Abstract
Understanding how non-invasive indicators of diet quality, such as faecal nitrogen (FN), relate to population performance is a valuable asset when assessing the management of wild ungulates. However, the relationships between FN and protein intake may depend on the ingestion of less palatable plants containing high quantities of tannin (which is known to reduce degradable proteins) and management schemes (such as supplemental feeding). In order to describe the factors and potential use of FN as an indicator of diet quality, we selected a range of red deer (Cervus elaphus) densities in Mediterranean habitats in South Central Spain, which were classified under different management regimens (with and without food supply). We specifically aimed to (i) identify the main population (management, density) and diet quality factors related to FN levels, (ii) assess the relationship between individual performance (body condition measured as kidney fat index, KFI) and FN and (iii) provide evidence of the modulating role that management may play in this relationship. FN was positively associated with the faecal tannin concentration and the density of deer. Interestingly, a positive correlation was observed between FN and nitrogen in plants on hunting estates with a supplemental food supply, whereas this relationship was not evident in the absence of supplemental feeding. Moreover, KFI was negatively associated with FN, and this negative association was more marked in the presence of supplemental feeding. This study exemplifies that caution is necessary when the interpretation of individual FN values is used to monitor the performance of red deer populations, particularly in high density contexts with a reduced quality of forage, and when supplemental food is provided. However, when FN is interpreted in concomitance with body condition, population parameters and diet quality, it has been proved to be a potentially simple, cost-effective and reliable indicator of biological performance and diet quality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When measuring physical performance in wild ungulates, it is necessary to consider the individuals’ body condition and health, along with estimates of population density and environmental conditions (Côté et al. 2004; Acevedo et al. 2008; Putman et al. 2011), since body condition and health interact with population density via the habitat-related factors of diet and nutrient intake (Santos et al. 2013).
Protein is a limiting nutrient for wild herbivores, and its availability in plants signifies that nitrogen intake is a potential indicator of diet quality (Putman 1984). Since protein content and digestibility are positively correlated in plants (Robbins 1983), faecal nitrogen (FN) is therefore a common index that is correlated with the intake of proteins and dietary digestibility (Leslie and Starkey 1985; Hodgman et al. 1996). FN has been widely used as an index of dietary quality in the nutritional ecology of free-ranging and captive ruminants (Hamel et al. 2009; Leslie et al. 2008). The relationship between proteins in diet and FN is, however, controversial. Tannins can bind to proteins during chewing and digestive processes (Mokoboki et al. 2011; Verheyden et al. 2011) and are then excreted in the faeces as tanning-protein complexes, thus leading to a decrease in protein digestibility and an increase in FN values (Mould and Robbins 1981; Robbins et al. 1987). What is more, some cervids have tannin-binding salivary proteins (Shimada 2006). It is therefore difficult to interpret the relationships between FN levels and diet quality.
Wild ungulates’ body conditions depend on the quality of their food (Santos et al. 2013). Whether and how diet quality indicators relate to individual performance (in terms of body condition), and which factors determine this relationship are key elements as regards understanding the practical use of FN. Body condition is affected by habitat quality (Taillon et al. 2011), population dynamics (Morellet et al. 2007) and extra food provided by wildlife managers (Santos et al. 2013). In the Iberian red deer (Cervus elaphus hispanicus) populations in Spain, high densities are favoured for hunting purposes, and this may impact on the conservation status of vegetation cover (Acevedo et al. 2008). Indeed, the use of supplementary feeding on some estates improves individual (trophy size and body condition) and population characteristics (density) (Vicente et al. 2007; Rodríguez-Hidalgo et al. 2010). Since supplemental feeding provides high-quality food in terms of nutritional content and palatability, it may have the potential to modulate the relationship between FN, pasture quality and body condition. The kidney fat index (KFI) has often been used to measure the physical condition of red deer and is widely used with ungulates (Dauphine 1975; Santos et al. 2013). This method is based on the assumption that the amount of perirenal fat is a reliable indicator of the total body fat, and thus of the body’s physical condition (Finger et al. 1981).
Nevertheless, there is little information that can be used to interpret individual and population nutritional indicators (such as FN and nitrogen in the diet) in wild ungulates, particularly in high density conditions and/or when supplemental feeding is provided. In order to describe the factors and potential use of FN as an indicator of diet quality, we selected a range of red deer (Cervus elaphus) densities in Mediterranean habitats in South Central Spain, which were classified under different management regimens (with and without food supply). We specifically aimed to (i) identify the main population (management, density) and diet quality factors related to FN levels, (ii) assess the relationship between individual performance (body condition measured as KFI) and FN and (iii) provide evidence of the modulating role that management may play in this relationship.
Material and methods
Study area
Data were collected from nine different hunting estates located in southern Spain in the province of Cordoba, in which the altitude ranges from 400 to 800 m a.s.l. The dominant vegetation includes tree species such as holm oak (Quercus ilex) and cork oak (Quercus suber), together with pine (Pinus pinea and Pinus pinaster), accompanied by scrub species dominated by Cystus spp., Erica spp., Pistacia spp., Phyllirea spp. and Rosmarinus officinalis with scattered pastures and small areas of crops. The study sites are principally used for the recreational hunting of wild boar and red deer.
Red deer density estimation
Deer abundance was estimated per hunting estate, and the estates were considered to be discrete management units. We performed two spotlight counts in August and September 2011, driving at 10–15 km/h. Each transect studied was, on average, 20.3 ± 2.34 km (S.E.) in length. The abundance of the deer populations was estimated by means of distance sampling (Buckland et al. 2004, Distance 5.0 software). Half-normal, uniform and hazard rate models for the detection function were fitted against the data using cosine, hermite polynomial and simple polynomial adjustment terms, which were fitted sequentially. The selection of the best model and adjustment term was based on Akaike’s information criterion (AIC). The best relative fit of the model and adjustment term for distance-sampling was the hazard rate cosine based on the lowest AIC score.
Sampling and analytical techniques
We used FN from fresh (soft, shiny and not dried) deer faeces collected from the ground. We performed two transects of 4 km in length per estate (n = 18) during the spring, from which deer droppings (10 stools/sample) were collected every 500 m (n = 8 per estate) in order to ensure that the samples were spatially independent. In the same transects, pasture samples (the main food resource during this season for Iberian red deer) (Verheyden-Tixier et al. 2008) were collected every 500 m within a randomly located ring of 0.5 m2, where the plants were cut for subsequent analysis (see below). A total of 144 samples of faeces and plants were obtained.
The concentrations of FN and nitrogen in plants were analysed using the EUROVECTOR EA 3000 elementary analyser, which determines the quantitative carbon, nitrogen, hydrogen and sulphur content of the samples. We thus obtained the percentage content of each element of the sample, which was in this case nitrogen-analysed with regard to weight (Acevedo et al. 2011).
Fifty-four red deer faecal samples (6 per estate) were randomly selected and analysed to obtain their concentration of tannin. Total extractable phenols (TEPH) were determined using Julkunen-Tiitto (1985).
Habitat structure and composition
Habitat composition per estate was determined by using GIS and land use 1/25,000 maps of Andalusia. We stratified 10 transects per estate, and the main habitats present were grouped into open land (composed of dehesa (savannah oak) and pastures) and woodland, composed of Mediterranean scrubland and forests. The transects were 50 m in length and were studied in May (maximum spring production, San Miguel et al. 1996) 2012 to estimate scrubland, woodland and pasture cover and the percentage of bare soil. A ruler was used to record the maximum height of the grass in each transect as an indirect measure of the availability of pasture (Lazo et al. 1992). We also calculated the percentage of cover occupied by herbaceous legumes [area occupied by legumes/area occupied by all herbaceous matter * 100] (Ritchie et al. 1998) in each transect.
Morphometric measurement
We collected the data regarding 103 hunted male deer in December 2011 and January 2012. The animals were assigned to three age classes: yearlings, sub-adults (<3 years old) and adults (≥4 years old) (Rodríguez-Hidalgo et al. 2010).
Nutritional status was determined using the KFI. This was obtained by weighing the perirenal fat which was then expressed as a percentage of the weight of that kidney [KFI = fresh weight of the perirenal fat/fresh weight of the kidney * 100] (Dauphine 1975; Riney 1955).
Statistical analyses
In order to provide evidence of the relationships between FN (N = 144) and forage quality, we designed a linear mixed model (LMM, normal error distribution and an identity link function, model 1) in which management (two levels: feeding supplement vs no feeding supplement) was included as a fixed factor. Plant nitrogen, red deer density, tannin content and the percentage of leguminosae, scrubland and woodland were included as co-variables. Since increased protein intake resulting from supplemental feeding could interfere in the relationships between FN and diet quality, the interactions between supplemental feeding and nitrogen in plants and tannins were also included in the model.
A second linear mixed model (normal distribution with an identity link function, model 2) was created to study the relationship between KFI (N = 103) (dependent variable) and FN. FN was included as a co-variable, whereas supplementary feeding and age class were treated as categorical variables with 2 and 3 levels, respectively. The interactions with supplemental feeding were also added. The population sampled was included as a random factor in both models. The assumptions of normality, homogeneity of variance and independence of residuals were confirmed in both models (Zuur et al. 2009), and the KFI was therefore log-transformed in order to fulfil normality.
Results
Red deer densities ranged from 25 to 68 deer/100 ha (average ± SD 39 ± 14), while the coefficients of variation of distance-sampling estimates ranged between 4.55 and 30.54 %. The FN values ranged from 1.7 to 3.4 % (average ± SD 2.47 ± 0.30), the nitrogen in plants ranged from 0.8 to 4.5 % (average ± SD 1.81 ± 0.53) and the concentration of faecal tannins ranged from 6.7 to 26.8 mg tannic acid/g sample (average ± SD 15.8 ± 4.1) (Table 1).
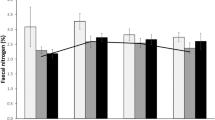
With regard to the factors affecting FN, model 1 showed a significant positive relationship between red deer density and tannin content in faeces (model 1, Table 2). There was also a significant interaction between supplemental feeding and nitrogen in plants, signifying that the FN increased in proportion with the amount of nitrogen in plants in the presence of supplemental feeding. No evidence of this relationship was found when the deer were not provided with supplementary food (Fig. 1). There was a negative relationship between FN and the percentage of scrubland and woodland.
With regard to model 2, which concerns individual KFI (Table 2), the interaction between supplementary feeding and FN was statistically significant (P = 0.02), showing that the negative association between FN and KFI was more marked when food was supplied (Fig. 2).
Discussion
This study has assessed FN as potential non-invasive indicator of diet quality and its association with individual performance traits (body condition) in contexts of high ungulate density. Interestingly, the relationships between FN and the availability of food and individual body condition were modulated by prevalent management (supplemental feeding).
The highest FN values were found in those areas with the highest red deer densities and tannin concentrations in faeces. At high densities, there may be fewer palatable scrub species as a consequence of over-browsing, thus favouring the growth of species that are unpalatable for herbivores (Acevedo et al. 2008; Suzuki et al. 2008). These plants have higher concentrations of tannins (Mould and Robbins 1981; Robbins et al. 1987) which, in plants, act as a defence mechanism (Perea and Gil 2014) against herbivores. Tannins may inhibit the digestion of protein and fibre which are excreted as a non-metabolic nitrogen (Frutos et al. 2004), thus reducing the amount of digestible protein in forage and increasing FN (Kariuki and Norton 2008). Although the digestibility of the N in diet was not assessed, we used the N content in forage as a practical proxy to diet quality. This could explain the higher FN values found in areas with higher deer densities and in which vegetation has high concentrations of tannin. Indeed, the positive association between FN and tannin content in faeces is consistent with the potential role played by tannins as binders of protein in the diet. The tannins in plants are tolerated by ungulates to some extent, but the animals avoid them once certain amounts have been ingested. Other studies on ungulates in different ecosystems have shown a negative relationship between density and FN (e.g. Asada and Ochiai 1999) and have argued that the consumption of plant species with a lower nutritive value as a result of the limited availability of high-quality forage leads to a decrease in FN. However, in Asada and Ochiai (1999), the densities ranged from 4.7 to 26.5 deer/100 ha, which were much lower than those found in our study area (25 to 68 deer/100 ha), and our results must therefore be interpreted in the context of a very high density in Mediterranean environments. An evaluation of a wider range of densities would probably evidence non-linear relationships between FN and diet quality.
The relationship between FN and N in diet was modulated by food supplementation. On the one hand, the results showed that FN was positively associated with N in plants, in populations in which supplemental food was provided. This positive association has been found in several other studies and is the basis of the belief that FN is a useful indicator of diet quality (e.g. Leslie and Starkey 1985; Ueno et al. 2007). Conversely, in non-supplemented populations, we speculate that most tannin originates from scrubs, which would be more frequently consumed when supplementary feeding is not practised and herbaceous plants are depleted. This may result in plant N being neutralised by tannins and thus in no association being found (Robbins et al. 1987) (Fig. 1). According to Mould and Robbins (1981), the relationship between FN and nitrogen in the diet of red deer is no longer linear when the diet contains large amounts of tannins. But when the diet is made up of lower tannin concentrations, this relationship remains linear and positive. This suggests that FN is not a straightforward positive indicator of diet quality in terms of protein intake, but probably results from the interaction between diet quality (protein content) and any anti-nutritive factor (such as tannins) that is able to reduce N digestibility.
Finally, our results suggest that individual body condition decreases with increased FN. This is also consistent with the fact that, in the context of high deer density, an increase in plant tannin contents may bind protein and form insoluble complexes that are excreted in faeces as non-metabolic nitrogen, and FN may therefore increase while protein assimilation decreases. This result suggests that animals that excrete both large amounts of N and large amounts of tannins in their faeces have a worse body condition. In supplemented populations, we found higher KFI scores at low FN content than in non-supplemented populations, but the KFI values become similar at high FN values (Fig. 2). Previous studies agree that supplementary feeding interferes with fitness indexes, thus improving the physical condition of individuals even at high densities (Rodríguez-Hidalgo et al. 2010).
The objective of this paper is to highlight the importance of simultaneously evaluating nutritional indicators, environmental factors and management parameters when monitoring ungulate populations. Caution is therefore needed when the interpretation of FN values is used to monitor the performance of red deer populations. However, FN proved to be a potentially simple, cost-effective and reliable indicator of diet quality once supplemental feeding practices, population density and tannin concentrations had been taken into account. Our study also suggests that, in the context of high ungulate density, the higher values of FN are associated with poor body condition, suggesting that the use of FN as an indicator of body condition should also be interpreted together with other factors such as tannin content and the game management regime.
References
Acevedo P, Ruiz-Fons F, Vicente J, Reyes-Garcia AR, Alzaga V, Gortazar C (2008) Estimating red deer abundance in a wide range of management situations in Mediterranean habitats. J Zool 277:37–47
Acevedo P, Santos JP, Real R, Vicente J (2011) Evaluación del estado de la población de cabra montés de los Montes de Toledo: relaciones con el ciervo. Pirineos 166:29–49
Asada M, Ochiai K (1999) Nitrogen content in feces and the diet of Sika deer on the Boso Peninsula, central Japan. Ecol Res 14:249–253
Buckland ST, Anderson DR, Burnham KP, Laake JL, Borchers DL, Thomas L (2004) Introduction to distance sampling: estimating abundance of biological population. Oxford University Press, Oxford
Côté SD, Rooney TP, Trembley JP, Dussault C, Waller DM (2004) Ecological impacts of deer overabundance. Annu Rev Ecol Evol Syst 35:113–147
Dauphine TC (1975) Kidney weight fluctuations affecting kidney fat index in caribou. J Wildl Manage 39:379–386
Finger SE, Brisbin IL, Smith MH, Urbston DF (1981) Kidney fat as a predictor of body condition in white-tailed deer. J Wildl Manag 45:964–968
Frutos P, Hervas G, Giraldez FJ, Mantecon AR (2004) Review. Tannins and ruminant nutrition. Spanish J Agric Res 2:191–202
Hamel S, Garel M, Festa-Bianchet M, Gaillard JM, Côté SD (2009) Spring Normalized Difference Vegetation Index (NDVI) predicts annual variation in timing of peak faecal crude protein in mountain ungulates. J Appl Ecol 46:582–589
Hodgman TP, Davitt BB, Nelson JR (1996) Monitoring mule deer diet quality and intake with fecal indices. J Range Manage 49:215–222
Julkunen-Tiitto R (1985) Phenolics constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33:213–217
Kariuki IW, Norton BW (2008) The digestion of dietary protein bound by condensed tannins in the gastro-intestinal tract of sheep. Anim Feed Sci Technol 142:197–209
Lazo A, Delecourt C, Soriguer RC (1992) Evaluation of hare abundance allowed by their use of attraction points. Z Saugetierkd 57:373–379
Leslie DM, Starkey EE (1985) Fecal indices to dietary quality of cervids in old-growth forests. J Wildl Manag 49:142–146
Leslie DM, Bowyer RT, Jenks J (2008) Facts from feces: nitrogen still measures up as a nutritional index for mammalian herbivores. J Wildl Manag 72:1420–1433
Mokoboki HK, Ndlovu LR, Malatje MM (2011) Intake and relative palatability indices of acacia species fed to sheep and goats. Agrofor Syst 81:31–35
Morellet N, Gaillard JM, Hewison AJM, Ballon P, Boscardin Y, Duncan P, Klein F, Maillard D (2007) Indicators of ecological change: new tools for managing populations of large herbivores. J Appl Ecol 44:634–643
Mould ED, Robbins CT (1981) Nitrogen metabolism in elk. J Wildl Manag 45:323–334
Perea R, Gil L (2014) Tree regeneration under high levels of wild ungulates: The use of chemically vs. physically-defended shrubs. For Ecol Manag 312:47–54
Putman RJ (1984) Facts from faeces. Mammal Rev 14:79–97
Putman R, Langbein J, Green P, Watson P (2011) Identifying threshold densities for wild deer in the UK above which negative impacts may occur. Mammal Rev 41:175–196
Riney T (1955) Evaluating condition of free-ranging red deer (Cervus elaphus) with special reference to New Zealand. NZJ Sci Technol 36:429–463
Ritchie ME, Tilman D, Knops JMH (1998) Herbivore effects on plant and nitrogen dynamics in oak savanna. Ecology 79:165–177
Robbins CT (1983) Wildlife feeding and nutrition. Academic Press, New York, USA, 343 pp
Robbins CT, Hanley TA, Hagerman AE, Hjeljord O, Baker DL, Schartz CC, Mautz WW (1987) Role of tannins in defending plants against ruminants: reduction in protein availability. Ecology 68:98–107
Rodríguez-Hidalgo P, Gortazar C, Tortosa FS, Rodriguez-Vigal C, Fierro Y, Vicente J (2010) Effects of density, climate, and supplementary forage on body mass and pregnancy rates of female red deer in Spain. Oecologia 164:389–398
San Miguel A, Rodriguez VC, Sanz V (1996) Ordenación del monte mediterráneo para la caza mayor. Ecosistemas 16:7–13
Santos JPV, Fernández-de-Mera IG, Acevedo P, Boadella M, Fierro Y, Vicente J, Gortázar C (2013) Optimizing the sampling effort to evaluate body condition in ungulates: A case study on red deer. Ecol Indic 30:65–71
Shimada T (2006) Salivary proteins as a defense against dietary tannins. J Chem Ecol 32:1149–1163
Suzuki M, Miyashita T, Kabaya H, Ochiai K, Asada M, Tange T (2008) Deer density affects ground-layer vegetation differently in conifer plantations and hardwood forests on the Boso Peninsula, Japan. Ecol Res 23:151–158
Taillon J, Brodeur V, Festa-Bianchet M, Côté SD (2011) Variation in body condition of migratory caribou at calving and weaning: which measures should we use? Ecoscience 18:295–303
Ueno M, Nishimura C, Takahashi H, Kaji K, Saitoh T (2007) Fecal nitrogen as an index of dietary nitrogen in two sika deer cervus nippon populations. Acta Theriol 52:119–128
Verheyden H, Aubry L, Merlet J, Petibon P, Chauveau-Duriot B, Guillon N, Duncan P (2011) Faecal nitrogen, an index of diet quality in roe deer Capreolus capreolus? Wildl Biol 17:166–175
Verheyden-Tixier H, Renaud PC, Morellet N, Jamot J, Besle JM, Dumont B (2008) Selection for nutrients by red deer hinds feeding on a mixed forest edge. Oecologia 156:715–726
Vicente J, Höfle U, Fernandez-De-Mera IG, Gortazar C (2007) The importance of parasite life history and host density in predicting the impact of infections in red deer. Oecologia 152:655–664
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed Effects Models and Extension in Ecology with R. Springer, New York
Acknowledgments
We would like to thank D. Carpio and E. Lopez, and the students Pilar, Jesus, Sara and especially Marta for their help in collecting field data. We would also like to thank Julia for her assistance in the laboratory and E. Molina for her helpful comments. We should also thank Sally Newton and Joel Walmsley for editing our English text. Finally, we are very grateful to the ‘Consejeria de Medio Ambiente de la Junta de Andalucía’, the estate owners and the estate keepers for their hospitality and assistance during the fieldwork.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Gortázar
Rights and permissions
About this article
Cite this article
Carpio, A.J., Guerrero-Casado, J., Ruiz-Aizpurua, L. et al. Interpreting faecal nitrogen as a non-invasive indicator of diet quality and body condition in contexts of high ungulate density. Eur J Wildl Res 61, 557–562 (2015). https://doi.org/10.1007/s10344-015-0927-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10344-015-0927-3