Abstract
Large carnivore populations have suffered declines worldwide. For the African continent, these have been particularly strong in West and Central Africa. The Bénoué Complex in North Cameroon, located in Central Africa, is a key landscape for their conservation. We determined spatiotemporal trends in lion (Panthera leo), leopard (Panthera pardus) and spotted hyaena (Crocuta crocuta) abundance, using repeated spoor counts on transects from 2007 to 2015. Results of the temporal analysis indicate that lion and spotted hyaena abundance reduced over time across the complex, whereas leopards only declined in the last 2 years and primarily in the Faro Block. From the spatial analysis, it became clear spoor abundances differ between areas within the Bénoué Complex and between management types: Spoor densities were especially higher in Bouba Ndjida National Park and the hunting zones around Faro. This effect is most probably related to a more effective management strategy in these areas. Our fine-scale long-term monitoring technique provides a low-cost, easy to implement, multi-scale and effective tool for the identification of both regional and range-wide carnivore conservation hotspots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Large carnivores are among the most threatened taxa due to their large ranges, low densities and propensity for conflict with humans, and as keystone species, their disappearance can have cascading effects on entire ecosystems (Estes et al. 2011; Ripple et al. 2014; Le Roux et al. 2018). Habitat degradation, fragmentation, prey depletion and human-carnivore conflicts are the main causes of large carnivore declines in Africa (Woodroffe et al. 2005; Lindsey et al. 2017; Green et al. 2018; Everatt et al. 2019). Human populations have grown rapidly in the last century, which caused drastic declines in both distributional range and numbers throughout the continent (Hofer and Mills 1998; Riggio et al. 2013; Jacobson et al. 2016).
Monitoring of large carnivores is often challenging, due to their low densities, rarity and cryptic behaviour, and long-term repeated surveys to monitor trends are generally not considered feasible (Bauer et al. 2015a). Spoor surveys have been widely used as a method that can be applied over large areas (Winterbach et al. 2016), but data are typically insufficient to determine spatial variation at the level of individual management units (Bauer et al. 2017).
Compared with elsewhere on the African continent, large carnivore declines have been stronger in West and Central Africa, where fragmented populations are now largely restricted to protected areas (Craigie et al. 2010; Henschel et al. 2010; Toni and Lodé 2013; Henschel et al. 2014; Bauer et al. 2015a; Bohm and Höner 2015; Brugiere et al. 2015; Jacobson et al. 2016). As a result, lions meet the criteria for being listed as Regionally Critically Endangered in West Africa and Regionally Endangered in Central Africa (Bauer et al. 2015a). Despite the lack of accurate population estimates of leopards and spotted hyaenas in a large part of their distributional range, studies revealed significant population declines and even their disappearance from many areas in their former range (Bohm and Höner 2015; Stein et al. 2016).
The Bénoué Complex in Cameroon is a regional stronghold for several species of carnivore, including lion, leopard and spotted hyaena. The Central African savanna ecosystem extends into Gashaka Gumti National Park (NP) in Nigeria and Sena Oura NP in Chad, where impacts from, e.g., livestock and agricultural encroachment as well as bushmeat poaching and illegal artisanal mining for gold have substantially degraded the habitat (Croes et al. 2011; Scholte and Iyah 2016). The most recent population estimates for large carnivores in the Bénoué Complex suggest a total of 250 lions, 316 leopards and 1376 spotted hyaenas (Bauer et al. 2015b). In designated hunting zones around the three national parks of the complex, quotas are assigned annually that allow professional hunters to hunt several species. While no trophy hunting quotas are set for leopards, and there is only limited demand for the available quota of spotted hyaena, lion quota in Cameroon has historically been around 30 lions per annum. In response to a 2015 survey by Bauer et al. (2015b), the lion quota was cut to 11, but recently raised again to 14 lions per year.
To ensure effective conservation actions for the management of this key stronghold for large carnivores in West and Central Africa, long-term monitoring of population abundance indices is vital. Building on the work of Croes et al. (2011), we extended the large carnivore survey in the Bénoué Complex to cover more successive years (until 2015) and a larger area. In this current study, we will answer two different questions, based on two different analyses. (1) What are the trends in spoor densities of lion leopard and spotted hyaena, in relation to area and management type? (2) How is the dispersal of the current populations of lion, leopard and spotted hyaena throughout the complex and how is spoor density linked to area and management type? Based on both analyses, we are aiming to gain new insights on conservation effectiveness.
Materials and methods
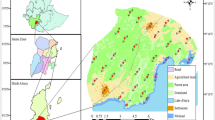
The Bénoué Complex in North Cameroon consists of three national parks (NPs): Faro NP, Bénoué NP and Bouba Ndjida NP, covering 7580 km2 in total. Each of the three NPs is surrounded by its own hunting zones (HZs) (covering 15,814 km2), making up three sub-areas that constitute one contiguous protected complex. The Sudanian wooded savanna landscape has a sloping character, with scattered rocky outcrops rising to 500 m above sea level and two major waterways (the Bénoué and Faro rivers) that hold water year-round. The Bénoué Complex has a distinct wet (May–September) and dry seasons (October–April) with an average temperature of 25 °C (maximum 41 °C). The average annual precipitation is 1200–1500 mm which falls mainly in the wet season. Human habitation is only allowed outside the NP perimeters, but villages have been slowly expanding along the dirt roads interconnecting the HZs and the NPs’ entrances (Croes et al. 2011). In 2006, the human population around Bénoué NP was estimated at around 100,000, with > 60% migrants from neighbouring countries such as Chad and the Central African Republic (Endamana et al. 2007). This was similar around Bouba Ndjida NP and Faro NP. Socio-economic conditions are poor in the region, with low-quality education, a low level of health infrastructure and scarcity of drinking water in the dry season (Endamana et al. 2007). Local populations are highly dependent on natural resources in their daily life, but in an unsustainable way (Endamana et al. 2007).
We used spoor surveys as population abundance indices for lion, leopard and spotted hyaena in space and time. Spoors were repeatedly counted on 25 km transects, one or two transects in each NP and one or two corresponding transects in an adjacent HZ (Fig. 1, Table 1). These transects were selected based on seasonal accessibility, substrate suitability, representativeness and position (within the Bénoué Complex). With the exception of one transect in HZ Voko Bantadje (in the Faro area), which had become a busy road and was therefore replaced by a comparable alternative, we repeated surveys using the same method and along the same transects that were used by Croes et al. (2011) during 2007–2011. Three transects were added in 2014 and 2015; one in a Faro HZ, one in a Bénoué HZ and one in Bouba Ndjida NP. These additional transects were not taken into account in analyses showing trends over time. Transects were distributed over the complex to optimise geographical coverage (i.e. with even distribution between periphery and core areas), but always such that they crossed habitat that was not notably different from the wider surroundings.
In 2007–2011, transect counts had been repeated several times within two periods of 3 weeks in the dry season, with a mean sampling interval of 3 days. In 2014 and 2015, each transect was sampled twice a month in the dry season. The number of repeats is therefore similar across all years, but less clustered in time for 2014–2015. Spoor counts were conducted by local research assistants sitting on the front of a car or on a motorcycle, driving approximately 10 km/h. Surveys were done in the early morning when spoors are most visible and to avoid disturbance from traffic, if any. To each survey zone, a team of two research assistants was assigned, who received a training prior to the surveys to optimise spoor identification accuracy and to reduce observer bias over time and between each protected area category (i.e. NP vs HZ). Following the protocol of Funston et al. (2010), we counted all large carnivore spoors, except when detected within 500 m (measured in a straight line) from another spoor of the same species that was not ostensibly a different individual, to prevent double counting. Because spoors on transects were only sampled with an interval of at least 3 days, and spoors were only counted if they were fresh (< 24 h), tracks were not double counted between transect repeats. Spoor age was estimated by the clarity of the spoors (sharpness of the edges and the amount of debris in spoor), all in accordance with standard protocol (Funston et al. 2020). In the field, each spoor encountered, species, location, date/time stamp, substrate type and spoor age were recorded, and at least one picture was taken for later verification and/or correction purposes. Based on the spoor, the large carnivore species was identified by visual appearance and measurements (size and shape) in the field and photographed by the field assistants. All pictures were verified by the authors and later double checked by an expert.
To answer the research questions, we did two separate analyses. The two analyses used different datasets, which were partly overlapping (see Table 1). The first analysis focused on the trends in time, taking into account differences in area and management type, by using a dataset that includes data from 2007 to 2011 (not every area has data in each year), 2014 and 2015. The second analysis focused on the effects area and management type, by using a (balanced) dataset of only 2014 and 2015. For both analyses, a similar statistical approach was used. We used track counts of lion, leopard and spotted hyaena per year and transect as response variable, assuming that they were normally distributed. As explanatory variables, we used area (factor with three levels: Faro, Bénoué and Bouba Ndjida) and management type (factor with two levels: hunting zone (HZ), national park (NP)) and time (continuous:Year). We used a normal linear model (lm), and started with a maximum model with all possible interactions. We simplified this model to a minimum adequate model by stepwise removing non-significant terms, taking into account the marginality principle. Depending on the results, which differed per species and analysis, a Tukey post hoc test was performed to determine differences between the three research areas. For the significant results, predictions (with procedure LSMEANS) were made for the relevant variables, assuming other variables as constant. All presented means are calculated on measured values. If necessary, means of means were taken.
Whether explanatory variables should be considered as fixed or random variables are in the first place is a matter of the research focus. Since one can argue that research area in both analyses and year in the second analysis (and the interaction year: area) could be considered random explanatory variables, we did a preliminary analysis with a mixed model, using GLMM. From this preliminary analysis, it became clear that the conclusions of the research remain the same, and we choose to use the GLM, because of the possibility to extend our conclusions also to differences between research areas.
All statistical analyses were run in R 3.3.2. (October, 2016), using a significance threshold of p < 0.05.
Results
During the 5-year study period, a total of 1089 spoors were recorded on 9600 km of transects. Table 1 shows the survey frequency and timing for each of the transects.
The first analyses which had a focus on the temporal differences in spoor density showed that for neither of the large carnivore species (lion, leopard and spotted hyaena), a significant interaction effect was found between year with area or management type, indicating that if effects in time were found they were consistent through the entire complex. Lions showed a significant effect in year (F(1) = 8.238, p = 0.015) (Table 2). A reduction of 12.9% per year was found in the lion spoor density in the measured period. For leopard, there were no effects of time on the spoor densities in the entire period of 2007 till 2015 (F(1) = 1354, p = 0.269) (Table 2). However, based on our second analysis, where only the years 2014 and 2015 were taken into account, a significant interaction effect of time and area for leopards was found (F(2) = 12,108, p = 0.003) (Table 3). Post hoc test showed a 68% reduction of spoor densities between 2014 and 2015, only the Faro area. Last, for spotted hyaena, an effect of time was found (F(1) = 8.200, p = 0.013) (Table 2), where a reduction of 6.8% per year was found in the spoor density in the measured period. In addition, our second analysis, where only the years 2014 and 2015 were taken into account, an effect of time was found (F(1) = 6.4461, p = 0.023). Spotted hyaena spoor densities showed a 68% reduction throughout the Bénoué Complex in those years.
The second analyses, which had a focus on the spatial differences in spoor density, showed that there was a significant interaction between area and management type for lions (F(2) = 14.552, p < 0.001) and leopards found (F(2) = 12.108, p = 0.003) (Table 4). The post hoc showed that in the Bouba Ndjida area, lions spoor densities were 11.8 times higher in the NP compared with the HZs and leopards spoor densities were 3.1 times higher in the NP compared with the HZs. In contrast, in the Faro area, lion spoor densities were 3.7 times higher in the HZs compared with the NP and leopard spoor densities 1.6 times higher in HZs as compared with NP. In the Bénoué area, the spoor densities of both species were low, but did not differ between NP and HZ. For spotted hyaenas, there was only an effect on area found (F(2) = 17.027, p = < 0.001). No interaction effect with management type or year has been found (Table 4). Post hoc test showed that the spotted hyaena spoor density was 3 to 4 times higher in the Faro area as compared with the rest of the complex (Bénoué area and Bouba Ndjida area).
Discussion
We found declines in spoor densities of lion and spotted hyaena throughout the complex which could signal trophic downgrading (Estes et al. 2011). The strong decline of spotted hyaena in 2014 and 2015 may indicate that the trophic downgrading is accelerating, but it still needs to be interpreted with care due to natural yearly fluctuations in populations. Lions in particular show strong declines in the Bénoué Area, which is bisected by a North-South national highway and livestock corridor that are likely to have contributed to this population’s fragility. And while spotted hyaenas were not affected by differences in management practices between our study areas, lion and leopard abundance did vary depending on management strategy. Leopards appear to be more resilient; we found no significant changes in leopard relative abundances measured between 2007 and 2015, possibly due to their high level of adaptability to ecosystem change (Jacobson et al. 2016; Stein et al. 2016; Hayward et al. 2006), or because they cannot be hunted because Cameroon has no hunting quota for leopard. However, the observed leopard decline in Faro NP during 2014–2015 suggests that this resilience is not without limits. We do not know the cause of this decline and we need to interpret it with care, but poaching may be a factor and in the perspective of the alarming rate at which leopard numbers are declining across the continent, it should not be overlooked (Stein et al. 2020).
Since our study aimed at detecting spatiotemporal trends in large carnivore abundance indices between differently managed zones rather than estimating their densities, we did not rely on published regression between spoor and carnivore densities. As a result, the uncertainties that have increasingly been raised in recent years (e.g. Winterbach et al. 2016; Bauer et al. 2017; Elliot and Gopalaswamy 2017; Belant et al. 2019) with respect to the reliability of using spoor counts for density estimates were eliminated. This makes our method a low-cost, easy to implement, multi-scale and effective tool for the carnivore population monitoring.
It is not surprising to find an overall decline in large carnivore numbers in an ecosystem that for decades suffered from intense and growing anthropogenic pressure, such as poaching, retaliatory killings, illegal cattle herding, mining and agricultural encroachment. We suggest that escalation of threats is also linked to human and livestock immigration from surrounding countries such as Nigeria, Chad and the Central African Republic (Endamana et al. 2007). In recent years, the strain on the natural resources of the Bénoué Complex has become even higher due to the expansion of old villages and creation of new villages. Without a transition towards more sustainable land use practices, this resulted in massive encroachment deep into protected areas. These pessimistic observations were shared by Lindsey et al. (2017), who stated that the scores across all threats of all three NPs were among the highest in Africa. Similarly, a 2015 aerial survey by WCS revealed over 500,000 heads of cattle present in the protected area complex, numerous gold mining camps in the Bénoué and Bouba Ndjida areas and a devastating impact of poaching on the elephant (Loxodonta africana) population (WCS, Unpubl.).
This aerial survey report mentions higher wildlife numbers in several ‘pockets’, with Bouba Ndjida NP and the HZs close to Faro NP still providing the least degraded habitat, similar to our findings. Details on conservation management investments across the complex are largely lacking, but anecdotal information suggests that these two hotspots have long been more effectively managed. Areas with trophy hunting do thus not necessarily have lower carnivore abundances. Anti-poaching efforts and actions against other illegal intrusions have been more frequent and stringent here than in the other parts of the protected areas. Increased funding and support for conservation efforts following the ‘elephant massacres’ (Platt 2012) in Bouba Ndjida NP have allowed the NP’s management to implement several protective measures that are now ensuring an important prey base for large carnivores. The higher lion and leopard spoor densities we found in Bouba Ndjida NP compared with the adjoining HZs may have been a direct result of this increased prey availability. Our finding that spotted hyaena spoor densities did not follow this trend is consistent with the slow recovery of this species after substantial population reduction (Smuts 1978) and may be partly due to their proneness to interspecific competition with other large carnivores (Creel et al. 2001; Trinkel and Kastberger 2005. Since large carnivores have large ranges, populations typically occur across a variety of management regimes, and the ‘averaging out’ of trends prevents detection of meaningful trends. We show that monitoring is possible at a lower spatial scale, showing divergence in trends between sub-units and between species.
Our study findings are at least indicative of the recovery potential of the complex as a whole and emphasise the importance of continued efforts to harmonise anti-poaching strategies, avoid fragmentation and counteract the devastating impact of the growing human population across the ecosystem.
References
Bauer H, Chapron G, Nowell K, Henschel P, Funston P, Hunter LTB, MacDonald DW, Packer C (2015a) Lion (Panthera leo) populations are declining rapidly across Africa, except in intensively managed areas. PNAS 112:14894–14899
Bauer H, Kamgang SA, Kirsten I, Tumenta P, Saleh A, Henschel P, Sillero-Zubiri C (2015b) Large carnivore abundance in the Benoue ecosystem, North Cameroon. Afr J Ecol 54:235–237
Bauer H, Henschel P, Packer C, Sillero-Zubiri C, Chardonnet B, Sogbohossou E, De Iongh H, Macdonald D (2017) Lion trophy hunting in West Africa: a response to Bouché et al. PLoS One 12:e0173691
Belant JL, Bled F, Mkasanga IJ, Wilton CM, Mwampeta SB, Beyer DE, Mwakilema W, Fyumagwa R (2019) Track surveys do not provide accurate or precise lion density estimates in serengeti. Global Ecology and Conservation:e00651
Bohm T, Höner O (2015) Crocuta crocuta. The IUCN Red List of Threatened Species 9:2015–2013
Brugiere D, Chardonnet B, Scholte P (2015) Large-scale extinction of large carnivores (lion Panthera leo , cheetah Acinonyx jubatus and wild dog Lycaon pictus ) in protected areas of West and Central Africa. Trop Conserv Sci 8:513–527
Craigie ID, Baillie JEM, Balmford A, Carbone C, Collen B, Green RE, Hutton JM (2010) Large mammal population declines in Africa’s protected areas. Biol Conserv 143:2221–2228
Creel S, Spong G, Creel N (2001) Interspecific competition and the population biology of extinction-prone carnivores. In: Gittleman JL, Funk SM, Macdonald DW, Wayne RK (eds) Carnivore conservation. Cambridge Univ Press, Cambridge, pp 35–60
Croes BM, Funston PJ, Ramussen G, Buij R, Saleh A, Tumenta PN, De Longh HH (2011) The impact of trophy hunting on lions (Panthera leo) and other large carnivores in the B‚nou‚ Complex, northern Cameroon. Biol Conserv 144:3064–3072
Elliot NB, Gopalaswamy AM (2017) Toward accurate and precise estimates of lion density. Conserv Biol 31:934–943
Endamana D, Sayer J, Etog G, Bene Bene L (2007) Conservation and development: the influence of accessibility, participatory management and immigration around the Bénoué National Park in Cameroon. Nature and Faune 22:10–20
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soulé ME, Virtanen R, Wardle DA (2011) Trophic downgrading of planet earth. Science 333:301–306
Everatt KT, Moore JF, Kerley GI (2019) Africa’s apex predator, the lion, is limited by interference and exploitative competition with humans. Glob Ecol Conserv 20:e00758
Funston PJ, Frank L, Stephens T, Davidson Z, Loveridge A, Macdonald DM, Durant S, Packer C, Mosser A, Ferreira SM (2010) Substrate and species constraints on the use of track incidences to estimate African large carnivore abundance. J Zool 281:56–65 https://doi.org/10.1111/j.1469-7998.2009.00682.x
Green D, Johnson-Ulrich L, Couraud H, Holekamp K (2018) Anthropogenic disturbance induces opposing population trends in spotted hyaenas and African lions. Biodivers Conserv 27:871–889
Hayward MW (2006) Prey preferences of the spotted hyaena (Crocuta crocuta) and the degree of dietary overlap with the lion (Panthera leo). J Zool 270:606–614. https://doi.org/10.1111/j.1469-7998.2006.00183.x
Henschel P, Azani D, Burton C, Malanda G, Saidu Y, Sam M, Hunter L (2010) Lion status updates from five range countries in West and Central Africa. Cat News 52:34–39
Henschel P, Coad L, Burton C, Chataigner B, Dunn A, MacDonald D, Saidu Y, Hunter LT (2014) The lion in West Africa is critically endangered. PLoS One 9:e83500
Hofer H, Mills G (1998) Worldwide distribution of hyaenas. Hyaenas: status survey and conservation action plan, eds Mills G, Hofer H (International Union for the Conservation of Nature, Cambridge, UK):40-63
Jacobson AP, Gerngross P, Lemeris JR Jr, Schoonover RF, Anco C, Breitenmoser-Würsten C, Durant SM, Farhadinia MS, Henschel P, Kamler JF (2016) Leopard (Panthera pardus) status, distribution, and the research efforts across its range. PeerJ 4:e1974
Le Roux E, Kerley GI, Cromsigt JP (2018) Megaherbivores modify trophic cascades triggered by fear of predation in an African savanna ecosystem. Curr Biol 28:2493–2499.e2493
Lindsey PA, Petracca LS, Funston PJ, Bauer H, Dickman A, Everatt K, Flyman M, Henschel P, Hinks AE, Kasiki S, Loveridge A, Macdonald DW, Mandisodza R, Mgoola W, Miller SM, Nazerali S, Siege L, Uiseb K, Hunter LTB (2017) The performance of African protected areas for lions and their prey. Biol Conserv 209:137–149
Platt J (2012) Cameroon elephant massacre shows poaching, ivory trade require an international response. Scientific American http://blogs.Scientificamerican.com/extinctioncountdown/2012/03/20/cameroon-elephant-massacre-poaching-ivory-trade
Riggio J, Jacobson A, Dollar L, Bauer H, Becker M, Dickman A, Funston P, Groom R, Henschel P, de Iongh H (2013) The size of savannah Africa: a lion’s (Panthera leo) view. Biodivers Conserv 22:17–35
Ripple WJ et al (2014) Status and ecological effects of the world’s largest carnivores. Science 342(6167):1241484
Scholte P, Iyah E (2016) Declining population of the vulnerable common hippopotamus Hippopotamus amphibius in Bénoué National Park, Cameroon (1976–2013): the importance of conservation presence. Oryx 50:506–513
Smuts (1978) Effects of population reduction on the travels and reproduction of lions in Kruger National Park. Carnivore 1:61–72
Stein A, Athreya V, Gerngross P, Balme G, Henschel P, Karanth U, Miquelle D, Rostro S, Kamler J, Laguardia A (2016) Panthera pardus. The IUCN red list of threatened species 2016: e. T15954A50659089. Gland, Switzerland: IUCN
Stein AB, Athreya V, Gerngross P, et al (2020) Panthera pardus, Leopard. The IUCN red list of threatened species e.T15954A163991139
Toni P, Lodé T (2013) Fragmented populations of leopards in West-Central Africa: facing an uncertain future. Afr Zool 48:374–387
Trinkel M, Kastberger G (2005) Competitive interactions between spotted hyaenas and lions in the Etosha National Park, Namibia. Afr J Ecol 43:220–224
Winterbach CW, Ferreira SM, Funston PJ, Somers MJ (2016) Simplified large African carnivore density estimators from track indices. PeerJ 4:e2662
Woodroffe R, Thirgood S, Rabinowitz A, Woodroffe R, Thirgood S, Rabinowitz A (2005) People and wildlife: conflict or coexistence? Press, Cambridge University
Acknowledgements
We thank F. Tarla, B.Kadiri, M. Babalé, N. Nhiomog, P. Bour, M. Saleh and the lion guards Daïrou, Hayatou, Souaibou, Bobo, Aoudou, Issa and Hamidou. We also thank MINFOF and the Garoua Wildlife College for the permits and support, and the park wardens of Bouba Ndjida, Faro and Bénoué NP for their trust and cooperation and commitment.
Funding
This research was financially supported by the US Fish and Wildlife Service, World Wildlife Fund and Prins Bernhard Natuur Fonds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics declarations
The collection of the material and analyses presented here were conducted in line with the regulations in Cameroon and at the respective authors’ institutions and do not require additional ethical clearance.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bakker, C.E., Kirsten, I.E., Bauer, H. et al. Divergent trends of large carnivore populations within the Bénoué Complex, North Cameroon, shown by long-term fine-scale monitoring. Eur J Wildl Res 66, 82 (2020). https://doi.org/10.1007/s10344-020-01420-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-020-01420-y