Abstract
A hydroponic experiment was conducted to assess whether grafting with citroides rootstocks could improve the salt tolerance of cucumber. One cucumber cultivar (Mercur F1) was grafted onto six diploid and tetraploid (auto and allo) citroides genotypes and the commercial rootstocks Argentario and RS841. Plants were grown in hydroponic culture at two electrical conductivity (EC) levels (control at 1.5 dS m−1 and salt at 6.0 dS m−1). Hydroponic salt stress led to a significant reduction in biomass growth of both grafted and nongrafted cucumbers. However, the plants least affected by salt stress were those grafted onto tetraploid citroides rootstocks. The leaf nutrient uptake of cucumber plants was significantly (p < 0.001) affected by salt, graft combination, and the salt × graft interaction. Ion leakage was significantly increased by salt application, and rootstock genotypic variation was significant. While the highest amount of proline was measured in plants grafted onto RS841 and N7T, the lowest amount of proline was determined in nongrafted control plants. Antioxidative enzyme activities were significantly affected by rootstocks under both control and salt-stress conditions. In this study, all graft combinations showed increased superoxide dismutase, catalase, and peroxidase activities with salt application, which differed according to rootstock genotypes. Tetraploid citroides cultivars have high rootstock potential for cucumber and their significant contribution to salt tolerance was closely associated with inducing physiological and biochemical responses of scions. These traits could be useful for the selection and breeding of salt-tolerant rootstocks for sustainable agriculture in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Salinity is one of the most important abiotic stress factors preventing the expected yield in crop production worldwide. Salt-affected soil is increasingly exacerbated by agricultural practices such as improper irrigation and fertilization, especially in arid and semiarid regions (Villa-Castorena et al. 2003). Continuation of plant growth under salinity stress depends on continued water uptake from the growth medium, exclusion of toxic ions entering plant organs, transport of excess ions to the aged leaves, or a combination of these mechanisms (Berthomieu et al. 2003; Genc et al. 2007; Shabala and Cuin 2008; Plett and Møller 2010; Teakle and Tyerman 2010). Salt stress in plants affects cell division and elongation, causing a decrease in the number of cells in the root and stem and reducing mitotic activity and the cell division rate. The decrease in the amount of usable water in the rhizosphere causes cell expansion to decrease and shoot growth to slow down. In the ionic stress phase, which occurs due to prolongation of osmotic stress, nutrient deficiency or nutrient imbalance occurs in plants as Na+ and Cl− ions increase in competition with essential nutrients such as K+, Ca2+, and NO3 (Hu and Schmidhalter 2005). Salt stress triggers a change in lipid composition of the cell membrane, causing membrane damage (Huang 2006). Since salt stress disrupts cellular energy metabolism, it causes production of reactive oxygen species (ROS) which are harmful to cell organelles and cell metabolites. ROS in plant cells are a double-edged sword: they act as a signaling molecule at lower concentrations while damaging cells at higher concentrations (Gapper and Dolan 2006). Different studies have shown that one of the important mechanisms involved in salt tolerance is the antioxidant mechanism. To reduce oxidative damage initiated by ROS, plants have developed a complex antioxidative defense system including antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidases (POX), as well as low-molecular-mass antioxidants. SOD is the main O2 scavenger and its enzymatic action results in the formation of H2O2 and O2. The H2O2 produced is then cleared by CAT and various classes of peroxidases (POX; Noctor and Foyer 2003). The content of malondialdehyde (MDA), a product of lipid peroxidation, has been accepted as an indicator of oxidative damage; therefore, it has been widely used for cell membrane stability assessment and differentiation of salt-tolerant and salt-sensitive varieties (Hernández and Almansa 2002; Meloni et al. 2003).
Cucumber (Cucumis sativus L.) is one of the most important and widely grown vegetable crops in the world and is considered salt sensitive. It can tolerate an electrical conductivity (EC) of approximately 2.5 dS m−1 and fruit yield decreases by 13% with each unit increase in EC (Maas and Hoffman 1977; Chen et al. 2020). Since improvement of saline soils is very expensive and difficult, it is necessary to develop salt-resistant or salt-tolerant varieties and/or rootstocks for this type of soil. One of the approaches used to improve the performance of commercial cultivars susceptible to salinity in plants is the technique of grafting the targeted crop onto other salt-tolerant plants. Today, grafting in fruit-bearing vegetables is a common procedure to develop salt-tolerant plants in which both rootstock and scion affect salt tolerance in grafted plants (Etehadnia et al. 2008). In previous studies, it has been reported that grafting in cucumber increases plant vigor, provides earliness, and has positive effects on resistance to stress factors, depending on the rootstock genotypes used (Lee et al. 2010; Schwarz et al. 2010; Velkov and Pevicharova 2016). It was determined that the use of rootstock increased the yield of cucumber fruit, but lower yield values were obtained in plants grafted on weak rootstocks (Singh and Soltan 2016). It has also been shown by many researchers that grafting has a significant positive effect on quality parameters such as fruit dry matter and water-soluble sugar content under stress conditions (Zhong and Bie 2007; Junguo Zhou et al. 2010). Cucumber can be grafted onto open pollinated cultivars or intra-/interspecies hybrids of other species (Cucurbita maxima, C. moschata, C. ficifolia, Lagenaria spp., Lufta spp.) in the Cucurbitaceae family (Edelstein et al. 2011).
One way to solve the abovementioned quality, abiotic, and biotic problems in grafted cucumber production may be to use rootstocks developed from close genera such as Citrullus (Edelstein et al. 2014). The citroides group is an ancient cultivar group originating from southern Africa that can nowadays be found globally and is generally considered the ancestor of cultivated sweet watermelons (Paris 2015). Citron melons are grown worldwide mainly for animal feed and fruit preserves. Resistance to nematodes, expressed as less galling than that of Cucurbita hybrids and bottle gourd rootstocks (Thies et al. 2016), and Fusarium wilt (Patrick Wechter et al. 2012) has been reported in some citron melon accessions, suggesting that this group is a promising alternative source of rootstock for managing root-knot nematodes (RKNs) and Fusarium wilt in watermelon. It has been reported by different researchers that the use of citron rootstock in the production of grafted watermelon can be an interesting alternative to the currently used commercial rootstock (Edelstein et al. 2014; Levi et al. 2014; Fredes et al. 2016). In the literature review, no published data were found on the effects of salt stress (osmotic and ionic stress) on morphological, physiological, and biochemical mechanisms at the whole-plant, tissue, and cellular levels in cucumbers grafted onto citroides group rootstocks. It was determined that auto- and allotetraploid citroides genotypes developed in studies by the current authors produced higher biomass than both cultured watermelon and diploid citroides genotypes and increased shoot growth when used as rootstock for watermelon (unpublished data). The results of these previous studies on watermelon led the present authors to investigate whether citroides rootstocks with different ploidy levels can be used as rootstock for cucumber under salt-stress conditions.
Materials and Methods
Plant Material, Treatments, and Experimental Design
A hydroponic experiment was conducted during the 2021–2022 growing season in a Kırşehir Ahi Evran University agricultural research and application greenhouse. One cucumber cultivar (Mercur F1) was used as scion and six diploid and tetraploid (auto and allo) Citrullus lanatus var. citroides genotypes and commercial rootstocks Argentario (Lagenaria siceraria) and RS841 (C. maxima × C. moschata) were used as rootstock (Table 1). The auto- and allotetraploid citroides genotypes were developed in the authors’ watermelon rootstock development project. To develop allotetraploid genotypes, citroides genotypes were crossed with the Calhoun Gray watermelon cultivar and the hybrid individuals’ genomes were duplicated with colchicine treatment. The seeds were sown in multipots in a mixture of peat (pH 6.0–6.5) and perlite in a 2:1 ratio and appropriate seedlings were then selected for the grafting process using the procedure of “cleft grafting” described by Lee and Oda (2010), while nongrafted cucumbers were used as control plants. Plants were left to heal and acclimatize for 1 week in a wide container protected by double-layered plastic film and shade cloth in the climate chamber after grafting (Lee and Oda 2010). To improve healing, the container was closed for the first 3–4 days of the healing and acclimatization period to prevent grafted plants from wilting due to excessive transpiration. Opening and closing of the container was performed for the next 3–4 days, depending on the conditions of the grafted plants and the growth space. This was required for acclimatization of grafted plants to environmental conditions outside the container. The seedlings were transplanted to 136 L plastic pots after cleaning off the growth substrate by washing with tap water. The upper surface of the pots was covered with Styrofoam and the plants were placed in the holes drilled on the Styrofoam plate. The cultivation solution was constantly aerated with a pump. Fourteen plants were grown in each pot. The experiment was conducted with two different EC levels (1.5 dS m−1 and 6 dS m−1). The nutrient solution contained 1125 µM Ca(N03)2, 375 µM (NH4)2SO4, 750 µM K2SO4, 650 µM MgSO4, 500 µM KH2PO4, 10 µM H3BO3, 0.5 µM MnSO4, 0.5 µM ZnSO4, 0.4 µM CuSO4, 0.4 µM MoNa2O4, and 80 µM Fe EDDHA (Hoagland and Arnon 1950). The experiment was designed according to the randomized plot design with three replications and three plants in each replication. The study was continued for 30 days under controlled greenhouse conditions (22–24 °C daytime/16–18 °C nighttime and 60% relative humidity).
Plant Growth Measurements
After 4 weeks of growing, plants were harvested and separated into shoot and roots. Stem length (cm) was measured using a meter rule. Fresh weight of shoot and roots were determined (g). In order to determine shoot and root dry weight, plant materials were dried in a forced-air oven for 48 h at 70 °C. Root length, volume, and diameter of the plants were determined using the special software program WinRHIZO (Win/Mac RHIZO Pro V. 2002c Regent Instruments Inc., Quebec, Canada).
Determination of Chlorophyll Index
The FieldScout CM 1000 Chlorophyll Meter (Spectrum Technologies, Aurora, IL, USA) was used to determine leaf chlorophyll index. The chlorophyll index was determined in fully expanded leaves of all plants for each treatment by measuring twice with the chlorophyll meter during the growth period.
Determination of Ion Leakage
Ion leakage (IL) of three leaves per replication was measured. Samples were cut into equal-sized pieces (0.5 g per replication) and placed in a test tube containing 10 ml of distilled water, and then at 45 °C for 30 min in a water bath. The initial conductance of the solution was measured using a conductivity meter (model-146, Systronics India Limited, Mumbai, India). The tubes were kept in a boiling water bath for 10 min then cooled to room temperature, and their conductivity was measured once again. IL (%) was calculated by the following formula: IL = (initial EC/final EC) × 100.
Determination of Leaf Relative Water Content
Relative water content (RWC; %) in the leaves was established as 100 × (FM − DM)/(SM − DM), where FM represents the fresh mass of 10 leaf discs (diameter 10 mm), SM is the saturated mass of the same discs after their hydration in the dark for 4 h, and DM is the dry mass of these discs after they had been oven-dried at 105 °C for 48 h. RWC was determined in five repetitions.
Lipid Peroxidation Analysis
Lipid peroxidation level was determined as the content of malondialdehyde (MDA) using the thiobarbituric acid reaction as described by Madhava Rao and Sresty (2000).
Nutrient Analysis
After harvest, fresh plant material was divided into two parts. One part was frozen in liquid nitrogen and stored at 80 °C for later use. The remaining fresh plant material was dried at 70 °C for 24 h. For determination of N, Ca, Cl, Na, and K concentrations, 100 mg dried plant material was extracted by 1 h of boiling in 5 ml Milli-Q system (Millipore®, Berdford, USA). The solution was filtered through 0.2 mm filter paper (Whatman, Little Chalfont, England) and N, Ca, Cl, Na, and K contents in the filtrate were analyzed using high-performance liquid chromatography (HPLC). The HPLC system (Shimadzu, Kyoto, Japan) was equipped with a ø 4.6 mm 6125 mm Shodex IC YS-50 column (Showa Denko, Tokyo, Japan). As an eluent, 4.0 mM methane sulfonic acid was used in HPLC-grade H2O (J.T. Baker, Thermo Fisher Scientific Inc., Waltham, MA, USA) with a flowrate of 1 ml min−1. Final ion concentrations in the filtrate were calculated according to a calibration curve.
Determination of Proline
Proline concentration was determined according to the method of Troll and Lindsley (1955). Fresh samples (0.5 g) were ground and homogenized with one volume of 100 mM sodium phosphate buffer (pH 6.0). The samples were centrifuged for 10 min at 16,000 × g. The reaction proceeded for 1 h in a boiling water bath and the developed dye was extracted with 1 ml of toluene and measured in the UV–vis spectrophotometer (CT 200 spectrophotometer, Waltham, MA, USA) at 515 nm.
Antioxidant Enzyme Activity Analysis
CAT activity was determined based on the rate of H2O2 decomposition as described in Abedi and Pakniyat (2010), POD activity was measured at 436 nm with a UV/VIS spectrophotometer (Perkin Elmer Lambda 25, USA) according to its capability to turn guaiacol into tetraguaiacol, and SOD activity was measured at 560 nm based on inhibition of the photochemical diminution of nitro blue tetrazolium (NBT) as described by Abedi and Pakniyat (2010). Briefly, for SOD, POD, and CAT activities, frozen cucumber leaves were homogenized in 5 mL of 100 mM phosphate buffer (pH 7.0) containing 1% (w/v) PVPP at 4 °C. The homogenate was centrifuged at 15,000 × g for 15 min and the supernatant was directly examined for enzymatic activities. CAT activity was determined by the decrease in absorbance at 240 nm that was caused by hydrogen peroxide decomposition. The reaction mixture contained 50 mM phosphate buffer (pH 7.0), 10 mM H2O2, and 100 μL of the plant extract. The oxidation extinction coefficient for H2O2 was 39.4 mM cm−1. Total SOD activity was assayed by following the super-radical-induced reduction of NBT. Briefly, 200 μL of the reaction mixture (50 mM phosphate buffer pH 7.8, 0.1 mM EDTA, 63 μM NBT, 50 μM riboflavin, 13 mM methionine, and 50 μL of plant extract) was placed in the wells of a 96-well microplate under a 40‑W fluorescent lamp. After 10 min, the light was turned off and the absorbance recorded at 560 nm. A non-illuminated reaction mixture treated in the same manner was used as the blank. One unit of SOD was used, which produced a 50% inhibition of NBT reduction.
Statistical Analysis
The data were analyzed with SAS Statistical Software (SAS 9.0, SAS Institute Inc., Cary, NC, USA). A two-factorial analysis of variance was performed to study the effects of salinity (NaCl), rootstock, and salt × rootstock interactions on the variables. Levels of significance are represented by *p < 0.05, **p < 0.01, and ***p < 0.001, and n.s. means not significant. Differences between means were analyzed using the Duncan multiple test (p < 0.05). Correlation analysis was performed between plant growth parameters and biochemical data using SPSS software (version 22.0, IBM Corp., Armonk, NY, USA).
Results and Discussion
Main stem length and leaf number per plant were significantly affected by rootstock, salt stress, and the rootstock × salt interaction, while chlorophyll content was significantly affected by rootstock and salt stress (Table 1). Under control conditions, the longest main stem length was recorded in the N7T/Mercur graft combination and the plant grafted onto N5D and Calhoun Gray and nongrafted Mercur (M) had the shortest main stem length. Salt application caused a decrease in main stem length at different rates in all graft combinations and nongrafted control plants. All graft combinations, regardless of rootstock, had taller plants than the nongrafted control plants under salt-stress conditions. The longest main stem was measured in plants grafted on autotetraploid N7T (50.60 cm), while nongrafted control plants had the shortest stem (16.60 cm). The reduction in stem length due to salt stress ranged from 1.9 to 40%, and the largest decrease was observed in nongrafted control plants and the plants grafted onto Argentario. The number of leaves per plant was significantly affected by rootstock and salt stress and the rootstock × salt interaction. Under nonsaline conditions, the highest leaf number per plant was recorded in the N7D/M, N7T/M, and RS841/M graft combinations, while the N5D/Mercur graft combination had the lowest leaf number, with 7.8 leaves per plant. With salt application, there was a decrease in the number of leaves varying between 7% (CX7T/M) and 25% (control and Argentario/M) in all graft combinations. Under salt stress, all graft combinations produced more leaves than the nongrafted control plants except for the N5D/M graft combination. Under saline conditions, the highest leaf number was determined in the N7T/M graft combination with 16.2 leaves per plant, and the lowest leaf number was determined in the nongrafted control plants (8.2 leaves per plant) and the plants grafted on diploid N5D (7 leaves per plant). Leaf chlorophyll content, which was significantly affected by rootstock and salt application, was found to be lowest in nongrafted control plants under both control and salt-stress conditions. Under control conditions, all graft combinations had a higher leaf chlorophyll content ranging from 30 to 68%, while grafted plants had a higher leaf chlorophyll content ranging from 70 to 164% under salt stress. Salt application caused decreases in leaf chlorophyll content in all applications and the highest decrease was observed in nongrafted control plants with 30% (Table 2). In our study, it was observed that grafting on citroides rootstocks with different ploidy levels in cucumber reduces the negative effects of salt stress in terms of plant height, number of leaves per plant, and chlorophyll content. Chlorophyll index was positively correlated with main stem length, shoot and root fresh and dry weight, and root volume (Table 9). A possible explanation for this may be that grafted plants are stronger and have strong root systems compared to nongrafted plants, resulting in higher water and nutrient uptake, leaf count, plant height, and higher net CO2 assimilation rate (Rouphael et al. 2012; Amaro et al. 2014; Fan et al. 2017; Ulas et al. 2020). In Bayoumi et al. (2021), plant height and leaf area were generally higher in grafted plants compared to control plants. While the total chlorophyll content was 12.51 µmol m-2 in the control group in pepper plants subjected to salt stress, the chlorophyll content decreased to 5.41 µmol m-2 as a result of NaCl application (Tuna et al. 2016).
Shoot fresh weight was significantly affected by rootstock and salt application in both control and saline conditions (p < 0.001). Under control conditions, the rootstock effect on shoot fresh weight was found to be significant and five of the graft combinations produced higher shoot fresh weight than control plants, while three graft combinations produced lower or equal shoot fresh weight compared to the control plants. Under control conditions, shoot fresh weight ranged from 122.4 g per plant to 45.2 g per plant, and the highest shoot fresh weight was determined in plants grafted onto autotetraploid N7T (Table 3). Salt application caused significant reductions in shoot fresh weight in all graft combinations. All grafted plants produced higher shoot fresh weight than nongrafted control plants, ranging from 39 to 367%. The decrease in shoot fresh weight caused by salt stress ranged from 61 to 30% in grafted plants, while shoot fresh weight decreased by 72% in control plants. As for shoot fresh weight, shoot dry weight was also affected by the treatments and the rootstock × salt interaction was found to be significant. The shoot fresh weights of the graft combinations with high shoot fresh weight (e.g., N7T/M and N7D/M) were also high under control conditions. Shoot dry weight decreased by between 68 and 29% with salt application. All grafted plants produced a higher shoot dry weight (14–294%) than the nongrafted control plants under saline conditions, and the highest shoot dry weight was determined in the N7T/M graft combination.
Root fresh weight was significantly influenced by salt application, rootstock, and the salt rootstock × interaction under both control and saline conditions (Table 3). Under control conditions, root fresh weight ranged from 86.40 g per plant (CX7T/M) to 20.20 g per plant (CX7D/M), while all graft combinations produced higher root fresh weight than control plants except for CX7D/M. The mitigating effect of rootstocks was more pronounced under salt stress and all graft combinations produced higher root fresh weight than control plants. Nongrafted control plants produced 12 g per plant root dry weight in saline conditions, while grafted plants produced between 15 g per plant (N5D/M) and 49 g per plant (N7T/M) root fresh weight. The reduction in root fresh weight due to salt stress varied between 71% (CX7T/M) and 6% (CX7D/M) depending on the graft combinations. Root dry weight was also significantly affected by rootstock, salt application, and the rootstock × interaction. All grafted combinations produced higher root dry weight than that of the nongrafted control plants under both control and saline conditions. The highest and lowest root dry weight in both control and saline conditions were determined in the N7T/M graft combination (control: 6.92 g per plant; salt: 5.10 g per plant) and nongrafted control plants (control: 2.82 g per plant; salt: 1.32 g per plant), respectively. Root dry weight, which was adversely affected by salt application, decreased by 5 to 57% in saline conditions. It was observed that rootstocks with a strong root system increased shoot biomass under both control and salt stress conditions. Significant positive correlations (r = 0.730 for fresh weight and r = 0.855 for dry weight) were found between shoot biomass and root biomass. Shoot and root growth were adversely affected by hydroponic salt stress and, therefore, significant reductions were found in shoot (49.9%) and root (17.6%) dry matter and the shoot–root ratio (45.8%; Ulas et al. 2020). Plant fresh and dry weights decreased in cucumber, melon, watermelon, tomato, pepper, and eggplant plants grown under salt-stress conditions (del Amor et al. 1999; Yasar et al. 2006; Gong et al. 2013; al Rubaye et al. 2020). These effects of grafting on the development and growth of scions and rootstocks were probably the result of physiological relationships existing between the scions and rootstocks in the different grafting plants. The tetraploid citroides rootstocks used in the study showed positive effects on the fresh and dry weights of plants grown in salty conditions.
Root length, root volume, and root diameter of cucumbers grafted onto citroides rootstocks with different ploidy levels grown under control (1.5 dS m−1) and NaCl (6 dS m−1) conditions are presented in Table 4. The variation between graft combinations was significant for root length under both control and salt-stress conditions, and the effects of rootstock, salt, and the rootstock × salt interaction were found to be significant (P < 0.01). In general, grafted plants had longer roots under both control and salt-stress conditions. While the longest roots were measured in the N7T/M graft combination (12,198.65 cm), the shortest roots were determined in the nongrafted control plants. Salt treatment caused root length reductions in all graft combinations and control plants, ranging from 17 to 63%. The lowest reduction in root length was determined in the N7T/M graft combination, while the highest decrease was determined in the plants grafted onto N5D under salt stress. As in the control conditions, the longest roots were determined in the N7T/M graft combination under salt stress, while the shortest roots were measured in the nongrafted control plants. It is an important finding that plants grafted on autotetraploid N5T and N7T citroides genotypes produced longer roots under salt stress than commercial rootstocks (Table 4). Root volume was significantly affected by salt stress, rootstock, and the rootstock × salt stress interaction. Under control conditions, plants grafted onto citroides genotypes other than the CX7T/M graft combination had higher root volumes than nongrafted control plants and plants grafted onto commercial rootstocks. The root volume was reduced significantly by salt application and variation between graft combinations was significant. All grafted plants produced higher root volume than nongrafted control plants under salt stress. While the highest root volume was determined with 3.64 cm3 in the N7T/M graft combination, the lowest root volume was measured with 2.40 cm3 in nongrafted control plants. Under saline conditions, the N7T/M, N5D/M, and N5T/M graft combinations produced higher root volume than both nongrafted control plants and commercial rootstocks (Table 4). Variation in root diameter was found to be significant under both control and salt-stress conditions, and it was determined that graft combinations produced thicker roots than nongrafted control plants. Under salt stress, fine roots were formed regardless of graft combinations. Root diameter varied between 0.35 and 0.63 mm under salt stress. N5T/M and CX7T/M graft combinations produced the thickest (0.62–0.63 mm) roots, while the thinnest roots (0.35 mm) were detected in nongrafted control plants. Salt stress negatively affects the root morphology of plants in general (Ulas et al. 2020; Göçer et al. 2021). Salt stress had a negative effect on root morphological parameters in all graft combinations. However, the plants that are least affected by salt stress are those grafted onto tetraploid citroides rootstocks. For root length index, all the abovementioned concentrations of NaCl significantly affected all the cucumber genotypes. Root length index was reduced with an increased level of salt stress; the genotypes Valley and then HC-999 achieved a good length of plant root as compared to the rest (Marium et al. 2019).
The leaf nitrogen (N), potassium (K+), and calcium (Ca2+) uptake of cucumber plants was significantly (p < 0.001) affected by salt, graft combination, and the salt × graft combination interaction (Table 5). Leaf N content was significantly affected by rootstock, salt treatment, and the rootstock × salt interaction in both control and saline conditions. It was found that grafting onto different rootstocks increased leaf N content under control conditions and all graft combinations had higher N content than control plants. In addition, it was determined that plants grafted onto citroides genotypes had higher leaf N content than plants grafted onto commercial rootstocks. There was a significant decrease (6–60%) in the N content of leaves under salt stress. Under salt stress, the highest leaf N content was detected in plants grafted onto autotetraploid N5T and N7T, while the lowest leaf N content was determined in nongrafted plants. A significant positive correlation was found between leaf N content and main stem length, shoot and root fresh and dry weight, and root length (Table 9). Leaf K+ content was also significantly influenced by the applications and the interactions between applications. Grafted plants had higher leaf K content than nongrafted control plants under both control and salt-stress conditions. Under control conditions, the highest and lowest leaf K+ contents were determined as 3.57 and 1.62% in the N7T/M graft combination and nongrafted control plants, respectively. On the other hand, while the highest leaf K+ content under salt stress was determined in the N5T/M graft combination as 0.94%, the lowest leaf K+ content was determined in nongrafted control plants as 0.34%. Under salt stress, plants grafted onto citroides rootstocks had higher leaf K+ content than plants grafted onto commercial rootstocks, except for plants grafted onto N5D and N7D plants. Leaf K+ content was positively correlated with main stem length, shoot fresh and dry weight, root fresh weight, and root length (Table 9). As in leaf N and K+, Ca2+ was significantly affected by rootstock, salt, and the rootstock × salt interaction. Leaf Ca2+ content varied between 0.94 and 2.07% under control conditions, the highest and lowest leaf Ca2+ contents were determined in the CX7D/M graft combination and nongrafted control plants, respectively. Salt stress caused a significant reduction in the leaf Ca2+ content in all graft combinations, and the decrease in leaf Ca2+ content varied from 21% (N7T/M) to 83% (nongrafted control). Under salt stress, the highest leaf Ca2+ content was determined in plants grafted on autotetraploid N7T, and the lowest leaf Ca2+ content was determined in nongrafted control plants. A significant positive correlation was determined between leaf Ca2+ content and main stem length, shoot fresh and dry weight, root fresh and dry weight, and root length (Table 9). N and K+ concentrations in grafted melons’ and cucumbers’ leaves and roots were significantly affected by salinity, and the highest results were obtained in plants grown in salt-free environments compared to plants treated with NaCl. Cucumber plants grafted onto figleaf gourd and Chaofeng Kangshengwang had higher K+ contents in the fruits and leaves compared to self-grafted plants under NaCl stress (Huang et al. 2009). Parallel to the current results, salt stress has been associated with macronutrient deficiencies, such as Ca2+ and N deficiencies, caused by high NaCl concentrations in cucumbers and tomatoes. Salinity with a predominance of Na+ salts not only reduces the availability of Ca2+, but also reduces Ca2+ transport and mobility to the growing parts of the plant, which affects the quality of both vegetative and reproductive organs (Navarro et al. 2000; Cerda and Martinez 2015). Salinity can directly affect plant nutrient intake, such as Na+ reducing K+ intake or chloride reducing NO3 intake (Grattan and Grieve 1998).
The leaf chloride (Cl−), sodium (Na+), and K/Na contents were significantly affected by salt treatment, rootstock, and the salt × rootstock interaction (Table 6). Leaf Cl− content, which did not change significantly under control conditions, increased significantly with salt application, and significant differences were found among graft combinations. Under control conditions, the highest leaf Cl content was found in the nongrafted control, RS841/M, N7D/M, and N7T/M graft combinations. When looking at the Cl− and Na+ contents in the plant leaves, the highest were measured in nongrafted Mercur plants under salt-stress conditions (76.12 mg kg−1 and 14,382 mg kg−1, respectively). The lowest Cl− and Na+ contents were measured in the graft combination CX7T/M (20.99 mg kg−1 and 9924.92 mg kg−1, respectively). The highest increase in Na content with salt stress application was measured in nongrafted Mercur (90,126.87%) plants. The highest K+/Na+ ratio was measured in nongrafted mercury plants under control conditions, but the lowest K+/Na+ ratio was measured in nongrafted plants in saline conditions. With salt application, nongrafted plants accumulated less K+ than Na+ in leaf tissues compared to other graft combinations. The graft combination with the highest K+/Na+ ratio in leaf tissues in saline conditions was CX7D/M (0.0026; Table 6). Leaf Na+ and Cl− concentrations of melon plants increased by 1137.5 and 1392.3%, respectively, under salt stress compared to control conditions, regardless of the graft combination (Ulas et al. 2020). Plants grafted onto figleaf gourd and Chaofeng Kangshengwang had higher fruit number as well as marketable and total fruit yield than those of self-grafted plants under 0, 30, and 60 mM NaCl, which could be attributed at least in part to the higher K+ but lower Na+ and/or Cl− contents in the leaves (Huang et al. 2009). It is well known that the Na+ and Cl− uptake of leaves increases with increasing salinity levels. The concentration of Cl− in cucumber leaves increased by 300% with salt application, regardless of genotype (Colla et al. 2013). According to our study results, the Cl− and Na+ contents in the leaves of all grafted plants were lower than those of nongrafted plants.
The relative water content of the leaf was significantly affected by salt application and rootstock genotype in both control and salt-stress conditions. Overall, grafting of cucumbers onto citroides rootstocks improved the RWC of the plants, and all grafted plants had higher RWC than nongrafted control plants, except for the Argentario/M graft combination under control conditions. NaCl application reduced RWC regardless of graft combination, and the reduction in RWC ranged from 0.64 to 25%. Under saline conditions, all citroides–cucumber graft combinations except for CX7T/M and Argentario/M had a higher RCW than nongrafted control plants (Table 7). There was no significant variation in ion leakage (IL) under control conditions among graft combinations. Ion leakage was significantly increased by salt application and rootstock genotypic variation was significant. Ion leakage ranged from 44 to 253% under salt stress (Table 7). Malondialdehyde (MDA), as a lipid peroxidation marker, was also significantly affected by salt stress, rootstock, and the salt × rootstock interaction. The amount of MDA varying between 3.96 and 7.69 mmol kg−1 under control conditions differed depending on rootstocks. Under control conditions, the highest amount of MDA was obtained from plants grafted onto Argentario with 7.69 mmol kg−1, but nongrafted control plants, CX7T/M, CX7D/M, and N7T/M graft combinations had the lowest MDA content. The amount of MDA was increased in saline conditions. While the highest amount of MDA was determined in the Argentario/M graft combination under salt stress, the N7T/M and CX7D/M graft combinations produced the lowest MDA. Plants grafted onto CX7D, N7T, and N5T outperformed commercial rootstocks in terms of MDA content. The amount of proline was significantly affected by rootstocks under both salt-stress and control conditions. The amount of proline, varying between 0.04 and 0.06 mmol kg−1 under control conditions, increased in all graft combinations with salt application, while it decreased in nongrafted control plants. While the highest amount of proline was measured in plants grafted onto RS841 and N7T, the lowest amount of proline was determined in nongrafted control plants (Table 7). It is a well-known phenomenon that salt stress causes a reduction in RCW and increases in ion leakage in plants. Similar to the current study, decreased RCW and increased IL in cucumbers due to salt stress have been reported in previous studies (Furtana and Tipirdamaz 2010). Parallel to our results, grafted plants increased RWC compared to ungrafted plants (El-Shraiy and Mostafa 2008).
Electrolyte leakage was enhanced with increasing salinity levels as compared to the control cucumber plants. Similarly, Lechno et al. (1997) observed the same increasing trend of electrolyte leakage in salt-sensitive cucumber cultivar as compared to the salt-tolerant cultivar. In the present study, the MDA content increased significantly under NaCl stress, indicating that membrane stability had been destroyed and lipid peroxidation had occurred. Similar results were observed in mulberry (Sudhakar et al. 2001), tomato (Alpaslan and Gunes 2001) and Catharanthus roseus (Elkahoui et al. 2005). Ulas et al. (2020) reported that regardless of the graft combination, salt stress caused a significant increase in proline and MDA content and ion leakage in leaf and root of salt-treated melon plants compared to controls. The MDA reactive product (lipid peroxidation) showed a significant effect of treatments. Compared with the nongrafted plants, salinity increased the MDA content to a lesser extent in the grafted plants, especially in the leaf. The proline content in the leaves of cucumber plants generally increased with salt stress, but the proline content decreased in nongrafted plants.
The results of CAT, POD, and SOD are presented in Table 8 and antioxidative enzyme activities were significantly affected by rootstocks under both control and salt-stress conditions. Under control conditions, the highest CAT activity was determined in the plants grafted onto N7T and Argentario, while the lowest CAT activity was measured in RS841/M and N5D/M graft combinations. A significant increase (25–396%) in CAT activity was found in all graft combinations, whereas nongrafted control plants had the lowest CAT activity and CAT activity in control plants decreased compared to salt-free conditions. The highest CAT activity was determined in the graft combination of N7T/M, followed by Argentario/M and CX7D/M; nongrafted control plants produced the lowest CAT activity under salt stress. POD enzyme activity showed significant variation among graft combinations under control conditions (Table 8). Under the control conditions, the highest POD activity was determined in the N7T/M and Argentario/M graft combinations, while the RS41/M and N5D/M graft combinations had the lowest POD activities with 2207.30 and 2313.32 EU per g leaf tissue, respectively. As for CAT activity, POD activity also increased significantly in all graft combinations (2.8–9.8-fold compared to control conditions) with salt application. Under salt stress, all plants grafted onto different rootstocks showed significantly higher POD activities than nongrafted control plants and the lowest POD activity was determined in nongrafted control plants, with 12,837 EU per g leaf tissue. SOD activity was significantly affected by rootstock genotype under both control and salt-stress conditions. Under control conditions, the highest SOD activity was obtained from the Argentario/M and N7T/M graft combinations, while the plants grafted onto RS841 and N5D showed the lowest SOD activity. SOD activity, which varied from 380.4 to 759.4 EU per g leaf tissue under control conditions, ranged from 252.6 to 2539.4 EU per g leaf tissue under saline conditions. Under saline conditions, regardless of rootstock, SOD activity increased at different rates (1.9- and 3.4-fold compared to control conditions) in all grafted plants, while a decrease in SOD activity was detected in nongrafted control plants compared to salt-free conditions (Table 8). In this study, all graft combinations showed increased SOD, CAT, and POD activities, which differed according to rootstock genotype with salt application. Plants with high levels of antioxidants, either constitutive or induced, have been reported to have greater resistance to the oxidative damage caused by stress factors. In general, tetraploid and diploid citroides genotypes used as rootstock in this study showed higher antioxidant enzyme activities than C. maxma × C. moschata hybrid rootstock RS841, but they showed slightly lower than or equivalent antioxidant enzyme activity to Lageanaria rootstock Argentario. It suggests that the mechanism of protection against oxidative damage due to the stimulated activity of antioxidant enzymes may be better in citroides rootstocks than in commercial rootstock RS841. However, the inability of the Argentario/M graft combination, which has a high level of antioxidant enzyme activity, to show high plant growth performance may indicate that the damage caused by salinity stress cannot be prevented by the antioxidant enzyme system alone. In this study, increases in catalase activity, which eliminates H2O2 by decomposing it directly into water and oxygen, were determined in plants grafted onto citroides genotypes. CAT. This result may suggest that rootstocks have a more effective H2O2 dismutation capacity outside of chloroplasts under salinity. Our results are in agreement with previous studies (v. Madhava Rao and Sresty 2000; Sudhakar et al. 2001; Sairam et al. 2002). Rout and Shaw (2001) reported that salt-tolerant plants should have a better antioxidant defense system for effective removal of ROS, including antioxidant enzymes such as SOD, POD, and CAT. In our study, under 6 dS m−1 salt stress, nongrafted plants had lower SOD, POD, and CAT activities than grafted plants. Cucumber plants appear to have a higher antioxidant capacity to remove H2O2 when grafted onto relatively salt-tolerant rootstocks under NaCl stress than relatively salt-sensitive rootstocks, regardless of scion genotype. This result is consistent with those found in tomato (He et al. 2009). The enhanced salt tolerance could be partially attributed to the improved water status and POD activity in the leaf.
Correlation Analysis
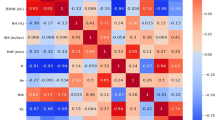
Stem length showed a positive correlation with chlorophyll index (0.400) and leaf N, (0.571), K (0.579), and Ca (0.603) content. Shoot and root fresh and dry weight also showed a positive correlation with chlorophyll index and leaf N, K, and Ca content. Root length showed a positive correlation with N (0.694), K (0.697), and Ca (0.732) content. Root volume positively correlated with chlorophyll index (0.395), MDH (0.598), proline (0.622), CAT (0.575), POD (0.500), and SOD (0.598), while leaf Cl content was negatively correlated with root volume (−0.586) and root diameter (−0.567; Table 9).
Conclusion
As one of the most prevalent abiotic stress factors, salinity usually has harmful effects on crop productive capacity by decreasing yield and quality, particularly in arid and semiarid regions of the world. To solve this problem, grafting with salt-tolerant rootstocks can be an effective management strategy for improving the salt tolerance of crop plants. Cucumber (Cucumis sativus L.) is one of the most important and widely grown vegetable crops in the world and is considered salt sensitive. One way to solve the quality, abiotic, and biotic problems in grafted cucumber production mentioned above may be to use rootstocks developed from close genera such as Citrullus. In this short-term hydroponic experiment, one cucumber (Cucumis sativus L.) cultivar (Mercur F1) was grafted onto six diploid and tetraploid (auto and allo) Citrullus lanatus var. citroides genotypes and the commercial rootstocks Argentario (Lagenaria siceraria) and RS841 (C. maxima × C. moschata). Plants were grown in hydroponic culture at two electrical conductivity (EC) levels (control at 1.5 dS m−1 and salt at 6.0 dS m−1). In our study, it was observed that grafting onto citroides rootstocks with different ploidy levels in cucumber reduces the negative effects of salt stress in terms of plant height, number of leaves per plant, and chlorophyll content. Chlorophyll index was positively correlated with main stem length, shoot and root fresh and dry weight, and root volume. It was observed that rootstocks with a strong root system increased shoot biomass under both control and salt-stress conditions. Significant positive correlations (r = 0.730 for fresh weight and r = 0.855 for dry weight) were found between shoot biomass and root biomass. The leaf nitrogen, potassium, calcium, chloride, and sodium uptake of cucumber plants was significantly (p < 0.001) affected by salt, graft combination, and the salt × graft combination interaction. NaCl application reduced RWC regardless of graft combination and the reduction in RWC ranged from 0.64 to 25%. Ion leakage ranged from 44 to 253% under salt stress. The amount of MDA was increased in saline conditions. While the highest amount of MDA was determined in the Argentario/M graft combination under salt stress, the N7T/M and CX7D/M graft combinations produced the lowest MDA. While the highest amount of proline was measured in plants grafted onto RS841 and N7T, the lowest amount of proline was determined in nongrafted control plants.
In this study, all graft combinations showed increased SOD, CAT, and POD activities, which differed according to rootstock genotypes with salt application. Plants with high levels of antioxidants, either constitutive or induced, have been reported to have greater resistance to the oxidative damage caused by stress factors. In general, tetraploid and diploid citroides genotypes used as rootstock in this study showed higher antioxidant enzyme activities than C. maxma X C. moschata hybrid rootstock RS841.
In this study, it has been shown that tetraploid (auto and allo) genotypes of the Citrullus genus can increase plant growth and plant nutrient uptake when used as rootstock. Therefore, in terms of vegetative growth and nutrient uptake, tetraploid (allo and auto) rootstocks developed from the Citrullus genus are expected to become commercially available for different horticultural crops in the near future. More detailed studies are needed to determine the effect of tetraploid rootstocks on fruit yield and quality characteristics, plant nutrient metabolism, and stress tolerance of the scion when used as rootstocks for cultivated plants.
References
Abedi T, Pakniyat H (2010) Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J Genet Plant Breed 46:27–34. https://doi.org/10.17221/67/2009-CJGPB
Alpaslan M, Gunes A (2001) Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants
Amaro ACE, Macedo AC, Ramos ARP et al (2014) The use of grafting to improve the net photosynthesis of cucumber. Theor Exp Plant Physiol 26:241–249. https://doi.org/10.1007/S40626-014-0023-1/TABLES/2
del Amor FM, Martinez V, Cerdá A (1999) Salinity duration and concentration affect fruit yield and quality, and growth and mineral composition of melon plants grown in perlite. HortScience 34:1234–1237. https://doi.org/10.21273/HORTSCI.34.7.1234
Bayoumi Y, Abd-Alkarim E, El-Ramady H et al (2021) Grafting improves fruit yield of cucumber plants grown under combined heat and soil salinity stresses. Horticulturae 7:61. https://doi.org/10.3390/HORTICULTURAE7030061
Berthomieu P, Conéjéro G, Nublat A et al (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22:2004–2014. https://doi.org/10.1093/EMBOJ/CDG207
Cerda A, Martinez V (2015) Nitrogen fertilization under saline conditions in tomato and cucumber plants. Journal of horticultural science 63:451–458. https://doi.org/10.1080/14620316.1988.11515878
Chen TW, Pineda IMG, Brand AM, Stützel H (2020) Determining Ion toxicity in cucumber under salinity stress. Agronomy 10:677. https://doi.org/10.3390/AGRONOMY10050677
Colla G, Rouphael Y, Jawad R et al (2013) The effectiveness of grafting to improve NaCl and CaCl2 tolerance in cucumber. Sci Hortic 164:380–391. https://doi.org/10.1016/J.SCIENTA.2013.09.023
Edelstein M, Plaut Z, Ben-Hur M (2011) Sodium and chloride exclusion and retention by non-grafted and grafted melon and Cucurbita plants. J Exp Bot 62:177–184. https://doi.org/10.1093/jxb/erq255
Edelstein M, Tyutyunik J, Fallik E et al (2014) Horticultural evaluation of exotic watermelon germplasm as potential rootstocks. Sci Hortic 165:196–202. https://doi.org/10.1016/J.SCIENTA.2013.11.010
El-Shraiy AM, Mostafa MA (2008) Enhancing salt tolerance of cucumber using grafting and some bioregulators. Middle East J Agric Res
Elkahoui S, Hernández JA, Abdelly C et al (2005) Effects of salt on lipid peroxidation and antioxidant enzyme activities of Catharanthus roseus suspension cells. Plant Sci 168:607–613. https://doi.org/10.1016/J.PLANTSCI.2004.09.006
Etehadnia M, Waterer DR, Tanino KK (2008) The method of ABA application affects salt stress responses in resistant and sensitive potato lines. J Plant Growth Regul 27:331–341. https://doi.org/10.1007/S00344-008-9060-9
Fan HF, Ding L, Xu YL, Du CX (2017) Antioxidant system and photosynthetic characteristics responses to short-term PEG-induced drought stress in cucumber seedling leaves. Russ J Plant Physiol 64:162–173. https://doi.org/10.1134/S1021443717020042
Fredes A, Roselló S, Beltrán J et al (2016) Fruit quality assessment of watermelons grafted onto citron melon rootstock. J Sci Food Agric 97:1646–1655. https://doi.org/10.1002/jsfa.7915
Furtana GB, Tipirdamaz R (2010) Physiological and antioxidant response of three cultivars of cucumber (Cucumis sativus L.) to salinity. Turk J Biol 34:287–296. https://doi.org/10.3906/biy-0812-10
Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345. https://doi.org/10.1104/PP.106.079079
Genc Y, McDonald GK, Tester M (2007) Reassessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant Cell Environ 30:1486–1498. https://doi.org/10.1111/J.1365-3040.2007.01726.X
Göçer H, Yetişir H, Ulaş A et al (2021) Plant Growth, Ion Accumulation and Essential Oil Content of Salvia officinalis Mill. and S. tomentosa L. Grown under Different Salt Stress. Ksu Tarım ve Doga Dergısı-Ksu. J Agrıculture Nat 24:505–514. https://doi.org/10.18016/KSUTARIMDOGA.VI.730477
Gong B, Wen D, VandenLangenberg K et al (2013) Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci Hortic 157:1–12. https://doi.org/10.1016/J.SCIENTA.2013.03.032
Grattan SR, Grieve CM (1998) Salinity–mineral nutrient relations in horticultural crops. Sci Hortic 78:127–157. https://doi.org/10.1016/S0304-4238(98)00192-7
He Y, Zhu Z, Yang J et al (2009) Grafting increases the salt tolerance of tomato by improvement of photosynthesis and enhancement of antioxidant enzymes activity. Environ Exp Bot 66:270–278. https://doi.org/10.1016/J.ENVEXPBOT.2009.02.007
Hernández JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant 115:251–257. https://doi.org/10.1034/J.1399-3054.2002.1150211.X
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn 347
Hu Y, Schmidhalter U (2005) Drought and salinity: A comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549. https://doi.org/10.1002/jpln.200420516
Huang B (2006) Cellular membranes in stress sensing and regulation of plant adaptation to abiotic stresses. Plant Environ Interact. https://doi.org/10.1201/9781420019346.CH1
Huang Y, Tang R, Cao Q, Bie Z (2009) Improving the fruit yield and quality of cucumber by grafting onto the salt tolerant rootstock under NaCl stress. Sci Hortic 122:26–31. https://doi.org/10.1016/J.SCIENTA.2009.04.004
Junguo Zhou, Huiling Hu, Xinzheng Li et al (2010) Effects of rootstock on fruit yıeld and quality of hydroponically cultivated grafted cucumber under Nacl stress. Acta Hortic. https://doi.org/10.17660/ACTAHORTIC.2010.871.6
Lechno S, Zamski E, Tel-Or E (1997) Salt stress-induced responses in cucumber plants. J Plant Physiol 150:206–211. https://doi.org/10.1016/S0176-1617(97)80204-0
Lee J‑M, Oda M (2010) Grafting of herbaceous vegetable and ornamental crops. Hortic Rev. https://doi.org/10.1002/9780470650851.CH2
Lee JM, Kubota C, Tsao SJ et al (2010) Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci Hortic 127:93–105. https://doi.org/10.1016/J.SCIENTA.2010.08.003
Levi A, Thies JA, Wechter PW et al (2014) USVL-360, a novel watermelon tetraploid germplasm line. HortScience 49:354–357. https://doi.org/10.21273/HORTSCI.49.3.354
López-Gómez E, San Juan MA, Diaz-Vivancos P et al (2007) Effect of rootstocks grafting and boron on the antioxidant systems and salinity tolerance of loquat plants (Eriobotrya japonica Lindl.). Environ Exp Bot 60:151–158. https://doi.org/10.1016/J.ENVEXPBOT.2006.10.007
v. Maas E, Hoffman GJ (1977) Crop salt tolerance—current assessment. J Irrigation Drainage Div 103:115–134. https://doi.org/10.1061/JRCEA4.0001137
v. Madhava Rao K, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128. https://doi.org/10.1016/S0168-9452(00)00273-9
Marium A, Kausar A, Shah ASM et al (2019) Assessment of cucumber genotypes for salt tolerance based on germination and physiological indices. Dose Response. https://doi.org/10.1177/1559325819889809
Meloni DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76. https://doi.org/10.1016/S0098-8472(02)00058-8
Navarro JM, Martínez V, Carvajal M (2000) Ammonium, bicarbonate and calcium effects on tomato plants grown under saline conditions. Plant Sci 157:89–96. https://doi.org/10.1016/S0168-9452(00)00272-7
Noctor G, Foyer CH (2003) Ascorbate and glutathione: keeping active oxygen under control. Annual review of plant biology 49:249–279. https://doi.org/10.1146/ANNUREV.ARPLANT.49.1.249
Paris HS (2015) Origin and emergence of the sweet dessert watermelon, Citrullus lanatus. Ann Bot 116:133–148. https://doi.org/10.1093/aob/mcv077
Plett DC, Møller IS (2010) Na+ transport in glycophytic plants: what we know and would like to know. Plant Cell Environ 33:612–626. https://doi.org/10.1111/J.1365-3040.2009.02086.X
Rouphael Y, Cardarelli M, Rea E, Colla G (2012) Improving melon and cucumber photosynthetic activity, mineral composition, and growth performance under salinity stress by grafting onto Cucurbita hybrid rootstocks. Photosynthetica. https://doi.org/10.1007/S11099-012-0002-1
Rout NP, Shaw BP (2001) Salt tolerance in aquatic macrophytes: possible involvement of the antioxidative enzymes. Plant Sci 160:415–423. https://doi.org/10.1016/S0168-9452(00)00406-4
al Rubaye OM, Yetisir H, Ulas F, Ulas A (2020) Growth of pepper inbred lines as affected by rootstocks with vigorous root system under salt stress conditions. Acta Hortic 1273:479–485. https://doi.org/10.17660/ACTAHORTIC.2020.1273.60
Sairam RK, Rao K, Srivastava GC (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046. https://doi.org/10.1016/S0168-9452(02)00278-9
Schwarz D, Rouphael Y, Colla G, Venema JH (2010) Grafting as a tool to improve tolerance of vegetables to abiotic stresses: thermal stress, water stress and organic pollutants. Sci Hortic 127:162–171. https://doi.org/10.1016/J.SCIENTA.2010.09.016
Shabala S, Cuin TA (2008) Potassium transport and plant salt tolerance. Physiol Plant 133:651–669. https://doi.org/10.1111/J.1399-3054.2007.01008.X
Singh H, Soltan M (2016) Vegetable grafting—a tool to improve vegetable productivity. Adv Plants Agric Res. https://doi.org/10.15406/apar.2016.04.00143
Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–619. https://doi.org/10.1016/S0168-9452(01)00450-2
Teakle NL, Tyerman SD (2010) Mechanisms of Cl- transport contributing to salt tolerance. Plant Cell Environ 33:566–589. https://doi.org/10.1111/J.1365-3040.2009.02060.X
Thies JA, Ariss JJ, Kousik CS, Hassell RL, Levi A (2016) Resistance to Southern Root-knot Nematode () in Wild Watermelon (var.). Journal of Nematology 48(1):14–19. https://doi.org/10.21307/jofnem-2017-004
Troll W, Lindsley J (1955) A photometric method for the determination of proline. J Biol Chem 215:655–660
Tuna GS, Keleş H, Göçmen D et al (2016) Flow Sitometri ile Çok Yıllık Buğdaygil Yem Bitkisi Genetik Kaynaklarının Karakterizasyonu. Tarla Bitkileri Merkez Arastırma Enstitüsü Derg 25:7–12. https://doi.org/10.21566/TARBITDERG.281591
Ulas A, Aydin A, Ulas F et al (2020) Cucurbita rootstocks improve salt tolerance of melon scions by inducing physiological, biochemical and nutritional responses. Horticulturae 6:1–13. https://doi.org/10.3390/HORTICULTURAE6040066
Velkov N, Pevicharova G (2016) Skiepijimo įtaka agurkų derliui ir juslinėms savybėms. Zemdirbyste 103:405–410. https://doi.org/10.13080/Z-A.2016.103.052
Villa-Castorena M, Ulery AL, Catalán-Valencia EA, Remmenga MD (2003) Salinity and nitrogen rate effects on the growth and yield of Chile pepper plants. Soil Sci Soc Am J 67:1781–1789. https://doi.org/10.2136/SSSAJ2003.1781
Wechter PW, Kousik C, McMillan M, Levi A (2012) Identification of Resistance to Fusarium oxysporum f. sp. niveum Race 2 in Citrullus lanatus var. citroides Plant Introductions. HortScience 47:334–338. https://doi.org/10.21273/HORTSCI.47.3.334
Yasar F, Ellialtioglu S, Kusvuran Çankırı Karatekin Üniversitesi S et al (2006) Ion and lipid peroxide content in sensitive and tolerant eggplant callus cultured under salt stress Determination of physiological and biochemical tolerance level in different pepper genotypes under salt and drought View project GA3 Hormon Uygulamasının Kadmium (Cd) Stresi Altındaki Biber Bitkilerinin Gelişimi, View project Ion and Lipid Peroxide Content in Sensitive and Tolerant Eggplant Callus Cultured under Salt Stress. Eur J Hort Sci 71:1611–4426
Zhong YQ, Bie ZL (2007) Effects of grafting on the growth and quality of cucumber fruits. Acta Hortic 761:341–347. https://doi.org/10.17660/ACTAHORTIC.2007.761.47
Acknowledgements
We thank all staff members of the R&D greenhouse of Kırşehir Ahi Evran University for the technical support and supplying all facilities during the experiments.
Funding
This research was funded by Erciyes University Scientific Research Projects Coordinator (project code FDK-2021-10629).
Author information
Authors and Affiliations
Contributions
The contributions of all authors are defined as follows: study concept and design: A. Aydın, H. Yetişir; data collection: A. Aydın; analysis and interpretation of results: A. Aydın, H. Yetişir; preparing a draft text: A. Aydın, H. Yetişir. All authors reviewed the results and approved the final version of the article.
Corresponding author
Ethics declarations
Conflict of interest
A. Aydın and H. Yetişir declare that they have no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aydın, A., Yetişir, H. Rootstock Effect of Auto- and Allotetraploid Citron (Citrullus lanatus var. citroides) on Hydroponically Grown Cucumber Under Salt Stress. Gesunde Pflanzen 75, 1193–1206 (2023). https://doi.org/10.1007/s10343-022-00782-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-022-00782-4