Abstract
The current study was conducted to evaluate the efficacy of entomopathogenic fungi (EPF) as an alternative strategy for the sustainable control of Nilaparvata lugens. Three species of EPF, Beauveria bassiana, Metarhizium anisopliae and Lecanicillium lecanii, were tested against N. lugens with two suspensions of each tested EPF applied in different treatments. The observed mortality of N. lugens adults during the overall exposure period for the lowest and highest concentrations of each EPF ranged from 0–100%. At both highest and lowest concentrations of EPF, when sprayed on adult, the mortality of N. lugens was higher as compared to when sprayed on the stem pieces as food. A higher mortality rate was observed when the stem pieces were absent than when stem pieces were present. Maximum percent mortality of N. lugens was recorded due to the spray of highest concentration of M. anisopliae on only stem pieces (82.67%) and on adult N. lugens provided with stem pieces as food (93.33%) after 14 days of exposure interval. On the other hand, B. bassiana and L. lecanii were also responsible to cause more than 50% mortality of N. lugens in both treatments after 14 days of exposure interval. Results also indicated that maximum and minimum percent mycosis and sporulation from dead cadavers of N. lugens were recorded in both conidial suspensions of L. lecanii and M. anisopliae, respectively, in all treatments. The high efficacy levels recorded in the current study indicates that M. anisopliae can be effective biological control agents against N. lugens.
Zusammenfassung
Die aktuelle Studie wurde durchgeführt, um die Wirksamkeit entomopathogener Pilze (EPF) als alternative Strategie zur nachhaltigen Bekämpfung von Nilaparvata lugens zu bewerten. Drei EPF-Arten, Beauveria bassiana, Metarhizium anisopliae und Lecanicillium lecanii, wurden gegen N. Lugens getestet, wobei zwei Suspensionen von jedem getesteten EPF in verschiedenen Behandlungen angewendet wurden. Die beobachtete Mortalität von adulten N. lugens während des gesamten Expositionszeitraums für die niedrigste und höchste Konzentration jedes EPF lag zwischen 0 und 100 %. Sowohl bei der höchsten als auch bei der niedrigsten EPF-Konzentration war die Mortalität von N. lugens beim Sprühen auf Adulte höher als beim Sprühen auf Stängelteile als Nahrung. Die Mortalität war höher, wenn Stängelteile vorhanden waren als wenn keine Stängelteile vorhanden waren. Die maximale prozentuale Mortalität von N. lugens wurde nach 14-tägiger Exposition erreicht durch das Sprühen mit der höchsten Konzentration von M. anisopliae nur auf Stängelteile (82.67 %) und auf adulte N. lugens, die mit Stängelteilen als Nahrung (93.33 %) versorgt wurden. Andererseits waren B. bassiana und L. lecanii auch dafür verantwortlich, dass bei beiden Behandlungen nach 14 Tagen Exposition eine Mortalität von mehr als 50 % bei N. lugens auftrat. Die Ergebnisse zeigten auch, dass die maximale und minimale prozentuale Mykose und Sporulation von toten Körpern von N. lugens in beiden konidialen Suspensionen von L. Lecanii und M. anisopliae bei allen Behandlungen registriert wurden. Die in der aktuellen Studie festgestellte hohe Wirksamkeit weist darauf hin, dass M. anisopliae ein wirksames biologisches Bekämpfungsmittel gegen N. lugens sein kann.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brown planthopper, Nilaparvata lugens, is a typical phloem sap feeder and one of the most serious and devastating rice pests in Asia (Normile 2008; Heong and Hardy 2009). In the case of epidemics, N. lugens causes losses of up to 60% (Srivastava et al. 2009; Kumar et al. 2012). In the rice blanket for Pakistan, the rice fields are prone to the attack of pests that may decrease the yield up to 7–10% annually. Sabir et al. (2019) stated that the average grain yield loss due to the attack of N. lugens was 10 maund/acre, amounting to 100 US-$/acre during the growing season 2017/2018.
It is difficult to monitor such pests regularly because both the nymphs and adults suck sap from the leaves and leaf sheaths of rice so that infested leaves become yellow, in addition to the reduction in plant tellering and plant height with more number of unfilled grain. Infestation with N. lugens also causes a decrease in chlorophyll and protein content of the leaves and the rate of photosynthesis, while the severe attack of N. lugens produces symptoms of ‘hopper burn’ (Liu et al. 2008; Horgan 2009; Vanitha et al. 2011). It also transmits viral diseases such as ragged stunt (Baehaki et al. 2017), grassy stunt (Chomchan et al. 2002) and wilted stunt (Chen et al. 1978).
Although many chemicals are recommended to control this pest (Sarao 2015), farmers are unable to control this pest effectively because of their feeding behavior at the lower part of the plant. As a result, farmers use large number of insecticides that often destroy the ecological balance of rice ecosystem and the treated insects become more resistant to insecticide application (Gorman et al. 2008; Matsumura et al. 2009). Extensive chemical use against N. lugens on rice can lead to serious problems, toxicity to natural enemies of brown planthopper (Wang et al. 2008), an increase in total production costs and possible long-term damage to the agro-ecosystem and human health (Rola and Pingali 1993; Huang et al. 2000).
Many entomopathogenic fungi (EPF) have been commercialized and offer environmentally safe and economically viable chemical control alternatives for many insect pests (Wraight et al. 2000; Neves et al. 2001; Scholte et al. 2005; Rizwan et al. 2019a, 2019b). Currently, Beauveria bassiana, Metarhizium anisopliae and Metarhizium flavoviride, after intensive research, have the potential to develop biological control of sucking pests such as aphids (Yeo et al. 2003; Saranya et al. 2010; Jandricic et al. 2014), leaf hoppers (Tounou et al. 2003; Pu et al. 2005; Toledo et al. 2011), and plant hopper (Li et al. 2012a, 2014; Shaikh and Mohite 2015; Mohan et al. 2016).
Although there are many reports of the use of entomopathogenic fungi in various treatments of insect pests, there is very little information on the simultaneous evaluation of their application methods against N. lugens. Reddy et al. (2013) reported that M. anisopliae and B. bassiana were found effective against N. lugens and less toxic to predators under field condition. Li et al. (2012b) also reported that isolates of B. bassiana were found highly infectious to N. lugens eggs.
The objectives of the present study were to test the insecticidal efficacy of B. bassiana, M. anisopliae and L. lecanii against N. lugens under different treatments under controlled conditions by using various concentrations of conidial suspension.
Materials and Methods
Plant Material
Fine rice Basmati-515 variety was used to evaluate the pathogenicity of entomopathogens (B. bassiana, M. anisopliae and L. lecanii) against N. lugens. Rice seedlings of 10-day-old were transplanted in plastic pots (20 × 20 cm), and grown for another 10 days under controlled conditions (25 ± 5 °C; 80% R. H. and 14:10 hrs. L:D photoperiod) for being used in bioassays.
Insect Material
Adult insects of N. lugens were used in the study. A stock culture of the N. lugens was maintained following Heinrichs and Medrano (1985) method. Adults of N. lugens were collected from the rice fields in Rice Research Institute, Kala Shah Kaku, Pakistan (31° 43′ 17″ N, 74° 16′ 14″ E) and released onto potted rice plants. The gravid adults were removed from these plants and released onto individual potted plants kept inside the oviposition cages (120 × 80 × 50 cm) for egg laying. These eggs developed into adult N. lugens at 25 ± 5 °C and 14:10 hrs. L:D photoperiod. Adults of 10-day-old were collected from the rice seedlings for bioassay. For this purpose, the adults were transferred into petri dishes.
Entomopathogenic Fungi
Three commercial formulations of the entomopathogenic fungi Beauveria bassiana, Metarhizium anisopliae and Lecanicillium (Verticillium) lecanii in talc powder were purchased from AgriLife SOM Phytopharma (India) Limited (www.agrilife.in). Two concentrations of each EPF formulation were tested against N. lugens: 1 × 108 and 1 × 106 CFU/ml. The concentration of fungal conidia in the conidial suspension was checked by using a hemocytometer. Potato Dextrose Agar (PDA) was used to determine the conidial germination. The conidial germination was measured, based on the counts of 200 random conidia per plate, 18 h post incubation at 25 ± 2 °C (Ayala-Zermeño et al. 2015; Rizwan et al. 2019b).
Preparation of Conidial Suspension
The conidial suspension of 1 × 106 conidia/ml of all tested EPF was prepared according to the method followed by Ali et al. (2018) by dissolving 1 g of EPF formulation in 100 ml of distilled water.
Bioassays
Three experimental treatments with the conidial suspensions of EPF were applied in bioassays as follows:
- i.
Pieces of rice stem were sprayed with the conidial suspensions of EPF then placed in petri dishes containing adults of N. lugens.
- ii.
Adults of N. lugens were sprayed with the conidial suspensions of EPF then placed in petri dishes without pieces of rice stem.
- iii.
Adults of N. lugens were sprayed with the conidial suspensions of EPF then placed in petri dishes with untreated pieces of rice stem.
The technique of treatment of rice stem pieces with the conidial suspensions of EPF was applied as follows: (i) applying 1 ml of the conidial suspension of each concentration of each EPF per replicate, (ii) spraying of the conidial suspension was carried out by the help of a Pump Pressure Sprayer (Hommold, Lahore) and then cleaned with acetone after application of each concentration. The sprayed stem pieces were then transferred into petri dishes (with 1.5 cm diameter holes in the middle), containing moistened filter paper at their bottom, to maintain the freshness and turgidity of stem pieces.
Adults of N. lugens were treated with the conidial suspensions of EPF according to the following technique: (i) placing the adults in cylindrical plastic jar (24 × 14 × 14 cm) with a top covered with muslin cloth for aeration and to prevent insects from escape, and held for 30 min to reduce their movement (Hluchý and Samšiňáková 1989), (ii) applying 1 ml of each suspension (one suspension for each concentration of each of the EPF) by the help of same device mentioned previously, (iii) transferring the treated adults into petri dishes by aspirator, then the dishes were sealed with teflon tape to prevent the insects from escaping and placed in incubator at 27 °C and 65% R. H. during the entire experimental period.
Assessment or Evaluation of the Treatment Efficacy
Adult mortality was determined by prodding with a camel hair brush to detect movement under stereomicroscope (Cole-Parmer 625 East Bunker Court Vernon Hills, IL60061 USA) after 1, 2, 6, 7, 10 and 14 days of exposure. Mycosis percentage was recorded from the dead cadavers of N. lugens after 14 days of exposure interval. These cadavers were preserved in sterile petri plates, washed thrice in sterile dH2O and surface sterilized by 0.05% sodium hypochlorite solution for 2–3 min. These cadavers were shifted to Sabouraud dextrose agar (SDA) plates for incubation at 25 ± 2 °C, 75 ± 5 RH for 7 days to detect the external white fungal growth under a stereomicroscope (Cole-Parmer 625 East Bunker Court Vernon Hills, IL, 60061, USA) (Beris et al. 2013; Rizwan et al. 2019a). Mycosed cadavers from each replication were mixed in a beaker with a drop of Tween 80 with 20 ml of dH2O for assessing sporulation (Tefera and Pringle 2003; Rizwan et al. 2019a). The solution was carefully stirred and the number of conidia was counted by using a haemocytometer under the stereomicroscope (Rizwan et al. 2019a).
The main variables studied here were the treatment, concentration and exposure interval Interaction between these variables such as treatment × concentration, exposure interval × treatment, exposure interval × concentration and exposure interval × treatment × concentration were taken in consideration. The percent mortality was the response of the variables.
Statistical Analysis
The mortality and mycosis rate was converted into percent. Data was analyzed using Statistix software (version 8.1) (Tallahassee, FL). Three-way ANOVA was applied to factorial experiments in CRD for percent mortality, percent mycosis and sporulation from dead cadavers of N. lugens to understand the main and interactions of the variables. The means were compared with Tukey’s HSD means separation test at P < 0.05 (Sokal and Rohlf 1995).
Results
Treatment Effect of Beauveria Bassiana Against the Adults of Nilaparvata Lugens
Mortality of N. lugens adults was significantly affected by the exposure interval (F5, 179 = 214.41, P < 0.01). Repeated measures variables, for main effects and their associate interactions, are presented in Table 1. Percent mortality of adult N. lugens was low for EFP sprayed on only stem pieces and sprayed on adults (with stem pieces) treatments and concentrations after 2 days exposure interval and did not exceed 10%. Percent mortality was significantly increased at 2 days exposure interval when B. bassiana was sprayed on adults (without stem pieces), while 6 days exposure interval when B. bassiana was sprayed on adults (with stem pieces) as compared with when it was sprayed on only stem pieces. All adults died in both concentration treatments at 6 days exposure interval in the absence of stem pieces. Percent mortality of adult N. lugens was significantly increased after 10 days exposure interval and exceeded 25% in both concentration in which the B. bassiana was sprayed on only stem pieces (Table 2).
Treatment Effect of Metarhizium Anisopliae Against the Adults of Nilaparvata Lugens
Mortality of N. lugens adults was significantly affected by the exposure interval (F5, 179 = 172.92, P < 0.01). Repeated measures variables, for main effects and their associate interactions, are presented in Table 1. Percent mortality of adult N. lugens was low for EFP sprayed on only stem pieces and sprayed on adults (with stem pieces) treatments and concentrations after 2 days exposure interval and did not exceed 15%. Percent mortality was significantly increased at 2 days exposure interval when M. anisopliae was sprayed on adults (without stem pieces), while 6 days exposure interval when M. anisopliae was sprayed on adults (with stem pieces) as compared with when it was sprayed on only stem pieces. All adults died in both concentration treatments at 6 days exposure interval in the absence of stem pieces. Percent mortality of adult N. lugens was significantly increased after 10 days exposure interval and exceeded 30% in both concentration in which the M. anisopliae was sprayed on only stem pieces (Table 3).
Treatment Effect of Lecancillium Lecanii Against the Adults of Nilaparvata Lugens
Mortality of N. lugens adults was significantly affected by the exposure interval (F5, 179 = 135.51, P < 0.01). Repeated measures variables, for main effects and their associate interactions, are presented in Table 1. Percent mortality of adult N. lugens was low for EFP sprayed on only stem pieces and sprayed on adults (with stem pieces) treatments and concentrations after 2 days exposure interval and did not exceed 7%. Percent mortality was significantly increased at 2 days exposure interval when L. lecanii was sprayed on adults (without stem pieces), while 6 days exposure interval when L. lecanii was sprayed on adults (with stem pieces) as compared with when it was sprayed on only stem pieces. All adults died in both concentration treatments at 6 days exposure interval in the absence of stem pieces. Percent mortality of adult N. lugens was significantly increased after 10 days exposure interval and exceeded 15% in both concentration in which the L. lecanii was sprayed on only stem pieces (Table 4).
Comparison of the Treatment Effects of Three Entomopathogenic Fungi Against the Adult Nilaparvata Lugens
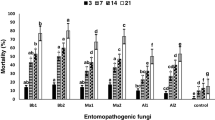
The maximum percent mortality of adults of N. lugens caused at higher concentration of B. bassiana, M. anisopliae and L. lecanii after 14 days of exposure were 86.67, 93.33 and 80.00%, respectively, when adults were sprayed with the conidial suspension of EPF and feed on untreated pieces of rice stem. While maximum percent mortality of adults of N. lugens caused at same concentration of B. bassiana, M. anisopliae and L. lecanii after 14 days of exposure were 72.00, 82.67 and 54.67%, respectively when only stem pieces were sprayed with conidial suspension of EPF. M. anisopliae was responsible for highest mortality of N. lugens as compared with B. bassiana and L. lecanii (Fig. 1).
Mycosis and Sporulation from the dead Cadavers of Nilaparvata Lugens
The main effect of treatment methods of all entomopathogenic fungi on mycosis (F2, 89 = 27.95) and sporulation (F2, 89 = 180.92) from dead cadavers of N. lugens was highly significant (P < 0.01). Similarly the main effect of all entomopathogenic fungi on mycosis (F2, 89 = 137.92) and sporulation (F2, 89 = 841.64) was highly significant (P < 0.01). Likewise the main effect of different concentrations of entomopathogenic fungi on mycosis (F1, 89 = 45.41) and sporulation (F1, 89 = 363.52) was highly significant (P < 0.01). Moreover, highly significant effect of interactions among the treatment methods × entomopathogenic fungi were also observed (P < 0.05) on mycosis (F4, 89 = 2.79) and sporulation (F4, 89 = 9.92). Non-significant effect of interactions among the treatment methods × concentration and entomopathogenic fungi × concentration was observed (P > 0.05) on mycosis (F2, 89 = 0.39 and F2, 89 = 0.43, respectively) and sporulation (F2, 89 = 0.39 and F2, 89 = 1.51, respectively). Non-significant and significant effect of interactions among the treatment methods × entomopathogenic fungi × concentration was observed on mycosis (F4, 89 = 2.23, P > 0.05) and sporulation (F4, 89 = 16.78, P < 0.01), respectively (Table 5).
Maximum mycosis from dead cadavers of N. lugens was recorded in both conidial suspension of L. lecanii sprayed on adults with the provision of stem pieces as food (96.0 and 88.0%), while similar trend was also observed on other two treatments, conidial suspension of L. lecanii sprayed on adults without the provision of stem pieces as food (89.6 and 80.8%) and conidial suspension of L. lecanii sprayed on stem pieces (84.8 and 76.8%). While, minimum mycosis from dead cadavers of N. lugens was recorded in both conidial suspension of M. anisopliae sprayed on adults with the provision of stem pieces as food (68.8 and 56.0%), while similar trend was also observed on other two treatments, conidial suspension of M. anisopliae sprayed on adults without the provision of stem pieces as food (64.8 and 64.0%) and conidial suspension of M. anisopliae sprayed on stem pieces (60.0 and 52.0%) (Table 6).
Similarly, maximum sporulation from dead cadavers of N. lugens was recorded in both conidial suspension of L. lecanii sprayed on adults with the provision of stem pieces as food (205.8 and 184.6 conidia ml−1), while similar trend was also observed on other two treatments, conidial suspension of L. lecanii sprayed on adults without the provision of stem pieces as food (195.4 and 172.4 conidia ml−1) and conidial suspension of L. lecanii sprayed on stem pieces (179.0 and 161.4 conidia ml−1). While, minimum mycosis from dead cadavers of N. lugens was recorded in both conidial suspension of M. anisopliae sprayed on adults with the provision of stem pieces as food (153.4 and 124.6%), while similar trend was also observed on other two treatments, conidial suspension of M. anisopliae sprayed on adults without the provision of stem pieces as food (141.8 and 139.4 conidia ml−1) and conidial suspension of M. anisopliae sprayed on stem pieces (135.2 and 116.2 conidia ml−1) (Table 7).
Discussion
There are numerous studies on the use of EPF for the management of Nilaparvata lugens (Hywel-Jones and Gillespie 1990; Jin et al. 2008; Song and Feng 2011). Adult insects were used in this study on the basis of previous study of Geng and Zhang (2004) who reported that adult N. lugens were more susceptible to EPF infection than their nymphs and the young nymphs were most resistant to the fungal infection. Based on the present studies, M. anisopliae proved effectiveness against N. lugens and responsible to caused 82.67–93.33% mortality, so these results are in accordance with the findings of Mohan et al. (2016) who reported that highest conidial concentration of Metarhizium (M1) strain (NBAIR) (a strain of M. anisopliae) is responsible to cause 76.67% mortality of N. lugens under in vitro conditions. Similar findings were also reported by Li et al. (2012a) that maximum mortality of N. lugens was recorded due to the M. flavoviride (Mf82) and gradually increased with the increase in exposure interval. This virulent isolate of M. flavoviride is concluded as a promising candidate for microbial control of N. lugens. Shaikh and Mohite (2015) reported that field application of M. anisopliae with highest conidial concentration was the most consistently effective for the control of N. lugens. Kirkland et al. (2004) reported that M. anisopliae and B. bassiana have the potential for controlling populations of Ixodes scapularis and Rhipicephalus sanguineus, and nymphal and adult mortality of these ticks due to these EPF was increased as the exposure interval increased.
Results indicated that maximum mortality of adult N. lugens was observed when EPF was sprayed on adult body and provided stem pieces as a food. The application method of EPF formulation is the most important factor in its efficacy against insect pests. Mortality was faster when the fungus was applied directly on the adult body (Kavallieratos et al. 2014). Similar finding was also reported by Rizwan et al. (2019a) that 74% mortality of Cnaphalocrocis medinalis larvae was recorded when dipped in M. anisopliae concentration for 10 s after 10 days. Likewise, Kavallieratos et al. (2014) reported that when higher concentration of EPF sprayed on adult Sitophilus oryzae, it caused 100% mortality after 14 days exposure interval. Kassa et al. (2002) found that adult Sitophilus zeamais dipped in B. bassiana suspension for 5 s caused 100% mortality after 4 days exposure interval. Cherry et al. (2005) reported that when adults of Callosobruchus maculatus were dipped in conidial suspension of M. anisopliae or B. bassiana for 5 s, 100% mortality was recorded after 6 and 8 days exposure interval. Rizwan et al. (2019b) found that higher concentration of B. bassiana caused 31.67% mortality of Tribolium castaneum after 21 days exposure interval.
Results from the application methods of EPF in this study indicated that direct application of the EPF on the adult body of N. lugens is more effective as compared to contact of the insect body with the EPF-treated substrate (stem pieces) may be due to the penetration of these pathogens in insect body through their cuticle. This difference is more apparent after 6 days exposure interval, while all treatments had similar efficacy level at shorter exposure intervals. Therefore, on the basis of the present findings, it is assumed that at lengthier exposure interval (14 days), the application of M. anisopliae on stem pieces could result in 93% mortality, at least in the cases of B. bassiana and L. lecanii. These findings were in accordance with Kavallieratos et al. (2014) that maximum mortality of S. oryzae adults was recoded when B. bassiana, M. anisopliae and Isaria fumosorosea were applied on insect body rather than sprayed on food and the efficacy of these EPF were increased as the exposure interval increased. A huge number of publications are dealing with the mode of action and the infection process of EPF (Sanjaya et al. 2013, 2015; Ramirez et al. 2018). The mode of action is very unique as conidial germination starts when these pathogens attach to the outer surface of insect cuticle (Liu et al. 2009; Ment et al. 2010; Leao et al. 2015) and producing germ tube (Lovett and Leger 2015). This tube can penetrate the insect cuticle towards the insect hemocoel where the fungal cells attain yeast like forms called as “hyphal bodies” that attack the host insect throughout a sequential process and insect becomes mummified (Mannino et al. 2019). These cells have the ability to secrete small toxic molecules that aids as immunosuppressive compounds, facilitating fungal infection (Pedrini 2018). Numerous studies are also supporting the infection of these EPF by oral ingestion route as an alternate to the cuticle penetration for entrance to the insect body (Wei et al. 2017; Batta 2018; Rafaluk-Mohr et al. 2018) which ultimately increases the significance of these pathogens.
Insecticidal activity of all tested EPF had increased with the exposure interval which ultimately increases the mortality rate of N. lugens. Viability of fungal conidia weakened with time (Moore et al. 2000). Batta (2004) found that the fungal conidial viability of M. anisopliae decreased with time when used against S. oryzae, but its insecticidal efficacy was not severely affected. Results from present study indicated that percent mycosis and sporulation (conidia ml−1) achieved the maximum in treatment where EPF were applied at low conidial suspension. Among them, maximum results of both parameters were recorded in L. lecanii while minimum results were recorded in M. anisopliae. These results are in accordance with Riasat et al. (2011) who reported that maximum mycosis and sporulation was observed in the dead cadavers of Rhyzopertha dominica at the lowest conidial suspension of B. bassiana. These results are also supported by other scientists (Sufyan et al. 2019; Tefera and Pringle 2003). The lowest percent mycosis and sporulation at higher conidial suspensions may be attributed to the self-inhibiting mechanism of fungal spores at higher suspensions (Garraway and Evans 1984; Tefera and Pringle 2003).
Conclusion
The results indicated that all tested EPF, B. bassiana, M. anisopliae and L. lecanii were effective against N. lugens but M. anisopliae was more effective than B. bassiana and L. lecanii. Furthermore, the application of the EPF directly on insect bodies is more effective than application on stem pieces. Such application would also be more consumer friendly than the “pathogen-treated food” approach.
References
Ali S, Farooqi MA, Sajjad A, Ullah MI, Qureshi AK, Siddique B, Waheed W, Sarfraz M, Asghar A (2018) Compatibility of entomopathogenic fungi and botanical extracts against the wheat aphid, Sitobion avenae (Fab.) (Hemiptera: Aphididae). Egypt J Biol Pest Co 28:97
Ayala-Zermeño MA, Gallou A, Berlanga-Padilla AM, Serna-Domínguez MG, Arredondo-Bernal HC, Montesinos-Matías R (2015) Characterisation of entomopathogenic fungi used in the biological control programme of Diaphorina citri in Mexico. Biocontrol Sci Technol 25(10):1192–1207
Baehaki SE, Iswanto EH, Munawar D, Song Y‑H, Choi IR, Park H‑H (2017) Abilities of brown planthopper immigrant transmits rice ragged stunt virus on rice of some district of Java-Indonesia. Sch J Agric Vet Sci 4(8):300–310
Batta YA (2004) Control of the rice weevil (Sitophilus oryzae L., Coleoptera: Curculionidae) with various formulations of Metarhizium anisopliae. Crop Prot 23:103–108
Batta YA (2018) Efficacy of two species of entomopathogenic fungi against the stored-grain pest, Sitophilus granarius L. (Curculionidae: Coleoptera), via oral ingestion. Egypt J Biol Pest Co 28:44
Beris EI, Papachristos DP, Fytrou A, Antonatos SA, Kontodimas DC (2013) Pathogenicity of three entomopathogenic fungi on pupae and adults of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). J Pest Sci 86:275–284
Chen CC, Ko VH, Chiu RJ (1978) Rice wilted stunt its transmission by the brown planthopper, Nilaparvata lugens (Stål). Plant Prot Bull Taiwan 20:374–376
Cherry AJ, Abalo P, Hell K (2005) A laboratory assessment of the potential of different strains of the entomopathogenic fungi Beauvaria bassiana (Balsamo) Vuillemin and Metarhizium anisopliae (Metschnikoff) to control Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in stored cowpea. J Stored Prod Res 41:295–309
Chomchan P, Miranda GJ, Shirako Y (2002) Detection of rice grassy stunt tenuivirus nonstructural proteins p2, p5 and p6 from infected rice plants and from viruliferous brown planthoppers. Arch Virol 147(12):2291–2300
Garraway MO, Evans RC (1984) Fungal nutrition and physiology. John Wiley & Sons, New York
Geng B‐W, Zhang R‐J (2004) Pathogenicity of Metarhizium anisopliae Var. Acridu to the deveolpmental stages of brown planthopper Nilaparvata lugens Stål and Sogatella furcifera (Horvath). Insect Sci 11(2):89–97
Gorman K, Liu Z, Denholm I, Bruggen KU, Nauen R (2008) Neonicotinoid resistance in rice brown planthopper, Nilaparvata lugens. Pest Manag Sci 64:1122–1125
Heinrichs EA, Medrano FG (1985) Influence of N fertilizer on the population development of brown planthopper (BPH). Int Rice Res Newsl 10:20–21
Heong KL, Hardy B (2009) Planthoppers: new threats to the Sustainability of intensive rice production systems in asia. International Rice Research Institute, Los Baños, pp 1–460
Hluchý M, Samšiňáková A (1989) Comparative study on the susceptibility of adult Sitophilus granarius (L.) (Coleoptera: Curculionidae) and larval Galleria mellonella (L.) (Lepidoptera: Pyralidae) to the entomogenous fungus Beauveria bassiana (Balsamo) Vuillemin. J Stored Prod Res 25:61–64
Horgan F (2009) Mechanisms of resistance: A major gap in understanding planthopper-rice interactions. In: Heong KL, Hardy B (eds) Planthoppers: New threats to the sustainability of intensive rice production systems in Asia. International Rice Research Institute, Los Baños, pp 281–302
Huang J, Qiao F, Zhang L, Rozelle S (2000) Farm pesticide, rice production and human health. Chinese Academy of Sciences, Beijing
Hywel-Jones NL, Gillespie AT (1990) Effect of temperature on spore germination in Metarhizium anisopliae and Beauveria bassiana. Mycol Res 94(3):389–392
Jandricic SE, Filotas M, Sanderson JP, Wraight SP (2014) Pathogenicity of conidia-based preparations of entomopathogenic fungi against the greenhouse pest aphids Myzus persicae, Aphis gossypii, and Aulacorthum solani (Hemiptera: Aphididae). J Invertebr Pathol 118:34–46
Jin S‐F, Feng M‐G, Chen J‐Q (2008) Selection of global Metarhizium isolates for the control of the rice pest Nilaparvata lugens (Homoptera: Delphacidae). Pest Manag Sci 64(10):1008–1014
Kassa A, Zimmermann G, Stephan D, Vidal S (2002) Susceptibility of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) and Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) to entomopathogenic fungi from Ethiopia. Biocontrol Sci Technol 12:727–736
Kavallieratos NG, Athanassiou CG, Aountala MM, Kontodimas DC (2014) Evaluation of the entomopathogenic fungi Beauveria bassiana, Metarhizium anisopliae, and Isaria fumosorosea for control of Sitophilus oryzae. J Food Prot 77(1):87–93
Kirkland BH, Westwood GS, Keyhani NO (2004) Pathogenicity of Entomopathogenic Fungi Beauveria bassiana and Metarhizium anisopliae to Ixodidae tick species Dermacentor variabilis, Rhipicephalus sanguineus, and Ixodes scapularis. J Med Entomol 41(4):705–711
Kumar H, Maurya RP, Tiwari SN (2012) Studies on antibiosis mechanism of resistance in rice against brown planthopper, Nilaparvata lugens (Stal.). Ann Plant Prot Sci 28:98–101
Leao MP, Tiago PV, Andreote FD, de Araujo WL, de Oliveira NT (2015) Differential expression of the pr1A gene in Metarhizium anisopliae and Metarhizium acridum across different culture conditions and during pathogenesis. Genet Mol Biol 38:86–92
Li M, Lin H, Li S, Chen P, Jin L, Yang J (2012a) Virulence of entomopathogenic fungi to adults and eggs of Nilaparvata lugens Stal (Homopera: Delphacidae). Afr J Agric Res 7(14):2183–2190
Li MY, Li SG, Xu AM, Lin HF, Chen DX, Wang H (2014) Selection of Beauveria isolates pathogenic to adults of Nilaparvata lugens. J Insect Sci 14:32
Li MY, Lin HF, Li SG, Xu AM, Feng MF (2012b) Efficiency of entomopathogenic fungi in the control of eggs of the brown planthopper Nilaparvata lugens Stål (Homopera: Delphacidae). Afr J Microbiol Res 6(44):7162–7167
Liu JL, Yu JF, Wu JC, Yin JL (2008) Physiological responses to Nilaparvata lugens in susceptible and resistant rice varieties: Allocation of assimilates between shoots and roots. J Econ Entomol 101:384–390
Liu W, Xie Y, Xue J, Gao Y, Zhang Y, Zhang X, Tan J (2009) Histopathological changes of Ceroplastes japonicas infected by Lecanicillium lecanii. J Invertebr Pathol 101:96–105
Lovett B, Leger RJS (2015) Stress is the rule rather than the exception for Metarhizium. Curr Genet 61:253–261
Mannino MC, Huarte-Bonnet C, Davyt-Colo B, Pedrini N (2019) Is the insect cuticle the only entry gate for fungal infection? Insights into alternative modes of action of entomopathogenic fungi. J Fungi 5:33
Matsumura M, Takeuchi H, Satoh M, Sanasa-Morimura S, Otuka A, Watanabe T, Thanh DV (2009) Current status of insecticide resistance in rice planthoppers. In: Heong KL, Hardy B (eds) Planthoppers: New threats to the sustainability of intensive rice production systems in Asia. International Rice Research Institute, Los Baños, pp 233–244
Ment D, Gindin G, Rot A, Soroker V, Glazer I, Barel S, Samish M (2010) Novel technique for quantifying adhesion of Metarhizium anisopliae conidia to the tick cuticle. Appl Environ Microbiol 76:3521–3528
Mohan C, Sridhar RP, Nakkeeran S (2016) Studies on efficacy of entomopathogenic fungi Metarhizium anisopliae (Metchnikoff) Sorokin against Nilaparvata lugens (Stål). Int J Agric Sci Res 6(6):227–234
Moore D, Lord JC, Smith SM (2000) Pathogens. In: Subramanyam B, Hagstrum DW (eds) Alternatives to pesticides in stored-product IPM. Kluwer, Dordreecht, pp 193–227
Neves PMOJ, Hirose E, Tchujo PT, Moino AJR (2001) Compatibility of entomopathogenic fungi with neonicotinoid insecticides. Neotrop Entomol 30(2):263–268
Normile D (2008) Reinventing rice to feed the world. Science 321:330–333
Pedrini N (2018) Molecular interactions between entomopathogenic fungi (Hypocreales) and their insect host: Perspectives from stressful cuticle and hemolymph battlefields and the potential of dual RNA sequencing for future studies. Fungal Biol 122:538–545
Pu X‑Y, Feng M‑G, Shi C‑H (2005) Impact of three application methods on the field efficacy of a Beauveria bassiana-based mycoinsecticide against the false-eye leafhopper, Empoasca vitis (Homoptera: Cicadellidae) in the tea canopy. Crop Prot 24(2):167–175
Rafaluk-Mohr C, Wagner S, Joop G (2018) Cryptic changes in immune response and fitness in Tribolium castaneum as a consequence of coevolution with Beauveria bassiana. J Invertebr Pathol 152:1–7
Ramirez JL, Dunlap CA, Muturi EJ, Barletta ABF, Rooney AP (2018) Entomopathogenic fungal infection leads to temporospatial modulation of the mosquito immune system. PLoS Negl Trop Dis 12(4):e6433
Reddy AV, Devi RS, Dhurua S, Reddy DVV (2013) Study on the efficacy of some entomogenous fungi against brown plant hopper, Nilaparvata lugens Stal in irrigated rice. J Biopestic 6(2):139–143
Riasat T, Wakil W, Ashfaq M, Sahi ST (2011) Effect of Beauveria bassiana mixed with diatomaceous earth on mortality, mycosis and sporulation of Rhyzopertha dominica on stored wheat. Phytoparasitica 39:325–331
Rizwan M, Atta B, Rizwan M, Sabir AM, Shah ZU, Hussain M (2019b) Effect of the entomopathogenic fungus, Beauveria bassiana combined with diatomaceous earth on the red flour beetle, Tribolium castaneum (Herbst) (Tenebrionidae: Coleoptera). Egypt J Biol Pest Co 29:27
Rizwan M, Atta B, Sabir AM, Yaqub M, Qadir A (2019a) Evaluation of the entomopathogenic fungi as a non-traditional control of the Rice leaf roller, Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae) under controlled conditions. Egypt J Biol Pest Co 29:10
Rola AC, Pingali LP (1993) Pesticides, rice productivity, and farmers’ health: An economic assessment. International Rice Research Institute, Los Baños
Sabir AM, Shah ZU, Sabar M, Rizwan M, Atta B, Qadir A, Asghar M (2019) Rice Planthoppers: Potential threat to the sustainable rice production in the Punjab, Pakistan. In: 39th Pakistan Congress of Zoology, Department of Zoology, Islamia College University, Peshawar, Pakistan, p 229
Sanjaya Y, Ocampo VR, Caoili BL (2013) Infection process of entomopathogenic fungi Metarhizium anisopliae in the Tetrancyhus kanzawai (Kishida) (Tetranychidae: Acarina). Agrivita J Agric Sci 35(1):64–72
Sanjaya Y, Ocampo VR, Caoili BL (2015) Infection process of entomopathogenic fungi Beauveria bassiana in the Tetrancyhus kanzawai (Kishida) (Tetranychidae: Acarina). Arthropods 4(3):90–97
Saranya S, Ushakumari R, Jacob S, Philip BM (2010) Efficacy of different entomopathogenic fungi against cowpea aphid, Aphis craccivora (Koch). J Biopestic 3:138–142
Sarao PS (2015) Integrated Management of insect-pests of rice and basmati. Prog Farm 51:9–12
Scholte E‑J, Nghabi K, Kihonda J, Takken W, Paaijmans K, Abdulla S, Killeen GF, Knols BGJ (2005) An Entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308(5728):1641–1642
Shaikh SH, Mohite P (2015) Effect of entomopathogenic fungi against brown plant hopper, Nilaparvata lugens (Stal.) (Hemiptera: Delphacidae) infesting rice. Int J Sci Res 4(10):905–907
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W. H. Freeman, New York
Song T‑T, Feng M‑G (2011) In vivo passages of heterologous Beauveria bassiana isolates improve conidial surface properties and pathogenicity to Nilaparvata lugens (Homoptera: Delphacidae). J Invertebr Pathol 106(2):211–216
Srivastava C, Chander S, Sinha SR, Palta RK (2009) Toxicity of various insecticides against Delhi and Palla population of brown planthopper (Nilaparvata lugens). Ind J Agric Sci 79:1003–1006
Sufyan M, Abbasi A, Wakil W, Gogi MD, Arshad M, Nawaz A, Shabbir Z (2019) Efficacy of Beauveria bassiana and Bacillus thuringiensis against maize stem borer Chilo Partellus (Swinhoe) (Lepidoptera: Pyralidae). Gesunde Pflanz. https://doi.org/10.1007/s10343-019-00465-7
Tefera T, Pringle KL (2003) Effect of exposure method to Beauveria bassiana and conodia concentration on mortality, mycosis, and sporulation in cadavers of Chilo partellus (Lepidoptera: Pyralidae). J Invertebr Pathol 84:90–95
Toledo AV, Alippi AM, de Remes Lenicov AMM (2011) Growth inhibition of Beauveria bassiana by bacteria isolated from the cuticular surface of the corn leafhopper, Dalbulus maidis and the planthopper, Delphacodes kuscheli, two important vectors of maize pathogens. J Insect Sci 11(1):29
Tounou A‑K, Agboka K, Poehling H‑M, Raupach K, Langewald J, Zimmermann G, Borgemeister C (2003) Evaluation of the entomopathogenic fungi Metarhizium anisopliae and Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) for control of the green leafhopper Empoasca decipiens (Homoptera: Cicadellidae) and potential side effects on the egg parasitoid Anagrus atomus (Hymenoptera: Mymaridae). Biocontrol Sci Technol 13(8):715–728
Vanitha K, Suresh S, Gunathilagaraj K (2011) Influence of brown planthopper Nilaparvata lugens (Stal.) feeding on nutritional biochemistry of rice plant. Oryza 48:142–146
Wang HY, Yang Y, Su JY, Shen JL, Gao CF, Chu YZ (2008) Assessment of the impact of insecticides on Anagrus nilaparvatae (Pang et Wang) (Hymenoptera: Mymanidae), an egg parasitoid of the rice planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Crop Prot 27(3–5):514–522
Wei G, Lai Y, Wang G, Chen H, Li F, Wang S (2017) Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc Natl Acad Sci Usa 114:5994–5999
Wraight SP, Carruthers RI, Jaronski ST, Bradley CA, Garza CJ, Galaini-Wraight S (2000) Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biol Control 17:203–217
Yeo H, Pell JK, Alderson PG, Clark SJ, Pye BJ (2003) Laboratory evaluation of temperature effects on the germination and growth of entomopathogenic fungi and on their pathogenicity to two aphid species. Pest Manag Sci 59:156–165
Author information
Authors and Affiliations
Contributions
B. Atta, M. Rizwan and A.M. Sabir planned the research and designed the methodology; B. Atta conducted the experiments; B. Atta and M. Rizwan wrote the manuscript; M.D. Gogi and M.A. Farooq statistically analyzed the data; A.M. Sabir, M.D. Gogi, M.A. Farooq and Y.A. Batta reviewed the manuscript and gave suggestions and comments for its improvement. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
B. Atta, M. Rizwan, A.M. Sabir, M.D. Gogi, M.A. Farooq and Y.A. Batta declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Atta, B., Rizwan, M., Sabir, A.M. et al. Efficacy of Entomopathogenic Fungi Against Brown Planthopper Nilaparvata Lugens (Stål) (Homoptera: Delphacidae) Under Controlled Conditions. Gesunde Pflanzen 72, 101–112 (2020). https://doi.org/10.1007/s10343-019-00490-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-019-00490-6
Keywords
- Rice stem
- Nilaparvata lugens
- Beauveria bassiana
- Metarhizium anisopliae
- Lecanicillium lecanii
- Biocontrol agents
- Virulence