Abstract
We studied the seasonal activity pattern of the Kuhl’s pipistrelle Pipistrellus kuhlii in eight sites of the Bou Hedma National Park, central Tunisia, from June 2010 to June 2011, using both mistnetting and acoustic bat detection. Pipistrelles were captured all year-round only at water bodies. Captures peaked in late spring and early autumn. Pregnant females were observed from March to June, lactating females only in June, and flying juveniles from June to August. Echolocation calls and buzzes were recorded in all sites throughout the year. However, activity varied significantly among months and sites. Activity peaked in June and September after a noteworthy decline in August; the minimum was recorded in December and January. Bats mainly foraged at water bodies and around streetlamps. This result supports the previously reported major role of water bodies for bats in arid environments. Bats also foraged in the open acacia forest in summer, during the flowering period of Acacia Acacia tortilis raddiana. The steppe was the least used habitat. Social calls were mainly recorded in autumn around streetlamps, suggesting a swarming behaviour. Despite being a common bat species in the area, P. kuhlii should deserve conservation efforts that would benefit other bat species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Kuhl’s pipistrelle, Pipistrellus kuhlii (Kuhl 1817), is a Western Palaearctic species-group whose range extends from the Mediterranean area, expanding to the North, over the whole Arabian Peninsula to Kazakhstan and Pakistan (Simmons 2005; Dietz et al. 2007). Despite clear morphological differences, the former desert pipistrelle Pipistrellus deserti Thomas, 1902, is considered to be an arid ecotype of P. kuhlii by Benda et al. (2015). In North Africa, P. kuhlii is represented by a genetic clade that is widespread across most of Europe (see Çoraman et al. 2013 for a large geographic sample). This species is widely distributed from Morocco (Aulagnier et al. 2017) to Egypt (Qumsiyeh 1985), across Algeria (Kowalski and Rzebik-Kowalska 1991) and Libya (Hanák and Elgadi 1984; Van Cakenberghe and Benda 2013a, b; Benda et al. 2014). In Tunisia, it was noted for the first time by Fitzinger (1870). Since then, P. kuhlii was reported from Galite Island in the north to Douirat in the south, and is the most recorded bat species in the north and centre of Tunisia (Dalhoumi et al. 2011; Puechmaille et al. 2012). The range of this species covers a gradient of bioclimatic zones from the humid Mediterranean mountains to the Saharan desert, including arid zones where the ecology of P. kuhlii is mostly unknown.
In the northern part of its range, P. kuhlii is reported to hibernate from November to March (Strelkov et al. 1985); however, some populations are active along the year, mainly in the Mediterranean area such as in Italy (Lanza 2012), Lebanon (Lewis and Harrison 1962), and Israel (Carmel and Safriel 1998), the threshold temperature for the winter activity being lower than 5 °C in Morocco (Aulagnier et al. 2017). In Algeria and Tunisia, some animals were mistnetted in winter, either in the Mediterranean or in the pre-Saharan areas (Deleuil and Labbé 1955; Gaisler 1983–84; Gaisler and Kowalski 1986; Dalhoumi et al. 2015). Parturition time also varies over the range, mainly influenced by climate conditions, from early April in Iran (Benda et al. 2012) to late June in northern France (Pottier et al. 1996).
This pipistrelle is mainly found in open habitats and has been frequently recorded foraging at streetlamps (Haffner and Stutz 1985–86; Vernier 1989; Russo and Jones 1999; Vernier and Ruggieri 1999; Feldman et al. 2000; Rainho 2007; Polak et al. 2011; Lanza 2012), and also in parks or over water bodies (Dietz et al. 2007; Rudolph et al. 2010; Bilushenko 2013). Nevertheless, this is a very flexible species, associated with a wide range of habitat types mainly below 1000 m a.s.l., including riparian habitats, open oak woodlands, low intensity farmland, and urban settlements (Carmel and Safriel 1998; Russo and Jones 2003; Rainho 2007; Di Salvo et al. 2009; Georgiakakis et al. 2010; Lisón and Calvo 2013). Considering its wide range in arid North-African areas where human settlements are scarce and streetlamps are rare, we can expect an even broader niche, and possibly differences in habitat use in winter when feeding is considerably reduced.
The aim of our study was to investigate the adaptation of P. kuhlii to an arid area of Tunisia by monitoring the seasonal activity of this bat species in various habitats. We hypothesised (1) a low but continual winter activity and a peak around parturition, including foraging activity determined by buzzes, and social activity and (2) a use of all the different sites along the year, with a possible higher activity over water bodies used in summer for foraging and drinking versus around streetlamps which could concentrate more insects during winter.
Materials and methods
Study area
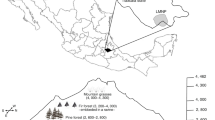
Sampling was conducted inside and nearby zone A of the Bou Hedma National Park, central Tunisia (34° 27′–34° 32′ N, 9° 23′–9° 41′ E). Established in 1980, this park (16,488 ha) is located in the transition zone between the southern boundary of the Orbata–Bou Hedma mountains and the low southern plains of Tunisia. It includes very steep slopes in the northern part and a plain in the southern part. At less than 50 km west to the Mediterranean Sea, the park ranges from 100 to 790 m a.s.l.
The climate is arid Mediterranean with temperate winters. The average temperature ranged from 3.9 °C (January) to 36.2 °C (August) (Noumi et al. 2010), negative temperatures down to −4 °C having been recorded in December, January, and February. The average annual rainfall ranges from 150 mm in the plain to 300 mm on the northern flanks of the mountain; the dry period can last up to 10 months per year (Karem et al. 1993). These are characteristics of the arid bioclimate (Chaieb and Boukhris 1998).
The Bou Hedma National Park was created in order to preserve the northernmost station of Acacia tortilis raddiana and reduce desert conversion (Gupta et al. 1981; Zaafouri et al. 1997). Vegetation is a pseudo-savannah of Acacia tortilis raddiana associated with more than 500 species, including 8 species with priority protection: Acacia tortilis raddiana, Juniperus phoenicea, Pistacia atlantica, Thymelia semperverens, Tetrapogon villosus, Tricholaena teneriffae, Cenchrus ciliaris, and Digitaria nodosa.
Six sites were sampled in zone A where 23,191 acacia trees are distributed over the 5114 ha (Noumi 2010), bordj basin, Nouh basin, Bou Hedma wadi, open acacia forest, dense acacia forest, and steppe, and two sites, sandy plain and Aiidi streetlamps, were sampled nearby (Fig. 1, Supplementary Table a).
Data collecting
Each site has been monitored once per month from June 2010 to June 2011 under favourable climate conditions (no rain, null, or weak wind), for 3 h starting 15 min after sunset. We used two techniques, bat call recording and captures with one mistnet (Flaquer et al. 2007; Hayes et al. 2009; Kunz et al. 2009). Water bodies were sampled in successive nights, whereas other sites were sampled simultaneously in nearby places. The mistnet (3 m × 12 m; 16 mm × 16 mm) was placed just above the water surface at the water bodies (bordj basin, Nouh basin, and Bou Hedma wadi), along furrows in the steppe, gaps in the clear and dense acacia forests, paths in the sandy plain, and below Aiidi streetlamps.
At the beginning of each session and every 30 min, we measured air temperature (°C) and humidity (%) using a C.A. 846 pocket thermo-hygrometer (Chauvin Arnoux, Taiwan, precision ± 0.5 °C and ± 2.5% RH). We calculated a mean value of these variables for each session to perform analyses.
Bat calls were recorded at one fixed point near water bodies (bordj basin, Nouh basin, and Bou Hedma wadi) and below streetlamps, and along one 300–400 m transect per site walking up and down at a constant speed of 2 km per hour in the steppe, the open and dense acacia forests, and the sandy plain.
Manual recordings of 3.4 s each were made with a D240X time expansion bat detector (Petterson Electronik AB, Uppsala, Sweden) connected to a digital audio recorder (Edirol R-09HR, Roland Corporation, China).
Species identification
Mistnetted specimens were identified using keys and field guides (Dietz and Helversen 2004; Dietz 2005; Dietz et al. 2007; Aulagnier et al. 2009), sexed (reproductive status of females was assessed), weighed, measured, and immediately released. The capture licence was provided by the Department of Forest (Direction Générale de Forêts), Ministry of Agriculture.
Recorded calls were analysed using the real-time analysis software BatSound, v. 3.10 (Petterson Electronik AB, Uppsala, Sweden) for spectrogram analyses. We used a sample frequency of 44,100 samples/s, 16 bits/sample, and selected a 512 pt. FFT with a Hamming window for analysis (Russ 1999). We used the shape of the signal, maximum energy peak frequency, and the end frequency for species identification. Reference calls of local P. kuhlii were obtained by recording bats which were positively identified in the hand and released (Dalhoumi unpublished data). P. kuhlii echolocation calls consist of a short frequency-modulated (FM) component followed by a terminal part of almost quasi-constant frequency (QCF) with end frequency at 35.8 ± 1.9 (mean ± SD) kHz and frequency of maximum energy at 39.8 ± 1.6 kHz. These calls can be separated clearly from those of other bat species, with the exception of Nathusius’ pipistrelle Pipistrellus nathusii (Russo and Jones 2002; Zsebők et al. 2012), which has not been recorded in Tunisia (Dalhoumi et al. 2011; Puechmaille et al. 2012). In Tunisia, Pipistrellus pipistrellus and Hypsugo savii emit calls with end frequency at 43.2 ± 1.5 and 29.6 ± 1.2 kHz, respectively, and frequency of maximum energy at 46.0 ± 1.2 and 32.0 ± 1.3 kHz (Dalhoumi unpublished data). We also identified P. kuhlii social calls; they started at 41.2 ± 7.4 kHz (23.4–68.5 kHz), lasted 45.8 ± 15.3 ms (22.3–95.8 ms), peaked at 14.3 ± 1.2 kHz (11.8–19.6 kHz), and ended at 11.2 ± 1.8 kHz (8.1–20.5 kHz). These calls have longer duration and lower values of peak and end frequencies than those of P. pipistrellus, which helps to discriminate both species (Russo and Jones 1999). We also used the sound library of Barataud (1999, 2002, 2012) to ascertain some identification.
Data analysis
Due to the skewed distribution of data, the seasonality of captures was tested using a chi-square test. Relation between the number of captures and air temperature and humidity were tested using Spearman correlation.
Flight activity was estimated by counting the number of bat passes (Russo and Jones 2003). We counted one pass every 37.4 s (3.4 s recording time of the bat detector; 34 s recording time of the portable digital tape) for each animal. Within each 3-h night session, bat passes were counted every period of 30 min; the number of bat passes recorded during each period was used as a proxy of bat activity (variable activity). Foraging activity was assessed by counting the number of feeding or drinking buzzes (Russo et al. 2016) simultaneously (variable buzzes).
We used generalised linear models to investigate variation in bat activity and buzzes among the eight sites, including month (13 consecutive months) and period (6 consecutive 30 min periods) in the model. Because our dependent variables were count data and have very different ranges among sites leading to overdispersion, we used a gamma distribution with a log link function. As this distribution does not allow zeros, we arbitrarily added 1 to activity and buzzes. We expected differences in activity and buzzes among sites and seasons, and potentially among periods; therefore, we considered those models which incorporated a difference among sites (two-way interactions between site and month and between site and period), and among months (two-way interaction between month and period), and all possible combinations of these hypotheses. All models specified with a two-way interaction also included main effects. We also considered models incorporating only main effects, and the null model (no difference of activity or buzzes). We did not consider the three-way interaction as we had no specific hypothesis to such a complex relationship. The models were fitted with the glm function in the stats package of R for windows version 3.1.2 (R Core Team 2014). We considered 17 models (Supplementary material) and used the second-order Akaike’s Information Criterion (AICc; Burnham and Anderson 1998) and the Akaike weight (AICcWt) to select the model with the most support. To interpret and visualise the effects incorporated in the retained models, we plotted the predicted effects and their associated 95% confidence intervals. In order to better estimate the confidence intervals, we considered the maximum likelihood estimate of the shape parameter of the gamma distribution, obtained from the library MASS (Venables and Ripley 2002) to calculate the dispersion parameter used in the predict function of the retained glm model. Pairwise comparisons among periods were computed using a sign test (Siegel and Castellan 1988). Flight activity and buzzes were related to air temperature and humidity using Spearman correlations.
The number of social calls was too low for computing such models, so differences among months were compared using a Friedman test and among sites using a Kruskal-Wallis test. Correlations between log-transformed numbers of captures and bat passes or buzzes were tested by Pearson coefficients. These analyses were performed using TANAGRA (Rakotomalala 2005).
Results
Seasonality of captures and bat reproduction
A total of 123 Pipistrellus kuhlii were mistnetted during 104 nights; ca. 80% of nights (n = 71) were unsuccessful. Bats were netted only at three localities: bordj basin (91.9%), Nouh basin (4.9%), and Bou Hedma wadi (3.2%). No bat was captured either around streetlamps or in acacia forests where nets were set near trees and across pathways. P. kuhlii were captured every month with a highly significant seasonal variation in the number of captures (chi-square test = 118.3; df = 12; P < 0.001; Fig. 2).
Captures peaked in early summer (June, n = 27 in 2010; n = 30 in 2011), and reached a minimum in December and January, and in August (n = 2). The relation between the number of captures and air temperature and humidity was not significant (ρ = 0.410; df = 11; P = 0.164; ρ = 0.188; df = 11; P = 0.538, respectively). However, the power to detect an effect is very low for such a small sample.
Among 105 captured adult P. kuhlii, females were more numerous than males (n = 72 vs 33), even if adult males were captured every month except in August 2010 and February 2010 (Table 1). Pregnant females were netted in March (n = 1) and particularly in May (n = 8) and June 2011 (n = 9).
Lactating females (n = 23) were all netted in June (2010 and 2011). Subadults (11 females and 7 males) were netted in June, July, and August.
Variations of P. kuhlii activity
Pipistrellus kuhlii was acoustically recorded at the eight sites every month, except in steppe (January and February) and open acacia forest (December). We recorded 11,144 P. kuhlii passes, with an outranked maximum of 1499 passes at bordj basin in September, and a total of 1140 buzzes; on average, 10.2% of bat passes included a buzz.
For bat activity, the best model contained the two-way interaction between site and month (AICcWt = 1) and period (Table 2). The unique contribution of bordj basin to the model was dismissed by modelling bat activity without this site, which gave similar results (supplementary material). Hence, the retained model supported the hypothesis of differential activity among sites along the year (Fig. 3a, b). Most activity was recorded from April to September at water bodies (except in August at Nouh basin) and from June to November around streetlamps; these sites gathering also most of the low winter activity. Sandy plain was mainly used from June to August, open acacia forest in July and August, dense acacia forest in September and steppe in October; these sites were rarely used during the rest of the year. In steppe, echolocation calls were detected particularly near an isolated acacia tree, along dry wadis and rocky lines where vegetation is somewhat more present. Activity differed among periods of the night with higher levels in the second and third periods (Fig. 4); pairwise comparisons using a sign test were significant between period 2 and periods 1 and 6 (z = 3.328; P < 0.001 and z = 2.773; P = 0.006), between period 3 and periods 1, 4, and 6 (z = 2.773, P = 0.006; z = 2.773, P = 0.006; and z = 2.219, P = 0.027). At last, activity was positively correlated with air temperature (r = 0.754; df = 11; P = 0.003), and not with air humidity (r = 0.143; df = 11; P = 0.641).
Monthly activity of Pipistrellus kuhlii (bat passes) at eight sites of the Bou Hedma National Park (Tunisia) from June 2010 to June 2011: a Entire plot of bat passes, b magnified lower half of the plot of bat passes (mean ± se, estimated using the first model of Table 2); c plot of buzzes (mean ± se, estimated using the first model of Table 3). Values are shown for the first period of the night which is representative of the whole recording (for abbreviations, see Fig. 1)
Variation of Pipistrellus kuhlii activity among 30-min periods starting after sunset in the Bou Hedma National Park (Tunisia) from June 2010 to June 2011 (mean ± se, estimated using the first model of Table 3)
For buzzes, the best model contained only the two-way interaction between site and month (AICcWt = 0.8, Table 2). The pattern of differential activity among sites along the year is similar to activity (r = 0.787; df = 11; P = 0.001; Fig. 3c), with slight differences. The maximum number of buzzes was recorded at Nouh basin in June 2011, this water body sharing with bordj basin the highest foraging activities from April to September. Buzzes were less numerous at Bou Hedma wadi and around streetlamps with maxima in July and August, June to November, respectively. At last, some buzzes were recorded in June in sandy plain, and July and August in open acacia forest where bats flew around trees; they were accidental in dense acacia forest and steppe. Winter activity was mainly restricted to bordj basin, Nouh basin, and around streetlamps. As activity, buzzes were positively correlated with air temperature (r = 0.750; df = 11; P = 0.003), and not with air humidity (r = 0.120; df = 11; P = 0.695).
We also recorded 392 social calls at the eight sites during the field study (Table 4), particularly in October (47.2%), November (36.5%), and additionally in December (6.9%) and January (6.1%). Social calls were very rarely emitted during spring and summer; the difference among months was significant (Friedman test, H = 26.77; df = 12; P = 0.008). Most of these calls were emitted around the streetlamps (90.6%), the difference among sites was significant (Kruskal-Wallis test, H = 14.43; df = 7; P = 0.044).
Variation in habitat use: captures vs acoustic data
Whereas echolocation calls and even rarer social calls were recorded in the eight sites, captures were restricted to water bodies where activity and buzzes were among the most numerous. In these sites, captures were highly log-correlated to both activity (r = 0.541; df = 38; P < 0.001) and buzzes (r = 0.494; df = 38; P = 0.001). On the contrary, no bat was captured around streetlamps despite a second level of activity after bordj basin and a third number of buzzes after bordj basin and Nouh basin.
Discussion
Both sampling methods showed a noteworthy occurrence of Pipistrellus kuhlii in the Bou Hedma National Park all the year. This was the second most captured bat after the isabelline serotine Eptesicus isabellinus (Dalhoumi et al. 2015) and the most acoustically recorded species, as well as in Italy (Russo and Jones 2003), Greece (Davy et al. 2007), and Israel (Razgour et al. 2010), contrary to Spain (Lisón and Calvo 2013, 2014). As it was reported by previous authors (O’Farrell and Gannon 1999; Flaquer et al. 2007), the two methods provide complementary results. Acoustic bat detection succeeded in recording P. kuhlii in all sites, even when bats were less active. In the surveyed quite open habitats, this bat was easily recorded when capture was not always successful due to netting effort at the ground level only and/or avoidance of the net by a highly manoeuvrable flight. Indeed, P. kuhlii is known to forage between 2 and 14 m above the ground depending on the season and the habitat (Masson and Sagot 1985; Vernier 1989; Barataud 1992; Gaisler 1994; Grodzinski et al. 2009), and could easily avoid the single 3-m-high mistnet, whereas they lower their flight for drinking at water bodies. On the other hand, capture, an invasive technique, provided information on the gender, biometrics, and reproductive status of animals.
Biological cycle and activity
As well as in Saudi Arabia (Alagaili 2008), females were more captured than males, mainly in May and June, when they were late pregnant or lactating in the Bou Hedma National Park. These months also provided high level of acoustic activity and (feeding and drinking) buzzes, supporting a high energy requirement. Indeed, pregnant females were recorded from March to May and early June, lactating females were caught only in June, and flying juveniles from June to August. This reproduction period, which is shorter than in Israel and Jordan where lactating females were recorded from May to July (Barak and Yom-Tov 1991, Benda et al. 2010), is later than in Iran where lactating females were caught in early April (Benda et al. 2012) or Saudi Arabia and Egypt where females gave birth from late April to early May (Gaisler et al. 1972; Alagaili et al. 2011). As well as in Italy, Lebanon, Syria, and Libya (Lewis and Harrison 1962; Vernier 1995; Benda et al. 2006, 2014), countries of the Mediterranean region (Blondel and Aronson 1999), parturition is earlier than in the more northern Azerbaijan where it peaks in middle June (Rakhmatulina 1983), and than in Armenia and Atlantic France where it starts in early June (Yavrouyan 1989; Touzot 2014). Gravid females were still recorded in June in Turkey (Baydemir and Albayrak 2006) and lactating females in early August in northern France (Pottier et al. 1996). Some of these differences should be related to climatic conditions as lactating females were recorded in early June in southern Tunisia (Baker et al. 1974) and late July in northern Tunisia (Deleuil and Labbé 1955) where spring temperatures are colder and vegetation (and insects) later.
In the Bou Hedma National Park, P. kuhlii were active throughout the year including winter when captures and acoustic activity were low but steady, particularly at water bodies and around streetlamps. Buzzes showed that bats were foraging even if insects are rare at that time. This result supports previous records of Gaisler (1983-84) and Gaisler and Kowalski (1986) who reported a low number of winter captures in Algeria and also many direct observations all over most of the southern range, Iraq (Weber 1955; Al Robbae 1966), Lebanon (Lewis and Harrison 1962), Morocco (Aulagnier et al. 2017), Israel (Carmel and Safriel 1998), Saudi Arabia (Alagaili 2008), and Italy (Vernier 1995, 1998; Lanza 2012). Whereas winter activity has been reported in temperate bat species for a long time (e.g. Verschuren 1949; Hooper and Hooper 1956), foraging activity has only been recorded more recently through diet analysis (e.g. Kaňuch et al. 2005; Miková et al. 2013; Hope et al. 2014) or acoustic bat detection that allows recording feeding attempts (Avery 1985; Ceľuch and Kaňuch 2005; Zahn and Kriner 2016).
Captures and acoustic activity, including buzzes, both peaked in late spring and early summer when females were pregnant or lactating. Their energy requirement (Neuweiler 2000) was then met by intense foraging behaviour, whereas water requirement for lactation (Adams and Hayes 2008) could explain the peak of capture in June. After a dramatic decrease in August, activity peaked again in early autumn when bats emitted a large number of buzzes too. The low summer activity could be due to their scattering in the wide flowering acacia forest or to a shallow torpor during the hottest days that proved to reduce respiratory and cutaneous water loss (Muñoz-Garcia et al. 2012). After the hot and dry summer, September offers to bats the opportunity to store reserves, and regain a heavy body mass (Alagaili 2008; Dalhoumi et al. 2016). Then, autumn was characterised by a decreasing activity but was also the main season for social calls which started and peaked in October, during the mating season (Barak and Yom-Tov 1991).
Despite huge variations of temperature along the year, we did not detect any seasonal influence among periods of the night on both activity and buzzes. Activity was higher 30 min after sunset and decreased after 90 min. This pattern is quite similar to Algerian records of captures (Gaisler and Kowalski 1986), and could suggest an absence of competitors (Razgour et al. 2011). P. kuhlii is reported to emerge early at sunset or before sunset after studies conducted at roosts (Rakhmatulina 1983; Vernier 1989; Yavrouyan 1989; Alagaili 2008; Rudolph et al. 2010; Maxinová et al. 2016). As recorded activity on foraging grounds starts early after sunset in our study, we reasonably suppose that bats roost nearby in rock crevices, tree holes, or even between the scales of date palms as it was reported by Dietz et al. (2007). However, following Hizem and Allegrini (2009), we only found 3 colonies of 3, 6, and 14 (including 12 young) specimens in December, January, and May, respectively, after the fall of Acacia peeling bark.
Habitat use
Whereas captures were restricted to water bodies along the year, echolocation calls and buzzes were recorded in all sites, even if they were rarely reported in winter in steppe, sandy plain, and dense acacia forest. The putative higher bat activity (Fisher-Phelps et al. 2017) detected by mobile acoustic transects in these sites relative to stationary acoustic counts at water bodies and streetlamps was overpassed in our study. As for Eptesicus isabellinus (Dalhoumi et al. 2017), the bordj basin was the most attractive in all seasons, followed by streetlamps and the two other water bodies. Contrary to Ancillotto et al. (2017), such a high activity of P. kuhlii near water bodies has been previously reported in different countries of southern Europe (Russo and Jones 2003; Pocora and Pocora 2008; Lisón and Calvo 2011, 2014; Maxinová et al. 2016), North Africa, and the Middle East (Carmel and Safriel 1998; Benda et al. 2010, 2014), including Tunisia (Dalhoumi et al. 2014). More generally in deserts, bats tend to concentrate at water bodies (Korine and Pinshow 2004; Rebelo and Brito 2006; Razgour et al. 2010) and some species can only live in oases, such as P. kuhlii, in southern Libya (Benda et al. 2014). Everywhere, bats use water bodies for drinking and/or foraging (Vaughan et al. 1997; Grindal et al. 1999; Adams and Simmons 2002; Tuttle et al. 2006; Geluso and Geluso 2012; Bilushenko 2013; Seibold et al. 2013). Indeed, some P. kuhlii captured at bordj basin were wet. Moreover, buzzes peaked at the three water bodies, suggesting both activities there. Using a night filming mode, Razgour et al. (2010) recorded a medium frequency of drinking, higher than that of desert-dwelling species, which was associated with a foraging activity. Indeed, water bodies provide also food for this flexible aerial hawker (Norberg and Rayner 1987) despite a generalist diet including a wide range of prey categories, e.g. Lepidoptera, Coleoptera, Hymenoptera, and Diptera in Libya (Benda et al. 2014), that could hardly explain the importance of this habitat.
Differences of activity and buzzes among water bodies can be related partly to their size (Rabe and Rosenstock 2005; Francl 2008) and partly to the permanence of water (Razgour et al. 2010). In Negev desert, P. kuhlii is largely associated with small temporary ponds. Yet it avoids large permanent ponds, which are usually occupied by competitors, Bodenheimer’s pipistrelle Hypsugo bodenheimeri and Rüppell’s pipistrelle Vansonia rueppellii (Razgour et al. 2011). In Bou Hedma National Park, the significant interaction between month and site for both activity and buzzes suggest that bats actively foraged at water bodies during part of the year, namely during spring and summer. Buzzes peaked in June 2010 at Nouh basin which is a built basin closely surrounded by date and gum trees where the level of water rarely drops down from the margin. On the contrary, water is not either running or standing in Bou Hedma wadi along the year, except during spring and autumn floods.
At streetlamps, bat activity and buzzes arouse from June to November. Most of the previous studies reported that P. kuhlii are particularly active near streetlamps in towns and villages that are with more intense illumination (Haffner and Stutz 1985–86; Barak and Yom-Tov 1989; Vernier 1989; Zava et al. 1994; Carmel and Safriel 1998; Russo and Jones 1999; Feldman et al. 2000; Ancillotto et al. 2015; Maxinová et al. 2016). Along with other bat species (Rydell 1992; Rydell and Racey 1995), P. kuhlii prefer lamps with high UV-proportion and luminosity which proved more attractive for insects (Haffner and Stutz 1985–86). Barataud (1992) watched that P. kuhlii circle streetlamps half-height or just over the light, which explain that they were not mistnetted at Aiidi streetlamps. A similar result was reported by Korine and Pinshow (2004).
The high number of social calls at streetlamps could support the tendency of P. kuhlii to forage in group (Barak and Yom-Tov 1989); however, these calls were only recorded in autumn; a result which could suggest an additional swarming activity more than a food patch defence such as in P. pipistrellus (e.g. Racey and Swift 1985; Budenz et al. 2009) due to the local abundance of insects. The mating system described by Barak and Yom-Tov (1991) includes a songflight display that could be related to these social calls, but both frequencies (between 30 and 45 kHz) and time (behaviour taking place during late night) are different. Following Russo and Jones (1999), these social calls should be further investigated.
At last, we recorded a high level of activity and buzzes in the open acacia forest in July and August which is the flowering period of acacia trees (Derbel et al. 2007). At the same time, activity was low in the main foraging sites, water bodies (except bordj basin), and streetlamps, suggesting that bats foraged on a temporary source of prey. Indeed, acacia trees were reported of unique importance to the community of desert-dwelling bats by Hackett et al. (2013), the health of the trees being crucial to their value as a foraging resource.
Conclusion
Bat monitoring in the Bou Hedma National Park supported a low but continual winter activity of Pipistrellus kuhlii in an arid region situated between the Mediterranean and Saharan areas. Activity peaked around parturition time, with a recrudescence in early autumn after the more quiet hot and dry summer, whereas social calls were mainly recorded during the mating period at streetlamps. The eight sampled sites have been visited along the year; however, water bodies, together with streetlamps, concentrated most of the activity. Contrary to our expectation, activity did not peak in summer, even at bordj basin when water is less available or attainable at the two other water sites. The protection of these sites should deserve the first conservation priorities.
P. kuhlii, the most common bat species in the Bou Hedma National Park, is not threatened. This favourable status does not preclude preserving the best quality of natural habitats inside the park and restoring the degraded habitats outside the protected area. Such conservation efforts would benefit other bat species, and the whole biodiversity.
References
Adams RA, Hayes MA (2008) Water availability and successful lactation by bats as related to climate change in arid regions of western North America. J Anim Ecol 77(6):1115–1121
Adams RA, Simmons JA (2002) Directionality of drinking passes by bats at water holes: is there cooperation? Acta Chiropt 4(2):1–5
Al Robbae K (1966) Untersuchungen der Lebensweise irakischer Fledermäuse. Säugetierkd Mitt 14:177–211
Alagaili AN (2008) Biological, ecological, and conservational study of Kuhl’s bat (Pipistrellus kuhlii) from Unizah Province, Saudi Arabia. Ph.D. Thesis, Arkansas University, Fayetteville
Alagaili AN, James DA, Mohammed OB (2011) Timing and pattern of molt in Kuhl's bat, Pipistrellus kuhlii, in Saudi Arabia. Acta Chiropt 13(2):465–470
Ancillotto L, Tomassini A, Russo D (2015) The fancy city life: Kuhl’s pipistrelle, Pipistrellus kuhlii, benefits from urbanisation. Wildl Res 42(7):598–606
Ancillotto L, Ariano A, Nardone V, Budinski I, Rydell J, Russo D (2017) Effects of free-ranging cattle and landscape complexity on bat foraging: implications for bat conservation and livestock management. Agri Ecosyst Environ 241:54–61
Aulagnier S, Haffner P, Mitchell-Jones T, Moutou F, Zima J (2009) Mammals of Europe, North Africa and the Middle East. A & C Black, London
Aulagnier S, Cuzin F, Thévenot M (2017) Chiroptera. In: Aulagnier S, Cuzin F, Thévenot M (eds) Mammifères sauvages du Maroc. Peuplement, répartition, écologie. Société Française pour l’Etude et la Protection des Mammifères, Paris, France, pp 117–154
Avery MI (1985) Winter activity in pipistrelle bats. J Anim Ecol 54(3):721–738
Baker RJ, Davis BL, Jordan RG, Binous A (1974) Karyotypic and morphometric studies of Tunisian mammals: bats. Mammalia 38(4):695–705
Barak Y, Yom-Tov Y (1989) The advantage of group hunting in Kuhl’s bat Pipistrellus kuhli (Microchiroptera). J Zool (Lond) 219(4):670–675
Barak Y, Yom-Tov Y (1991) The mating system of Pipistrellus kuhli (Microchiroptera) in Israel. Mammalia 55(2):285–292
Barataud M (1992) L'activité crépusculaire et nocturne de 18 espèces de Chiroptères, révélée par marquage luminescent et suivi acoustique. Rhinolophe 9:33–57
Barataud M (1999) Ballades dans l'inaudible. 3ème édition augmentée. Sittelle, Mens, France
Barataud M (2002) Méthode d'identification acoustique des Chiroptères d'Europe. Mise à jour printemps 2002. Sittelle, Mens, France
Barataud M (2012) Ecologie acoustique des Chiroptères d'Europe. Identification des espèces, étude de leurs habitats et comportements de chasse. Biotope-Publications Scientifiques du Muséum, Mèze-Paris, France
Baydemir N, Albayrak I (2006) A study on the breeding biology of some bat species in Turkey (Mammalia: Chiroptera). Turk J Zool 30(1):103–110
Benda P, Andreas M, Kock D, Lučan RK, Munclinger P, Nova P, Obuch J, Ochman K, Reiter A, Uhrin M, Weinfurtova D (2006) Bats (Mammalia: Chiroptera) of the Eastern Mediterranean. Part 4. Bat fauna of Syria: distribution, systematics, ecology. Acta Soc Zool Bohem 70:1–329
Benda P, Lučan RK, Obuch J, Reiter A, Andreas M, Bačkor P, Bohnenstengel T, Eid EK, Ševčík M, Vallo P, Amr ZS (2010) Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 8. Bats of Jordan: fauna, ecology, echolocation, ectoparasites. Acta Soc Zool Bohem 74:185–353
Benda P, Faizolâhi K, Andreas M, Obuch J, Reiter A, Ševčík M, Uhrin M, Vallo P, Ashrafi S (2012) Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 10. Bat fauna of Iran. Acta Soc Zool Bohem 76:163–582
Benda P, Spitzenberger F, Hanák V, Andreas M, Reiter A, Ševčík M, Šmíd J, Uhrin M (2014) Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 11. On the bat fauna of Libya II. Acta Soc Zool Bohem 78:1–162
Benda P, Andriollo T, Ruedi M (2015) Systematic position and taxonomy of Pipistrellus deserti (Chiroptera: Vespertilionidae). Mammalia 79(4):419–438
Bilushenko AA (2013) The current status of Kuhl’s pipistrelle Pipistrellus kuhlii (Chiroptera, Vesprtilionidae) in the central forest-steppe of Ukraine. Vestn Zool 47(4):343–349
Blondel J, Aronson J (1999) Biology and wildlife of the Mediterranean region. Oxford University Press, New York
Budenz T, Heib S, Kusch J (2009) Functions of bat social calls: the influence of local abundance, interspecific interactions and season on the production of pipistrelle (Pipistrellus pipistrellus) type D social calls. Acta Chiropterol 11(1):173–182
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information-theoretic approach. Springer, New York
Carmel Y, Safriel U (1998) Habitat use by bats in a Mediterranean ecosystem in Israel—conservation implications. Biol Conserv 84(3):245–250
Ceľuch M, Kaňuch P (2005) Winter activity and roosts of the noctule (Nyctalus noctula) in an urban area (Central Slovakia). Lynx n.s 36:39–45
Chaieb M, Boukhris M (1998) Flore succincte et illustrée des zones arides et sahariennes de Tunisie. L’Or du Temps, Tunis
Çoraman E, Furman A, Karataş A, Bilgin R (2013) Phylogeographic analysis of Anatolian bats highlights the importance of the region for preserving the chiropteran mitochondrial genetic diversity in the Western Palaearctic. Conserv Genet 14(6):1205–1216
Dalhoumi R, Aissa P, Aulagnier S (2011) Taxonomie et répartition des Chiroptères de Tunisie. Rev Suisse Zool 118(2):265–292
Dalhoumi R, Hedfi A, Aissa P, Aulagnier S (2014) Bats of Jebel Mghilla National Park (Central Tunisia): first survey and habitat-related activity. Trop Zool 27(2):53–62
Dalhoumi R, Aissa P, Aulagnier S (2015) Cycle annuel d’activité des Chiroptères du Parc National de Bou-Hedma (Tunisie). Rev Ecol (Terre Vie) 70(3):261–270
Dalhoumi R, Aissa P, Aulagnier S (2016) Seasonal variations of sexual size dimorphism in two Mediterranean bat species from Tunisia: the Kuhl’s pipistrelle (Pipistrellus kuhlii) and the Isabelline serotine (Eptesicus isabellinus). Folia Zool 65(2):157–163
Dalhoumi R, Morellet N, Aissa P, Aulagnier S (2017) Seasonal activity pattern and habitat use by the Isabelline serotine bat (Eptesicus isabellinus) in an arid environment of Tunisia. Acta Chiropterol 19:141–153
Davy CM, Russo D, Fenton MB (2007) Use of native woodlands and traditional olive groves by foraging bats on a Mediterranean island: consequences for conservation. J Zool (Lond) 273(4):397–405
Deleuil R, Labbé A (1955) Sur la variabilité de la Pipistrelle de Kuhl (Pipistrellus kuhli). Bull Soc Sci nat Tunisie 8:237–242
Derbel S, Noumi Z, Anton KW, Chaieb M (2007) Life cycle of the coleopter Bruchidius raddianae and the seed predation of the Acacia tortilis subsp. raddiana in Tunisia. CR Biol 330(1):49–54
Di Salvo I, Russo D, Sará M (2009) Habitat preferences of bats in a rural area of Sicily determined by acoustic surveys. Hystrix It J Mammal 20(2):137–146
Dietz C (2005) Illustrated identification key to the bats of Egypt, version 1.0. Electronic publication: http://www.mammalwatching.com/Palearctic/Otherreports/Bat%20Key%20for%20Egypt.pdf
Dietz C, Von Helversen O (2004) Illustrated identification key to the bats of Europe. Electronic publication: http://www.mammalwatching.com/palearctic/otherreports/batkey.pdf
Dietz C, Von Helversen O, Nill D (2007) Handbuch der Fledermäuse Europas und Nordwestafrikas. Franckh-Kosmos, Stuttgart
Feldman R, Whitaker JO Jr, Yom-Tov Y (2000) Dietary composition and habitat use in a desert insectivorous bat community in Israel. Acta Chiropt 2(1):15–22
Fisher-Phelps M, Schwilk D, Kingston T (2017) Mobile acoustic transects detect more bat activity than stationary acoustic point counts in a semi-arid and agricultural landscape. J Arid Environ 136:38–44
Fitzinger LJ (1870) Kritische Durchsicht der Ordnung der Flatterthiere oder Handflüger (Chiroptera). Familie der Fledermäuse (Vespertiliones). IV Abtheilung. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Math Naturwiss Kl 62:211–317
Flaquer C, Torre I, Arrizabalaga A (2007) Comparison of sampling methods for inventory of bat communities. J Mammal 88(2):526–533
Francl KE (2008) Summer bat activity at woodland seasonal pools in the northern Great Lakes region. Wetlands 28(1):117–124
Gaisler J (1983–84) Bats of northern Algeria and their winter activity. Myotis 21–22: 89–95
Gaisler J (1994) The bats Pipistrellus kuhlii and Hypsugo savii on the island of Rab (Croatia). Folia Zool 43:279–280
Gaisler J, Kowalski K (1986) Results of the netting of bats in Algeria (Mammalia, Chiroptera). Vĕst čs Společ zool 50:161–173
Gaisler J, Madkour G, Pelikán J (1972) On the bats (Chiroptera) of Egypt. Acta Sci Nat Acad Sci Bohem Brno 6:1–40
Geluso KN, Geluso K (2012) Effects of environmental factors on capture rates of insectivorous bats, 1971-2005. J Mammal 93:161–169
Georgiakakis P, Vasilakopoulos P, Mylonas M, Russo D (2010) Bat species richness and activity over an elevation gradient in Mediterranean shrublands of Crete. Hystrix It J Mammal 21(1):43–56
Grindal SD, Morissette JL, Brigham RM (1999) Concentration of bat activity in riparian habitats over an elevational gradient. Can J Zool 77(6):972–977
Grodzinski U, Spiegel O, Korine C, Holderied MW (2009) Context-dependent flight speed: evidence for energetically optimal flight speed in the bat Pipistrellus kuhlii? J Anim Ecol 78(3):540–548
Gupta JP, Aggarwal RK, Raiky NP (1981) Soil erosion by wind from bare sandy plain in western Rajasthan. Ind J Arid Environ 4(1):15–20
Hackett TD, Korine C, Holderied MW (2013) The importance of Acacia trees for insectivorous bats and arthropods in the Arava desert. PLoS One 8(2):e52999
Haffner M, Stutz HP (1985–86) Abundance of Pipistrellus pipistrellus and Pipistrellus kuhlii foraging at street lamps. Myotis 23–24: 167–168
Hanák V, Elgadi A (1984) On the bat fauna (Chiroptera) of Lybia. Vĕst čs Společ zool 48:165–187
Hayes JP, Ober HK, Sherwin RE (2009) Survey and monitoring of bats. In: Kunz TH, Parsons S (eds) Ecological and behavioral methods for the study of bats, 2nd edn. The Johns Hopkins University Press, Baltimore, pp 112–129
Hizem MW, Allegrini B (2009) Contribution à la connaissance des Chiroptères du Parc National de bou Hedma (Tunisie). Poiretia 1:5–9
Hooper JHD, Hooper WM (1956) Habits and movements of cave dwelling bats in Devonshire. Proc Zool Soc Lond 127(1):1–26
Hope PR, Bohmann K, Gilbert MTP, Lisandra Ezpeda-Mendoza M, Razgour O, Jones G (2014) Second generation sequencing and morphological faecal analysis reveal unexpected foraging behaviour by Myotis nattereri (Chiroptera, Vespertilionidae) in winter. Front Zool 11(1):39
Kaňuch P, Janečková K, Krištín A (2005) Winter diet of the Noctule bat (Nyctalus noctula). Folia Zool 54(1–2):53–60
Karem A, Ksantitini M, Soenberger T, Waibel T (1993) Contribution à la régénération de la végétation dans les parcs nationaux en Tunisie aride. Ministère de l’Agriculture, Tunis
Korine C, Pinshow B (2004) Guild structure, foraging space use, and distribution in a community of insectivorous bats in the Negev Desert. J Zool (Lond) 262(2):187–196
Kowalski K, Rzebik-Kowalska B (1991) Mammals of Algeria. Ossolineum, Wrocław
Kunz TH, Hodkison R, Weise CD (2009) Methods of capturing and handling bats. In: Kunz TH, Parsons S (eds) Ecological and behavioral methods for the study of bats, 2nd edn. The Johns Hopkins University Press, Baltimore, pp 3–35
Lanza B (2012) Fauna d’Italia. Mammalia V. Chiroptera. Calderini, Milano
Lewis RE, Harrison DL (1962) Notes on bats from the Republic of Lebanon. Proc Zool Soc Lond 138(3):473–486
Lisón F, Calvo JF (2011) The significance of water infrastructures for the conservation of bats in a semiarid Mediterranean landscape. Anim Conserv 14(5):533–541
Lisón F, Calvo JF (2013) Ecological niche modelling of three pipistrelle bat species in semiarid Mediterranean landscape. Acta Oecol 47:68–73
Lisón F, Calvo JF (2014) Bat activity over small ponds in dry Mediterranean forests: implications for conservation. Acta Chiropt 16(1):95–101
Masson D, Sagot F (1985) Groupe de travail Chiroptères: synthèse des observations réalisées entre mars 1984 et février 1985. Lutreola 2:13–36
Maxinová E, Kipson M, Naďo L, Hradická P, Uhrin M (2016) Foraging strategy of Kuhl’s pipistrelle at the northern edge of the species distribution. Acta Chiropt 18(1):215–222
Miková E, Varcholová K, Boldogh S, Uhrin M (2013) Winter diet analysis in Rhinolophus euryale (Chiroptera). Cent Eur J Biol 8(9):848–853
Muñoz-Garcia A, Ben-Hamo M, Pinshow B, Williams JB, Korine C (2012) The relationship between cutaneous water loss and thermoregulatory state in the Kuhlʼs pipistrelle, Pipistrellus kuhlii, a widely distributed vespertillionid bat. Physiol Biochem Zool 85(5):516–525
Neuweiler G (2000) The biology of bats. Oxford University Press, New York
Norberg U, Rayner JMV (1987) Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Phil Trans R Soc Lond B Biol Sci 316(1179):335–427
Noumi Z (2010) Acacia tortilis subsp. raddiana en Tunisie pré-saharienne: structure du peuplement, réponses et effets biologiques et environnementaux. Thèse de doctorat, Université de Sfax-Université Bordeaux 1, Sfax-Bordeaux, Tunisia-France
Noumi Z, Ouled Dhaou S, Chaieb M (2010) Seed germination characteristics of Periploca angustifolia Labill. and Rhus tripartita (Ucria) Grande: effects of temperature, salinity and water stress. Acta Bot Gall 157(2):317–327
O’Farrell MJ, Gannon WL (1999) A comparison of acoustic versus capture techniques for the inventory of bats. J Mammal 80(1):24–30
Pocora I, Pocora V (2008) Abundance and habitat use of bat (Chiroptera) species in the Letea forest area (Danube delta). Sci Ann Danube Delta Inst 14:57–64
Polak T, Korine C, Yair S, Holderied MW (2011) Differential effects of artificial lighting on flight and foraging behaviour of two sympatric bat species in a desert. J Zool 285(1):21–27
Pottier T, Leugé F, Joly JM (1996) Reproduction de la Pipistrelle de Kuhl (Pipistrellus kuhli Natterer, 1819) en Normandie. Petit Lérot 52:9–11
Puechmaille S, Hizem WM, Allegrini B, Abiadh A (2012) Bat fauna of Tunisia: review of records and new records, morphometrics and echolocation data. Vespertilio 16:211–239
Qumsiyeh MB (1985) The bats of Egypt. Spec Publ Mus Texas Tech Univ 23:1–102
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rabe MJ, Rosenstock SS (2005) Influence of water size and type on bat captures in the lower Sonoran desert. West N Am Nat 65(1):87–90
Racey PA, Swift SM (1985) Feeding ecology of Pipistrellus pipistrellus (Chiroptera: Vespertilionidae) during pregnancy and lactation. 1. Foraging behaviour. J Anim Ecol 54(1):205–215
Rainho A (2007) Summer foraging habitats of bats in a Mediterranean region of the Iberian Peninsula. Acta Chiropt 9(1):171–181
Rakhmatulina IK (1983) Ekologija sredizemnomorskogo netopyrja (Vespertilio kuhlii Kuhl, 1819) v Azerbajdžane. Bjul Mosk Obšč Ispyt Prir. Otd Biol 88:33–42
Rakotomalala R (2005) TANAGRA: un logiciel gratuit pour l’enseignement et la recherche. ERIC, Université Lumière Lyon 2, Bron, France
Razgour O, Korine C, Saltz D (2010) Pond characteristics as determinants of species diversity and community composition in desert bats. Anim Conserv 13(5):505–513
Razgour O, Hanmer J, Jones G (2011) Does interspecific competition drive patterns of habitat use in desert bat communities? Oecologia 167(2):493–502
Rebelo H, Brito JC (2006) Bat guild structure and habitat use in the Sahara desert. J Afr Zool 45(2):228–230
Rudolph BU, Lichti H, Liegl C, Pichl S (2010) Verbreitung, Status und erste Erkenntnisse zum Verhalten und zur Ökologie der Weißrandfledermaus, Pipistrellus kuhlii (Kuhl, 1817), in Bayern. Nyctalus 15:195–212
Russ J (1999) The bats of Britain and Ireland: echolocation calls, sound analysis, and species identification. Alana Ecology Books, Belfast
Russo D, Jones G (1999) The social calls of Kuhl’s pipistrelles Pipistrellus kuhlii (Kuhl, 1819): structure and variation. J Zool (Lond) 249(4):476–481
Russo D, Jones G (2002) Identification of twenty-two bat species (Mammalia: Chiroptera) from Italy by analysis of time-expanded recordings of echolocation calls. J Zool (Lond) 258(1):91–103
Russo D, Jones G (2003) Use of foraging habitats by bats (Mammalia: Chiroptera) in a Mediterranean area determined by acoustic surveys: conservation implications. Ecography 26(2):197–209
Russo D, Ancillotto L, Korine C (2016) The buzz of drinking on the wing in echolocating bats. Ethology 122(3):226–235
Rydell J (1992) Exploitation of insects around streetlamps by bats in Sweden. Funct Ecol 6(6):744–750
Rydell J, Racey PA (1995) Street lamps and the feeding ecology of insectivorous bats. Symp Zool Soc Lond 67:291–307
Seibold S, Buchner J, Bässler C, Müller J (2013) Ponds in acidic mountains are more important for bats in providing drinking water than insect prey. J Zool (Lond) 290(4):302–308
Siegel S, Castellan NJ (1988) Nonparametric statistics for the behavioral sciences, 2nd edn. McGraw-Hill Book Company, New York
Simmons NB (2005) Order Chiroptera. In: Wilson DE, Reeder DM (eds) Mammal species of the world. A taxonomic and geographic reference, 3rd edn. The Johns Hopkins University Press, Baltimore, MD, pp 312–359
Strelkov PP, Unkurova VI, Medvedeva GA (1985) Novye dannye o netopyre Kulâ (Pipistrellus kuhlii) i dinamike ego areala v SSSR. Zool Zh 64:87–97
Touzot O (2014) Pipistrelle de Kuhl Pipistrellus kuhlii (Kuhl, 1817). In: Ruys T (coord) Atlas des Mammifères sauvages d’Aquitaine. Tome 4: Les Chiroptères. Cistude Nature, Le Haillan. pp 170–174
Tuttle SR, Chambers CL, Theimer TC (2006) Potential effects of livestock water-troughs on bats in northern Arizona. Wildl Soc Bull 34(3):602–608
Van Cakenberghe V, Benda P (2013a) Pipistrellus deserti Desert pipistrelle. In: Happold M, Happold DCD (eds) Mammals of Africa. Volume IV. Hedgehogs, shrews and bats. Bloomsbury, London, pp 619–621
Van Cakenberghe V, Benda P (2013b). Pipistrellus kuhlli Kuhl’s pipistrelle. In Happold M, Happold DCD. (eds) Mammals of Africa. Volume IV. Hedgehogs, shrews and bats. Bloomsbury, London, pp 633–635
Vaughan N, Jones G, Harris S (1997) Habitat use by bats (Chiroptera) assessed by means of a broad band acoustic method. J Appl Ecol 34(3):716–730
Venables W N, Ripley BD (2002) Modern applied statistics with S. Fourth edition. Springer, New-York
Vernier E (1989) Ecological observations on the evening flights of Pipistrellus kuhlii in the town of Padova. In: Hanák V, Horáček I, Gaisler J (eds) European bat research 1987. Charles University Press, Praha, pp 537–541
Vernier E (1995) Seasonal movements of Pipistrellus kuhli: 18 years of observation on a single colony in Padova (N.E. Italy). Myotis 32-33:209–214
Vernier E (1998) Attività invernale di pipistrelli nella città di Padova. Amb Ris Sal 59:43–44
Vernier E, Ruggieri A (1999) Presenza, coesistenza e vicarianza di due specie di Pipistrellus (Pipistrellus kuhlii e Pipistrellus pipistrellus) in volo di caccia sotto i lampioni stradali in Provincia di Piacenza. In: Dondini G, Papalini O, Vergari S (eds) Atti del I° Convegno Italiano sui Chirotteri. Castell'Azzara (Grosseto), 28–29 marzo 1998. Gruppo Speleo. L'Orso, Castell'Azzara, Italy, pp 229–239
Verschuren J (1949) L’activité et les déplacements hivernaux des Chiroptères en Belgique. Bull Inst R Sci Nat Belgique 25:1–7
Weber NA (1955) Notes on Iraq. Insectivora and Chiroptera. J Mammal 36(1):123–126
Yavrouyan EG (1989) Ecology of Pipistrellus pipistrellus and Pipistrellus kuhlii in Armenia and Nachitschevan. In: Voprosy biolorii. Vypousk 5. Nzdatipbstvo erevanskogo Ouniversiteta, Erevan, Armenia, pp 88–99
Zaafouri MS, Zouaghi M, Akrimi N, Jeder H (1997) La forêt steppe à Acacia tortilis subsp. raddiana var. raddiana de la Tunisie aride: dynamique et évolution. Rev Rég arides n.s. 258–271
Zahn A, Kriner E (2016) Winter foraging activity of central European Vespertilionid bats. Mammal Biol 81(1):40–45
Zava B, Violani C, Mannino G (1994) Bats of Sicilian islands. II–Ustica. Mammalia 58(2):261–268
Zsebők S, Estók P, Görföl T (2012) Acoustic discrimination of Pipistrellus kuhlii and Pipistrellus nathusii (Chiroptera: Vespertilionidae) and its application to assess changes in species distribution. Acta Zool Acad Sci Hung 58(2):199–209
Acknowledgments
The field work was facilitated by Lazher Hamdi, manager of the Bou Hedma National Park, and the forest rangers. This work would not have been possible without the inestimable help of Frédéric Leblanc for training RD and analysing some echolocation calls. Authors also thank Aiidi ben Ali Rajeb for valuable help in the field work in the sandy plain and around streetlamps, Ioseb Natradze and Lena Godlevska for translations, and anonymous reviewers for their comments which improved this manuscript. Bat conservation International and Eurobats funded the equipment used in this study.
Funding
Bat Conservation International and Eurobats funded the equipment used in this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 206 kb)
Rights and permissions
About this article
Cite this article
Dalhoumi, R., Morellet, N., Aissa, P. et al. Seasonal activity pattern and habitat use by the Kuhl’s pipistrelle (Pipistrellus kuhlii) in an arid environment. Eur J Wildl Res 64, 36 (2018). https://doi.org/10.1007/s10344-018-1193-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-018-1193-y