Abstract
In 2011, the invasive American mink Neovison vison became the most acute threat to the globally critically endangered Hooded Grebe Podiceps gallardoi (global population <800 individuals) when mink killed over 4 % of their global population. The Hooded Grebe is endemic to the Argentinean Austral Patagonia. In 2014, we established a control program in the Buenos Aires Lake Plateau area; the first attempt to systematically control mink in Patagonia. Our aim was to preserve the Hooded Grebes throughout the reproductive season by eradicating mink from the highland lakes and the rivers that mink use as corridors. We used a combination of methods (live trapping, lethal trapping and hunting) to maximize mink removal during the short climate window that permits work in the area. Control effort in the summer seasons of 2014 and 2015 involved 47–91 traps working for 128–137 days and we also hunted for mink along 186 km of river. No mink predation on grebes has been observed since the beginning of the control program and 71 mink were removed from the area. Percentage of sites occupied by mink decreased after the first control season (occupancy estimation decreased ca. 50 %). However, there was also a decline in the number of mink trapped, indicating that mink removal was more difficult in the second control season. We show that mink culling can be established successfully in an area with challenging logistics, avoiding negative impacts on non-target native species and providing positive outcomes for a species of global conservation importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Hooded Grebe (Podiceps gallardoi) is endemic to Santa Cruz Province, Argentina (Roesler 2015). The species has declined by 80 % in the last 25 years and there are now less than 400 reproductive pairs remaining; the species was therefore up-listed to Critically Endangered in 2012 (Birdlife International 2013). Conservation of the Hooded Grebe is now a priority for national NGOs, governmental agencies, and international conservation programs.
Between October and April, the Hooded Grebe is found at shallow, fishless lakes on highland plateaus, breeding on floating nests between December and January. During April, the grebe migrates to estuaries on the Atlantic coast to overwinter (Roesler et al. 2012b). Fifty percent of the Hooded Grebe population breed at Buenos Aires Lake Plateau (Roesler et al. 2012b). Several threats to the species have been identified: drought, increases in wind speed (over 80 km/h) and gust frequency due to global climate change, predation of eggs and chicks by Kelp gulls (Larus dominicanus), and modification of the limnic food chains by introduced salmonids (Roesler et al. 2012a; Izaguirre and Saad 2014; Lancelotti et al. 2016).
American mink (Neovison vison) have recently become the most acute threat for juveniles and adults (Roesler et al. 2012a). In 2011, 33 Hooded Grebes’ nests were destroyed and incubating adults killed by an American mink (Roesler et al. 2012a). A single event with devastating effect on the species, with less than 800 grebes left and the 4 % of its population and their nests gone in 2 days. American mink is a well-known predator of other grebe species in its native range, where grebes have adaptive strategies to prevent its predation (e.g., night egg neglect and prolonged incubation; Nuechterlein and Buitron 2002). Despite their strategies, they cannot avoid the effects of this predator (Breault and Cheng 1988). The Hooded Grebe’s only known native predators are birds (falcons and harriers) (Beltrán et al. 1992), thus the species is naïve to amphibious mammalian predators. Also, surplus killings (Kruuk 1972) are common in mink (Macdonald and Harrington 2003). This behavior is more frequent in communities where the predator and prey have not evolved together, and such events can have rapid adverse effects on prey populations (Short et al. 2002). The 2011 event caused a 4 % reduction of the global Hooded Grebe population. Two other surplus killing events in 2012 in two different lakes produced an extra loss of almost 50 individuals overnight, including adults, chicks, and juveniles (Roesler unpublished data). Given the current numbers of grebes, repeat in surplus killing events can drive this species to extinction.
The American mink is a generalist predator native to North America. It feeds on mammals, birds, fish, and crustaceans by hunting on land, climbing on trees, swimming, or diving and the relative importance of food items on its diet is dependent on prey availability (Macdonald and Harrington 2003). The American mink was first introduced to Argentina between 1930 and 1940 for fur farming and in recent decades several studies have confirmed the presence of wild populations in most of the Patagonian region (Fasola et al. 2011; Valenzuela et al. 2014). American mink were first reported in the area of the Buenos Aires Lake Plateau (BALP) in 2011 (Fasola et al. 2011). The expansion of the mink population has raised concern about possible negative impacts on native species (Aued et al. 2003; Ibarra et al. 2009; Schüttler et al. 2009; Peris et al. 2009) especially given evidence that the mink has negative impacts on local biodiversity in Europe (Bonesi and Palazon 2007).
In this work, we evaluate the efficiency of a new control program at reducing mink population and improving Hooded Grebe reproductive success. The control program started in 2014 at Buenos Aires Lake Plateau, an area that has held the most persistent Hooded Grebe numbers since the 1980s and remains an important breeding site for the species.
Methodology
Area under control
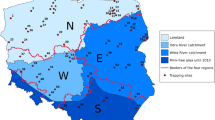
The area selected for the control program (ca. 3000 km2) encompasses the Buenos Aires Lake Plateau (hereafter BALP) and surroundings (Fig. 1). This is one of the biggest and highest plateaus of Santa Cruz Province, ranging from ca. 900 m in the east up to 1600 m in the west, with scattered higher peaks (2700 m, Zeballos ridge). The habitat on the plateau is part of the High Andes District (Cabrera 1971) and it is an extremely dry steppe. On the plateau, there are over 270 lakes but only 30 of them have been occupied by Hooded Grebes (HG from now onwards). Nine lakes are known to be breeding sites but only El Cervecero Lake has been a breeding site every season since 2011, for 30 to 50 pairs of HG (Roesler et al. 2012b). Therefore, most of the control program focused on avoiding mink in this lake. El Cervecero is a 12-ha crystal-clear water lake, c. 40 % covered by water-milfoil Myriophyllum quitense, and with a perimeter surrounded by a 10-m tall cliff. It is located on the southern half of the BALP and it is 900 m away from the Ecker River, which runs from west to east along the south edge of the plateau before descending and approaches other lakes that occasionally support a HG colony (e.g., Don Ferret, only 400 m away). The Ecker river is the most important of the 11 watercourses that act as corridors for mink. Six of these watercourses were chosen for mink control efforts based on their proximity to other important lakes for the Grebe (Fig. 1).
Most of the permanent watercourses in the BALP are predominantly shallow streams ca. 1–3 m wide, originating from springs, but there are some wider rivers (Ecker–Pinturas, El Correntoso, Columna, and Los Antiguos). Most watercourses run along deep valleys, surrounded by rocky cliffs or rocky areas, thus providing high-quality shelter for mink. Thus, all these rivers and streams are like oases crossing a desert. This “area under control” was delimited on the basis of logistical opportunities to access the different sites by an imaginary “ring” formed by the routes surrounding around the plateau (Fig. 1).
Mink removal methods
We selected objectives and methodologies that were appropriate for the extreme weather conditions and the remoteness of Patagonian plateaus. Mink are found at low elevations throughout the year but are not present in the highlands until the late summer when the young males disperse (Fasola and Roesler unpublished data). Therefore, the first objective was the complete removal of American mink from the upper parts of the area under control and the second to reduce mink numbers in the lower parts in order to keep dispersing juveniles near the source.
We used a combination of passive and active methodologies to maximize the impact of limited resources (traps, vehicles and trap operators) and to improve time efficiency during the seasons when access to the sites was possible (during winter, access to the mid- and upper parts is not viable). We applied both passive and active methods during two control seasons: 2014 (October 2013–April 15, 2014) and 2015 (December 2014–April 30, 2015).
For the passive methodology, we used live and lethal traps. Live traps demand daily examination and posterior humane disposal of trapped individuals or release of non-target species (see below). We live trapped only at the lakes with HG colonies that are constantly monitored (Roesler unpublished data). Live trapping was conducted using wire mesh single door cage traps (15 × 15 × 60 cm) set 200 m apart (Yamaguchi and Macdonald 2003) (Fig. 2a) around El Cervecero Lake (six traps—2014, 2015) and Don Ferret (four traps—2014). Bait (meat or fish) was replaced every 3 days and no attractant was supplemented in the lakes. Live traps were set as soon as HG started a colony (between 15 and 30 December) and removed in April (between 10 and 15).
Lethal traps were set on rivers and streams on areas where daily access was not possible. By-catch of small and medium-sized native terrestrial mammals (i.e., South American Gray Fox Pseudalopex griseus, Patagonian Hog-nosed Skunk Conepatus humboldtii, Patagonian Weasel Lyncodon patagonicus, and the Lesser Grisson Galictis cuja) was avoided by placing the traps on wooden and polystyrene floating rafts. The rafts were 80 × 50 × 7 cm supporting a wooden box 17 × 17 × 45 cm with an open entrance (guillotine closure with a 7 cm diameter open circle), facing downstream and anchored to the riverbank (Fig. 2b) and equipped with a conibear trap (110 model, single spring). The system was an adaptation from Reynolds et al. (2004) and Davis et al. (2011). These traps were baited in the same way as the live traps but we also added an attractant (scent from female mink anal glands, collected in a fur farm, diluted with liquid petroleum jelly).
Lethal traps were set along 50 km of the Ecker River both years, in a systematic approach using floating rafts spaced by 200–2000 m. We placed traps at other four rivers (Los Antiguos; Pinturas; Telken and Columna) in both years, and at two rivers (Page Chico, Correntoso) only in 2014 (these were not revisited in 2015 due to long distances and excessive time consumption). Experience gained in the first season let us increase the total number of operating traps in 2015 (∼93 % increase) by shortening distance between traps at most of the trapping accesses while the capture coverage along the rivers held.
For the active methodology we used spotlights and firearms (night patrols). We tested this methodology towards the end of the 2013 season, following the detection of mink in two lakes (El Cervecero and C199 at La Siberia Plateau, 250 km to the south of BALP) and we found it effective for detecting and removing mink in a localized area. The methodology consisted of periodical patrolling (every 2–4 nights) until mink were detected, when the frequency of patrols was increased to every night until the animal was killed. The patrols in 2014 and 2015 were conducted around El Cervecero Lake and ran all through the season. To increase early detection of mink, riversides close to lakes were frequently patrolled (twice a week) at mink dispersal period. Additionally, we patrolled stretches at sites where we opportunistically detected mink by means of fresh signs or camera traps.
Caught animals were sexed and aged whenever possible depending on the rate of decay of the specimen.
Measuring effectiveness
We used two measures of the effectiveness of the efforts applied with lethal trapping. For the higher elevations (above 900 m), we compared the sum of animals culled above this elevation (on corridor rivers and lakes) between seasons. Below 900-m altitude—the source of mink-, we estimated occupancy and detectability from trapping data and compared the percentage of occupied trapping points at the beginning of each season, accounting for the probability of detection (MacKenzie et al. 2006). In order to run the models for 2014 and 2015, we built the detection history for both years by splitting the ca. 140 days of trapping of both years into periods of ten days. As we wanted to assess occupation at the beginning of the trapping season, we only considered the first capture per trap and conducted removal sampling design (Mackenzie and Royle 2005). No covariates were included, as we wanted to obtain an estimate of initial occupancy for each trapping season. Cumulative detectability was calculated following Mackenzie and Royle (2005). Models were run using the Unmarked package for R (Fiske and Chandler 2011).
People involved
Different people were designated to the activities of checking traps and patrolling. Only people in charge of daily monitoring of Hooded Grebe colonies were involved simultaneously in both patrolling and live traps checking. People in charge of each activity were replaced along the season (∼15 days) to avoid boredom and keep them enthusiastic about fieldwork.
Results
No mink predation was recorded in the lakes with HG colonies in the area for the reproductive seasons in 2014–2015. Together, the active and passive trapping methods covered ca. 104 km of waterline in 2014 and ca. 89 km in 2015. Since 2014, we removed 71 mink from the area. 67 % of the animals could be effectively sexed and we obtained a rate of females to males of 0.83:1 and 0.15:1 for 2014 and 2015 respectively. All 5 mink hunted or trapped above 900 m were young males. The total effort applied to achieve this result is summarized in Table 1 and spatial allocation of resources is shown in Fig. 1.
We did not record trapped animals on the live traps set on the lakes. Total live trapping effort in lakes was lower the second season as the Hooded Grebe only nested in El Cervecero Lake in 2015.
Overall, 94.3 % of trapped mink were caught in lethal traps set on floating rafts. Every trap was checked between 14 and 20 times along the season. The number of animals caught dropped ca. 76 % from the first year. Also, for the second year, the number of females captured dropped by 70 %. The two rivers that were excluded the second year, recorded only 2 captures in 2014 (less than the 3 % of total captures recorded).
Patrolling exercises proved successful at the lakes. Two mink were hunted by patrols at El Cervecero Lake in 2014 (2 and 17 days after the mink was detected), and four by landowners who became informally involved in our efforts to control the mink. Along the Ecker River, mink were detected based on signs and camera traps (one in 2014 and two in 2015) in three sites that were then patrolled for two consecutive nights for 2 km around the detection point with no positive results.
Occupancy estimations were improved when corrected for detection probability (Table 2). The model based on the detection history until the first captures (Table 2) revealed that both the occupation as well as the detectability decreased from 2014 to 2015. Thus, the proportion of sites occupied in the second year was half of the initial situation, and the time lapse until first capture (detection by traps) was longer, as we reached the 50 % of probability of capture at the fifth session in 2014 and the ninth in 2015 (Fig. 3). The estimated mink densities based on removed animals were one individual every 2.5 km and 3.1 km for 2014 and 2015, respectively.
Total people involved in the mink control activities were lower than in experiences elsewhere. Two people were checking live traps and patrolling at nights at lakes. A group of two to three people was in charge of checking lethal traps on rivers and only occasionally two groups were operating the same day. Two people conducted opportunistic patrols along rivers typically.
Discussion
The present work describes the rationale and the results of the first 2 years of control in Austral Patagonia to stop American mink predation at one of the main breeding sites of the globally Critically Endangered Hooded Grebe and to mitigate its effect on the whole population. This is the first program to control American mink in Argentina and Patagonia (Chile and Argentina) with a tangible conservation purpose and it is among the few attempts at controlling this invader in a mainland area (ca. 3000 km2) naturally open to immigration from nearby sources (Northern Spain, 174 km2 Zuberogoitia et al. 2010; Northeastern Scotland, 5500 km2 Bryce et al. 2011; western England, 420 km2 Reynolds et al. 2013).
This control program relies on a combination of removal methodologies to address local peculiarities, rather than using a single methodology as deemed appropriate elsewhere. Aquatic habitat types, long distances between trapping sites, climatic constraints, the native carnivore community, and more importantly, the sensitivity of the focal endangered species demanded a different management strategy to remove minks in our study area. By planning a combination of methodologies and applying them adaptively (e.g., increasing trap numbers in later years), we could eliminate predation effects in two consecutive seasons. The analysis of the first two years suggests that this positive result can be maintained and that lessons learned one season help planning means of reducing efforts for the next one.
As climate conditions between May and November make it difficult to access to trap sites, we concentrated actions during late spring, summer and early autumn, which coincide with HG’s reproductive season. One disadvantage of this approach is that we are not trapping during the mink mating season, which is when activity of the species peaks (Yamaguchi and Macdonald 2003; Harrington et al. 2009). Instead, we extended the trapping period in order to overlap as much as possible with the dispersal period, which is the mink’s second activity peak.
The use of lethal traps along rivers allowed checking schedules of 5 to 10 days instead of daily schedules. Use of floating rafts, as platforms for the traps, effectively avoided by-catch of other native small and medium-sized carnivores throughout the period. On three occasions in 2015 we captured Patagonian Hog-nosed Skunks in the live traps at El Cervecero Lake, and they were released in less than 24 h in good condition. At lakes, permanent detection effort guaranteed absence of mink near colonies, an action supported by live traps along the shore. This was fundamental as mink surplus killing behavior has an impact on grebes that is independent from mink numbers (Roesler et al. 2012a).
The number of animals trapped above 900 m was reduced in 2015, which is important to maintain an absence of dispersing minks from grebe colonies. This could be the result of the new trap arrangement in 2015 and also the decrease of the occupation of the lower areas achieved after the 2014 season. Occupancy estimation in the lower parts (source of animals) was lower in 2015. Also, detection with traps decreased from one year to the second; this was an expected outcome as detection probability depends on abundance (Mackenzie 2005) and trapping efficiency is known to decay with increase of percentage of culled animals (Zabala et al. 2010; Bryce et al. 2011). All this supports the conclusion that 2014 efforts were successful in reducing occupancy of mink, as estimated the second year.
There was a reduction in the proportion of females in the animals extracted in 2015 compared to 2014. We assume that after the first year of mink removal, differential dispersal capability between sexes (males move more than females) explains the higher proportion of males among the immigrants from areas downstream of the controlled area. The same pattern was found in Scotland, where the sex ratio favored males with increasing altitude (Bryce et al. 2011). Additional local evidence supports this pattern, as all animals (n = 5) removed from 900 m were young males that reached this altitude after February (late Austral Summer) when young animals (3–4 months old) are expected to disperse following independence from the mother.
A positive impact on the success of Hooded Grebe colonies
The American mink surplus killings recorded on El Cervecero Lake destroyed 4 % and 2 % of the global population of Hooded Grebe. Since the last incident in 2013, when a single mink killed 17 adults and three juveniles during the breeding season (Roesler unpublished data), no depredation of HG by mink has been recorded. The permanent presence of the staff at lakes and patrols around the lake seem to prevent the use of the lake by mink as source of food. These results show an absolute improvement on the situation at the BALP. In addition, a parallel experience at a distant lake (C199) located in La Siberia Plateau (ca. 250 km south from the controlled area) supports the strategy. There, a single young male mink attacked ca. 3 % of the global population of the Hooded Grebe in March 2013 but the permanent presence of staff on the following seasons prevented further attacks.
Next phase
For the seasons to come, we plan to continue adapting this control plan, based on experience. Captures should continue moving downstream (Bryce et al. 2011), so control effort can be focalized in areas of ease and permanent access. More importantly, we seek to increase capture of females as a strategy to keep the source of young, highly mobile individuals progressively farther from the sensitive spots. We expect the number of individuals trapped to continue decreasing in consecutive years with the increasing number of removed mink. By modeling detection probability we estimate that, after 14 trapping periods of 10 days, our detectability was close to 90 % and in the second year this estimate dropped to less than 70 % by the end of the season. Use of sniffer dogs is planned to support detection activities in order to improve trap allocation when mink density becomes lower or for situations where mink are naturally hard to detect (Roy 2011), especially for detecting females at the beginning of the season when their mobility is reduced at late pregnancy or early rearing period.
By working on mink control at low elevations, the source of dispersing mink, we will support the conservation of the Hooded Grebe; some lakes on the lowlands could be used by the species as obligated stopovers while waiting for favorable conditions to ascend to the lakes at higher elevations. Also, other species associated with aquatic habitats also sensitive to mink impact will benefit from predator control, such as the threatened Austral rail (Rallus antarcticus) (Mazar Barnett et al. 2014).
While efforts continue in Buenos Aires Lake Plateau, plans have started to control mink and prevent further predation of grebes at other three plateaus.
References
Aued MB, Chéhebar C, Porro G et al (2003) Environmental correlates of the distribution of southern river otters Lontra provocax at different ecological scales. Oryx 37:413–421
Beltrán J, Bertonatti C, Johnson A et al (1992) Actualizaciones sobre la distribucion, biologia y estado de conservacióon del maca tobiano (Podiceps gallardoi). Hornero 13:193–199
BirdLife International. 2013. Podiceps gallardoi. The IUCN Red List of Threatened Species 2013:e.T22696628A48032621. doi:10.2305/IUCN.UK.2013-2.RLTS.T22696628A48032621.en. Downloaded on 15 October 2015
Bonesi L, Palazon S (2007) The American mink in Europe: status, impacts, and control. Biol Conserv 134:470–483
Breault AM, Cheng KM (1988) Surplus killing of Eared Grebes, Podiceps nigricollis, by mink, Mustela vison, in Central British Columbia. Can Field Nat 102:738–739
Bryce R, Oliver MK, Davies L et al (2011) Turning back the tide of American mink invasion at an unprecedented scale through community participation and adaptive management. Biol Conserv 144:575–583
Cabrera AL (1971) Fitogeografía de la República Argentina. B Soc Argent Bot 14:1–42
Davis EF, Anderson CB, Valenzuela AEJ et al (2011) American mink (Neovison vison) trapping in the Cape Horn Biosphere Reserve: enhancing current trap systems to control and invasive predator. Ann Zool Fenn 49:18–22
Fasola L, Muzio J, Chehébar C et al (2011) Range expansion and prey use of American mink in Argentinean Patagonia: dilemmas for conservation. Eur J Wildl Res 57:283–294
Fiske I, Chandler R (2011) Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23
Harrington LA, Harrington AL, Moorhouse T et al (2009) American mink control on inland rivers in southern England: an experimental test of a model strategy. Biol Conserv 142:839–849
Ibarra JT, Fasola L, MacDonald DW et al (2009) Invasive American mink Mustela vison in wetlands of the Cape Horn Biosphere Reserve, southern Chile: what are they eating? Oryx 43:87–90
Izaguirre I, Saad JF (2014) Phytoplankton from natural water bodies of the Patagonian Plateau. Adv Limnol 65:309–319
Kruuk H (1972) Surplus killing by carnivores. J Zool 166:233–244
Lancelotti JL, Marinone MC, Roesler I (2016) Rainbow trout effects on zooplankton in the reproductive area of the critically endangered hooded grebe. Aquat Conserv Mar Freshw Ecosyst. doi:10.1002/aqc.2629
MacDonald DW, Harrington LA (2003) The American mink : the triumph and tragedy of adaptation out of context. New Zeal J Zool 30:421–441
Mackenzie DI (2005) What are the issues with presence—absence data for wildlife managers ? special section : the value and utility of presence – absence data to wildlife monitoring and research. J Wild Manage 69:849–860
Mackenzie DI, Royle JA (2005) Designing occupancy studies : general advice and allocating survey effort. J App Ecol 42:1105–1114
Mackenzie DI, Nichols JD, Royle JA et al (2006) Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. Elsevier, London
Mazar Barnett J, Imberti S, Roesler I (2014) Distribution and habitat use of the Austral Rail (Rallus antarcticus) and perspectives on its conservation. Bird Conserv Int 24:114–125
Nuechterlein GL, Buitron D (2002) Nocturnal egg neglect and prolonged incubation in the red-necked grebe. Waterbirds 25:485–491
Peris SJ, Sanguinetti J, Pescador M (2009) Have Patagonian waterfowl been affected by the introduction of the American mink Mustela vison? Oryx 43:648–654
Reynolds JC, Short MJ, Leigh RJ (2004) Development of population control strategies for mink Mustela vison, using floating rafts as monitors and trap sites. Biol Conserv 120:533–543
Reynolds JC, Richardson SM, Rodgers BJE, Rodgers ORK (2013) Effective control of non-native American mink by strategic trapping in a river catchment in mainland Britain. J Wild Manage 77:545–554
Roesler I (2015) The status of hooded grebe (Podiceps gallardoi) in Chile. Ornitol Neotrop 26:255–263
Roesler I, Imberti S, Casañas H, Volpe N (2012a) A new threat for the globally Endangered Hooded Grebe Podiceps gallardoi: the American mink Neovison vison. Bird Conserv Int. 22:383-388
Roesler I, Imberti S, Casañas H et al. (2012b) Hooded Grebe Podiceps gallardoi population decreased by eighty percent in the last twenty-five years. Bird Conserv Int 22:371–382.
Roy S (2011) Strategies to improve landscape scale management of mink populations in the west coast of Scotland: lessons learned from the Uists 2001–2006. In: Veitch CR, Clout MN, Towns DR (eds) Island invasives: eradication and management. IUCN, Gland Switzerland, pp 114–117
Schüttler E, Klenke R, Mcgehee S et al (2009) Vulnerability of ground-nesting waterbirds to predation by invasive American mink in the Cape Horn Biosphere Reserve, Chile. Biol Conserv 142:1450–1460
Short J, Kinnear JE, Robley A (2002) Surplus killing by introduced predators in Australia—evidence for ineffective anti-predator adaptations in native prey species? Biol Conserv 103:283–301
Valenzuela AEJ, Anderson CB, Fasola L, Cabello JL (2014) Linking invasive exotic vertebrates and their ecosystem impacts in Tierra del Fuego to test theory and determine action. Acta Oecol 54:110–118
Yamaguchi N, MacDonald DW (2003) The burden of Co-occupancy: intraspecific resource competition and spacing patterns in American Mink, Mustela Vison. J Mammal 84:1341–1355
Zabala J, Zuberogoitia I, González-Oreja JA (2010) Estimating costs and outcomes of invasive American mink (Neovison vison) management in continental areas: a framework for evidence based control and eradication. Biol Invasions 12:2999–3012
Zuberogoitia I, González-Oreja JA, Zabala J, Rodríguez-Refojos C (2010) Assessing the control/eradication of an invasive species, the American mink, based on field data; how much would it cost? Biodivers Conserv 19:1455–1469
Acknowledgments
We thank the staff and volunteers of the Hooded Grebe Project involved in the intense fieldwork (H. Casañas, P. Hernández, S. Imberti, M. Figuera Tomas, M. I. Pereda, D. Friedrich, M. Mc Namara, L. Rosset, A. De Miguel, C. Ferreyra, J. Klavins, L. Pagano, P. Buchanan, P. Irazoqui, E. Tiberi, J.S. Verón, among others). National Parks Administration (J. Cuhna and J. Zottola), Flora y Fauna Foundation (P. Díaz and G. Vittone), and Consejo Agrario Provincial of Santa Cruz (C. Ruiz, Los Antiguos) also supported fieldwork. Dr. L and H. Harrington revised and discussed the original control plan and Sabin Foundation supported their visit to the area. We thank Dr. M. L. Guichón and an anonymous reviewer for valuable comments on the manuscript. C. Grey and R. Gums (EDGE ZSL) reviewed the English. For logistical assistance and allowance to access trapping sites, we thank Sres. Pedro, Juan and Norma Garitaonandia from “Estancia La Vizcaína” and “Estancia San Carlos,” Ing. María Rosa Couto and sons from “Estancia El Rincón,” Sr. Lazcano from “Estancia Laurak Bat,” Dr. Puricelli from “Estancia La Paloma,” Sr. M. García from “Estancia El Unco” and Sr. Cesar Castillo from “Estancia La Lucha,” Flia. Czetanovic from “Estancia El Correntoso,” Sr. Ericksen from “Estancia Manantiales,” Sr. Santiago from “Estancia La Madrugada,” and Sr. Sayhueque from “Estancia La Buitrera.” We thank Idea Wild for donation of equipment (camera traps). Sr. Madsen provided source of mink attractant. Main Sources of financial support: ICFC Canada, PanAmerican Energy, Toyota Argentina, CREOI, Rufford Small Grants. The Hooded Grebe Project belongs to Aves Argentinas (Buenos Aires—Argentina) and Asociación Ambiente Sur (Santa Cruz—Argentina). This is the scientific publication #8 of the Proyecto Macá Tobiano.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fasola, L., Roesler, I. Invasive predator control program in Austral Patagonia for endangered bird conservation. Eur J Wildl Res 62, 601–608 (2016). https://doi.org/10.1007/s10344-016-1032-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-016-1032-y