Abstract
Dispersal is a key process affecting the dynamic and genetic structure of populations. To increase our knowledge on first-year dispersal in the white-tailed eagle (WTE), 35 nestlings in the Czech Republic, Hungary, and Austria were equipped with GPS/GSM loggers while on the nest between 2016 and 2019. Twenty-nine individuals surviving until March 31 were used to assess post-fledging areas, first-year dispersal distance and direction, temporary settlement areas, and habitat selection. The first flight from the nest was observed between May 19 and July 8. Start of dispersal from post-fledging areas occurred between July 1 and November 14. Post-fledging areas were significantly larger in males (p = 0.001, median 1236 km2, n = 15) than females (median 139 km2, n = 12). Maximal dispersal distance ranged from 93 to 433 km from the native nest (median = 187 km) and did not correlate with Simpson’s Diversity Index computed for habitats in individual 100% minimum convex polygons (MCP). Median sizes of minimum convex polygons were 26,888 km2 for 100% MCP and 13,376 km2 for 95% MCP (n = 29). Median sizes of kernel density estimates (KDE) were 3393 km2 for 80% KDE and 1137 km2 for 50% KDE. After start of dispersal, young WTEs returned to the proximity of the parental nest for night roosting sporadically. No sex-specific differences in dispersal distance were recorded. While young individuals from the three subpopulations are likely to occur in the same area during the first year of life, future nesting site identification will enable us to assess real gene flow and the connectivity level among them. As this study has illustrated, future conservation efforts to protect young WTEs will require cross-border cooperation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dispersal behaviour is an important process shaping population structure of species through its effect on population size, dynamics, and genetics (Prugnolle and de Meeus 2002). An understanding of dispersal rules, especially in long-lived species with delayed maturity, can markedly contribute to effective conservation of endangered species. The dispersal patterns of raptors over a large spatial scale (as with migration patterns) remained hidden to biologists until the development of appropriate individual marking methods. Use of conspicuous tags, such as coloured rings or wing tags, with a unique code has enabled researchers to concentrate on raptor dispersal at an individual level, covering subjects such as adult breeding dispersal and also juvenile dispersal (Whitfield et al. 2009a; Forero et al. 2002). From many perspectives, the development of animal-borne telemetry devices has revolutionised our ability to study animals in the wild (Cagnacci et al. 2010; Kays et al. 2015; Hooten et al. 2017). Radio-tracking methods have increased the efficiency of finding of the same individual in time and space, enabling us to collect large datasets for individuals. One drawback from such studies, however, was the fact that signals from radio-tracked birds of prey sometimes ‘disappeared’ from the study area due to the bird having an unexpectedly large action radius, especially in the case of non-nesters (Nygård et al. 2000; Penteriani et al. 2011). Current telemetry methods give us opportunity to obtain detailed information also on young individuals during the process of achieving independence from parents, including the most distant locations of their first-year dispersal. Technological improvements in telemetry, such as the development of GPS/GSM loggers, now provide volumes of data previously considered inconceivable (López-López 2016), and the current challenge facing biologists is how best to analyse the large amounts of data received from GPS/GSM loggers.
The classic concept of animal home ranges is based on minimum convex polygons (MCP) and kernel density estimates (KDE) computed from all the locations obtained for an individual; however, this may not be the most appropriate method to describe the spatio-temporal activities of juvenile, subadult, and non-breeding adult eagles. Initially, fledglings occur in a post-fledging area (PFA) that surrounds the nesting area of the parental pair, defined as the area used by the family group from the time the young fledge until they are no longer dependent on the adults for food (Tapia and Zuberogoitia 2018). This may be a sensitive period for induction of natal habitat preference (Davis and Stamps 2004). Later, young birds perform long-distance movements and occupy one or more temporary settlement areas (TSA; del Mar Delgado et al. 2009; Nemček et al. 2016; Morandini et al. 2020), also called dispersal areas (Cadahía et al. 2010) or staging areas (Mellone et al. 2011). These sites are used repeatedly by the individuals and are used for the longest period throughout the dispersal process (Cadahía et al. 2010). To date, however, there is no unified definition of how best to identify and delineate TSAs, though species-specific differences can be expected.
The white-tailed eagle (WTE; Haliaeetus albicilla) is a non-migratory species that, aside from some northern European and Asian populations (Helander and Stjernberg 2002), does not regularly overwinter outside of its breeding distribution range. As such, it is an ideal species for studying the effects of resource availability (e.g. habitat, food, supply of free mates) on settlement behaviour in young individuals. In WTEs, natal dispersal, defined as the movement of wandering individuals from birthplace to first breeding location (Greenwood 1980; Ronce 2007; Penteriani and del Mar Delgado 2009), has a greater effect on gene flow and population demography than breeding dispersal (Whitfield et al. 2009b). WTEs usually breed for the first time at 5 years of age (Cramp and Simmons 1982), though earlier successful nestings (e.g. at 3 years) have also been recorded (Struwe-Juhl and Grünkorn 2007; Tihanyi et al. 2009; Škorpíková and Tunka 2013; Nygård and Mee 2017). Dispersal movements generally comprise three stages: (1) departure or emigration, (2) a vagrant stage, and (3) settling or immigration (Ronce 2007). Note, however, that the beginning of dispersal has been understood differently in different studies on WTEs (Nygård et al. 2000; Balotari-Chiebao et al. 2016; Tikkanen et al. 2018). The wandering phase can be relatively long in WTEs, as with other similar species with delayed sexual maturity. Though reproduction is presumed not to occur in the second and third calendar year of life in WTEs, Whitfield et al. (2009a) found a significant positive relationship between maximum juvenile dispersal distance in the first 2 years of life and natal dispersal distance, suggesting that first-year dispersal data could be sufficient to predict natal dispersal distance in young WTEs.
A number of previous studies have examined the genetic structure of WTEs from a range of European subpopulations to verify the origin of individuals recolonising Central Europe (e.g. Literák et al. 2007; Nemesházi et al. 2016). Nemesházi et al. (2016) recognised at least three genetic clusters in Central European countries: (1) a ‘northern cluster’ corresponding to genetic signatures for northern European WTEs from Finland and Lithuania; (2) a ‘central cluster’ mainly containing WTEs originating from Germany, Poland, and northern Austria; and 3) a ‘southern cluster’ unifying WTEs from the Pannonian Basin (e.g. from Hungary, Slovakia, Serbia) with north-eastern Austria. As individuals from all three clusters occur in the Czech Republic and Hungary, with individuals from two of the clusters in Austria (Nemesházi et al. 2016), these three countries may represent an interesting study area for further telemetry research aimed at WTE natal dispersal. Following the population bottleneck that persisted until the 1970s, WTEs began recolonising the Czech Republic in the 1980s, with breeding occurring in two core areas in the southwestern (South Bohemia) and southeastern parts (South Moravia) of the Czech Republic (Bělka and Horal 2009). By 2016, the population size had reached 116 pairs (Bělka 2017), and by 2019 it was estimated at ca 130–140 pairs (Bělka pers. comm.). Despite a large decline in numbers reported between the 1950s and 1970s, the species probably never became extinct in Hungary, and following a conservation programme launched in 1987, population numbers have increased substantially (Horváth 2009), with as many as 360–380 territorial pairs estimated in 2017 (Szelényi in Demeter et al. 2017). In Austria, the WTE has gone extinct twice, the first time in the 19th century and again in the late 1950s (Probst and Peter 2009). In 1999, the species started to breed in Austria once again (Probst 2009), and by 2017, its population size had risen to 30 pairs (Probst and Pichler 2017), increasing to ca. 40 pairs in 2019 (Probst pers. comm.). At present, there is a general lack of data on levels of connectivity between these three subpopulations of the European metapopulation and on the dispersal of young WTEs from the natal nests. For example, it is not known whether a more homogenous environment will result in longer dispersal distances in WTEs and whether fledged individuals return for night roosting to the vicinity of the parental nest.
The main aim of this study was to answer a series of questions concerning the dispersal of young WTEs during their first year of life. These cover five main areas: (1) How large are PFAs, when exactly does dispersal begin, and how frequently does the individual return to the nest? (2) How far do fledglings disperse from the hatching site, which direction do they take (azimuth), and how large is their total action radius over one year? (3) Are there any sex-specific differences in dispersal distance, dispersal direction, or PFA size? (4) What habitats do individuals prefer and does habitat diversity affect maximal dispersal distance? (5) What areas are occupied by birds originating from different subpopulations?
Material and methods
Tagging of individuals
Between 2016 and 2019, a total of 35 WTE nestlings (7–8 weeks old) were tagged with a GPS/GSM/GPRS logger in nests in the Czech Republic, Hungary, and Austria (Table 1). GPS/GSM tags were fixed to the bird’s back (as a backpack) using a harness consisting of two 6 mm Teflon ribbons encircling the body (one loop around the base of each wing, both loops joined in front of the breastbone). Between 2016 and 2017, 23 individuals were fitted with SKUA-H LF = KITE-H LF Ecotone loggers (Poland), while 12 individuals were fitted with OT-E50B-3GC Ornitela loggers (Lithuania) between 2018 and 2019. All Ecotone loggers were set to collect one location each 3 h from midnight to 21 PM (UTC), while one location per hour was used from Ornitela loggers.
Sex determination
Nestling tarsus thickness (cut-off point 14.15 mm) and weight (cut-off point 4300 g) were measured using a digital calliper and digital hanging scale, the measurements subsequently being used for sex identification based on the methods of Helander (1981) and Helander et al. (2007). Feather samples were also taken from Czech nestlings (stored in 96% ethanol) in order to confirm sex determination genetically, based on PCR using the primers CHD1-i16F and CHD1-i16R (Suh et al. 2011) and visualisation using horizontal agarose gel electrophoresis illuminated with a UV-transilluminator (Rymešová et al. 2020). DNA was first isolated using the Tissue Genomic DNA Mini Kit (Geneaid Biotech, Taiwan), following the manufacturer’s protocol. Conditions for PCR were set as follows: (1) 2 min at 94°C; (2) 35 cycles: (a) 30 s at 94°C, (b) 30 s at 56°C, and (c) 80 s at 72°C; (3) 5 min at 72°C; and (4) 10°C. Sex was determined based upon the occurrence of CHD-genes (CHD-Z and CHD-W) after PCR reaction (PPP Master Mix (Top-Bio, CZ) 10 μL, PCR water 4 μL, primer CHD1-i16F 2 μL, primer CHD1-i16R 2 μL, DNA 2 μL). Molecular sex determination confirmed that all but one male nestling from Czech nests could be successfully sexed on the basis of weight and tarsus thickness measurement at the thinnest point alone. Two nestlings tagged in 2016 (BS0041, BS0042) were not measured or sampled for DNA, and their sex is unknown.
GIS analysis and dispersal definitions
CSV files containing individual locations and sampling dates and times were downloaded onto a personalised provider’s website and further analysed in ArcMap 10.1 with Spatial Analyst extension (ESRI, USA). The data were first checked for possible errors (e.g. points with longitude = 0 and latitude = 0 simultaneously), and these outliers were removed from the dataset. Night roosting sites were selected in a separate layer as a list of first locations from each day (close to 0:00 UTC). Trajectories from successive points were created and home range sizes calculated using the free extension ArcMET 10.1.1. and Home Range Tools for ArcGIS 10.1 (HRT) in the Projected Coordinate System (WGS 1984 UTM Zone 33 N). Home range areas were calculated from all obtained locations (including night locations) from the tagging day to March 31 of the next calendar year (1-year-old birds in their second calendar year of life; 2CY) for 29 individuals (in six cases to an earlier date; Table 2). Occupied areas were defined from several points of view: (1) overall area used, defined as 100% minimum convex polygon (MCP); (2) overall area used without the most extreme exploratory flights (95% MCP); (3) core area of occurrence sensu lato (80% Kernel Density Estimate = KDE); (4) core area of occurrence sensu stricto (50% KDE); and (5) temporary settlement areas (TSAs), representing the most ‘important’ or ‘preferred’ areas with repeated occurrence of an individual. Each MCP was created with the floating mean method. The fixed kernel method with a reference bandwidth was used for KDE creation. TSAs were defined for each individual on the basis of a subsample of points selected according to the following criteria. First, we only worked with the layer that contained the earliest night roosting location for every day (close to 0:00 UTC). We then looked for clusters of points where night locations were maximally 10 km distant from each other (using the ArcGIS Buffer tool to create borders with a 5 km radius from each point and analysing any overlaps) and, simultaneously, where individuals occurred on at least ten nights. After identifying these point clusters, all daily locations with the same dates were added to the selected night roosting location, and the 95% MCP of these points was taken to represent a TSA. The first TSA containing the native nest was termed the post-fledging area (PFA).
Maximal dispersal distance was measured as a line between the most distant location and the native nest. Dispersal direction was measured in three ways. First, the azimuth to north between the natal nest and the most distant location (in degrees) was measured using the ArcGIS COGO tool. Second, the azimuth was measured between the natal nest and the centroid of the 50% KDE polygon (using the ArcGIS Feature to Point tool). Third, the azimuth was measured between the natal nest and the centroid of the 100% MCP from March locations. Similarly, distance between this March centroid and natal nest was measured to assess final occurrence of each individual. We computed the ratio of 100% MCP area from March locations to overall area used as an individual measure of final sedentarity at the end of observation. The date of first flight from the nest was determined as the first day when distance of the individual from the nest exceeded 200 m. Dispersal date was based on the definition of Balotari-Chiebao et al. (2016). When an individual occurred more than 5 km from the nest on at least eleven consecutive days, the first day of reaching of this distance was regarded as the beginning of dispersal (i.e. dispersal date) from the parental nest. This date simultaneously corresponded to the date, following which there was no subsequent return to the nest (closer than 200 m) in most individuals (77%). March 31 was used as the universal limit representing the end of the first year of life in all individuals studied, based on the common nesting cycle of WTE in the Czech Republic where eggs are most often laid during February and March (Kloubec et al. 2015). The last dates of occurrence closer than 200 m and closer than 5 km from the nest were recorded too.
Habitat classification and analysis
Habitat preferences were analysed through compositional analysis (Aebischer et al. 1993) using the AdehabitatHS package in R 3.6.2 (R Core Team 2019), based on a dataset comprising all 35 individuals. The percentage of different habitat areas (measured in km2) inside individual 100% MCPs represented the available habitat, and the percentage of individual locations in the habitats of 100% MCPs was regarded as the used habitat. Unique values were obtained for each individual studied. Preliminary classification of habitats in individual 100% MCPs and in individual locations was based on the 2018 CORINE land cover layer (https://land.copernicus.eu/pan-european/corine-land-cover). Thirty-three CORINE habitat categories were found in all 100% MCPs (categories no. 111, 112, 121-124, 131-133, 141, 142, 211, 212, 213, 221, 222, 231, 242, 243, 311-313, 321, 322, 324, 331-334, 411, 412, 511, 512; Bossard et al. 2000; Büttner and Kosztra 2017). Next, we reclassified the original categories into eight groups in order to obtain the most frequent habitats across all individuals (Table 3). Five habitat categories were not included in the compositional analysis (322, moors and heathland; 331, beaches, dunes, and sand plains; 332, bare rocks; 333, sparsely vegetated areas; 334, burnt areas) due to their low representation in MCPs and locations (medians equal to zero). Finally, compositional analysis was also undertaken on datasets of males and females separately (n = 33: 17 females, 16 males, sex undetermined in two cases; later reclassified as 27 individuals surviving the whole year: 13 females, 14 males).
Statistical analysis
As a first step, all datasets were checked for normal distribution of values with Shapiro-Wilk tests in Unistat 6.5. As normal distribution was not confirmed (variables were maximal dispersal distance, number of locations (overall and per day), number of tracking days, areas of 50% and 80% KDEs and 100% and 95% MCPs), non-parametric statistics were used for further dataset analysis. We computed basic descriptive statistics (median, min, max, standard error) for 29 individuals surviving until March 31. Dispersal date and date of first flight were computed for the whole dataset (n = 35 individuals) as both events occurred before eventual signal loss. The Mann-Whitney U test was used to compare home range sizes, dispersal dates, maximal dispersal distances, and azimuths between sexes, while the Spearman correlation was used to test the relationship between maximal dispersal distance and 100% MCP, 95% MCP, 80% KDE, 50% KDE, and Simpson’s Diversity Index. The ‘compana’ function in R was used for testing random or non-random utilisation of habitats by WTEs, based on the following settings: test = c(‘randomisation’), rnv = 0.01, nrep = 500, and alpha = 0.05. Simpson’s Diversity Index (1-D) was computed for each individual on the basis of available habitat percentage (Index value ranges between 0 and 1; the higher the value, the greater the habitat diversity in each 100% MCP).
Results
Beginning of dispersal and PFA size
After excluding six individuals with signal loss before March 31, we obtained 1488–5879 locations per individual (median = 2026 locations) over 304–330 tracking days (median = 314 days; Table 2). Individual frequency of localisation ranged from five to 18 locations per day, with a median of seven locations per day. First flight from the nest was performed between May 19 and July 8, with a median date of June 5 (n = 35 individuals). Dispersal began between July 1 and November 14, with a median date of August 30 (Table 2). No sex-specific differences were found in both these variables (Mann-Whitney U test: p > 0.05). The length of the period between first flight from the nest and the beginning of dispersal was 8–165 days (median 82 days). After the beginning of dispersal, young WTEs only sporadically returned to the proximity of the parental nest for night roosting. This behaviour was only observed in eight of 35 tracked individuals and most of them (six individuals) spent only one night inside the 200 m radius from the native nest (twice in August, twice in September, once in December, once in February). Only one female and one male were recorded for two nights inside this distance, the female on two consecutive nights in October, and the male in October and March. Post-fledging area size was 0.4–2963 km2, with a median of 383 km2 (n = 29 individuals; Table 4). There was a significant difference in post-fledging area size between sexes (Mann-Whitney U test: U = 26, p = 0.001; n = 27 individuals), being significantly larger in males (median 1151 km2; n = 14) than females (median 149 km2; n = 13).
Dispersal distance and direction and action radius
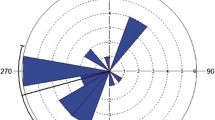
Maximal dispersal distance ranged from 93 to 433 km from the native nest, with a median of 187 km (n = 29 individuals; Fig. 1). Individuals occurred in the most distant point between July 6 and March 31 (median January 30, Table 4). Azimuth directions between the native nest and the furthest location appeared to be random (Table 4 - Azimuth 1). The highest number of individuals moved to the south (6 of 29 individuals, 20.7%), followed by the southwest (5 individuals, 17.2%) or northwest (5 individuals, 17.2%) for the furthest exploratory flights. Other directions were also recorded, though less frequently (Fig. 1; 4 x southeast, 4 x west, 3 x east, 1 x north, 1 x northeast). Though these azimuths appear to represent random exploratory flight directions rather than a shift in the core area of all locations obtained, a final summarisation for azimuths between the native nest and the centroid of the 50% KDE provided very similar results (Table 4 - Azimuth 2; 7 x south, 6 x west, 5 x northwest, 4 x southwest, 3 x east, 3 x southeast, 1 x north).
Overall area used (100% MCP) during the first year of life ranged from 2777 to 111249 km2 (median = 26888 km2, n = 29; Table 4). The median of the 95% MCP (100% MCP excluding the most extreme exploratory flights) was 13,376 km2 (min-max: 2178–103,497 km2), while that of the core area sensu lato (80% KDE) was 3393 km2 (min-max: 447–47,802 km2) and that of the core area sensu stricto (50% KDE) was 1137 km2 (min-max: 138-15,825 km2). There was no significant difference between males and females for maximal dispersal distance, azimuth to north and MCP or KDE size (n = 27, i.e. 29 minus two individuals of unknown sex, Mann-Whitney U test: p > 0.05). Excluding PFA, each individual occurred in 0–7 TSAs (median = 2 TSAs; Table 4) with mean sizes from 97 to 2277 km2, with a median of 514 km2.
Habitat selection
Compositional analysis with 35 individuals and eight habitat types confirmed significantly non-random utilisation of habitats by young WTEs during their first year of life (Lambda = 0.030; p < 0.002). Water areas were identified as the most preferred habitat type, followed by forests and shrubs and meadows (Table 5). While there was a non-significant preference for forests over shrubs (p > 0.05), differences in preference or avoidance in all other habitats were significant (p < 0.05). Sex-specific compositional analysis showed no difference in the final rank of selected habitats (Table 5). Simpson’s Diversity Index ranged from 0.46 to 0.75 (median 0.68), with no correlation observed with maximal dispersal distance (rS = 0.21; p > 0.05).
Final occurrence and connectivity between subpopulations
The last occurrence of individuals closer than 200 m from the native nest was observed between July 1 (of the first calendar year of life: 1CY) and March 5 of the second calendar year of life (2CY; median September 5; Table 6). The last occurrence closer than 5 km from the native nest was between August 14 (1CY) and March 31 (2CY; median March 7). Concerning the level of sedentarity at the end of observation, 100% MCP computed from March locations ranged from 482 to 62128 km2 (median 7424 km2), representing 2.4–88.1% of the overall area used (median 30.1%). Distance between the native nest and the centroid of March 100% MCP ranged from 0 to 179 km (median 63 km).
We recorded high connectivity between subpopulations of 1-year old WTEs from the Czech Republic, Austria, and Hungary (Fig. 2), although we do not know their future nest sites yet. Austrian WTEs were also recorded in Slovakia and Poland during their first year of life (Fig. 2b), while young WTEs from Czech nests were also recorded in Slovakia, Poland, and Germany (Fig. 2c). In addition to Austria, the Czech Republic, and Slovakia, Hungarian birds were also tracked in Croatia, Serbia, Romania, and Bosnia and Herzegovina (Fig. 2d).
Discussion
Sex-specific size of PFA
As far as we know, this is the first study revealing sex-specific PFA sizes in WTEs. From a parental point of view, providing longer post-fledging periods increases offspring survival and thereby parental fitness (López-Idiáquez et al. 2018). In this study, however, larger post-fledging areas in males could not be explained by different timing of dispersal from the natal nest site as no sex-specific differences in dispersal date were found. Reversed sexual size dimorphism is typical for most birds of prey, including WTEs. Thus, we believe that smaller males may be competitively forced to displace by their larger female siblings or conspecifics. A further explanation could be connected with different foraging strategies in comparison with females. Males usually catch smaller and more agile prey, which may require prolonged gathering of more prey items over a larger area compared to females in order to cover their daily energetic costs. We also cannot exclude an active role of parents in the differing feeding efforts of different sexed siblings.
No sex-specific dispersal
In most birds, natal dispersal distance is greater for females than males (Clarke et al. 1997; Kingma et al. 2017; Végvári et al. 2018). This sex-biased dispersal is usually explained by the ‘inbreeding avoidance’ or ‘resource-holding potential’ hypotheses (Greenwood 1980; Kingma et al. 2017). Surprisingly, maximal dispersal distance of WTE females and males did not differ in this study. In raptors (Accipitriformes), both female-biased (e.g. Eurasian sparrowhawk Accipiter nisus) and male-biased (e.g. northern goshawk Accipiter gentilis) natal dispersals have previously been described; however, there have also been previous cases where no effect of sex has been observed on natal dispersal, e.g. hen harrier Circus cyaneus (Clarke et al. 1997). No significant sex-specific differences in maximal dispersal distance have also been recorded in the Spanish Imperial Eagle Aquila adalberti (Ferrer 1993).
It is possible that a longer period or more detailed time measurements may be necessary to assess the role of sex in dispersal in WTEs. Whitfield et al. (2009a, 2009b), who studied WTE juvenile dispersal (up to age 48 months) and natal dispersal using coloured rings and patagium wing tags rather than telemetry, recorded significantly shorter natal dispersal distances in males than females in a reintroduced population of WTE in Scotland (Whitfield et al. 2009b) when measuring the distance between release site and the first breeding site of wing-tagged individuals. Whitfield et al. (2009a) stated that males initially dispersed farther than females in the first year of life, but that females occurred farther from the natal site in their second year. As breeding age approached, males were found closer to the natal site than females (Whitfield et al. 2009a). In a radio telemetry study, Nygård et al. (2000) observed females farther away from their natal site than males in winter. We cannot confirm these sex-specific findings on the basis of our dataset using molecular sexing. Maximal dispersal distances, used as a measure of dispersal in this study, were computed until March 31 of the second calendar year of life, and these need not correspond to the future nesting site, though Whitfield et al. (2009a) found a significant positive relationship between maximum juvenile dispersal distance in the first 2 years of life and natal dispersal distance. A longer tracking period than that described in this study may yet confirm their observations. According to Whitfield et al. (2009a), maximum juvenile dispersal distance was not affected by individual fledging date, body size, or native brood size, and we also observed no effect of fledging date on dispersal distance in this study. Similarly, dispersal distance in the Spanish Imperial Eagle was also not related to the date of departure from the natal population (Ferrer 1993).
Timing of dispersal
With improvements in molecular biology methods (Nemesházi et al. 2018; Rymešová et al. 2020) and animal tracking technologies, the importance of non-nesting WTEs (juvenile, subadult, and non-nesting adults) for intraspecific interactions and the dynamics of breeding population has been reassessed (Penteriani and del Mar Delgado 2009). Though dispersal is an essential process in population biology, with three natal dispersal phases usually recognised (emigration, transfer, and immigration), the beginning of the emigration phase has been defined differently by different authors (e.g. Cadahía et al. 2008; Soutullo et al. 2006a). A post hoc examination of locations is most frequently used for determining the end of the post-fledging period, which represents the first limit for the emigration phase (Cadahía et al. 2008). Due to different definitions and methods used by authors, or poor descriptions of the methods used, it is often not possible to compare the results of previously published studies on the same species. Similar inconsistencies also arise in definitions of home range size or TSAs. In this study, we used the same definition for the beginning of dispersal as Balotari-Chiebao et al. (2016), who, along with Tikkanen et al. (2018), stated that departure of young WTEs from their natal areas was indicated when individuals spent more than 10 consecutive days farther than 5 km from their natal nest. Similarly, Nygård et al. (2000, 2003) radio tracked 41 young WTEs in Norway and defined the beginning of dispersal as occurring with locations more than 4 km away from the nest with no subsequent return that season. Nygård et al. (2003) recorded permanent departure from the natal area on a median date of 1 October for males (estimated age 161 days) and 22 October for females (age 181 days), with non-significant differences between the sexes. Similarly, in Poland, Mirski et al. (2017) recorded young WTEs as finally leaving the parental territory at the end of October. Another contribution mentions September 29 as average date of leaving (July 27–January 20; Mirski 2017). We recorded a lower median for the beginning of dispersal (August 30), indicating an earlier departure of young WTEs from the PFA (July 1–November 14).
WTE dispersal distance in the first year
In this study, the median maximal dispersal distance was 187 km (93–433 km). Whitfield et al. (2009a) recorded a maximum juvenile dispersal distance ranging from 18 to 200 km, while Struwe-Juhl and Grünkorn (2007) recorded a median natal dispersal distance (on the basis of ringing) of 89 km, with a maximum of 450 km. This maximum recorded distance is similar to that observed in this study, at 433 km. On the other hand, a young WTE female from Russia spent her first winter 1100–1330 km away from the natal nest (Babushkin et al. 2017). Previous studies based on satellite telemetry usually have a very low sample size (e.g. three individuals: Mirski et al. 2017, Bekmansurov et al. 2018). Mirski et al. (2017), for example, tracked three young WTEs in Poland from fledging to November and recorded a female moving 723 km from the natal nest to Hungary, whereas a male moved only about 100 km. Mirski et al. (2017) stated that juveniles moved on average up to 367 km from nest (143–785 km). Bekmansurov et al. (2018) studied dispersal distances over a 2-month period after tagging on the nest and recorded distances from the native nest of 505 km and 260 km (without sex determination). Young WTEs from Schleswig-Holstein in Germany (n = 328 ringed nestlings) dispersed in all directions, with birds in the first, second, and third calendar year wandering widely (mostly alone) and returning to the natal location only occasionally (Struwe-Juhl and Grünkorn 2007), while older birds in their fourth and fifth calendar year occurred closer to their natal site, apparently searching for a breeding territory. Finally, in Norway, two young WTEs evidently did not leave the natal area at all during their first winter (Nygård et al. 2000, 2003). The same was also observed in some individuals in Finland (Saurola 2017).
Overall area used and TSAs
Bekmansurov et al. (2018) estimated the natal areas of three young WTEs tagged on the nest and tracked between May and August as ranging from 2.6 to 19.5 km2 (95% MCP). Balotari-Chiebao et al. (2016) tracked 14 WTE from fledging until the onset of dispersal from the natal area (up until December of the first calendar year) and described mean natal home range sizes as 0.67 km2 (50% KDE) and 7.83 km2 (95% KDE). The home range size of WTE was assessed for the first time by GPS telemetry by Krone et al. (2009), in a study tracking a 12-year-old female. Using a sample regime of three locations per day enabled them to obtain 475 positions for the female from July to January, which corresponded to a home range size of 4.53 km2 by 95% KDE, or 8.22 km2 by 95% MCP (Krone et al. 2009). In comparison to our own results for juveniles, adult nesting females appear to have a very limited action radius. Similarly, GPS data loggers placed on four adult WTEs (> 5 years old) in Germany (Krone et al. 2013) indicated a 100% MCP size of up to 669.7 km2 and a 50% fixed KDE of 2.97 km2. However, in this case, Krone et al. (2013) removed all outlying positions at a distance larger than six times the mean to the activity centre prior to MCP and KDE construction. Soutullo et al. (2006b) observed no statistical difference between the sexes in total area explored (MCP) of golden eagles (Aquila chrysaetos) during their first year of life.
Bragin et al. (2018) described three periods on the basis of different movement patterns of young WTEs after fledging: (1) the nest period, (2) the transitory period, and (3) the stopover period (defined as movements within a 30 km radius between transitory periods). Similarly, Cadahía et al. (2010) distinguished three phases of dispersal in young Bonelli’s eagles Aquila fasciata: (1) a dependence period, (2) departure from the parental territory, and (3) settlement in dispersal areas. For the first time, we attempted to define TSAs of WTEs in their first year of life as the most important stopover area for juveniles, with the aim of encouraging conservation of these sites. We suggest that future long-term studies should further assess the applicability of the TSA definition used in this study for WTE conservation management. While the method used in this study satisfactorily delimited the main clusters of individual locations, the resultant TSA sizes were not comparable between individuals due to differing numbers of night roosting sites and different numbers and dates of included tracking days. Nevertheless, the number of TSAs appears to be a good measure of individual sedentarity. Our data indicated that the TSAs of all the individuals studied showed a particular overlap around the nesting sites. Cadahía et al. (2010) found great individual variation in dispersal areas for Bonelli’s eagles, which only seldom overlapped, and recommended that conservation efforts should focus on the whole landscape matrix or preferred habitats rather than on a few clearly delimited geographical areas (Cadahía et al. 2010).
Habitat selection
Individual variation in habitat preference can play a role in WTE metapopulation dynamics (Davis and Stamps 2004). Bragin et al. (2018) used satellite telemetry and recorded highest habitat preference in WTEs for water areas, and this was confirmed by our own results. This preference fully corresponds with the WTE foraging strategy, which is based on prey consisting mainly of waterfowl and other birds, fishes, and smaller mammals (Sulkava et al. 1997; Sándor et al. 2015). Tikkanen et al. (2018) stated that subadult WTEs preferred the coastline and archipelagos along the Finnish coast close to their natal sites and avoided the open sea, urban areas, other constructed areas, and agricultural fields. Evans et al. (2010) examined nesting habitats of WTEs. Davis and Stamps (2004) noted that environmental heterogeneity may play an important role in the maintenance of genetic variation as different genotypes are likely to be favoured in different environment types. Tucker et al. (2019) revealed that environmental heterogeneity could affect dispersal distances. In this study, however, habitat diversity in 100% MCPs had no apparent effect on maximal dispersal distance in young WTEs. We suggest that detailed data on the habitat requirements of WTEs during their first year of life could be used together with future data from later years in a study exploring whether natal habitat preference induction plays a role in this species.
Final occurrence and future research
Ferrer (1993) noted that natal and breeding dispersal influences gene flow between populations or subpopulations. Unfortunately, the data obtained in this study were limited to a short period covering the first year of life and, as such, should be treated with caution as sites explored during the first year of life need not coincide with the final settlement and nesting area chosen over the next 4 years. Though young individuals from the three subpopulations studied occurred in the same areas during the first year of life, future nesting site identification will enable us to assess real gene flow and connectivity levels between subpopulations. Despite the above mentioned limitations, our study brought new data about post-fledging areas, overall area used, maximal dispersal distances, and returns to the native nest in WTEs during their first year of life. Obtained results are unique, at least for three countries, where the tagging was conducted. Data will be used in conservation of species.
Based on our results, we are of the opinion that an international approach is necessary for effective conservation of WTEs on a European-wide scale. The attachment of GPS/GSM loggers to WTE nestlings gave us a unique opportunity to study movements and behaviour of future nesting and non-nesting individuals, thereby enabling us to reliably estimate the floater-to-breeder ratio in the breeding age. Where floaters and dispersers constitute a substantial part of an endangered population, then it becomes essential that they are taken into account in conservation biology. Any future conservation efforts on endangered species with small population sizes such as the WTE should aim to protect not only traditional nesting sites but also the most important TSAs, which could attract many non-nesting individuals.
Data availability
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
Change history
16 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10344-021-01512-3
References
Aebischer NJ, Robertson PA, Kenward RE (1993) Compositional analysis of habitat use from animal radio-tracking data. Ecology 74:1313–1325
Babushkin MV, Kuznetsov AV, Demina OA (2017) White-tailed eagle on the Rybinsk reservoir: abundance, ecology, migration and wintering sites. In: The collection of abstracts and short notes of the Seaeagle 2017 conference, October 5-7. Roosta, Estonia. Eagle Club Estonia, pp 14–16
Balotari-Chiebao F, Villers A, Ijäs A, Ovaskainen O, Repka S, Laaksonen T (2016) Post-fledging movements of white-tailed eagles: conservation implications for wind-energy development. Ambio 45(7):831–840
Bekmansurov RH, Dzhamirzoev GS, Karyakin IV (2018) Tracking the white-tailed eagle movements by means of GPS/GSM transmitters. Raptors Conservation 1(Suppl. 1):30–32
Bělka T (2017) [White-tailed Eagle (Haliaeetus albicilla) – 2016 report]. Zpravodaj SOVDS 17:7–8
Bělka T, Horal D (2009) The white-tailed eagle (Haliaeetus albicilla) in the Czech Republic. In Probst R (ed) [The White-tailed Eagle in the heart of Europe]. Proceedings of the WWF Austria White-tailed Eagle Conference, November 17-18. Illmitz Denisia 27:65–77
Bossard M, Feranec J, Otahel J (2000) CORINE land cover technical guide – Addendum 2000. European Environment Agency, Copenhagen
Bragin EA, Poessel SA, Lanzone MJ, Katzner TE (2018) Post-fledging movements and habitat associations of white-tailed sea eagles (Haliaeetus albicilla) in Central Asia. Wilson Journal of Ornithology 130(3):784–788
Büttner G, Kosztra B (2017) CLC2018 Technical Guidelines. Environment Agency, Austria, 60 pp
Cadahía L, López-López P, Urios V, Negro JJ (2008) Estimating the onset of dispersal in endangered Bonelli’s Eagles Hieraaetus fasciatus tracked by satellite telemetry: a comparison between methods. Ibis 150:416–420
Cadahía L, López-López P, Urios V, Negro JJ (2010) Satellite telemetry reveals individual variation in juvenile Bonelli’s eagle dispersal areas. Eur J Wildl Res 56:923–930
Cagnacci F, Boitani L, Powell RA, Boyce MS (2010) Animal ecology meets GPS-based radiotelemetry: a perfect storm of opportunities and challenges. Phil Trans Soc B 365:2157–2162
Clarke AL, Sæther B-E, Røskaft E (1997) Sex biases in avian dispersal: a reappraisal. Oikos 79:429–438
Core Team R (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria https://www.R-project.org/
Cramp S, Simmons KEL (1982) The birds of the Western Palearctic, vol 2. Oxford University Press
Davis JM, Stamps JA (2004) The effect of natal experience on habitat preferences. Trends Ecol Evol 19(8):411–417
del Mar Delgado M, Penteriani V, Nams VO, Campioni L (2009) Changes of movement patterns from early dispersal to settlement. Behav Ecol Sociobiol 64:35–43
Demeter I, Horváth M, Prommer M (2017) Summary of population monitoring programmes run by MME / BirdLife Hungary´s Raptor Conservation Department (RCD) in 2017. Heliaca 15:74–75
Evans RJ, Pearce-Higgins J, Whitfield DP, Grant JR, MacLennan A, Reid R (2010) Comparative nest habitat characteristics of sympatric white-tailed Haliaeetus albicilla and Golden Eagles Aquila chrysaetos in western Scotland. Bird Study 57:473–482
Ferrer M (1993) Ontogeny of dispersal distances in young Spanish imperial eagles. Behav Ecol Sociobiol 32:259–263
Forero MG, Donázar JA, Hiraldo F (2002) Causes and fitness consequences of natal dispersal in a population of Black Kites. Ecology 83:858–872
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162
Helander B (1981) Nestling measurements and weights from two White-tailed Eagle populations in Sweden. Bird Study 28:235–241
Helander B, Stjernberg T eds. (2002) Action plan for the conservation of white-tailed sea eagle (Haliaeetus albicilla). BirdLife International Sweden
Helander B, Hailer F, Vilà C (2007) Morphological and genetic sex identification of white-tailed eagle Haliaeetus albicilla nestlings. J Ornithol 148:435–442
Hooten MB, Johnson DS, McClintock BT, Morales JM (2017) Animal movement: statistical models for telemetry data. CRC Press – Taylor & Francis Group, Boca Raton
Horváth Z (2009) White-tailed Eagle (Haliaeetus albicilla) populations in Hungary between 1987–2007. In: Probst R (ed) [The White-tailed Eagle in the heart of Europe]. Proceedings of the WWF Austria White-tailed Eagle Conference, November 17-18, Illmitz. Denisia 27:85-95
Kays R, Crofoot MC, Jetz W, Wikelski M (2015) Terrestrial animal tracking as an eye on life and planet. Science 348(6240):aaa2478
Kingma SA, Komdeur J, Burke T, Richardson DS (2017) Differential dispersal costs and sex-biased dispersal distance in a cooperatively breeding bird. Behav Ecol 28(4):1113–1121
Kloubec B, Hora J, Šťastný K (2015) Birds of South Bohemia. Jihočeský kraj, České Budějovice
Krone O, Berger A, Schulte R (2009) Recording movement and activity pattern of a white-tailed sea eagle (Haliaeetus albicilla) by a GPS datalogger. J Ornithol 150:273–280
Krone O, Nadjafzadeh M, Berger A (2013) White-tailed sea eagles (Haliaeetus albicilla) defend small home ranges in north-east Germany throughout the year. J Ornithol 154(3):827–835
Literák I, Mrlík V, Hovorková A, Mikulíček P, Lengyel J, Šťastný K, Cepák J, Dubská L (2007) Origin and genetic structure of white-tailed sea eagles (Haliaeetus albicilla) in the Czech Republic: an analysis of breeding distribution, ringing data and DNA microsatellites. Eur J Wildl Res 53:195–203
López-Idiáquez D, Vergara P, Fargallo JA, Martínez-Padilla J (2018) Providing longer postfledging periods increases offspring survival at the expense of future fecundity. PLoS One 13(9):e0203152
López-López P (2016) Individual-based tracking systems in ornithology: welcome to the era of the big data. Ardeola 63(1):103–136
Mellone U, Yáñez B, Limiñana R, Muñoz A-R, Pavón D, González J-M, Urios V, Ferrer M (2011) Summer staging areas of non-breeding short-toed snake eagles Circaetus gallicus. Bird Study 58(4):516–521
Mirski P (2017) Spatial ecology of white-tailed eagle in north-eastern Poland. In: The collection of abstracts and short notes of the Seaeagle 2017 conference, October 5-7, Roosta, Estonia. Eagle Club Estonia, 83
Mirski P, Anderwald D, Lewandowski S, Pieczyński P, Zawadzka D (2017) Movements of juvenile white-tailed eagles from Wigry National Park after fledgling. Studia i Materiały CEPL 53(4):56–66
Morandini V, Gonzáles E, Bildstein K, Ferrer M (2020) Juvenile dispersal in an uninhabitat continent: young Spanish Imperial Eagles in Africa. J Ornithol 161:373–380
Nemček V, Uhrin M, Chavko J, Deutschova L, Maderič B, Noga M (2016) Habitat structure of temporary settlement areas of young Saker Falcon Falco cherrug females during movements in Europe. Acta Ornithologica 51(1):93–103
Nemesházi E, Kövér S, Zachos FE, Horváth Z, Tihanyi G, Mórocz A, Mikuska T, Hám I, Literák I, Ponnikas S, Mizera T, Szabó K (2016) Natural and anthropogenic influences on the population structure of white-tailed eagles in the Carpathian Basin and central Europe. J Avian Biol 47:795–805
Nemesházi E, Szabó K, Horváth Z, Kövér S (2018) The effects of genetic relatedness on mate choice and territorial intrusions in a monogamous raptor. J Ornithol 159:233–244
Nygård T, Mee A (2017) The reintroduction of the white-tailed eagle to Ireland. In: The collection of abstracts and short notes of the Seaeagle 2017 conference, October 5-7. Roosta, Estonia. Eagle Club Estonia, pp 87–88
Nygård T, Kenward R, Einvik K (2000) Radiotelemetry studies of dispersal and survival in juvenile white-tailed sea eagles Haliaeetus albicilla in Norway. In: Chancellor RD, Meyburg B-U (eds) Raptors at risk. WWGBP, Hancock House, pp 487–497
Nygård T, Kenward R, Einvik K (2003) Dispersal in juvenile white-tailed sea eagles in Norway shown by radio-telemetry. In: Helander, B., Marquiss, M. & Bowerman, W. (eds) Sea Eagle 2000. Proceedings from an International Conference at Björkö, Sweden, 13–17 September 2000:191–196. Swedish Society for Nature Conservation, Stockholm
Penteriani V, del Mar Delgado M (2009) Thoughts on natal dispersal. J Raptor Res 43(2):90–98
Penteriani V, Ferrer M, del Mar DM (2011) Floater strategies and dynamics in birds, and their importance in conservation biology: towards an understanding of nonbreeders in avian populations. Anim Conserv 14:233–241
Probst R (2009) [The white-tailed eagle (Haliaeetus albicilla) in Austria: the white-tailed eagle project of WWF Austria]. In: Probst R (ed) [The White-tailed Eagle in the heart of Europe]. Proceedings of the WWF Austria White-tailed Eagle Conference, November 17-18, Illmitz. Denisia 27:29-50
Probst R, Peter H (2009) [The white-tailed eagle (Haliaeetus albicilla) in Austria: a revision of historical data]. In: Probst R (ed) [The White-tailed Eagle in the heart of Europe]. Proceedings of the WWF Austria White-tailed Eagle Conference, November 17-18, Illmitz. Denisia 27:19-28
Probst R, Pichler C (2017) The White-tailed Eagle in Austria: distribution and numbers, productivity and migration. In: The collection of abstracts and short notes of the Seaeagle 2017 Conference, 5-7 October, Roosta, Estonia. Eagle Club Estonia, 91
Prugnolle F, de Meeus T (2002) Inferring sex-biased dispersal from population genetic tools: a review. Heredity 88:161–165
Ronce O (2007) How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu Rev Ecol Evol Syst 38:231–253
Rymešová D, Pavlíček D, Kirner J, Mráz J, Papoušek I, Literák I (2020) Parentage analysis in white-tailed eagle Haliaeetus albicilla: are moulted feathers from nest sites a reliable source of parental DNA? Acta Ornithol 55:41–52
Sándor AD, Alexe V, Marinov M, Doroşencu A, Domşa C, Kiss BJ (2015) Nest-site selection, breeding success, and diet of white-tailed eagles (Haliaeetus albicilla) in the Danube Delta, Romania. Turkish J Zool 39:300–307
Saurola P (2017) Movements of Finnish sub-adult white-tailed eagles tracked by satellites 2009-2017. In: The collection of abstracts and short notes of the Seaeagle 2017 conference, October 5-7. Roosta, Estonia. Eagle Club Estonia, pp 94–95
Škorpíková V, Tunka Z (2013) The successful breeding of a three-year-old female of the white-tailed eagle (Haliaeetus albicilla). Crex 32:65–70
Soutullo A, Urios V, Ferrer M, Peñarrubia SG (2006a) Post-fledging behaviour in Golden Eagles Aquila chrysaetos: onset of juvenile dispersal and progressive distancing from the nest. Ibis 148:307–312
Soutullo A, Urios V, Ferrer M, Peñarrubia SG (2006b) Dispersal of Golden Eagles Aquila chrysaetos during their first year of life. Bird Study 53:258–264
Struwe-Juhl B, Grünkorn T (2007) Results of colour-ringing white-tailed sea eagles Haliaeetus albicilla in Schleswig-Holstein: site fidelity, movements, dispersal, age of first breeding, age structure and breeding of siblings. Vogelwelt 128:117–129
Suh A, Kriegs JO, Brosius J, Schmitz J (2011) Retroposon insertions and the chronology of avian sex chromosome evolution. Mol Biol Evol 28(11):2993–2997
Sulkava S, Tornberg R, Koivusaari J (1997) Diet of the white-tailed eagle Haliaeetus albicilla in Finland. Ornis Fenn 74:65–78
Tapia L, Zuberogoitia I (2018) Breeding and nesting biology in raptors. In: Sarasola JH, Grande JM, Negro JJ (eds) Birds of prey: biology and conservation in the XXI century. Springer, Cham
Tihanyi G, Dudás M, Horváth T (2009) First breeding record of colour ringed white-tailed eagle in Hungary. Heliaca 7:87–88
Tikkanen H, Balotari-Chiebao F, Laaksonen T, Pakanen V-M, Rytkönen S (2018) Habitat use of flying subadult white-tailed eagles (Haliaeetus albicilla): implications for land use and wind power plant planning. Ornis Fenn 95:137–150
Tucker MA, Alexandrou O, Bierregaard RO Jr et al (2019) Large birds travel farther in homogeneous environments. Glob Ecol Biogeogr 28(5):576–587. https://doi.org/10.1111/geb.12875
Végvári Z, Katona G, Vági B, Freckleton RP, Gaillard J-M, Székely T, Liker A (2018) Sex-biased breeding dispersal is predicted by social environment in birds. Ecol Evol 8(13):6483–6491
Whitfield DP, Duffy K, McLeod DRA, Evans RJ, MacLennan AM, Reid R, Sexton D, Wilson JD (2009a) Juvenile dispersal of white-tailed eagles in western Scotland. J Raptor Res 43(2):110–120
Whitfield DP, Douse A, Evans RJ, Grant J, Love J, McLeod DRA, Reid R, Wilson JD (2009b) Natal and breeding dispersal in a reintroduced population of white-tailed eagles Haliaeetus albicilla. Bird Study 56(2):177–186
Acknowledgements
We thank the following ornithologists and their cooperators for monitoring white-tailed eagles and their nest sites in the field: K. Makoň, H. Matušík, J. Mráz, and M. Váczi. We thank Kevin Roche for language editing. We also thank the editor and the anonymous referees for their valuable comments.
Code availability
Not applicable
Funding
This research was financially supported by the IGA VFU Brno (project no. 216/2019/FVHE).
Author information
Authors and Affiliations
Contributions
RR and IL designed the study and provided legislative permissions. RR, IL, PS, DR, and DH participated in the tagging of nestlings and regular checks of locations recorded and individual fates. DR, with VM, DD, and LR, analysed the data in GIS. DR contributed most to the writing of the paper, which was reviewed and approved by the other co-authors.
Corresponding author
Ethics declarations
Ethics approval
All animal manipulation and tagging were consistent with the laws of the Czech Republic, Austria, and Hungary. Tagging was allowed on the basis of the following permissions: (1) Czech Republic: JMK 48995/2017 (S-JMK 25358/2017 OŽP/Ško), JMK 18112/2018 (S-JMK 176395/2017 OŽP/Ško), KUCJK 40400/2017 (OZZL 25808/2017); (2) Austria: GFL2-J-107/014; (3) Hungary: tagging was performed by the ringer Péter Spakovszky under general deliverance PE-KTF/97-13/2017 of the Hungarian Bird Ringing Centre (MME/BirdLife).
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rymešová, D., Raab, R., Machálková, V. et al. First-year dispersal in white-tailed eagles Haliaeetus albicilla. Eur J Wildl Res 67, 44 (2021). https://doi.org/10.1007/s10344-021-01490-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-021-01490-6