Abstract
Even though lead (Pb) poses a hazard to the plants, an eco-friendly strategy in reducing Pb toxicity in wheat has received little attention. The purpose of this work was to define the effect of sulfur (S), a critical nutrient element, in reducing Pb toxicity in wheat plants. S reduced the adverse effects of Pb on root biomass, cellular integrity, redox status and chlorophyll score. The exogenous S also restored the Fe status accompanied by the upregulation of TaNAS1 and TaDMAS1 genes in roots. The ICP-MS analysis revealed that Pb concentrations increased in the root, shoot, and vacuole but not in the cell wall subjected to Pb-toxicity compared to the plants cultivated without Pb and S. In addition, cysteine, glutathione, and phytochelatin were increased together with the induction of TaGST and TaPCS1 genes in roots subjected to the dual application of Pb and S. It implies that higher glutathione levels caused by S may allow phytochelatin to bind with excess Pb, resulted in the subcellular sequestration in the root vacuole. S can also stimulate the S‑metabolites in Pb-exposed wheat to restore redox equilibrium. These findings can be used to promote the usage of S and to develop Pb-free wheat through breeding and transgenic initiatives.
Zusammenfassung
Obwohl Blei (Pb) eine Gefahr für die Pflanzen darstellt, wurde einer umweltfreundlichen Strategie zur Verringerung der Pb-Toxizität in Weizen bisher wenig Aufmerksamkeit geschenkt. Ziel dieser Arbeit war es, die Wirkung von Schwefel (S), einem kritischen Nährstoff, bei der Verringerung der Pb-Toxizität in Weizenpflanzen zu bestimmen. Schwefel verringerte die nachteiligen Auswirkungen von Pb auf die Wurzelbiomasse, die zelluläre Integrität, den Redoxstatus und den Chlorophyllwert. Der exogene Schwefel stellte auch den Fe-Status wieder her, begleitet von einer Hochregulierung der Gene TaNAS1 und TaDMAS1 in den Wurzeln. Die ICP-MS-Analyse ergab, dass die Pb-Konzentrationen in der Wurzel, im Spross und in der Vakuole, nicht aber in der Zellwand, die der Pb-Toxizität ausgesetzt war, im Vergleich zu den ohne Pb und S kultivierten Pflanzen zunahmen. Außerdem stiegen Cystein, Glutathion und Phytochelatin zusammen mit der Induktion der Gene TaGST und TaPCS1 in den Wurzeln an, die der doppelten Anwendung von Pb und S ausgesetzt waren. Das bedeutet, dass die durch Schwefel verursachten höheren Glutathionwerte es Phytochelatin ermöglichen, überschüssiges Pb zu binden, was zu einer subzellulären Sequestrierung in der Wurzelvakuole führt. Schwefel kann auch die S‑Metaboliten in Pb-belastetem Weizen stimulieren, um das Redox-Gleichgewicht wiederherzustellen. Diese Erkenntnisse können genutzt werden, um die Verwendung von Schwefel zu fördern und durch Züchtung und transgene Initiativen einen Pb-freien Weizen zu entwickeln.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lead (Pb) is a pollutant that pollutes the environment and has an adverse effect on the growth of plants. Improper management of industrial waste and agrochemicals causes a rapid increase in Pb in soil and water system (Li et al. 2020). The bioavailability of Pb is determined by the amount of Pb present in the soil, the physical and chemical condition of the soil, and the exact genotype of specific plant species (Peralta-Videa et al. 2009; Li et al. 2020). Pb is immobilized in the soil as a result of its complicated formation, and as a result, it is inaccessible for plant absorption (Peralta-Videa et al. 2009). Pb is taken up by the root epidermal cells and then transported to the root xylem vessels, where it is distributed to the rest of the plant’s organs. All transporter proteins in plants are membrane proteins, and they are all responsible for maintaining Pb homeostasis (Romanowska et al. 2002). Pb-toxicity in soil caused poor biomass, reduced growth, chlorosis, photosynthetic disturbance, and alteration in water balance, nutrient uptake, and maturity in plants (Pourrut et al. 2011). Early on in the course of Pb poisoning, the production of reactive oxygen species (ROS) such as H2O2 increases, resulting in secondary oxidative stress and growth inhibition (Yang et al. 2010). According to Mroczek-Zdyrska and Wójcik (2012), Pb toxicity reduces the number of mitochondria in the cell, which negatively impacts the photosynthesis and respiration processes by diminishing oxidative phosphorylation potential.
Plants absorb sulfur (S) as sulfate, which is required for the biosynthesis of proteins, co-enzymes, prosthetic groups, vitamins, amino acids as well for the tolerance of abiotic and metabolic stress (Yoshimoto et al. 2003). Synthetic S‑containing molecules such as the amino acids cysteine and methionine, as well as proteins and lipids as well as coenzymes and many secondary metabolites of sulfur, can be produced using this method (Saito 2004; Siddiqui et al. 2020). S is linked to co-enzymes, proteins, vitamins, anti-oxidants, glutathione, methionine, cysteine, and phytochelatin, as well as glutathione and methionine synthesis (Yoshimoto et al. 2003; Kabir et al. 2016). Furthermore, glutathione serves as a precursor for phytochelatin (PC) production, and it is produced by the glutathione synthetase (GS) enzyme (Ortega-Villasante et al. 2007). The glutathione S‑Transferase (GST) has been shown to defend plants against some abiotic stressors, including heavy metals (Zhang et al. 2013). The biosorption capacity of Pb can be found within the root cell wall, which can help to reduce heavy metal translocation. Metals are sequestered intracellularly mediated by PC, which is commonly observed in the detoxification process (Zhang et al. 2013). PC’s ability to bind to metal is mostly determined by the occurrence or lack of metal, which is commonly located in the root vacuole of the cell (Bari et al. 2019). The PCS gene is activated, on the other hand, and is principally responsible for PC accumulation in plants, resulting in the accumulation of heavy metal chelates (Piotrowska-Niczyporuk et al. 2020).
Excessive ROS in plant cells is a well-known side effect of Pb toxicity (Gechev and Petrov 2020). Furthermore, plants contain adaptive systems for scavenging ROS, which are generally controlled by glutathione (Rahman et al. 2020). To detoxify ROS molecules, glutathione interacts with them and also regulates enzymes that combat free radicals in the body (Kabir et al. 2016). Furthermore, various endogenous metabolites (such as cysteine, glycine and alanine) play crucial roles in decreasing metal stress damage, according to the study (Kabir 2016). Aside from enzymes, the amino acids cysteine and methionine have also been linked to the lowering of ROS in plants, according to research. Furthermore, methionine is essential for the production of nicotianamine, which is beneficial in maintaining metal homeostasis (Bonneau et al. 2016).
Wheat (Triticum aestivum L.) is a grass species that is one of the world’s oldest and most important cereal crops, with great nutritional benefits. It is also one of the most widely planted crops in the world. It is believed that the addition of excess S helps to protect plants against the toxicity of heavy metals (Park et al. 2012). However, the mechanisms behind S‑mediated Pb-toxicity detoxification in wheat are unknown. Therefore, we investigated whether and how exogenous S decreases Pb toxicity in wheat plants. We also performed a series of physiological, biochemical, and molecular techniques to investigate the mechanistic basis of S‑mediated Pb detoxification in wheat.
Material and Methods
Plant Cultivation
Wheat seeds (BARI Gom-26) were disinfected with 70% ethyl alcohol for three minutes before being rinsed twice with distilled water. At room temperature, the sterilized seeds sprouted on a germination plate containing moist tissue paper. Plants that are 2 days old were subsequently transported into 2 liters of half-strength aerated Hoagland solution (Hoagland and Arnon 1950). In addition to these components, the following was done with Pb and S: control (without 500 μM Pb(NO3)2 and 2.5 mM K2SO4), +Pb (500 μM Pb(NO3)2), +Pb +S (500 μM Pb(NO3)2 and 2.5 mM K2SO4), and +S (2.5 mM K2SO4). The pH of the nutrient solution was adjusted to 6.0 using 1 M KOH. Plants were grown for 7 days after transferring to the hydroponic conditions under 10 h of light and 14 h of darkness (550–560 μmol s−1 perμA) in the growth room before analyzing data.
Measurement of Morphological Parameters and Chlorophyll Score
The length of the longest root and shoot of each sample was measured using a digital caliper. Before being weighed dry, the root and shoot were dried in an electric oven for three days at 80 °C. A chlorophyll meter (atLEAF CHL STD, Wilmington, Delaware, USA) was used to measure the chlorophyll content of juvenile leaves.
Determination of Electrolyte Leakage
The electrolyte leakage, an indicator of the cell membrane integrity, was determined using a conductivity meter (Lutts et al. 1996). Briefly, contaminants on the root and shoot surface were removed by repeated washing with distilled water. Following that, the new samples were moved to a beaker containing distilled water (20 mL) and maintained at 25oC for 2 h. Finally, the electrical conductivity of the solution was calculated as a percentage of its initial and final conductivity values at the beginning and end of the incubation time.
Measurement of Cell Death (%)
Evans blue was used to calculate the cell death (%) (Zhao et al. 2005). Briefly, the entire fresh root and shoot were placed into an Evans blue mixture tube that contained 2 mL of water and incubated for 15 min. The samples were then transferred to 80% ethanol and kept for 10 min. The tubes were then incubated in a water bath for 15 min at 50 °C and subsequently centrifuged for 10 min at 12,000 rpm. The absorbance of the supernatant was measured at 600 nm. Finally, the total cell loss % in the root and shoot tissues was determined based on the fresh weight.
H2O2 Measurement
As previously mentioned, the H2O2 content in roots was measured using a spectrophotometric technique (Sayed and Gadallah 2019). After cleaning with deionized water, fresh root and shoot were homogenized with 0.1% trichloroacetic acid. To separate the aqueous component, the liquid extract was centrifuged for 15 min at 10,000 rpm. To allow for a 1 h reaction, 10 mM potassium phosphate (pH 7.0) and 1 M KI were added to the top aqueous fraction. Finally, at 390 nm, the absorbance was measured.
\(\text{O}_{2}^{-\cdot}\) Measurement
The superoxide (\(\text{O}_{2}^{-\cdot}\)) concentration was determined by utilizing an extinction coefficient of 2.16 × 104 M−1 cm−1 (Hu et al. 2012). In a nutshell, the samples of root and shoot were rinsed with water and then homogenized using cold K‑phosphate buffer (10 mM) before being centrifuged for 10 min at 12,000 rpm at 4oC. Subsequently, the translucent supernatant was combined with a supplementary test solution containing 0.25 mM XTT sodium salt (2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) and 50 mMTris-HCl, followed by centrifugation (pH). Lastly, the optical densities of the solutions were measured at 580 nm.
Determination of Pb and Fe Concentration
To remove exterior elements, roots were rinsed once with CaSO4 (0.1 mM) and numerous times with Milli‑Q water. The roots and shoots were then individually desiccated in an oven for 72 h at 70 ºC. The dried materials were then weighed and dissolved in a 1:3 v/v suspension of HClO4/HNO3, and the Pb and Fe concentrations in the digested solution were determined using ICP-MS (inductively coupled plasma mass spectrometry) based on the standard known solution of these components (Agilent 7700, ICP-MS). The centrifugation procedures mentioned before were utilized to measure Pb compartmentalization in the cell wall and root vacuole (Pourghasemian et al. 2019). In summary, root fresh weight was obtained and mashed using a mortar and pestle (chilled) in 1 ml extraction buffer (500 mM sucrose, 50 mM HEPES, 5.0 mM ascorbic acid, 1.0 mM DTT (dithiothreitol), 1.0% (w/v) PVP) after multiple washes with Mili Q water (polyvinylpyrrolidone). The homogenate was sifted using a nylon cloth (10 mm), and the residue on the nylon fabric was rinsed twice with the same homogenization buffer (1 ml) and labeled as the cell wall fraction. The precipitate was labeled as the fraction of the vacuole after centrifuging the first filtrate at 4000 rpm for 10 min. The particles were dried in an oven after the supernatant was discarded. ICP-MS was used to calculate the Pb concentrations in the cell wall and vacuole.
Cys, GSH and PC Determination
Empower3TM software and high-performance liquid chromatography (HPLC) at 280 and 360 nm with a dual Waters 2489 detector were used to analyze cysteine, glutathione, and phytochelatin in wheat roots and shoots (Kabir et al. 2016). As gradient conditions, a C18 reverse-phase column was employed with 100% acetonitrile as the mobile phase (Kabir et al. 2016). Before injection (0.22 mm Minisart Syringe Filters), the extracts and samples were diluted (100 times) and filtered. For identifications, known GSH and PC3 standards were employed (Greger et al. 2016). By comparing the standard’s peak area and retention time, the concentration of the unidentified samples was determined.
Expressions of Genes
In response to Pb stress, the genes involved in vacuolar storage (TaNAS1 and TaDMAS1) and sulfate transport (TaGST and TaPCS1) in wheat plants were found in the ENSEMBL (https://plants.ensembl.org/Triticum aestivum/Info/Index) and NCBI databases. As previously disclosed, total RNA was extracted from wheat plant root tissues using the SV total RNA isolation system kit (Promega, USA). In a nutshell, RNA extraction buffer was used to homogenize 80 mg of root samples from each condition in a mortar pestle, and 1% (v/v) β‑mercaptoethanol was added before centrifuging at 12,000 rpm for 2 min. After the washing processes were completed, additional RNase-free water was used to recover RNA from the RNA spin columns, and the RNA’s final yield was measured using a Nanodrop Spectrophotometer. The first-strand cDNA was generated from RNA using the PrimeScriptTM RT kit (Takara, United States). The real-time PCR was done on a CFX-96TM real-time PCR (BIORAD, United States) using gene-specific primers created using the NCBI primer designing tool (Supplementary Table S1) modeled on the database of sorghum genes. The PCR protocol was as follows: 95oC for 30 sec, followed by 40 cycles of 95oC for 5 sec and 60oC for 30 sec. The expression level of the target gene was measured using Actin as an internal control (Livak and Schmittgen 2001). Three independent replications were performed for each condition.
Statistical Analysis
All studies were carried out using three distinct biological replications. The significance level for analysis of variance (ANOVA) was set at 5%, which was subsequently followed by Duncan’s Multiple Ranking Test (DMRT) (SPSS Statistics 20 software). The GraphPad Prism 6 software produced graphic figures.
Results
Chlorophyll Score and Morphological Parameters
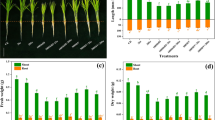
The chlorophyll score was significantly reduced in leaves of wheat owing to the toxicity of Pb in contrast to controls (Fig. 1). The exogenous S combined with Pb showed a significant increase in chlorophyll score in contrast to Pb-exposed wheat. Plants only cultivated with S displayed a similar chlorophyll score compared to the rest of the parameters except for Pb-exposed plants (Fig. 1b). Pb-stressed plants showed adverse phenotypic effects including reduced root dry weight and root length than the plants cultured with Pb and S (Fig. 2a,b). However, exogenous S applied with Pb-stressed plants caused a significant improvement in these root morphological characteristics contrasting with Pb-stressed wheat. The addition of S merely demonstrated resembling morphological features compared to the controls and plants exposed to Pb with S (Fig. 2a–d). However, there were no significant differences in shoot height or dry weight when Pb or S was present or not (Fig. 2c,d).
Phenotype (a) and leaf chlorophyll (b) score in wheat plants cultivated in different growth conditions: control (without K2SO4 and PbSO4), +Pb (500 µM PbSO4), +Pb +S (500 µM PbSO4 and 2.5 mM K2SO4) and +S (2.5 mM K2SO4). Data represent means ± SD of three independent biological samples. Different letters indicate significant differences at P < 0.05 level, where applicable
Root length (a), root dry weight (b), shoot height (c) and shoot dry weight (d) in wheat plants cultivated in different growth conditions: control (without K2SO4 and PbSO4), +Pb (500 µM PbSO4), +Pb +S (500 µM PbSO4 and 2.5 mM K2SO4) and +S (2.5 mM K2SO4). Data represent means ± SD of three independent biological samples. Different letters indicate significant differences at P < 0.05 level
Changes in Stress Indicators
Wheat plants that exposed to Pb manifested a considerable rise in electrolyte leakage, H2O2 and \(\text{O}_{2}^{-\cdot}\) concentration in roots in comparison to the controls (Fig. 3a,c,d). Despite this, the addition of both S and Pb resulted in a significant reduction in root electrolyte leakage, H2O2 and \(\text{O}_{2}^{-\cdot}\) concentrations compared to the plants exposed to Pb (Fig. 3a,c,d). Additionally, plants treated alone with S had similar electrolyte leakage, H2O2 and \(\text{O}_{2}^{-\cdot}\) concentrations in the roots relative to controls (Fig. 3a,c,d). However, no significant changes were found in electrolyte leakage, H2O2, or \(\text{O}_{2}^{-\cdot}\) concentrations in the shoot between the treatments (Fig. 3a,c,d). Besides this, the cell death (%) was significantly increased due to Pb-toxicity both in root and shoot compared to controls (Fig. 3b). However, when S was combined with Pb, there was a considerable reduction in root and shoot cell death (%) compared to plants exposed to Pb. Plants treated alone with S had the similar root and shoot cell death as controls (Fig. 3b).
Electrolyte leakage (a), cell death % (b), H2O2 (c) and \(\text{O}_{2}^{-\cdot}\) (d) in wheat plants cultivated in different growth conditions: control (without K2SO4 and PbSO4), +Pb (500 µM PbSO4), +Pb +S (500 µM PbSO4 and 2.5 mM K2SO4) and +S (2.5 mM K2SO4). Data represent means ± SD of three independent biological samples. Different letters indicate significant differences at P < 0.05 level
The Concentration of Pb and Fe
When compared to untreated controls, the concentration of Pb in the roots and shoots of wheat plants increased considerably as a result of Pb stress (Fig. 4a,b). Plants grown with Pb and S showed no significant changes in Pb concentration in the root, but the Pb concentration in the shoot decreased significantly as compared to plants grown with Pb-toxicity (Fig. 4a,b). Furthermore, Pb concentrations in root vacuoles increased in Pb-stressed plants compared to untreated controls (Fig. 4c). Moreover, S added with Pb further displayed a significant increase of Pb in root vacuoles relative to Pb-stressed wheat (Fig. 4c). Pb concentration in the root cell wall increased significantly when Pb was added alone or in combination with S. (Fig. 4d). Pb concentrations in the root, shoot, vacuoles, and cell walls of wheat plants treated separately with S were similar to that of controls (Fig. 4a–d).
Pb concentration in root (a), Pb concentration in shoot (b), Pb concentration in vacuole (c) and Pb concentration in cell wall (d), Fe concentration in root (e) and Fe concentration in shoot (f) of wheat plants cultivated in different growth conditions: control (without K2SO4 and PbSO4), +Pb (500 µM PbSO4), +Pb +S (500 µM PbSO4 and 2.5 mM K2SO4) and +S (2.5 mM K2SO4). Data represent means ± SD of three independent biological samples. Different letters indicate significant differences at P < 0.05 level
In this experiment, Fe concentration significantly declined because of the detrimental effect of Pb as opposed to untreated controls in both root and shoot. Subsequently, Fe concentration was strongly inversed due to exogenous S together with Pb (Fig. 4e,f). Plants grown alone with S had Fe levels in the root and shoot that were similar to controls (Fig. 4e,f).
S-metabolites and Phytochelatin Analysis
Among the metabolites, cysteine and glutathione concentrations in roots decreased significantly due to Pb-toxicity in comparison to the untreated controls (Fig. 5a,b). However, plants cultivated in the presence of Pb with S demonstrated significantly increased cysteine and glutathione levels in roots compared to Pb-exposed conditions. Furthermore, plants grown alone with S had similar cysteine and glutathione levels in the roots relative to controls (Fig. 5a,b). PC content, on the other hand, demonstrated no significant alterations in response to Pb stress compared to controls (Fig. 5c). In contrast to the other treatments (control, +Pb, and +S), the addition of S with Pb resulted in a significant accumulation of PC in roots (Fig. 5c).
Cysteine (a), GSH (b) and PC concentration (c) in the roots of wheat plants cultivated in different growth conditions: control (without K2SO4 and PbSO4), +Pb (500 µM PbSO4), +Pb +S (500 µM PbSO4 and 2.5 mM K2SO4) and +S (2.5 mM K2SO4). Data represent means ± SD of three independent biological samples. Different letters indicate significant differences at P < 0.05 level
Expression Analysis of Candidate Genes
When roots were exposed to Pb, the expression of TaNAS1 decreased considerably compared to controls (Fig. 6). In comparison to Pb-exposed plants, exogenous S with Pb resulted in a significant induction of the TaNAS1 gene. TaNAS1 expression in the roots of plants grown solely with S was similar to that of controls (Fig. 6). Furthermore, the expression of the TaDMAS1 gene in wheat roots was dramatically reduced due to Pb compared to controls. When compared to Pb-exposed plants, Pb with exogenous S showed a substantial increase in TaDMAS1 expression in roots. Further, the expression of TaDMAS1 showed similar expression pattern under exogenous S in the roots to that of controls (Fig. 6). Finally, there were no significant differences in the expression of TaGST and TaPCS1 genes in roots between controls and Pb-toxic conditions (Fig. 6). The addition of S with Pb showed a significant upregulation of TaGST and TaPCS1 genes in roots to that of Pb-exposed plants. However, the expression of TaGST and TaPCS1 showed similar expression patters in roots to those of controls and Pb-toxic plants (Fig. 6).
Quantitative gene expression analysis in the roots of wheat plants cultivated in different growth conditions: control (without K2SO4 and PbSO4), +Pb (500 µM PbSO4), +Pb +S (500 µM PbSO4 and 2.5 mM K2SO4) and +S (2.5 mM K2SO4). Data represent means ± SD of three independent biological samples. Different letters indicate significant differences at P < 0.05 level
Discussion
Pb is a hazardous contaminant that causes cell damage and an increase in ROS, resulting in diminished growth and development in plants (Mroczek-Zdyrska and Wójcik 2012). S is an essential element that plays a key role in physiological function as well as resistance to a variety of abiotic stimuli, including heavy metal stress (Saifullah et al. 2016; Das et al., 2021). In this investigation, exogenously adding S into the culture medium resulted in improvements in the morphological and physiological properties of wheat that had been exposed to Pb. We revealed how S indirectly promotes Pb detoxifying mechanisms in wheat plants by fine-tuning of physiological and molecular features.
In wheat, S supplementation resulted in a considerable increase in root biomass and length. The decrease in plant biomass is reported in several plants when exposed to heavy metals due to cell injury (Rucinska-Sobkowiak 2016; Das et al. 2021). However, we did not see growth retardation in shoot parameters in Pb-stressed wheat. This is possibly due to the early age of the wheat plants. In sorghum, the early stage Fe-deficiency also caused no damage in shoot (Prity et al. 2021). Heavy metals disrupt the equilibrium of ions and the osmosis of plants (Roy et al. 2016). The morphological improvement in wheat plants following S addition in the presence of Pb is compatible with decreased cell death and electrolyte leakage; in addition, S addition improves the redox state in the roots and shoots. As a result, S can aid wheat plant recovery from Pb-induced morphological retardation and cell damage.
Photosynthesis is extremely vulnerable to stress caused by the environment and metals, and it is essential for plant development (Das et al. 2021). Chlorophyll degradation, photosystem damage, photosynthetic electron flow arrest, and lower PSI and PSII quantum yields are all symptoms of Pb poisoning in plants (Mroczek-Zdyrska and Wójcik 2012; Mallhi et al. 2019). In this investigation, the presence or absence of Pb and S revealed a substantial connection between chlorophyll score and photosynthesis. Under Pb stress, wheat plants were unable to regulate the photosynthetic activity, as evidenced by the decrease in chlorophyll score. Surprisingly, when wheat plants were supplied with S under Pb stress, chlorophyll scores were restored. These findings are in agreement with Cd-stressed alfalfa in which S surplus showed substantial improvement in chlorophyll synthesis and photosynthesis biophysical traits (Das et al. 2021). In another study, S fertilization in the presence of Pb boosted photosynthetic and transpiration rates, resulting in higher wheat straw and grain yields in a pot experiment (Saifullah et al. 2016). Given the fact that Pb toxicity is detrimental to photosynthesis, we further demonstrated the decrease of Fe status in root and leaves of Pb-exposed wheat. Thus, it may also be possible the Pb-induced chlorophyll reduction is related to the decreased Fe accumulation in wheat. This phenomenon is further supported by the restoration of Fe status accompanied by the upregulation of TaNAS1 and TaDMAS1 genes in roots following S addition in Pb-exposed wheat.
Wheat plants’ roots and shoots accumulated a significant amount of Pb in response to Pb treatment. Surprisingly, S in addition to Pb supplementation increased Pb concentration in the root, but subsequent Pb translocation into the shoot in Pb-stressed wheat plants showed no significant changes. This shows that there is an excess of Pb in the root system, resulting in a Pb status in the shoot that is harmless. Although the molecular rationale was not explored, S fertilization was revealed to be crucial in lowering Pb in wheat by increasing Pb accumulation by aboveground plant parts (Saifullah et al. 2016). Thus, we performed an in-depth Pb analysis in vacuole and cell wall of wheat plants to whether any of these organs do have any association with the excess Pb accumulation even after S addition. Excess metals are retained in roots by vacuolar sequestration, which has been observed in some metal-tolerant plant species (Bari et al. 2019). Our analysis showed that it is the vacuole not the cell wall of the root system that showed a significant increase of Pb in response to the dual application of Pb and S compared to Pb-stressed wheat. To understand more about the mechanisms impacted by S, we observed that the concentration of S‑metabolites (cysteine, GSH, and PC) in the root was considerably increased when wheat plants were treated with Pb and S at the same time. It suggests that S supplementation induces an increase in S‑metabolites, which causes PC levels to rise, allowing more Pb to bind to it. However, whether PC-induced Pb retention happens in plants’ vacuoles or cell walls is unknown (Bari et al. 2019; Das et al. 2021). According to Carrier et al. (2003), the negatively charged cell walls bond to the positively charged metal via the ability to exchange cations. S‑mediated Pb accumulation in wheat roots is linked to increased Pb accumulation in vacuoles rather than cell walls, according to our findings. This demonstrates that in wheat plants, the reduction of Pb toxicity is followed by an increase in Pb deposition in the vacuole, which is mediated by PC. To further validate these findings, we performed real-time PCR analysis of candidate genes responsible for GSH and PC synthesis in wheat plants. While Pb and S were used to treat wheat plants at the same time, the TaGST and TaPCS1 genes were found to be upregulated in the roots. The influence of S‑induced PC buildup on Cd detoxification in Tartary buckwheat was also reported by Lu et al. (2019), albeit this result was not supported by molecular data. Overexpression of arsenic-phytochelatin synthase 1 (AsPCS1) and yeast cadmium factor 1 (YCF1) in Arabidopsis thaliana boosted tolerance to Cd and As, as well as the ability to accumulate more metals (Shukla et al. 2013; Guo et al. 2012). Thus, PCS can be a promising candidate gene to increase resistance in plants to heavy metals.
Conclusion
The addition of exogenous S significantly increases the root biomass, cellular integrity, and redox status under Pb stress in wheat. In addition, the S supplementation in Pb-exposed wheat improved the chlorophyll score and Fe status in the roots and shoots, accompanied by the upregulation of Fe-related genes (TaNAS1 and TaDMAS1). Furthermore, S treated with Pb revealed a considerable increase in Pb in the vacuole but not in the cell wall, indicating that safe Pb deposition in the root vacuole inhibits long-distance Pb transport in wheat (Fig. 7). Concomitantly, the increase of glutathione and phytochelatin accompanied by the upregulation of TaGST and TaPCS1 expression in root confirm the PC-driven vacuolar sequestration of Pb due to S supplementation in wheat. The findings further suggest that S‑metabolites have a role in scavenging Pb-induced oxidative damage in wheat. These conclusions provide vital information to lessen the environmental and health risks of bioremediation of Pb in wheat and other crops.
References
Bari MA, Akther MS, Reza MA, Kabir AH (2019) Cadmium tolerance is associated with the root-driven coordination of cadmium sequestration, iron regulation, and ROS scavenging in rice. Plant Physiol Biochem 136:22–33
Bonneau J, Baumann U, Beasley J, Li Y, Johnson AA (2016) Identification and molecular characterization of the nicotianamine synthase gene family in bread wheat. Plant Biotechnol J 14(12):2228–2239
Carrier P, Baryla A, Havaux M (2003) Cadmium distribution and microlocalization in oilseed rape (Brassica napus) after long-term growth on cadmium contaminated soil. Planta 216:939–950
Das U, Rahman MA, Ela EJ, Lee K, Kabir AH (2021) Sulfur triggers glutathione and phytochelatin accumulation causing excess Cd bound to the cell wall of roots in alleviating Cd-toxicity in alfalfa. Chemosphere 262:128361
Gechev T, Petrov V (2020) Reactive oxygen species and abiotic stress in plants. Int J Mol Sci 21(20):7433
Guo J, Xu W, Ma M (2012) The assembly of metals chelation by thiols and vacuolar compartmentalization conferred increased tolerance to and accumulation of cadmium and arsenic in transgenic Arabidopsis thaliana. J Hazard Mat 199/200:309–313
Greger M, Kabir AH, Landberg T, Maity PJ, Lindberg S (2016) Silicate reduces cadmium uptake into cells of wheat. Environ poll 211:90–7
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circ Calif Agric Exp Stat 347
Hu L, Li H, Pang H, Fu J (2012) Responses of antioxidant gene, protein and enzymes to salinity stress in two genotypes of perennial ryegrass (Lolium perenne) differing in salt tolerance. J Plant Physiol 169:146–156
Kabir AH, Hossain MM, Khatun MA, Mandal A, Haider SA (2016) Role of Silicon Counteracting Cadmium Toxicity in Alfalfa (Medicago sativa L.). Front Plant Sci 7:1117
Li J, Wang SL, Zhang J, Zheng L, Chen D, Wu Z (2020) Coconut-fiber biochar reduced the bioavailability of lead but increased its translocation rate in rice plants: elucidation of immobilization mechanisms and significance of iron plaque barrier on roots using spectroscopic techniques. J Hazard Mater 389:122117
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2‑∆∆CT method. Methods 25:402–408
Lu Y, Wang QF, Li J, Xiong J, Zhou LN, He SL, Zhang JQ, Chen ZA, He SG, Liu H (2019) Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Sci Rep 9(1):7397
Lutts S, Kinet JM, Bouharmont J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Mallhi ZI, Rizwan M, Mansha A, Ali Q, Asim S, Ali S, Hussain A, Alrokayan SH, Khan HA, Alam P, Ahmad P (2019) Citric acid enhances plant growth, photosynthesis, and phytoextraction of lead by alleviating the oxidative stress in castor beans. Plants 8(11):525
Mroczek-Zdyrska M, Wójcik M (2012) The influence of selenium on root growth and oxidative stress induced by lead in Vicia faba L. minor plants. Biol Trace Elem Res 147:320–328
Ortega-Villasante C, Hernández LE, Rellán-Álvarez R, Del Campo FF, Carpena-Ruiz RO (2007) Rapid alterration of cellular redox homeostasis upon exposure to cadmium and mercury in alfalfa seedlings. New Phytol 176:96–107
Park J, Song WY, Ko D, Eom Y, Hansen TH, Schiller M, Lee TG, Martinoia E, Lee Y (2012) The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J 69:278–88
Peralta-Videa JR, Lopez ML, Narayan M, Saupe G, Gardea-Torresdey J (2009) The biochemistry of environmental heavy metal uptake by plants: implications for the food chain. Int J Biochem Cell Biol 41:1665–1677
Piotrowska-Niczyporuk A, Bajguz A, Kotowska U, Zambrzycka-Szelewa E, Sienkiewicz A (2020) Auxins and cytokinins regulate phytohormone homeostasis and thiol-mediated detoxification in the green alga Acutodesmus obliquus exposed to lead stress. Sci Rep 10(1):10193
Pourghasemian N, Landberg T, Ehsanzadeh P, Greger M (2019) Different response to cd stress in domesticated and wild safflower (Carthamus spp.). Ecotoxicol Environ Saf 171:321–328
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity, and detoxification in plants. Rev Environ Contamin Toxicol 213:113–136
Prity SA, El-Shehawi AM, Elseehy MM, Tahura S, Kabir AH (2021) Early-stage iron deficiency alters physiological processes and iron transporter expression, along with photosynthetic and oxidative damage to sorghum. Saudi J Biol Sci 28(8):4770–4777
Rahman MA, Kabir AH, Mandal A, Roy SK, Song Y, Ji HC, Lee KW (2020) Glutathione restores Hg-induced morpho-physiological retardations by inducing phytochelatin and oxidative defense in alfalfa. Biology 9(11):364
Romanowska E, Wasilewska W, Fristedt R, Venerand AV, Zienkiewicz M (2002) Phosphorylation of PSII proteins in maize thylakoids in the presence of Pb ions. J Plant Physiol 169:345–352
Roy SK, Cho SW, Kwon SJ, Kamal AH, Kim SW, Oh MW, Lee MS, Chung KY, Xin Z, Woo SH (2016) Morpho-physiological and proteome level responses to cadmium stress in sorghum. PLoS ONE 11(2):e150431
Rucinska-Sobkowiak R (2016) Water relations in plants subjected to heavy metal stresses. Acta Physiol Plant 38:257
Saifullah KMN, Iqbal M, Naeem A, Bibi S, Waraich EA, Dahlawi S (2016) Elemental sulfur improves growth and phytoremediative ability of wheat grown in lead-contaminated calcareous soil. Int J Phytoremediat 18(10):1022–1028
Saito K (2004) Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol 136:2443–2450
Sayed S, Gadallah M (2019) Hydrogen peroxide supplementation alleviates the deleterious effects of cadmium on photosynthetic pigments and oxidative stress and improves growth, yield and pods quality of pea (Pisum sativum L.) plants. Acta Physiol Plant 41:113
Shukla D, Tiwari M, Tripathi RD, Nath P, Trivedi PK (2013) Synthetic phytochelatins complement a phytochelatin-deficient Arabidopsis mutant and enhance the accumulation of heavy metal(loid)s. Biochem Biophys Res Commun 434(3):664–669
Siddiqui MH, Alamri S, Alsubaie QD, Ali HM, Khan MN, Al-Ghamdi A, Ibrahim AA, Alsadon A (2020) Exogenous nitric oxide alleviates sulfur deficiency-induced oxidative damage in tomato seedlings. Nitric Oxide 94:95–107
Yang Y, Wei X, Lu J, You J, Wang W, Shi R (2010) Lead-induced phytotoxicity mechanism involved in seed germination and seedling growth of wheat (Triticum aestivum L.). Ecotoxicol Environ Saf 73:1982–1987
Yoshimoto N, Inoue E, Saito K, Yamaya T, Takahashi H (2003) Phloem-localizing sulfate transporter, Sultr1;3, mediates re-distribution of sulfur from source to sink organs in Arabidopsis. Plant Physiol 131:1511–1517
Zhang Y, Liu J, Zhou Y, Gong T, Wang J, Ge Y (2013) Enhanced phytoremediation of mixed heavy metal (mercury)—organic pollutants (trichloroethylene) with transgenic alfalfa co-expressing glutathione S‑transferase and human P450 2E1. J Hazard Mater 260:1100–1107
Zhao J, Fujita K, Sakai K (2005) Oxidative stress in plant cell culture: a role in production of β‑thujaplicin by Cupresssus lusitanica suspension culture. Biotechnol Bioeng 90:621–631
Acknowledgements
We are grateful to Jessore University of Science & Technology for the ICP-MS service. The work was supported by the grant (5/52/RU/Bio-20/2019–2020) provided by the University of Rajshahi, Bangladesh. In addition, the work was funded by Taif University Researchers Supporting Project number (TURSP—2020/75), Taif University, Taif, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.M. Rahman, A. Swaraz, A.M. El-Shehawi, M.M. Elseehy, M.F. Alam and A.H. Kabir declare that they have no competing interests.
Supplementary Information
Rights and permissions
About this article
Cite this article
Rahman, M.M., Swaraz, A.M., El-Shehawi, A.M. et al. The Mechanistic Basis of Sulfur-mediated Alleviation of Pb Toxicity in Wheat. Gesunde Pflanzen 74, 571–581 (2022). https://doi.org/10.1007/s10343-022-00632-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-022-00632-3