Abstract
Two independent field experiments (2017 and 2019) were conducted to evaluate the effects of plant growth promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi (AMF; AMF1: Rhizophagus irregularis strain and AMF2: AMF consortium) and compost (Comp) in comparison to chemical NPK fertilizers on growth and yield of lettuce plants and soil properties. The biofertilizers-biostimulants were applied alone or in combinations and increased significantly the lettuce dry weight (DW), number of leaves, and yield compared to the control. In the first experiment, the highest plant DW was obtained by NPK, PGPR + AMF2 + Comp and PGPR treatments recording an increase of 109, 109, and 95%, respectively, compared to the control plants. In the second experiment the highest plant DW was obtained by the NPK (77%), followed by Comp and PGPR + AMF1 + Comp treatments increasing the plant DW by 52 and 51%, respectively, compared to the control. Concerning to lettuce yield, in the first experiment, the highest yields were obtained by NPK, PGPR + AMF2, PGPR + AMF1 + Comp, PGPR, AMF2 + Comp, AMF1 + Comp and AMF2 treatments recording an enhancement of 68, 64, 63, 58, 57, 57, and 55%, respectively. In the second experiment, the application of NPK based fertilizers resulted in the highest yield (77%), followed by PGPR + AMF1 + Comp, PGPR + AMF2 + Comp, AMF1 + Comp, and AMF2 + Comp treatments, increasing the yield by 61, 61, 54, and 55%, respectively, compared to the control. Concerning the soil organic matter (OM), the applied treatments had significantly increased the amount of the OM compared to the control. The highest amounts of OM were obtained by the PGPR + AMF2 + Comp treatment in the first experiment and the PGPR + AMF1 + Comp treatment in the second experiment. The available phosphorus (P) was significantly increased by the application of all treatments. The highest records were obtained by the application of Comp, PGPR + AMF1 and PGPR + AMF1 + Comp treatment after the first experiment. In the second experiment, the highest amount of P was obtained by PGPR + AMF2 + Comp treatment. Application of biofertilizers-biostimulants in combination proved to be beneficial for the improvement of the tested culture yield.
Zusammenfassung
In zwei unabhängigen Feldversuchen (2017 und 2019) wurden die Auswirkungen von pflanzenwachstumsfördernden Rhizobakterien (PGPR), arbuskulären Mykorrhizapilzen (AMF; AMF1: Rhizophagus irregularis-Stamm und AMF2: AMF-Konsortium) und Kompost (Comp) im Vergleich zu chemischen NPK-Düngern auf Wachstum und Ertrag von Salatpflanzen und Bodeneigenschaften untersucht. Die Biodünger-Biostimulanzien wurden allein oder in Kombinationen eingesetzt und steigerten das Trockengewicht (DW), die Anzahl der Blätter und den Ertrag von Salat im Vergleich zur Kontrolle deutlich. Im ersten Versuch erzielten die Behandlungen mit NPK, PGPR + AMF2 + Comp und PGPR die höchsten DW-Werte der Pflanzen mit einer Steigerung von 109 %, 109 % bzw. 95 % im Vergleich zu den Kontrollpflanzen. Im zweiten Versuch wurde das höchste Pflanzentrockengewicht durch die NPK-Behandlung (77 %) erzielt, gefolgt von den Behandlungen Comp und PGPR + AMF1 + Comp, die das Pflanzentrockengewicht um 52 % bzw. 51 % im Vergleich zur Kontrolle erhöhten. Was den Salatertrag betrifft, so wurden im ersten Versuch die höchsten Erträge mit den Behandlungen NPK, PGPR + AMF2, PGPR + AMF1 + Comp, PGPR, AMF2 + Comp, AMF1 + Comp und AMF2 erzielt, die eine Steigerung von 68 %, 64 %, 63 %, 58 %, 57 %, 57 % bzw. 55 % aufwiesen. Im zweiten Versuch führte die Anwendung von NPK-Dünger zum höchsten Ertrag (77 %), gefolgt von den Behandlungen PGPR + AMF1 + Comp, PGPR + AMF2 + Comp, AMF1 + Comp und AMF2 + Compt, die den Ertrag um 61 %, 61 %, 54 % bzw. 55 % im Vergleich zur Kontrolle erhöhten. Was die organische Substanz (OM) im Boden anbelangt, so hatten die angewandten Behandlungen einen signifikant höheren OM-Gehalt als die Kontrolle. Die höchsten OM-Mengen wurden durch die PGPR + AMF2 + Comp-Behandlung im ersten Versuch und durch die PGPR + AMF1 + Comp-Behandlung im zweiten Versuch erzielt. Der verfügbare Phosphor (P) wurde durch die Anwendung aller Behandlungen deutlich erhöht. Die höchsten Werte wurden durch die Anwendung der Behandlungen Comp, PGPR + AMF1 und PGPR + AMF1 + Comp im ersten Versuch erzielt. Im zweiten Versuch wurde der höchste P‑Gehalt durch die Behandlung PGPR + AMF2 + Comp erzielt. Der kombinierte Einsatz von Biodüngern und Biostimulanzien erwies sich als vorteilhaft für die Verbesserung des Ertrags der getesteten Kulturen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world population has reached 7.5 billion people, with a forecast of about 10 billion as the projected global population by 2050 (PRB 2017). At the present time, food security is considered as one of the most serious challenges facing society, which will further enhance demand on the production of global food (Raklami et al. 2019). Therefore, to increase yields, farmers applied intensively chemical products. The use of these chemicals (herbicides, insecticides and fungicides) has increased agricultural production (Raklami et al. 2019; Essalimi et al. 2022). However, these products have extremely polluted the soil and water resources (Riah et al. 2014). The use of pesticides has not only influenced the level of agricultural production and its sustainability, but also the health of users (mainly farmers), those living near to farms and consumers of food products containing pesticides residues (De Jaeger et al. 2012).
Therefore, reliable, environmentally friendly techniques are needed to sustainably meet growing global food demands. Some plant-microbe interactions like plant growth promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi (AMF) and compost has been widely used to enhance the plant growth by different mechanisms’ action (Boutasknit et al. 2020, 2021). In fact, PGPR bacteria are a group of microbes, which colonize the plant roots and improve the plant growth either directly or indirectly (Antoun 2013; Ahemad and Kibret 2014). These microorganisms can enhance the plant growth by atmospheric nitrogen fixation (Dixon and Kahn 2004). They are able to solubilize insoluble P, produce phytohormones such as indole acetic acid (IAA) and gibberellic acids (GAs), and have 1‑aminocycloprapane-1-carboxylic acid (ACC) deaminase activity (Mehnaz et al. 2010; Rokhbakhsh-Zamin et al. 2011; Sharma et al. 2013; Ahemad and Kibret 2014; Kang et al. 2014; Vurukonda et al. 2016; Khan et al. 2016). Moreover, the PGPR bacteria have the ability to produce suppressive metabolites (hydrogen cyanide, siderophores) to deleterious pathogen that act indirectly to enhance plant growth (Rolli et al. 2014; De Souza et al. 2015).
The mycorrhizal symbiosis is particularly important for improving the uptake of relatively immobile and insoluble phosphate ions in the soil (Fitter et al. 2011; Abdel-Salam et al. 2018). AMF can secrete phosphatases to hydrolyze phosphate from organic compounds (Koide and Kabir 2000; Li et al. 2015), and thus improve the crop productivity under P deficiency conditions (Smith et al. 2011; Ergin and Gülser 2016). AMF not only improve the plant nutrition (biofertilizers), but also interfere with the plant’s phytohormones synthesis balance, thereby influencing the plant development (bioregulators) and mitigating the effects of environmental stress (bioprotectors) (Ben-Laouane et al. 2020a; Toubali et al. 2020). This leads to increased biomass and yield, as well as changes in different quality parameters (Antunes et al. 2012). In addition to the above-mentioned benefits, AMF provide other important benefits such as environmental stress tolerance (Garg and Chandel 2011; Jayne and Quigley 2014; Rouphael et al. 2015; Ben-Laouane et al. 2020b; Anli et al. 2020).
However, the compost helps to fight against the degradation of the soil (Bresson et al. 2001), it provides to the plant the major nutrients essential for its growth, and improves the soil’s water balance (Anli et al. 2021). Indeed, compost improves the soil fertility sustainably and effectively by providing the essential elements, such as nitrogen, P, potassium and calcium (Barje et al. 2016; Ben-Laouane et al. 17,18,a, b; Raklami et al. 2020; Anli et al. 2020; Boutasknit et al. 2020). The beneficial effects of the composts on soil fertility include the accumulation of organic carbon included in the humin, humic and fulvic acids fractions (Gobat et al. 2010). These humic acids can positively influence the plant growth (Ekin 2019).
Recently, there are many researches concerning the application of different types of composts, as organic soil amendments, PGPR bacteria or AMF, as biostimulants, in different types of cultivation systems (field and greenhouse cultures) (Flores-Félix et al. 2013; Avio et al. 2017; Vukobratović et al. 2018; Raklami et al. 2019; Anli et al. 2020; Toubali et al. 2020). However, to our knowledge there is no reference dealing with different combination effects of PGPR bacteria, AMF and compost on open field cultivated lettuce.
Lettuce (Lactuca sativa L.) is a very popular vegetable all over the world with a worldwide production of more than 29 million tons grown each year (FAOSTAT 2019). In Morocco, lettuce production is at 5505 tons (FAOSTAT 2019). From a nutritional point of view, lettuce has an important place as it contains vitamins A, B (folic acid), C, E, and minerals such as calcium and iron (Govedarica-Lucic and Perkovic 2015). Due to its high content of vitamin C, lettuce is known for its resistance to infections and its ability to fight anemia. The phytochemicals of the plant L. sativa belong mainly to secondary metabolites that are synthesized during the normal growth of plants or in response to a number of environmental conditions. The plants have been used in traditional medicine since many decades for many ailments including inflammation, pain, stomach problems including indigestion and lack of appetite, bronchitis, and urinary tract infections (Ismail and Mirza 2015). Different reports documented the scientific evidence of its biological activities including antimicrobial, antioxidant, and neuroprotective (Noumedem et al. 2017).
Accordingly, this study aims at investigating the impact of selected biofertilizers-biostimulants: PGPR bacteria, AMF, compost in comparison to recommended NPK based fertilizers doses on the growth, the yield, and the soil physico-chemical properties after harvest of lettuce crop under field conditions.
Material and Methods
Study Sites and Experimental Design
To assess the effects of the selected biofertilizers-biostimulants on growth and yield of lettuce, two independent field experiments were performed:
-
The first experiment was performed in 2017 growing season. The field is located at 13 km south-west of Marrakesh (Commune Tassultant, Marrakesh region, Morocco), the geographical coordinates of the study site are the following: 31° 32′ 30″ N, 08° 01′ 14″ W and 511 m above sea level. The soil of this field had a sandy loam texture, pH: 8.12, EC: 0.138 mS cm−1, total organic carbon: 0.5%, organic matter: 0.87%, NTK: 0.67% and P‑Olsen: 58 mg kg−1 (Table 1).
-
The second experiment was performed in 2019 growing season. The field is located 15 km west of Marrakesh (Commune Essaada, Marrakesh region, Morocco). The geographical coordinates of the study site are the following: 31° 37′ 39.5″ N, 08° 07′ 46.7″ W and 449 m above the sea level. The soil of this field had a sandy clay loam texture, pH: 8.09, EC: 1.065 mS cm−1, total organic carbon: 1.53%, organic matter: 2.64%, NTK: 0.952% and P‑Olsen: 74 mg kg−1 (Table 1).
The climate of the two field experiments is semi-arid with an average annual temperature of 19.6 °C (from September to June) and an average annual rainfall of 250 mm (Boutasknit et al. 2020).
A completely randomized block design with four blocks, each block contains thirteen treatments, and each treatment is consisted of a plot of land of 1.2 m2 (1.5 × 0.8 m) of surface containing 8 plants of lettuce. For the same plot the distance between the two drip lines was 40 cm and between each two internal drippers. The drip hose used was equipped with suitable internal drippers (sheath), which released 8 L/h of water. The distribution of treatments within each block, was conducted randomly. The different applied treatments were as follows:
-
1.
Control plants without inoculation nor amendment (Control),
-
2.
Plants amended with compost (Comp),
-
3.
Plants inoculated with pure AMF strain (Rhizophagus irregularis) (AMF1),
-
4.
Plants inoculated with AMF1 in combination with compost (AMF1 + Comp),
-
5.
Plants inoculated with the AMF consortium alone (AMF2),
-
6.
Plants inoculated with AMF2 in combination with compost (AMF2 + Comp),
-
7.
Plants inoculated with PGPR bacteria (BS14 + BS36) alone (PGPR),
-
8.
Plants inoculated with PGPR bacteria in combination with compost (PGPR + Comp),
-
9.
Plants inoculated with PGPR bacteria and AMF1 (PGPR + AMF1),
-
10.
Plants inoculated with PGPR bacteria and AMF1 and amended with compost (PGPR + AMF1 + Comp),
-
11.
Plants inoculated with PGPR bacteria and AMF2 (PGPR + AMF2),
-
12.
Plants inoculated with PGPR bacteria and AMF2 and amended with compost (PGPR + AMF2 + Comp),
-
13.
Plants fertilized with recommended NPK based fertilizers doses (NPK)
The seedlings of lettuce (Lactuca sativa L. ‘Batavia’) were transferred to the field at the rate of eight plants of lettuce per plot in two rows. The planting distance was 0.4 m between plants and 0.2 m between the two rows. Each two plots were spaced by 0.4 m to avoid any possible contamination.
After two months, plants were harvested. Fresh weight, plant height and number of leaves were determined to assess plant growth. Samples of the harvested plants were frozen in liquid nitrogen and conserved at −20 °C for the biochemical analysis. The rest of the vegetal material was dried at 80 °C until constant weight for dry weight and nutrient determination. As lettuce is a leafy vegetable, the plant yield was evaluated by the fresh weight of the aerial part in tons ha−1.
PGPR Bacteria
The bacterial inoculum consisted of two PGPR bacteria: BS14 (Acinetobacter sp.) and BS36 (Rahnella aquatilis) strains isolated from the rhizosphere of bean plants (Vicia faba L.) in Ait Ourir region (31° 46′ 01″ N, 07° 67′ 65″ W). The inoculum was prepared by multiplication of the strains in Tryptic Soy Broth (TSB) medium and shacked for 2 to 3 days at 28 °C to obtain an optical density (OD = 1) at 600 nm (about 109 colony forming unit (CFU) mL−1). The inoculation of the plants was carried out by spraying 4 mL of the bacterial suspension formed from the two above mentioned strains into equal volumes as close as possible to the roots using a micropipette. A second inoculation (booster) was carried out after 15 days by spraying 8 mL of the bacterial suspension on the plant roots, to increase the rate of these bacteria in the soil and ensure the new formed roots infection.
The quantification in vitro of the plant growth promoting traits of the used strains were examined by standard protocols. Phosphate and potassium solubilization as described by Alikhani et al. (2006), siderophores production (Schwyn and Neilands 1987), exopolysaccharides production (Lee et al. 2007), IAA production (Bano and Musarrat 2003), HCN production (Lorck 1948) and atmospheric nitrogen fixation (Onyeze et al. 2013). The PGPR characteristics of the two bacterial strains are listed in Table 2.
Arbuscular Mycorrhizal Fungi
Two types of AMF were used: (i) pure AMF strain (AMF1, Rhizophagus irregularis), kindly provided by Dr. Hijri (Research Institute of Plant Biology, University of Quebec, Montreal, Canada) and (ii) consortium of arbuscular mycorrhizal fungi (AMF2). The used AMF consortium was isolated from the Tafilalet palm grove located at 500 Km southeast of Marrakesh, it contains a mixture of native species: (i) Glomus sp. (15 spores/g of soil), (ii) Sclerocystis sp. (9 spores/g soil) and (iii) Acaulospora sp. (one spore/g of soil) (Meddich et al. 2015). Corn (Zea mays L.) plants were used as a host plant to trap and multiply the native mycorrhizal complex naturally associated with date palm and G. irregularis species.
During transplantation, seedlings were inoculated with 2 g of sand containing spores and corn roots pre-mycorrhized by the above AMF. While, non-AMF plants received the same weigh of autoclaved inoculums. After harvest, the AMF frequency (F%) of lettuce roots was determined by the technique described by Phillips and Hayman (1970). Root tissues were cleared by 10% KOH and stained with 0.05% trypan blue in lactic acid (v/v). The AMF frequency was calculated according to the following formula:
Compost and NPK Based Fertilizers
The compost used in this work was obtained from the composting unit of the Faculty of Sciences Semlalia Marrakesh (FSSM) (Marrakesh, Morocco). Compost was produced from green waste selectively collected from gardens pruning. The compost was amended at the rate of 10 tons ha−1 (Boutasknit et al. 2021). Their main characteristics are reported in Table 1.
For the NPK based fertilizers, we used the mixture of N‑P‑K respecting the recommended doses: 80-60-190 Kg/ha (Pandorf et al. 2020) using for nitrogen (N) supplementation the Ammonitrate 33.5% at the rate of 28.65 g per plot (1.2 m2), for P fertilization we used the lime superphosphate 18% at the rate of 40 g per plot and for potassium (K) application we used the potassium sulphate 48% at the rate of 47.5 g per plot.
Biochemical Analyses
For sugar and protein content determination, extracts were prepared by grinding 0.5 g of the fresh leaves with 10 mL of 80% ethanol and centrifuged at 4000 rpm for 20 min. The extraction was done three times to have a final volume of 30 mL for each extract. The soluble proteins were determined according to the Bradford (1976) method. Briefly, 5 mL of Bradford reagent was supplemented with 0.1 mL of the ethanolic extract. After homogenization, the reaction mixture was placed for 30 min at 30 °C. Then the absorbance was read at 595 nm. The total sugar content was determined following the colorimetric method. For that, to 0.2 mL of supernatant, 200 µL of phenol (5%) and 1 mL of H2SO4 were added and stirred. After cooling, the absorbance at 485 nm was read (Dubois et al. 1956).
Polyphenol content was measured using the method described by Yamamoto et al. (1977) with slight modifications. One g of fresh leaves was ground in 8 mL of methanol (80%). To ensure a maximum extraction, two supplementary extraction was done by washing the residues with methanol (80%). The mixture of the filtrate and additional filtration were centrifuged at 1000 g for 5 min. 0.2 mL of the supernatant was supplemented to 0.4 mL Folin-Denis reagent and distilled water in a total reaction mixture of 3 mL. After 3 min, 1 mL of Na2CO3 saturated aqueous solution was added at ambient temperature for 1 h to complete the reaction. After that, the absorbance was determined at 765 nm using Gallic acid as standard.
Mineral Analyses
The sodium (Na+), potassium (K+), calcium (Ca2+) and P determination was carried out after plant material mineralization. Briefly, 0.5 g of plant shoot material (dry weight) was mineralized at 550 °C in a muffle furnace for 6 h. Then the samples were soaked for 1 h in 3 mL hydrochloric acid (5 N), filtered and diluted with distilled water up to 25 mL. P was determined according to Olsen et al. (1954). Na+, K+ and Ca2+ elements were determined by flame photometer (AFP 100 flame photometer).
Total nitrogen content in plants was determined using 0.5 g of dry matter. The samples were analyzed by the Kjeldahl method as described by Baize (2000). Briefly, 0.5 g dry weight was mixed with 0.5 g of a catalyst mixture (K2SO4, CuSO4.5H2O and Se) and treated with 5 mL of sulfuric acid (98%). After mineralization, the volume was adjusted to 100 mL with distilled water; 40 mL of the solution were transferred to Kjeldahl bottles containing few drops of NaOH (40%), and the resulting was distilled. The distillate was titrated with 0.02 N sulfuric acid. The nitrogen content was expressed as mg of nitrogen/plant.
Soil Physicochemical Properties
The soil physicochemical properties were analyzed before and after the field experimentations as follow: pH and electrical conductivity (EC) of soil samples (1:4; w:v) were estimated using a digital pH (pH21, Hanna Instruments, Romania) and conductivity meter (LF92, WTW, France), respectively. Soil texture was determined by Robinson’s method (Baize 2000). Total organic carbon (TOC) in the soil samples was estimated using 1 N potassium dichromate and then, back titrated with 0.5 N ferrous ammonium sulphate (Baize 2000). The available form of P was determined with 1 M acidic ammonium fluoride (Olsen et al. 1954) using colorimeter (VR-2000 Spectrophotometer, Selecta, Spain).
Statistical Analyses
The obtained results were analyzed statistically with the CoStat software version 6.400 (Copyright © 1998–2008 CoHort Software). The statistical treatments include an analysis of the variances (ANOVA) followed by a comparison of the means with the Least Significant Difference (LSD) test at P < 0.05. The principal component analyses (PCA) using XLStat software was performed by analyzing correlation between applied treatments and the measured parameters.
Results
Properties of PGPR Strains
The two tested strains showed significant PGPR activities (Table 2). They solubilized tricalcium phosphate. BS14 can also solubilize potassium. While, BS36 was able to fix atmospheric nitrogen. Furthermore, the rhizobacterial strains were able to produce exopolysaccharides up to 152.31 mg of Congo red/OD600 (BS36) and IAA of 12.69 µg/mL for BS36 and 195.64 µg/mL for BS14. However, no strain was able to produce siderophores and HCN.
Mycorrhization Frequency
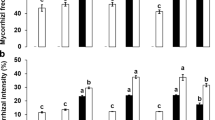
The effectiveness of the AMF mycorrhization was demonstrated by computing the infection frequency of lettuce root system after inoculation with Rhizophagus irregularis strain (AMF1) or with the AMF consortium (AMF2), alone or in various combinations with the PGPR bacteria and the compost. The findings showed the pre-existence of natural and autochthonous AMF in the non-inoculated plants with a frequency of 8.66 and 12.33% for the first experiment and the second experiment, respectively. However, all treatments amended with the AMF1 or the AMF2 alone or in combination with the PGPR bacteria and/or the compost had a mycorrhizal frequency higher than 60%, this proves that the used AMF were more infectious than the native one. For the two field experiments, we noted that the co-inoculation of the AMF consortium and the PGPR bacteria resulted in the highest AMF frequency exceeding 94%. However, the application of compost reduced the AMF frequency in all treatments (Fig. 1).
Effects of biofertilizers-biostimulants on the AMF frequency of lettuce root system. (Bars for each experiment sharing the same letters are not significantly different at P < 0.05 using Least significant (LSD) test. Data presented are means ± SD from three repetitions. Comp compost, AMF1 pure AMF strain, AMF2 AMF consortium, PGPR PGPR bacteria (BS14 + BS36), NPK recommended NPK based fertilizers doses)
Growth Parameters and Yield
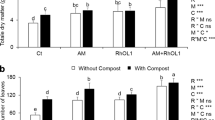
In the current study, we assessed the effects of PGPR bacteria, AMF, composts and an NPK based fertilizers on growth and yield of lettuce. In regards to lettuce total fresh weight (TFW), the obtained results showed that the biofertilizers-biostimulants (PGPR, AMF and compost) application had a positive and significant effect (P < 0.001). All the applied treatments resulted in a significant increase (P < 0.05) in the TFW compared to the control. In the first experiment, the highest TFW was recorded in NPK, PGPR + AMF2, PGPR + AMF1 + Comp, AMF1 + Comp, AMF2 and AMF2 + Comp treatments with an increment up to 67% (Table 3). However, in the second experiment the highest TFW was obtained by the NPK fertilization enhancing the TFW by 77% over the control, followed by the two tripartite combinations and the double combinations (AMF1 + Comp and AMF2 + Comp) with an enhancement up to 61% (Table 3).
As for TFW, the biofertilizers-biostimulants use had a positive and significant effect (P < 0.001) on the total dry weight (TDW) with a significant increase (P < 0.05) by the application of the different treatments compared to the control (Table 3). In the first field experiment, the highest TDW was obtained in plants treated by NPK, PGPR + AMF2 + Comp and PGPR treatments recording an enhancement up to 109% over the control plants. However, in the second experiment the highest TDW was obtained by the NPK (59% of increase than the control plants), followed by the Comp and the PGPR + AMF1 + Comp treatments increasing the TDW by 52 and 51%, respectively (Table 3).
Regarding the plant height, we found a significant effect (P < 0.01) of the biofertilizers-biostimulants use in the two field experiments. Except the AMF2 + Comp, PGPR + AMF1 and PGPR + AMF1 + Comp treatments in the first experiment and AMF2 treatment in the second experiment that had no significant effect on the plant height compared to the control plants, all the other treatments significantly (P < 0.05) enhanced this parameter (Table 3). The maximum plant height was recorded by the compost amendment (21% of increase than the control plants) in the first experiment. In the second experiment, NPK and Comp treatments resulted in an increased plant height (18 and 16%, respectively).
Concerning the leaf number, the use of biofertilizers-biostimulants had a significant effect (P < 0.001) on this parameter. All the studied treatments had significantly (P < 0.05) increased the leaf number than the control plants (Table 3). The highest increase of leaf number was recorded by the application of PGPR + AMF2 + Comp, AMF1 + Comp, AMF2 + Comp and AMF1 treatments in the first experiment exceeding the control plants by up to 81%. In the second experiment, the high leaf number was obtained by the NPK, PGPR + AMF2 + Comp, AMF2 + Comp and AMF1 + Comp treatments reaching up to 82% of enhancement (Table 3).
The application of the biofertilizers-biostimulants had a significant effect (P < 0.001) on the yield. As shown in Table 3, in all treatments there was a significant and positive effect (P < 0.05) of the applied biofertilizers-biostimulants alone or in the different combinations on the final yield of lettuce compared to the non-inoculated plants. In the first experiment, the highest yields were obtained from the application of NPK, PGPR + AMF2, PGPR + AMF1 + Comp, PGPR, AMF2 + Comp, AMF1 + Comp and AMF2 treatments, enhancing the yield over the control plants by 68, 64, 63, 58, 57, 57, and 55%, respectively. In the second experiment, the application of NPK based fertilizers resulted in the highest yield (77% of increment over the control plants), followed by the two tripartite combinations and the double combinations (AMF1 + Comp and AMF2 + Comp) (Table 3). Comparing the two experiment yields, we noted that the second experiment resulted in higher yields than the first experiment.
Biochemical Analyses
Sugar, protein and polyphenol concentrations were measured in leaves. We found a significant effect (P < 0.001) of the applied treatments.
The sugar content was significantly (P < 0.05) enhanced by the applied biofertilizers-biostimulants in the both experiments, except the AMF1 treatment in the second experiment, which had no significant difference compared to the control plants (Fig. 2). In the first experiment, AMF1 + Comp, AMF2 + Comp, PGPR, PGPR + AMF1 + Comp, PGPR + AMF2 and NPK treatments resulted in the highest sugar content with an increment up to 103% (Fig. 2). In the second experiment, the AMF1 + Comp, AMF2 + Comp, PGPR, PGPR + AMF1 + Comp, PGPR + AMF1, PGPR + AMF2 and NPK treatment had resulted in the high soluble sugar content (Fig. 2).
Effects of biofertilizers-biostimulants on soluble sugars content, protein content and polyphenol content of lettuce leaves. (Bars for each experiment sharing the same letters are not significantly different at P < 0.05 using Least significant (LSD) test. Data presented are means ± SD from three repetitions. Comp compost, AMF1 pure AMF strain, AMF2 AMF consortium, PGPR PGPR bacteria (BS14 + BS36), NPK recommended NPK based fertilizers doses)
Determination of protein content revealed that the applied biofertilizers-biostimulants significantly (P < 0.05) enhanced this parameter during the both experiments compared to the control plants (Fig. 2). Plants inoculated with the PGPR bacteria presented the highest protein content in the two experiments (Fig. 2; 96 and 55% over the control in the first experiment and the second experiment, respectively).
As for the two biochemical parameters, the polyphenols content was significantly (P < 0.05) enhanced over the control plants. In the first experiment, the NPK, PGPR + AMF1 + Comp and PGPR + AMF2 treatments showed the highest polyphenols content recording an enhancement up to 69%. However, in the second experiment, PGPR, NPK, the double combinations (AMF2 + Comp, AMF1 + Comp, PGPR + AMF2) and the two triple combinations showed the highest polyphenols content recording an enhancement up to 81% (Fig. 2).
Mineral Analyses
The analysis of plants mineral status showed that the applied treatments had a positive and significant effect on the P (P < 0.001), N (P < 0.001), Na+ (P < 0.001), K+ (P < 0.001) and the Ca2+ (P < 0.01) after the two experiments.
The P content of the lettuce plants was significantly (P < 0.05) enhanced by the applied treatments after the two field experiments, except in plants inoculated with AMF1, which had no significant difference compared to the control plants (Table 4). The NPK based fertilizers resulted in the high P content under the two experiments increasing this mineral element by 162 and 118% in the first experiment and the second experiment, respectively (Table 4). The PGPR bacteria allowed the second high P content in the first experiment with an increment of 134% over the control (Table 4).
Determination of the total nitrogen (N) using the Kjeldahl procedure showed that all treatments significantly (P < 0.05) increased the N content of plants (Table 4). As for the P content, the NPK based fertilizers resulted in the highest N content with an increment of 127 and 147% in the first experiment and the second experiment, respectively, followed by PGPR, PGPR + AMF1, PGPR + AMF1 + Comp, PGPR + AMF2 and PGPR + AMF2 + Comp treatments, which increased the N content up to 114% in the first experiment (Table 4). In the second experiment, PGPR bacteria and the two tripartite combinations presented the second high N level reaching an enhancement up to 114% (Table 4).
The concentration of three mineral elements (Na+, K+ and Ca2+) was measured in lettuce leaves. The findings displayed that the applied biofertilizers-biostimulants significantly (P < 0.05) enhanced these three elements compared to the control plants, except the AMF1 treatment in the first experiment and Comp and AMF1 treatments in the second experiment, which had no significant effect on the Ca2+ content compared to the control plants (Table 4). The highest Na+ amount was recorded by the application of PGPR, PGPR + AMF1 + Comp, PGPR + AMF2 and NPK treatments in the both field experiments (Table 4). Regarding the K+ content, the highest values were recorded by the application of PGPR, PGPR + AMF1 + Comp, PGPR + AMF2, PGPR + AMF2 + Comp and NPK treatments in the first experiment (Table 4). However, in the second experiment the high K+ level was recorded by the inoculation of PGPR bacteria in combination with compost (Table 4). With regard to Ca2+, the high values were recorded by the PGPR + AMF1 + Comp, PGPR + AMF2 and NPK treatments in the first experiment (Table 4). In the second experiment, in addition to the aforementioned treatments the PGPR treatment resulted in high Ca2+ content (Table 4).
The Principal Component Analysis (PCA)
Principal component analysis (PCA) showed the score plot of PCA for the applied treatments for two experiments: the first experiment (a) and the second experiment (b), according to the different measured variables (Fig. 3). For the PCA of the first experiment, the first two principal components are significant and explain 73.27% of the total inertia. PC1 presents 59.56% of the total inertia, whereas PC2 presents 13.71%. For the PCA of the second experiment, the first two principal components are significant and explain 72.88% of the total inertia. PC1 presents 57.09% of the total inertia, whereas PC2 presents 15.79%. Most of the treatments (in bold and surrounded by a rectangle) were pointed to the right of PC1 of the two PCAs, meaning that the applied treatments had large positive loadings on PC1. On the other hand, Control, AMF1, AMF2 and Comp treatments are presented on the left of PC1, meaning that they had large negative loadings on PC1. According to parameters contribution, plant fresh weight, plant dry weight, yield, sugar content, protein content and mineral contents could be considered as markers of the impact of the selected biofertilizers-biostimulants. Moreover, the PCA has revealed a distinct separation between the treatments according to their impact on the studied parameters. The PCA showed that treatments with higher growth, nutrition and yields were on the right, they corresponded to NPK, double and triple combinations between PGPR, AMF and compost (Fig. 3). Lower growth, nutrition and yield levels were on the left, they corresponded to the control without inoculation followed by AMF1, AMF2 and Comp treatments.
Principal component analysis (PCA) for the applied treatments for two experiments: the first experiment was carried out on 2017 culture season (a) and the second experiment carried out on 2019 culture season (b). (Comp compost, AMF1 pure AMF strain, AMF2 AMF consortium, PGPR PGPR bacteria (BS14 + BS36), NPK recommended NPK based fertilizers, LN leaves number, DW plant dry weight, FW plant fresh weight, PT plant total phosphorus content, EC electrical conductivity, TOC total organic carbon, OM organic matter, P‑Olsen soil available phosphorus content, NTK total Kjeldahl nitrogen)
Physicochemical Properties of the Soil
Several soil parameters (pH, EC, TOC, OM and P‑Olsen) were measured in soil fraction associated to the roots of all treatments (Table 5). In the first experiment, the application of compost alone or in the different combinations resulted in a decrease in the pH values of soil (P < 0.05; Table 5). Moreover, the PGPR inoculation alone or in combination with the AMF2 decreased the pH value compared to the control. In the second experiment, only the Comp and PGPR + AMF1 + Comp decreased (P < 0.05) the pH values than the control (Table 5).
The EC of soil after the experiments was significantly (P < 0.05) enhanced by the applied treatments compared to the control, except the EC in the soil inoculated with the AMF1 alone or in combination with PGPR bacteria and the soil treated with the tripartite combination (PGPR + AMF1 + Comp) in the first experiment and the soil amended with AMF2 alone or in combination in the second experiment (Table 5). The highest EC values were recorded in the soil treated with the double combination between PGPR + Comp and the triple combination between PGPR + AMF2 + Comp in the first experiment. However, under the second experiment only the triple combination (PGPR + AMF2 + Comp) resulted in the high EC value (Table 5).
Concerning the TOC% and the OM%, the applied treatments had significantly (P < 0.05) increased the amount of the TOC and the OM than the control, except the soil inoculated with the AMF2 in the first experiment and the soil fertilized with the NPK based fertilizers in the second experiment, which had no significant effect compared to the control (Table 5). In the first experiment, the high amounts of TOC and OM were obtained by the tripartite combination between the PGPR + AMF2 + Comp. However, in the second experiment the highest amount was recorded by the PGPR + AMF1 + Comp treatment.
The available P (P-Olsen) was significantly (P < 0.05) increased by the application of all treatments, except that one obtained by the application of AMF2 in the second experiment (Table 5). The highest records were obtained by the application of Comp, PGPR + AMF1 and PGPR + AMF1 + Comp treatment after the first experiment. In the second experiment the high amount of P‑Olsen was obtained by the triple combination between PGPR + AMF2 + Comp (Table 5).
Discussion
Biofertilizers-biostimulants based on PGPR bacteria, AMF and compost are promising strategies for integrated solutions to agro-environmental problems because inoculants and amendments possess the capacity to promote the plant growth, enhance the nutrient availability and uptake, and support the plants health (Gharib et al. 2008; Bouizgarne et al. 2015; De Souza et al. 2015; Meddich et al. 2015; Barje et al. 2016; Carnot et al. 2017; Anli et al. 2020; Toubali et al. 2020). They are widely known for their positive effect when applied to plants. In this context, this study was conducted to elucidate the impact of selected biofertilizers-biostimulants alone or in different combinations on the growth, yield and quality of lettuce grown in field and on the soil physico-chemical properties after harvest.
The tested rhizobacterial strains showed significant PGPR activities (Table 3). They can solubilize tricalcium phosphate and potassium. These PGPR activities may improve the plant growth and development by providing the essential nutrients such as nitrogen, P, and potassium. Furthermore, the tested rhizobacterial strains were able to produce exopolysaccharides and IAA, which can modulate the plant growth and maintain the water film necessary for the photosynthetic activity and the plant growth (Vurukonda et al. 2016; Khan et al. 2016).
Moreover, the applied AMF in our study are effective and they are adapted to the studied culture, with a high mycorrhization frequency when AMF were applied in combination with PGPR bacteria. The combined inoculation of plants with PGPR bacteria and AMF enhanced root colonization. Comparable results were found by Aalipour et al. (2020) who reported that the double inoculation of Pseudomonas fluorescens and the AMF induced a high AMF colonization (60%). Visen et al. (2017) showed that the simultaneous inoculation with PGPR bacteria considerably stimulates the AMF colonization of litchi plants in comparison to the mono-AMF inoculated plants. Similarly, Raklami et al. (2019) had also reported that the co-inoculation with rhizobacteria and AMF enhanced the AMF efficiency by over 90%. The mechanisms implicated in this phenomenon seem to be related to the PGPR ability to synthesize cell wall-degrading enzymes, which help in the establishment of AMF symbiosis (Visen et al. 2017; Diagne et al. 2020). In contrast, the application of compost reduced AMF colonization. This could result from the nutrient input provided by the compost, which could allow the plant to be less dependent on the AMF symbiosis to acquire sufficient amounts of nutrients (Bücking et al. 2012). The decrease of AMF root colonization by the compost supplementation has been reported by other researches (Ben-Laouane et al. 2020b; Ait-El-Mokhtar et al. 2020). The AMF structures formation can be influenced by the soil characteristics. The organic amendments application like compost, a source of mineral nutrients like P generally decrease AMF root colonization. This could be explained by the release of mineralized P in the soil, which can reduce colonization (Baslam et al. 2011). In disagreement with our results, Ben-Laouane et al. (2020a) showed that the application of compost increased the AMF frequency and intensity, with higher mycorrhization rate were recorded in the inoculated plants with AMF and rhizobia among the use of the compost, reaching 147 and 241% of increment in the frequency and intensity, respectively.
The inoculation with PGPR and/or AMF and/or compost amendment improve the plant growth and productivity of lettuce plants, compared to non-inoculated and non-amended plants. However, the best results were obtained by the application of the double and tripartite combination, which recorded the highest improvements in total fresh biomass, dry biomass, plant height, leaves number and yield of L. sativa plants. Our results are in agreement with those obtained by Anli et al. (2020), who reported that the amendment of compost alone or in combined use with AMF/PGPR bacteria increases the plant growth performance. This improvement of growth traits could be due to the growth promoting mechanisms employed by AMF and PGPR bacteria such as production of phytohormones and solubilization of minerals (Raklami et al. 2019; Anli et al. 2020; Toubali et al. 2020). Previous studies have shown that plants inoculated with AMF/PGPR bacteria and amended with compost accumulate more N and P in the leaves than non-inoculated and non-amended plants (Baslam et al. 2014; Raklami et al. 2019; Anli et al. 2020). These results could be explained by the ability of PGPR bacteria to act by their direct and indirect mechanisms on plants (Sharma et al. 2013; Ahemad and Kibret 2014; Kang et al. 2014; Rolli et al. 2014; De Souza et al. 2015). They have been described as solubilizing agents for the complex P sources, which is mostly unavailable for plants (Kang et al. 2009; Behera et al. 2017). Moreover, bacteria are considered as able agents to modulate the crop growth by the release of phytohormones (auxin, cytokinins) or other antimicrobial and/or antifungal substances for the control of the harmful effects of pathogens (Kang et al. 2009, 2014; Ahemad and Kibret 2014; Bouizgarne et al. 2015; De Souza et al. 2015). Some studies showed that PGPR could stimulate the growth and yield of some vegetable crops such as tomato, lettuce, and broccoli (Yildirim et al. 2008, 2011). Moreover, the AMF have the ability to improve the plants water and mineral status (Carnot et al. 2017), by developing an extensive hypha network that supply plants with water and nutrients, and thereby, enhance the soil structure (Raklami et al. 2019). They are able to solubilize P and mobilize macro-elements and micro-elements (Rouphael et al. 2015). In addition, compost is used as a soil amendment in agriculture to improve the supply of organic carbon and increase the storage capacity of water and nutrients in the soil, resulting in increased photosynthetic activity, growth and yields (Gharib et al. 2008; Anli et al. 2020; Ben-Laouane et al. 17,18,a, b). On the other hand, Bharti et al. (2016) studied the effect of combined application of AM fungus Glomus intraradices (Gi) and halotolerant plant growth promoting bacterium Dietzia natronolimnaea STR1 in promoting the plant growth under saline conditions along with the effect of vermicompost (VC) on the growth of Ocimum basilicum plants. They found that the combined application of STR1, Gi and VC improved the plant growth by 53% under non-saline conditions and recorded doubly increased fresh weight under saline conditions in comparison to the control (non-inoculated) and the bipartite applications of STR1, Gi, and VC. The combined application of STR1 + Gi + VC recorded maximum herb yield under both conditions; non saline and saline conditions.
The tested biofertilizers-biostimulants increased the levels of sugars, proteins and polyphenols content of L. sativa plants compared to the control, especially the double and triple combination between the applied biofertilizers-biostimulants. Accordingly, Anli et al. (2020) demonstrated that the application of biofertilizers-biostimulants based on PGPR, AMF and composts increased the amount of sugar and protein. The triple combination between the aforementioned biofertilizers-biostimulants resulted in the high protein and sugar (Anli et al. 2020). Similarly, Toubali et al. (2020) noted clearly that PGPR, AMF and compost alone or in the different combinations significantly increased the amount of sugar compared to the control plants. The highest sugar concentrations were recorded in the plants treated with the two tripartite combinations (PGPR + AMF1 + Comp and PGPR + AMF2 + Comp) with an enhancement of 73 and 71% respectively (Toubali et al. 2020). Our results are in agreement with those obtained by Raklami et al. (2019) and Boutasknit et al. (2020) who reported increased sugar and protein levels in bean, wheat and garlic under field conditions.
Our results showed an increase in the mineral elements concentration in the plants. The application of PGPR alone or in combination with AMF and/or with compost increased the amount of N, P, K, Na and Ca. This can be attributed to the richness of the compost in N and P (Table 1) and the effective phosphates solubilization of the PGPR strains used in this study. AMF are widely known for their ability to enhance the mineral nutrition of 80% of vascular plants (Smith and Read 2008). Other study showed that the radish seeds inoculation with PGPR bacteria EY37 and EY43 significantly increased concentration of P and N in the plants leaves (Yildirim et al. 2008). Phosphate solubilizing and N2 fixing bacteria can improve the nitrogen and the P nutrition of plants and stimulate the plant growth (Raklami et al. 2019; Anli et al. 2020). In the same way, results obtained by Anli et al. (2020) showed clearly and significantly the positive effect of the applied treatments on P and N content of date palm plants, especially the double and triple combination between composts (C1: grasses waste compost or C2: green waste compost) AMF (AMF1: exotic AMF strain or AMF2: native AMF consortium) and PGPR consortia (B1 or B2). The treatments, which increased the N and P concentrations in this previous study were the following: C1 + B1, AMF1, AMF1 + B2, AMF1 + C1 + B1, AMF2, AMF1 + B1 + C1AMF2 + C1 + B2, and AMF2 + C2 (Anli et al. 2020). Similarly, Raklami et al. (2019) showed that the application of PGPR, AMF and especially their combination increased the N, P, K, Na and Ca concentrations of faba bean cultivated under field conditions.
Our study revealed the improvement of physicochemical properties of soil after harvest. The application of biofertilizers-biostimulants, especially compost increased the quality of soil. In addition, the application of compost reduces soil pH. The application of compost alone or in the different combination resulted in a decrease in the pH values of soil. Moreover, PGPR inoculation alone or in combination with AMF2 decreased the pH value compared to the control. In the second experiment, only the Comp and PGPR + AMF1 + Comp decreased the pH values than the control. Under alkaline pH, the P is complexed with Ca2+ (McLaughlin et al. 2011). By their produced organic acids, PGPR, solubilize complex P while releasing Ca2+ and available P (Behera et al. 2017). AMF may also promote P uptake by increasing its solubility in soil through pH changes or by exudation of P mobilizing compounds like organic acids and phosphatases (Sharif and Claassen 2011). Our results are in accordance with those obtained by Anli et al. (2020) who revealed a decrease in the pH values after biofertilizers-biostimulants application. Significant increments in the OM and available P, especially with the application of compost alone or in combination with PGPR or AMF, were noted and resulted from the high content of OM and available P in the compost. Gaiotti et al. (2017) recorded similar results, showing that inoculation with microorganisms and compost application were very effective in improving the soil quality especially in OM and mineral nutrients (P and N). An increase in the organic matter was observed by the use of compost from vineyard pruning and compost from cattle manure, while the control un-amended stayed similar to those recorded at the beginning of the experiment (Gaiotti et al. 2017). Our results are in accordance with those found by Lashari et al. (2013), they showed that the use of Biochar-poultry manure compost (BPC) increased soil available P content. The available P was seen increased under BPC treatment by over 100% compared to the un-amended soil. Similar results were obtained by Toubali et al. (2020) who worked on date palm vitroplants, they revealed the positive effect of the application of compost, AMF and PGPR alone or in combined use by improving the quality of soil.
Conclusion
The two conducted field experiments after assessing the impacts of the biofertilizers-biostimulants on growth, yield and nutrition of lettuce crop and soil properties, allowed us to conclude that the organic and biological fertilizers used in our study gave noticeable results compared to the control plants. They increased the growth parameters of lettuce plants. Indeed, the amended compost, PGPR bacteria (BS14 and BS36) and AMF, alone or in the different combinations increased the yield and growth parameters and gave similar results to those obtained by the use of NPK based fertilizers. In light of the results obtained in this work, the tested biofertilizers-biostimulants can be a better alternative that is well adapted to reduce the use of chemical fertilizers.
References
Aalipour H, Nikbakht A, Etemadi N, Rejali F, Soleimani M (2020) Biochemical response and interactions between arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria during establishment and stimulating growth of Arizona cypress (Cupressus arizonica G.) under drought stress. Sci Hortic 261:108923. https://doi.org/10.1016/j.scienta.2019.108923
Abdel-Salam E, Alatar A, El-Sheikh MA (2018) Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J Biol Sci 25:1772–1780. https://doi.org/10.1016/j.sjbs.2017.10.015
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J King Saud Univ Sci 26(1):1–20. https://doi.org/10.1016/j.jksus.2013.05.001
Ait-El-Mokhtar M, Baslam M, Ben-Laouane R, Anli M, Boutasknit A, Mitsui T, Wahbi S, Meddich A (2020) Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front Sustain Food Syst 4:131. https://doi.org/10.3389/fsufs.2020.00131
Alikhani HA, Saleh-Rastin N, Antoun H (2006) Phosphate solubilization activity of rhizobia native to Iranian soils. Plant Soil 287(1–2):35–41. https://doi.org/10.1007/s11104-006-9059-6
Anli M, Baslam M, Tahiri A, Raklami A, Symanczik S, Boutasknit A, Ait-El-Mokhtar M, Ben-Laouane R, Toubali S, Ait Rahou Y, Ait Chitt M, Oufdou K, Mitsui T, Hafidi M, Meddich A (2020) Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front Plant Sci 11:1–21. https://doi.org/10.3389/fpls.2020.516818
Anli M, Symanczik S, El Abbassi A, Ait-El-Mokhtar M, Boutasknit A, Ben-Laouane R, Toubali S, Baslam M, Mäder P, Hafidi M, Meddich A (2021) Use of arbuscular mycorrhizal fungus Rhizoglomus irregulare and compost to improve growth and physiological responses of Phoenix dactylifera ‘Boufgouss’. Plant Biosyst 155:763–771. https://doi.org/10.1080/11263504.2020.1779848
Antoun H (2013) Plant-growth-promoting rhizobacteria. In: Brenner’s encycl genet. Elsevier, Québec, pp 353–355
Antunes PM, Franken P, Schwarz D, Rillig MC, Cosme M, Scott M, Hart MM (2012) Linking soil biodiversity and human health: do arbuscular mycorrhizal fungi contribute to food nutrition. In: Wall DH, Bardgett RD, Behan-Pelletier V, Herrick JE, Jones TH, Ritz K, Six J, Strong DR, van Der Putten WH (eds) Soil Ecol Ecosyst Serv, 1st edn. Oxford University Press, Oxford, pp 153–172
Avio L, Sbrana C, Giovannetti M, Frassinetti S (2017) Arbuscular mycorrhizal fungi affect total phenolics content and antioxidant activity in leaves of oak leaf lettuce varieties. Sci Hortic 224:265–271. https://doi.org/10.1016/j.scienta.2017.06.022
Baize D (2000) Guide des analyses en pédologie [Guide of analyzes in pedology], 2nd edn. Editions Quae, Paris
Bano N, Musarrat J (2003) Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr Microbiol 46(5):324–328. https://doi.org/10.1007/s00284-002-3857-8
Barje F, Meddich A, El Hajjouji H, El Asli A, Ait Baddi G, El Faiz A, Hafidi M (2016) Growth of date palm (Phoenix dactylifera L.) in composts of olive oil mill waste with organic household refuse. Compost Sci Util 24(4):273–280. https://doi.org/10.1080/1065657X.2016.1171738
Baslam M, Pascual I, Sánchez-Díaz M, Erro J, García-Mina JM, Goicoechea N (2011) Improvement of nutritional quality of greenhouse-grown lettuce by arbuscular mycorrhizal fungi is conditioned by the source of phosphorus nutrition. J Agric Food Chem 59(20):11129–11140. https://doi.org/10.1021/jf202445y
Baslam M, Qaddoury A, Goicoechea N (2014) Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees 28(1):161–172. https://doi.org/10.1007/s00468-013-0939-0
Behera BC, Yadav H, Singh SK, Mishra RR, Sethi BK, Dutta SK, Thatoi HN (2017) Phosphate solubilization and acid phosphatase activity of Serratia sp. isolated from mangrove soil of Mahanadi river delta, Odisha, India. J Genet Eng Biotechnol 15(1):169–178. https://doi.org/10.1016/j.jgeb.2017.01.003
Ben-Laouane R, Ait-El-Mokhtar M, Anli M, Boutasknit A, Ait Rahou Y, Raklami A, Oufdou K, Wahbi S, Meddich A (2020a) Green compost combined with mycorrhizae and rhizobia: a strategy for improving alfalfa growth and yield under field conditions. Gesunde Pflanz 73(2):193–207. https://doi.org/10.1007/s10343-020-00537-z
Ben-Laouane R, Baslam M, Ait-El-Mokhtar M, Anli M, Boutasknit A, Ait-Rahou Y, Toubali Y, Mitsui T, Oufdou K, Wahbi S, Meddich A (2020b) Potential of native arbuscular mycorrhizal fungi, rhizobia, and/or green compost as alfalfa (Medicago sativa) enhancers under salinity. Microorganisms 8(11):1695. https://doi.org/10.3390/microorganisms8111695
Bharti N, Barnawal D, Wasnik K, Tewari SK, Kalra A (2016) Co-inoculation of Dietzia natronolimnaea and Glomus intraradices with vermicompost positively influences Ocimum basilicum growth and resident microbial community structure in salt affected low fertility soils. Appl Soil Ecol 100:211–225. https://doi.org/10.1016/j.apsoil.2016.01.003
Bouizgarne B, Oufdou K, Ouhdouch Y (2015) Actinorhizal and rhizobial-legume symbioses for alleviation of abiotic stresses. In: Arora NK (ed) Plant microbes symbiosis appl facet. Springer India, New Delhi, pp 273–295
Boutasknit A, Ait-Rahou Y, Anli M, Ait-El-Mokhtar M, Ben-Laouane R, Meddich A (2020) Improvement of garlic growth, physiology, biochemical traits, and soil fertility by Rhizophagus irregularis and compost. Gesunde Pflanz 73(2):149–160. https://doi.org/10.1007/s10343-020-00533-3
Boutasknit A, Anli M, Tahiri A, Raklami A, Ait-El-Mokhtar M, Ben-Laouane R, Ait Rahou Y, Boutaj H, Oufdou K, Wahbi S, El Modafar C, Meddich A (2021) Potential effect of horse manure-green waste and olive pomace-green waste composts on physiology and yield of garlic (Allium sativum L.) and soil fertility. Gesunde Pflanz. https://doi.org/10.1007/s10343-020-00511-9
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bresson LM, Koch C, Le Bissonnais Y, Barriuso E, Lecomte V (2001) Soil surface structure stabilization by municipal waste compost application. Soil Sci Soc Am J 65(6):1804. https://doi.org/10.2136/sssaj2001.1804
Bücking H, Liepold E, Ambilwade P (2012) The role of the mycorrhizal symbiosis in nutrient uptake of plants and the regulatory mechanisms underlying these transport processes. In: Dhal NK, Sahu SC (eds) Plant Sci., 1st edn. IntechOpen, Rijika, pp 107–138
Carnot AC, Desire T, Caustel DKD, Armand N, Emmanuel EL, Ledoux NDG, Annie NN, Zachée A (2017) Effect of the hydric factor and arbuscular mycorrhizal fungi (AMF) on the severity of Phytophthora colocasiae. Plant 5(4):61–67. https://doi.org/10.11648/j.plant.20170504.11
Diagne N, Ndour M, Djighaly PI, Ngom D, Ngom MCN, Ndong G, Svistoonoff S, Cherif-Silini H (2020) Effect of Plant Growth Promoting Rhizobacteria (PGPR) and Arbuscular Mycorrhizal fungi (AMF) on salt stress tolerance of Casuarina obesa (Miq.). Front Sustain Food Syst 4:1–8. https://doi.org/10.3389/fsufs.2020.601004
Dixon R, Kahn D (2004) Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol 2(8):621–631. https://doi.org/10.1038/nrmicro954
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Ekin Z (2019) Integrated use of humic acid and plant growth promoting rhizobacteria to ensure higher potato productivity in sustainable agriculture. Sustainability 11:3417. https://doi.org/10.3390/su11123417
Ergin SF, Gülser F (2016) Effect of mycorrhiza on growth criteria and phosphorus nutrition of lettuce (Lactuca sativa L.) under different phosphorus application rates. Eurasian J Soil Sci 5:275. https://doi.org/10.18393/ejss.2016.4.275-278
Essalimi B, Esserti S, Rifai LA, Koussa T, Makroum K, Belfaiza M, Rifai S, Venisse JS, Faize L, Alburquerque N, Burgos L, El Jadoumi S, Faize M (2022) Enhancement of plant growth, acclimatization, salt stress tolerance and verticillium wilt disease resistance using plant growth-promoting rhizobacteria (PGPR) associated with plum trees (Prunus domestica). Sci Hortic 291:110621
FAOSTAT (2019) Food and agriculture organization of the United Nations statistics division. http://www.fao.org/faostat/en/#data/QC. Accessed 15 Feb 2021
Fitter AHH, Helgason T, Hodge A (2011) Nutritional exchanges in the arbuscular mycorrhizal symbiosis: Implications for sustainable agriculture. Fungal Biol Rev 25(1):68–72. https://doi.org/10.1016/j.fbr.2011.01.002
Flores-Félix JD, Menéndez E, Rivera LP, Marcos-García M, Martínez-Hidalgo P, Mateos PF, Martínez-Molina E, Velázquez ME, García-Fraile P, Rivas R (2013) Use of Rhizobium leguminosarum as a potential biofertilizer for Lactuca sativa and Daucus carota crops. J Plant Nutr Soil Sci 176(6):876–882. https://doi.org/10.1002/jpln.201300116
Gaiotti F, Marcuzzo P, Belfiore N, Lovat L, Fornasier F, Tomasi D, Bel N, Lovat L, Fornasier F, Tomasi D (2017) Influence of compost addition on soil properties, root growth and vine performances of Vitis vinifera cv Cabernet sauvignon. Sci Hortic 225:88–95. https://doi.org/10.1016/j.scienta.2017.06.052
Garg N, Chandel S (2011) Effect of mycorrhizal inoculation on growth, nitrogen fi xation, and nutrient uptake in Cicer arietinum (L.) under salt stress. Turkish J Agric 35:205–214. https://doi.org/10.3906/tar-0908-12
Gharib FA, Moussa LA, Massoud ON (2008) Effect of compost and bio-fertilizers on growth, yield and essential oil of sweet marjoram (Majorana hortensis) plant. Int J Agric Biol 10(4):381–382
Gobat J‑M, Aragno M, Matthey W (2010) Le sol vivant: bases de pédologie—Biologie des sols [Living soil: bases of pedology—Soil biology], 3rd edn. Les Presses polytechniques et universitaires romandes, Lausanne
Govedarica-Lucic A, Perkovic G (2015) Mineral content in a salad leaf (Lactuca sativa L.) depending of the genotype and applied agricultural measures. Genetika 47(3):951–958. https://doi.org/10.2298/GENSR1503951G
Ismail H, Mirza B (2015) Evaluation of analgesic, anti-inflammatory, anti-depressant and anti-coagulant properties of Lactuca sativa (CV. Grand Rapids) plant tissues and cell suspension in rats. BMC Complement Altern Med 15:199. https://doi.org/10.1186/s12906-015-0742-0
de Jaeger C, Voronska E, Fraoucene N, Cherin P (2012) Exposition chronique aux pesticides, santé et longévité. Rôle de notre alimentation. Med Longevite 4:75–92. https://doi.org/10.1016/j.mlong.2012.05.002
Jayne B, Quigley M (2014) Influence of arbuscular mycorrhiza on growth and reproductive response of plants under water deficit: a meta-analysis. Mycorrhiza 24(2):109–119. https://doi.org/10.1007/s00572-013-0515-x
Kang S‑M, Joo G‑J, Hamayun M, Na C‑I, Shin D‑H, Kim HY, Hong J‑K, Lee I‑J (2009) Gibberellin production and phosphate solubilization by newly isolated strain of Acinetobacter calcoaceticus and its effect on plant growth. Biotechnol Lett 31(2):277–281. https://doi.org/10.1007/s10529-008-9867-2
Kang S‑M, Khan AL, Waqas M, You Y‑H, J‑GJ‑H K, J‑GJ‑H K, Hamayun M, Lee I‑J (2014) Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9(1):673–682. https://doi.org/10.1080/17429145.2014.894587
Khan AL, Halo BA, Elyassi A, Ali S, Al-Hosni K, Hussain J, Al-Harrasi A, Lee I‑JJ (2016) Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron J Biotechnol 21:58–64. https://doi.org/10.1016/j.ejbt.2016.02.001
Koide RT, Kabir Z (2000) Extraradical hyphae of the mycorrhizal fungus Glomus intraradices can hydrolyse organic phosphate. New Phytol 148(3):511–517. https://doi.org/10.1046/j.1469-8137.2000.00776.x
Lashari MS, Liu Y, Li L, Pan W, Fu J, Pan G, Jufeng Z, Jinwei Z, Zhang X, Yu X (2013) Effects of amendment of biochar-manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from Central China Great Plain. F Crop Res 144:113–118
Lee VT, Matewish JM, Kessler JL, Hyodo M, Hayakawa Y, Lory S (2007) A cyclic-di-GMP receptor required for bacterial exopolysaccharide production. Mol Microbiol 65(6):1474–1484
Li S, Bi Y, Kong W, Yu H, Lang Q, Miao Y (2015) Effects of arbuscular mycorrhizal fungi on ecological restoration in coal mining areas. Russ J Ecol 46:431–437. https://doi.org/10.1134/S1067413615050173
Lorck H (1948) Production of hydrocyanic acid by bacteria. Physiol Plant 1(2):142–146. https://doi.org/10.1111/j.1399-3054.1948.tb07118.x
McLaughlin MJ, McBeath TM, Smernik R, Stacey SP, Ajiboye B, Guppy C (2011) The chemical nature of P accumulation in agricultural soils-implications for fertiliser management and design: an Australian perspective. Plant Soil 349(1):69–87. https://doi.org/10.1007/s11104-011-0907-7
Meddich A, Jaiti F, Bourzik W, El Asli A, Hafidi M (2015) Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palm (Phoenix dactylifera). Sci Hortic 192:468–474
Mehnaz S, Deeba NB, George L (2010) Genetic and phenotypic diversity of plant growth promoting rhizobacteria isolated from sugarcane plants growing in Pakistan. J Microbiol Biotechnol 20(12):1614–1623. https://doi.org/10.4014/jmb.1005.05014
Noumedem JAK, Djeussi DE, Hritcu L, Mihasan M, Kuete V (2017) Lactuca sativa. In: Medicinal spices and vegetables from Africa. Elsevier, Amsterdam, pp 437–449 https://doi.org/10.1016/B978-0-12-809286-6.00020-0
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. United States Department of Agriculture, Washington
Onyeze RC, Onah GT, Igbonekwu CC (2013) Isolation and characterization of nitrogen-fixing bacteria in the soil. Int J Life Sci Biotechnol Pharma Res 2(3):438–445
Pandorf M, Pourzahedi L, Gilbertson L, Lowry GV, Herckes P, Westerhoff P (2020) Graphite nanoparticle addition to fertilizers reduces nitrate leaching in growth of lettuce (Lactuca sativa). Environ Sci Nano 7:127–138. https://doi.org/10.1039/C9EN00890J
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55(1):158–161
Population Reference Bureau (2017) World population data sheet with a special focus on youth. http://www.worldpopdata.org. Accessed 15 Mar 2020
Raklami A, Bechtaoui N, Tahiri A‑I, Anli M, Meddich A, Oufdou K (2019) Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front Microbiol 10:1–11. https://doi.org/10.3389/fmicb.2019.01106
Raklami A, El Gharmali A, Ait Rahou Y, Oufdou K, Meddich A (2020) Compost and mycorrhizae application as a technique to alleviate Cd and Zn stress in Medicago sativa. Int J Phytoremediation 23(2):190–201. https://doi.org/10.1080/15226514.2020.1803206
Riah W, Laval K, Laroche-Ajzenberg E, Mougin C, Latour X, Trinsoutrot-Gattin I (2014) Effects of pesticides on soil enzymes: a review. Environ Chem Lett 12(2):257–273. https://doi.org/10.1007/s10311-014-0458-2
Rokhbakhsh-Zamin F, Sachdev D, Kazemi-Pour N, Engineer A, Pardesi KR, Zinjarde SS, Dhakephalkar PK, Chopade BA (2011) Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J Microbiol Biotechnol 21(6):556–566. https://doi.org/10.4014/jmb.1012.12006
Rolli E, Marasco R, Vigani G, Ettoumi B, Mapelli F, Deangelis ML, Gandolfi C, Casati E, Previtali F, Gerbino R, Cei FP, Borin S, Sorlini C, Zocchi G, Daffonchio D (2014) Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ Microbiol 17(2):316–331. https://doi.org/10.1111/1462-2920.12439
Rouphael Y, Cardarelli M, Colla G (2015) Role of arbuscular mycorrhizal fungi in alleviating the adverse effects of acidity and aluminium toxicity in zucchini squash. Sci Hortic 188:97–105. https://doi.org/10.1016/j.scienta.2015.03.031
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56. https://doi.org/10.1016/0003-2697(87)90612-9
Sharif M, Claassen N (2011) Action mechanisms of arbuscular mycorrhizal fungi in phosphorus uptake by Capsicum annuum L. Pedosphere 21(4):502–511. https://doi.org/10.1016/S1002-0160(11)60152-5
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2(1):1–14. https://doi.org/10.1186/2193-1801-2-587
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, New York
Smith SE, Jakobsen I, Gronlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156(3):1050–1057. https://doi.org/10.1104/pp.111.174581
de Souza R, Ambrosini A, Passaglia LMP (2015) Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol 38(4):401–419. https://doi.org/10.1590/S1415-475738420150053
Toubali S, Tahiri A, Anli M, Symanczik S, Boutasknit A, Ait-El-Mokhtar M, Ben-Laouane R, Oufdou K, Ait-Rahou Y, Ben-Ahmed H, Jemo M, Hafidi M, Meddich A (2020) Physiological and biochemical behaviors of date palm vitroplants treated with microbial consortia and compost in response to salt stress. Appl Sci 10(23):8665. https://doi.org/10.3390/app10238665
Visen A, Bohra M, Singh PN, Srivastava PC, Kumar S, Sharma AK, Chakraborty B (2017) Two pseudomonad strains facilitate AMF mycorrhization of litchi (Litchi chinensis Sonn.) and improving phosphorus uptake. Rhizosphere 3:196–202. https://doi.org/10.1016/j.rhisph.2017.04.006
Vukobratović M, Lončarić Z, Vukobratović Ž, Mužić M (2018) Use of composted manure as substrate for lettuce and cucumber seedlings. Waste Biomass Valor 9(1):25–31
Vurukonda SSKP, Vardharajula S, Shrivastava M, Sk ZA (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res 184:13–24. https://doi.org/10.1007/s12649-016-9755-2
Yamamoto H, Hokin H, Tani T, Kadota G (1977) Phenylalanine ammonia-lyase in relation to the crown rust resistance of oat leaves. J Phytopathol 90(3):203–211. https://doi.org/10.1111/j.1439-0434.1977.tb03238.x
Yildirim E, Karlidag H, Turan M, Dursun A, Goktepe F (2011) Promotion of broccoli by plant growth promoting rhizobacteria. Hort Sci 46(6):932–936
Yildirim E, Turan M, Donmez MF (2008) Mitigation of salt stress in radish (Raphanus sativus L.) by plant growth: promoting rhizobacteria. Rom Biotechnol Lett 13(5):3933–3943
Acknowledgements
The authors gratefully acknowledge the European Union’s Horizon 2020 research and innovation program under grant agreement N°862555 and the Socially Responsible Projects, Cadi Ayyad University UCAM/RSU 2018.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Tahiri, A. Raklami, N. Bechtaoui, M. Anli, A. Boutasknit, K. Oufdou and A. Meddich declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Tahiri, Ai., Raklami, A., Bechtaoui, N. et al. Beneficial Effects of Plant Growth Promoting Rhizobacteria, Arbuscular Mycorrhizal Fungi and Compost on Lettuce (Lactuca sativa) Growth Under Field Conditions. Gesunde Pflanzen 74, 219–235 (2022). https://doi.org/10.1007/s10343-021-00604-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-021-00604-z
Keywords
- Field
- Rhizophagus irregularis
- Plant growth promoting rhizobacteria
- Compost
- Growth
- Biofertilizers-biostimulants
- Lactuca sativa