4):331–340. https://doi.org/10.1007/s13364-017-0332-2
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1(1):3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
The authors would like to thank to TETRAO brand and IBO company, for providing part of the materials used in this study. The authors would like to thank Cobeñas’ logistic center for their help in receiving and storing materials, Susana and Eulogio for their help in preparing the gypsum-filled eggs, and Pelayo and Joaquin for providing part of the cameras. This study was partially supported by the Alt Pirineo Natural Park Research Observatory, the Institute for the Development and Promotion of the High Pyrenees and Aran, the Center d’Art i Natura de Farrera and the Fundació Catalunya La Pedrera, the Servei de Fauna I Flora i del Parc Natural De l’Alt Pirineu del Departament d’Accio Climàtica, Alimentació I Agenda Rural, and especially, the Salvador Grau i Tort scholarship which partially supports the research, and finally by the. We also would like to thank the Spanish Association of Terrestrial Ecology (AEET) and the project-call “Ganando independencia” which partially supported this research. Pablo Palencia received support from the University of Castilla-La Mancha through a contract Margarita Salas (2022-NACIONAL-110053) and from the University of Oviedo through a Juan de la Cierva contract JDC2022-048567-I supported by “Ministerio de Ciencia e Innovación”, “Agencia Estatal de Investigacion” and “NextGeneration EU” (MCIN/AEI/10.13039/501100011033). Patricia Barroso received support from the University of Castilla-La Mancha through a contract Margarita Salas (2022-NACIONAL-110053) and from the University of León through a Juan de la Cierva contract DC2022–049103-I.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was partially supported by the Alt Pirineo Natural Park Research Observatory, the Institute for the Development and Promotion of the High Pyrenees and Aran, the Center d’Art i Natura de Farrera and the Fundació Catalunya La Pedrera, the Servei de Fauna I Flora i del Parc Natural De l’Alt Pirineu del Departament d’Accio Climàtica, Alimentació I Agenda Rural, and especially, the Salvador Grau i Tort scholarship which partially supports the research, and finally by the. We also would like to thank the Spanish Association of Terrestrial Ecology (AEET) and the project-call “Ganando independencia” which partially supported this research. Pablo Palencia received support from the University of Castilla-La Mancha through a contract Margarita Salas (2022-NACIONAL-110053) and from the University of Oviedo through a Juan de la Cierva contract JDC2022-048567-I supported by “Ministerio de Ciencia e Innovación”, “Agencia Estatal de Investigacion” and “NextGeneration EU” (MCIN/AEI/10.13039/501100011033). Patricia Barroso received support from the University of Castilla-La Mancha through a contract Margarita Salas (2022-NACIONAL-110053) and from the University of León through a Juan de la Cierva contract DC2022–049103-I.
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
P. P.: Funding acquisition, Conceptualization, Data curation, Formal analysis, Writing – original draft, Project administration. P. B.: Conceptualization, Data curation, Writing – review & editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palencia, P., Barroso, P. Disentangling ground-nest predation rates through an artificial nests experiment in an area with western capercaillie (Tetrao urogallus) presence: martens are the key. Eur J Wildl Res 70, 87 (2024). https://doi.org/10.1007/s10344-024-01837-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-024-01837-9