Abstract
Regarding the low availability of phosphorus in soil, tree internal cycling of phosphorus through re-translocation among needles would be a good strategy for conifers to cope with soil phosphorus deficiency and to support new needles in annual growth. Therefore, the relationship between the amount of plant-available phosphorus in the soil and the differences in concentrations of phosphorus among first- and second-year needles (P-difference Ny1 − Ny2) of adult Norway spruce was examined. No significant correlation could be detected between the stocks of available phosphorus extracted using citric acid and P-difference Ny1 − Ny2, even for trees with deficient nutritional status. The temporal variations of P-difference Ny1 − Ny2 at single plots showed the same order of magnitude as the variability between plots. The typically lower concentrations of phosphorus in second-year needles result mainly from an increase in needle weight of older needles. The net phosphorus re-translocation into younger needles appears to be of minor importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorus is one of the most critical elements for forest ecosystem productivity (Mishra et al. 2017; Proe and Millard 1995; Vitousek et al. 2010). Impaired forest and tree conditions, as well as limitation of plant growth, have often been reported to be related to phosphorus deficiencies (Braun et al. 2010; Ewald 2000; Hüttl 1991). In recent years, there has been an increasing interest in identifying the tree phosphorus status and assessing the impact of available phosphorus in soils on trees growth, especially under nutrient-poor conditions. Forest ecosystems receive little phosphorus from atmosphere (Belyazid and Belyazid 2012); thus, soil weathering is the main source of phosphorus (Augusto et al. 2017; Prietzel et al. 2013). Phosphorus internal cycling within the tree, particularly for new organs, is also supposed to be a strategy that compensates for phosphorus demand or soil phosphorus shortfalls (Fraser 1956; Turner and Lambert 1986; Van den Driessche 1984).
Wyttenbach et al. (1995) studied phosphorus in first- and second-year needles of adult Norway spruce and assumed that the difference in phosphorus concentrations of needles is caused by a “re-translocation” from old needles for supporting new needles. The concept of phosphorus re-translocation from older tree organs is mainly based on the theory, that this phenomenon is related to the maintenance of nutrients and trees use this strategy to preserve growth efficiency in poor stands (Reemtsma 1966). However, Chapin and Kedrowski (1983) and Helmisaari (1992a) concluded that for many tree species, re-translocation of phosphorus from older foliage is not an important source of phosphorus on soils with low nutrient status. In a seedling experiment, Fife and Nambiar (1984) for Pine did not detect a dependency for phosphorus re-translocation between needles and soil fertility. The authors clarified, even when ecological conditions were suitable for element uptake, phosphorus in needles is partly shifted into the shoots. Element re-translocation between conifer needles has also been investigated by Fiedler et al. (1973), Nambiar and Fife (1987), and Proe and Millard (1995); however, most of these studies were carried out using seedlings or saplings and monitored only a relatively short growing period.
The phosphorus status is highly variable in Norway spruce and mostly depends on soil quality (Achat et al. 2016; Manghabati et al. 2018b). For example, the results of the Second National Forest Soil Inventory in Germany (BZE II) indicated that phosphorus status in trees on limed and calcareous sites is inferior compared to non-calcareous sites (Riek et al. 2016). Applying simple soil extraction methods (citric acid and sodium bicarbonate), Fäth et al. (2019) and Manghabati et al. (2018a, b) found a significant relationship for European beach and Norway spruce trees between soil-extractable phosphorus and phosphorus in foliage. Therefore, citric acid-extractable phosphorus is suggested as an appropriate indicator for phosphorus availability. However, since content of phosphorus in foliage have a large fluctuation among trees and among years, this variability demands sampling of a great number of trees in several subsequent years in order to thoroughly evaluate tree phosphorus nutrition (Chapin and Van Cleve 1989; Wehrmann 1959). If phosphorus re-translocation from older needles supports the growing needles to maximize growth every year, particularly under deficient conditions, site phosphorus fertility should have an influence on this process.
Recently, a positive relationship between the level of phosphorus internal cycling and soil phosphorus supply has been reported by Netzer et al. (2017) in beech forests. The authors identified bark and stem-wood as main phosphorus pools for tree internal cycling. In adult Norway spruce, a large proportion of the nutrient elements is stored in the needles (Weis et al. 2009); thus, this nutrient pool has a high potential for internal element re-translocation. Several studies have investigated the development of phosphorus in needles from buds to the end of the first growing season (e.g., Fiedler et al. 1973; Reemtsma 1966), whereas changes in phosphorus concentration after the end of the first vegetation period until waste of the needles are less investigated. According to Reemtsma (1966), in addition to first-year needles also older needles should be taken into consideration for whole tree nutrient balance. However, to which degree older needles contribute to the phosphorus demand of growing needles by re-translocation is still an open question. The results of most investigations to this question are uncertain because alterations of element content in needles have seldom been monitored in long-term studies. In this relation, Nambiar and Fife (1991) emphasized in their conclusion that, if the sequential foliage sampling is poorly coordinated, the probability of errors in results arises.
Furthermore, increasing the weight of older needles during growth periods is an important factor for evaluating the value of net phosphorus transfer. The lower concentrations of phosphorus in older needles, at least partly, are the result of accumulation of immobile elements, such as calcium (Göttlein et al. 2012). For example, a 7% increase in weight of Pine seedlings’ needles, during one-year growth, was reported by Cousens (1988). With regard to the above mentioned considerations, this paper assesses the relationship between soil-extractable phosphorus and the difference in concentration of phosphorus between first- and second-year needles (P-difference Ny1 − Ny2) in adult Norway spruce trees. We hypothesize a relationship between site-available phosphorus and phosphorus “re-translocation.” Moreover, the “dilution effect” which is caused by an increase in weight will be estimated.
Materials and methods
Data sources
To address different aspects of P-difference Ny1 − Ny2, data were taken from following sources:
I. For evaluating the influence of phosphorus availability in the soil on P-difference Ny1 − Ny2, we used the Bavarian BZE II-plots (Schubert et al. 1995). This dataset contains 272 Norway spruce sites, covering the big variety of parent materials and soils in Bavarian forests. In addition to the nutritional and soil scientific BZE II standard data, we could use the data of citric acid-extractable phosphorus, which was measured for all Bavarian BZE II plots described by Fäth et al. (2019).
II. To get an impression of the inter-annual variation of P-difference Ny1 − Ny2, we took soil and nutritional data (from 1987 to 2013) for 33 adult Norway spruce sites of the Bavarian forest monitoring program (Bodendauerbeobachtungsflächen, BDF, Schubert et al. 1995, 2015).
III. At the experimental site Kranzberger Forst (Pretzsch et al. 1998), which is located 40 km north of the city of Munich, a crane was available to sample needles directly from tree crowns. This unique situation allowed a repeated sampling of the same branches over several years. Data used in this study were derived from an intensive tree sampling of eight adult Norway spruce trees (age 53–55) over 9 years (from 1998 to 2007, except 2005).
Analytical methods
All soil samples had already been taken during the BZE II and BDF sampling campaign and have been prepared and analyzed according to the German handbook of forest analyses (König et al. 2005). In Bavaria, in addition to the standard analytical procedures, also values of phosphorus extracted by citric acid (Pcit) were available for all soil samples. Pcit proved to be the best predictor to determine the phosphorus nutritional status of adult Norway spruce, when calculating stocks down to 40 cm soil depth (Manghabati et al. 2018a, b). Sampling and analysis of needles in all three studies have been performed according to König et al. (2005). We used the concentration of phosphorus in the first-year needles to assess the tree nutritional phosphorus status according to Göttlein (2015). For needle samples of Kranzberger Forst, the weight of needles was also available. All statistical analyses were performed with SPSS 22 (IBM, 2013), using linear regressions and logarithmic curve fitting, as well as paired T test.
Results
Site dependent variation of P-difference Ny1 − Ny2 (Bavarian BZE II and BDF dataset)
As trees obtain their nutrients not only from a certain soil layer, we calculated the stocks of Pcit and aggregated them down to 40 cm, which is according to Manghabati et al. (2018a, b) an appropriate aggregation level.
As shown in Fig. 1a there is a relatively good logarithmic relationship between the stock of soil-extractable phosphorus and the concentration of phosphorus in first-year needles (R2 = 0.41) (see also Fäth et al. 2019). Principally, at each site first-year needles had higher phosphorus concentrations than second-year needles. Calculating P-difference Ny1 − Ny2 classified by the nutritional status according to Göttlein (2015), we expected that trees with lower available phosphorus in the soil and lower phosphorus concentration in first-year needles should show a higher tendency for phosphorus “re-translocation” (Fig. 1b). However, even in the deficiency range, no considerable relationships between soil-extractable phosphorus and P-difference Ny1 − Ny2 could be detected.
a Relation between the stock of citric acid-extractable phosphorus (Pcit) aggregated down to 40 cm (including organic layer) and phosphorus concentration in first-year needles of 305 forest sites in Bavaria (BZE II + BDF), logarithmic curve fit. b Linear regressions for relationships between the stock of citric acid-extractable phosphorus (Pcit) aggregated down to 40 cm soil depth (including organic layer) and difference in concentrations of phosphorus in first- and second-year needles (P-difference Ny1 − Ny2). Symbols show ranges for tree phosphorus nutritional status according to Göttlein (2015)
Inter-annual variation of P-difference Ny1 − Ny2 (BDF dataset)
Because the annual fluctuations of foliar nutrient concentration are a common phenomenon (Vitousek et al. 1995; Yang et al. 2016), we also assessed the temporal variations of P-difference Ny1 − Ny2 at the BDF monitoring plots (Fig. 2). The results of 5–8 years of needles sampling per plot showed that the temporal variability of the P-difference Ny1 − Ny2 at single plots displayed the same order of magnitude as the variability between plots (see Fig. 1b). Again, there is no interdependence between the amount of soil-extractable phosphorus and P-difference Ny1 − Ny2. Neither is there a tendency of higher P-difference Ny1 − Ny2 when in a single year at a particular site the nutritional status is deficient.
Year-to-year fluctuation of P-difference Ny1 − Ny2 at Bavarian BDF sites in dependence of their citric acid-extractable phosphorus (Pcit) aggregated down to 40 cm (including organic layer). Symbols show ranges for tree phosphorus nutritional status according to Göttlein (2015)
Detailed study at Kranzberger Forst
The repeated needle sampling at the same branches of adult Norway spruce at the Kranzberger Forst allows a more detailed study of P-difference Ny1 − Ny2.
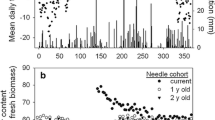
As expected, the intensive needle analysis indicated a decrease in phosphorus concentration when needles got 1 year older (Fig. 3a). This decrease mostly was coupled to an increase in the weight (Fig. 3b). In combination, these two effects resulted in the fact that there was no significant difference in the content of phosphorus for the two needle ages (Fig. 3c).
Phosphorus concentration (a), weight (b), and phosphorus content (c) of needles in the first year and second year of their life. On the left, the data (single values) of tree number 535 are given. On the right, all data from eight intensive monitored trees are given (error bars indicate the 95% confidence interval; significance of differences calculated by a paired T-test)
For all P-difference Ny1 − Ny2 values, the influence of an increase in weight on the phosphorus concentration in second-year needles was calculated. The result is a boxplot derived from 64 data pairs (Fig. 4). Variations in weight of needles explained between 0 and 100 percent of changes in phosphorus concentration in second-year needles. As median, 85% of changes in phosphorus concentration of second-year needles are attributed to a “dilution effect” and only 15% of changes are the result of net phosphorus “re-translocation” between needles.
Discussion
Phosphorus is well known as a mobile element in the plant (Fife et al. 2008; Helmisaari 1992b) and thus has the potential to move between tree organs. Thus, element re-translocation between tree components is regarded as strategy for nutrient conservation (Chapin and Kedrowski 1983; Machado et al. 2016). In particular, older needles may compensate for the demand of growing needles (Oren et al. 1989). As a consequence, tree species should be less influenced by variations in soil nutrient availability (Helmisaari 1992a). Hence, identifying phosphorus internal cycling through re-translocation between needles would confirm tree adaption to soil phosphorus deficiency, as well as shortages in critical years. Several studies have reported that the amount of nutrients reused (mobile elements N, P, Mg, K) in plant organs increases with a decrease in the soil nutrient availability (Grime 1979; Chapin 1980; Berendse and Aerts 1987). Therefore, nutrient re-translocation should be found to a greater extent at stands with lower soil fertility (Khanna et al. 2007). In contrast, the results of BZE II by Riek et al. (2016), however, illustrated that the differences in concentration of phosphorus between first- and second-year needles in luxury and normal nutrition ranges were higher than that in latent and deficiency ranges.
As shown in Figs. 1b and 3a, the concentration of phosphorus in first-year needles of Norway spruce was considerably higher than that of second-year needles. Almost all previous studies (e.g., Riek et al. 2016; Wyttenbach et al. 1995; Wyttenbach and Tobler 1988) observed this difference for needles of Norway spruce. We detected no significant correlation between the stocks of plant-available phosphorus and P-difference Ny1 − Ny2. This result reflects those of Chapin and Kedrowski (1983) and Lim and Cousens (1986), who also concluded that element re-translocation from foliage during the growing season supports new organ development without correlation to soil fertility. Degradation in tree growth rates was presented by Nambiar (1985) as the only adaptive mechanism that trees use to respond to low nutrient availability. In accordance with Aerts (1990), Nambiar and Fife (1991), and Turner and Olsen (1976), we observed that even in trees under the threshold for normal phosphorus nutrition (deficiency ranges) the site-available phosphorus is not related to the needle phosphorus P-difference Ny1 − Ny2 although stocks of Pcit in topsoil are significantly correlated with the phosphorus concentration in the needles (Fäth et al. 2019; Manghabati et al. 2018a, b). This means that despite the important role of root uptake on needle phosphorus nutrition, internal cycling of phosphorus, at least between needles, is not dependent on plant-available phosphorus at Norway spruce sites.
Concentrations of phosphorus in needles show a high year-to-year variation (Dambrine et al. 1995; Reemtsma 1966). As a consequence, also a high annual variation for P-difference Ny1 − Ny2 was observed. The magnitude of this annual difference at one site is comparable to the magnitude of variation between sites. Looking at the nutritional ranges (luxury, normal, and deficiency) Fig. 2 once more demonstrated that there is no dependence of P-difference Ny1 − Ny2 from soil-extractable phosphorus. The big advantage of the intensive sampling at the site Kranzberger Forst is that the second-year needles are collected from the same branches from which, in the previous year, first-year needles were collected and that needle weights are available. The mean concentrations of phosphorus in the second-year of needles’ life were always, and thus significantly, lower than that in first-year needles (Fig. 3a). However, a net phosphorus re-translocation into the first needles, as indicated by a reduction in the phosphorus content in the second year of needle life, was only observed in some single years. In total, there was no significant difference in the content of phosphorus between first and second year of needles’ life. These results seem to follow the observations of Nambiar and Fife (1987) for Pine, who stated that the content of foliage phosphorus (µg/needle) fluctuated internally between needles with remarkable steps of accumulation, re-translocation, and replenishment.
We also observed a strong variation of needles weight, which according to Timmer and Morrow (1984) can be explained by increasing accumulation of nutrients in needles, other than phosphorus. The great influence of variations in needles weight may also, at least partly, explain the results of the first part of our study, that displayed no relationship between soil-extractable phosphorus and P-difference Ny1 − Ny2. Net nutrient re-translocation from second-year to first-year needles for seedlings of Norway spruce and Pine seedlings was observed by Fiedler et al. (1973) and Nambiar and Fife (1987). In their studies during sprout of the new needles, phosphorus concentration in the previous-year needles decreased. Regarding the large physiological and biological dissimilarities between adult trees and seedlings or saplings (e.g., root extension, and rate of nutrient accumulation in needles) these results, however, as shown in our study, may not be transferable to adult trees. At the intensive sampled site Kranzberger Forst we found, that in average more than 80% of the decrease in the concentration of phosphorus in second-year needles is explained by an increase in weight of these needles. These findings also confirm the results of Göttlein et al. (2012), Linder (1995), and Nambiar and Fife (1987), who stated that the element concentration in needles mainly fluctuates due to variations in the dry matter content of needles, which varies seasonally and increases along with needle age. According to our dataset from the Kranzberger Forst, this statement is also true for magnesium, an element for which in the literature also a high amount of re-translocation is reported (Fiedler et al. 1973; Wolff et al. 1999).
Conclusion
The present study was designed to determine the impact of plant-available phosphorus on tree internal phosphorus cycling. Our results do not support the view often found in the literature that phosphorus re-translocation from older to new needles of adult Norway spruce is a targeted mechanism for countering soil phosphorus deficiency. Although there is a positive correlation between soil-extractable phosphorus (Pcit) and phosphorus concentration in needles, no such relation could be found for the difference in phosphorus concentration between needles’ year one and two. This concentration difference shows a high variation between sites, as well as at one site between different years and thus obviously is much more influenced by year-to-year differing growth conditions. This result is supported by the detailed data from the Kranzberger Forst, where on average 85% of the difference in phosphorus concentration was caused by an increase in the weight of second-year needles. Thus, the lower concentration of phosphorus in second-year needles is mainly a dilution effect and only to a very minor part caused by tree internal phosphorus re-translocation. Accordingly, there is no systematic pre-translocation between needles and this is the reason why there is no relationship between the concentration differences in needles and P availability in soil.
References
Achat DL, Pousse N, Nicolas M, Bredoire F, Augusto L (2016) Soil properties controlling inorganic phosphorus availability: general results from a national forest network and a global compilation of the literature. Biogeochemistry 127:255–272. https://doi.org/10.1007/s10533-015-0178-0
Aerts R (1990) Nutrient use efficiency in evergreen and deciduous species from heathlands. Oecologia 84:391–397
Augusto L, Achat DL, Jonard M, Vidal D, Ringeval B (2017) Soil parent material-A major driver of plant nutrient limitations in terrestrial ecosystems. Glob Chang Biol 23(9):3808–3824. https://doi.org/10.1111/gcb.13691
Belyazid UJ, Belyazid S (2012) Phosphorus cycling in boreal and temperate forest ecosystems. Belyazid Consulting and Communication, Malmö
Berendse F, Aerts R (1987) Nitrogen-use efficiency: a biologically-meaningful definition? Funct Ecol 1:293–296
Braun S, Thomas VF, Quiring R, Flückinger W (2010) Does nitrogen deposition increase forest production? The role of phosphorus. Environ Pollut 158(5):2043–2052. https://doi.org/10.1016/j.envpol.2009.11.030
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11:233–260
Chapin FS III, Kedrowski RA (1983) Seasonal changes in nitrogen and phosphorus fractions and autumn retranslocation in evergreen and deciduous taiga trees. Ecology 64(2):376–391
Chapin FS III, Van Cleve K (1989) Approaches to studying nutrient uptake, use and loss in plants. In: Pearcy RW, Ehleringer J, Mooney HA, Rundel PW (eds) Plant physiological ecology-field methods and instrumentation. Chapman and Hall, London, pp 187–207
Cousens JE (1988) Report of a twelve-year study of litter fall and productivity in a stand of mature Scots pine. Forestry 61(3):255–266
Dambrine E, Martin F, Carisey N, GranierJan A, Hällgren JE, Bishop K (1995) Xylem sap composition: a tool for investigating mineral uptake and cycling in adult spruce. Plant Soil 168:233–241. https://doi.org/10.1007/BF00029333
Ewald J (2000) Does phosphorus deficiency cause low vitality in European beech (Fagus sylvatica L.) in the Bavarian Alps? Eur J For Res 119:276–296. https://doi.org/10.1007/BF02769143
Fäth J, Kohlpaintner M, Blum U, Göttlein A, Mellert KH (2019) Assessing phosphorus nutrition of the main European tree species by simple soil extraction methods. For Ecol Manag 432:895–901. https://doi.org/10.1016/j-foreco.2018.10.007
Fiedler HJ, Nebe W, Hoffmann F (1973) Forstliche Pflanzenernährung und Düngung. Gustav Fischer, Stuttgart. ISBN 3-437-30165-9
Fife DN, Nambiar EKS (1984) Movement of nutrients in radiata pine needles in relation to the growth of shoots. Ann Bot 54:303–314
Fife DN, Nambiar EKS, Saur E (2008) Retranslocation of foliar nutrients in evergreen tree species planted in a Mediterranean environment. Tree Physiol 28(2):187–196
Fraser DA (1956) The translocation of minerals in trees. Government of Canada, Department of Northern Affairs and National Resources, Forestry Branch. Forest Research Division Technical Note No. 47
Göttlein A (2015) Ranges of threshold values for the nutritional assessment of the main tree species spruce, pine, oak and beech. Allg Forst Jagdztg 186:110–116
Göttlein A, Baumgarten M, Dieler J (2012) Site conditions and tree-internal nutrient partitioning in mature European beech and Norway Spruce at the Kranzberger forest. In: Matyssek R, Schnyder H, Oßwald W, Ernst D, Munch JC, Pretzsch H (eds) Growth and defence in plants, resource allocation at multiple scales. Ecological studies, Chap 9. Springer, pp 193–211
Grime JP (1979) Plant strategies and vegetation processes. Wiley, Chichester, p 222
Helmisaari HS (1992a) Nutrient retranslocation in three Pinus sylvestris stands. For Ecol Manag 51(4):347–367. https://doi.org/10.1016/0378-1127(92)90334-6
Helmisaari HS (1992b) Nutrient retranslocation within the foliage of Pinus sylvestris. Tree Physiol 10:45–58
Hüttl RF (1991) Die Nährelementversorgung geschädigter Wälder in Europa und Nordamerika. Dissertation Freiburger Bodenkundliche Abhandlungen, 28: 440. Institut für Bodenkunde und Waldernährungslehre der Albert-Ludwigs-Universität. Freiburg i. Br.
Khanna PK, Bauhus J, Meiwes KJ, Kohler M, Rumpf S, Schönfelder E (2007) Assessment of changes in the Phosphorus status of forest ecosystems in Germany-literature review and analysis of existing data: a report to the German Federal Ministry of food, agriculture and consumer protection. Göttinger Nordwestdeutsche Forstliche Versuchsanstalt, Göttingen
König N, Blum U, Symossek F, Bussian B, Ellinghaus R, Furtmann K, Gärtner A, Gutwasser F, Hauenstein M, Kiesling G (2005) Handbuch forstliche Analytik. Bundesministerium für Verbraucherschutz, Ernährung und Landwirtschaft, Bonn
Lim MT, Cousens JE (1986) The internal transfer of nutrients in a Scots pine stand. 2. The pattern of transfer and the effects of nitrogen availability. Forestry 59(1):17–27. https://doi.org/10.1093/forestry/59.1.17
Linder S (1995) Foliar analysis for detecting and correcting nutrient imbalances in Norway spruce. Ecol Bull 44:178–190
Machado MR, de Tarso Barbosa Sampaio P, Ferraz J, Camara R, Pereira MG (2016) Nutrient re-translocation in forest species in the Brazilian amazon. Acta Sci Agron 38:93–101. https://doi.org/10.4025/actasciagron.v38i1.26805
Manghabati H, Kohlpaintner M, Ettl R, Mellert KH, Blum U, Göttlein A (2018a) Correlating phosphorus extracted by simple soil extraction methods with foliar phosphorus concentrations of Picea abies (L.) H. Karst. and Fagus sylvatica (L.). Plant Nutr Soil Sci 181(4):547–556. https://doi.org/10.1002/jpln.201700536
Manghabati H, Weis W, Göttlein A (2018b) Importance of soil extractable phosphorus distribution for mature Norway spruce nutrition and productivity. Eur J For Res 137(5):631–642. https://doi.org/10.1007/s10342-018-1130-3
Mishra G, Debnath S, Rawat S (2017) Managing phosphorus in terrestrial ecosystem: a review. Eur J Biol Res 7:255–270. https://doi.org/10.5281/zenodo.854681
Nambiar EKS (1985) Critical processes in forest nutrition and their importance for management, Div Forest Research. In: Landsberg JJ, Parsons W (eds) Research for Forest Management. CSIRO, Canberra, pp 52–71
Nambiar EKS, Fife DN (1987) Growth and nutrient re-translocation in needles of Radiata pine in relation to nitrogen supply. Ann Bat 60:147–156
Nambiar EKS, Fife DN (1991) Nutrient re-translocation in temperate conifers. Tree Physiol 9:185–207
Netzer F, Schmid C, Herschbach C, Rennenberg H (2017) Phosphorus-nutrition of European beech (Fagus sylvatica L.) during annual growth depends on tree age and P-availability in the soil. Environ Exp Bot 137:194–207. https://doi.org/10.1016/j.envexpbot.2017.02.009
Oren R, Schulze ED, Werk KS, Meyer J (1989) Performance of two Picea abies (L.) Karst. stands at different stages of decline. Oecologia 77:163–173. https://doi.org/10.1007/BF00379182
Pretzsch H, Kahn M, Grote R (1998) Die Fichten-Buchen-Mischbestände des Sonderforschungs-bereiches „Wachstum und Parasitrenabwehr?“im Kranzberger Forst. Forstwiss CBL 117:241–257
Prietzel J, Dümig A, Wu Y, Zhou J, Klysubun W (2013) Synchrotron-based P K-edge XANES spectroscopy reveals rapid changes of phosphorus speciation in the topsoil of two glacier foreland chronosequences. Geochim Cosmochim Acta 108:154–171. https://doi.org/10.1016/j.gca.2013.01.029
Proe MF, Millard P (1995) Effect of P supply upon seasonal growth and internal cycling of P in Sitka spruce (Picea sitchenis (Bong.) Carr.) seedlings. Plant Soil 168–169:313–317
Reemtsma JB (1966) Untersuchungen über den Nährstoffgehalt der Nadeln verschiedenen Alters an Fichte und anderen Nadelbaumarten. Flora Abt B Bd 156:105–121
Riek W, Talkner U, Dammann I, Kohler M, Meiwes KJ, Göttlein A (2016) Waldernährung. In: Wellbrock N, Bolte A, Flessa H (eds) Dynamik und räumliche Muster forstlicher Standorte in Deutschland: ergebnisse der Bodenzustandserhebung im Wald 2006 bis 2008. Thünen Report 43. Johann Heinrich von Thünen-Institut, Braunschweig, Germany, pp 245–291
Schubert A, Butz-Braun R, Schöpke K, Mellert KH (1995) Waldboden-Dauerbeobachtungsflächen in Bayern. Standarduntersuchungen, Tonmineralische Untersuchungen, Aufnahme der Humusformen, Aufnahme der Bodenfauna. Berichte aus der Bayerischen Landesanstalt für Wald und Forstwirtschaft, LWF, Freising. Nr. 4. 80 S. ISSN 0945-8131
Schubert A, Falk W, Stetter U (2015) Waldböden in Bayern. Ergebnisse der BZE II Forstliche Forschungsberichte München 213
Timmer VR, Morrow LD (1984) Predicting fertilizer growth responses and nutrient status of Jack pine by foliar diagnosis, p 335–351. In: Stone EL (ed) Forest soils and treatment impacts. Proceeding of 6Ih North American forest soils conference department of forest wildlife and fish. University of Tennessee, Knoxville
Turner I, Lambert MJ (1986) Nutrition and nutritional relationships of Pinus radiata. Ann Rev Ecol Syst 17:325–350
Turner J, Olsen PR (1976) Nitrogen relations in a Douglas-fir plantation. Ann Bot 40:1185–1193
Van den Driessche R (1984) Nutrient storage, retranslocation and relationship of stress to nutrition. In: Bowen GD, Nambiar EKS (eds) Nutrition of plantation forests. Academic Press, London, pp 181–209
Vitousek PM, Turner DR, Kitayama K (1995) Foliar nutrients during long-term soil development in Hawaiian montane rain forest. Ecology 76:712–720
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15. https://doi.org/10.1890/08-0127.1
Wehrmann J (1959) Methodische Untersuchungen zur Durchführung von Nadelanalysen in Kiefernbeständen. Forstwissenschaftliches Zentralblatt 78:77–97. https://doi.org/10.1007/bf01822233
Weis W, Gruber A, Huber C, Göttlein A (2009) Element contents and storage in the above ground biomass of limed and unlimed Norway spruce trees at Höglwald. Eur J For Res 128:437–445. https://doi.org/10.1007/s10342-009-0291-5
Wolff B, Riek W, Baritz R, Henning P (1999) Deutscher Waldbodenbericht 1996. Ergebnisse der bundesweiten Bodenzustandserhebung im Wald von 1987–1993 (BZE), vol 1. BML, Bonn
Wyttenbach A, Tobler L (1988) The seasonal variation of 20 elements in 1st and 2nd year needles of Norway spruce, Picea abies (L.) Karst. Trees 2:52–64. https://doi.org/10.1007/BF00196980
Wyttenbach A, Schleppi P, Tobler L, Bajo S, Bucher J (1995) Concentration of nutritional and trace elements in needles of Norway spruce (Picea abies [L.] Karst.) as function of the needle age class. Plant Soil 168–169:305–312
Yang Y, Yania RD, Fatemi FR, Levine CR, Lilly PJ, Briggs RD (2016) Sources of variability in tissue chemistry in northern hardwood species. Can J For Res 46:285–296
Acknowledgements
We thank the LWF (Bayerische Landesanstalt für Wald und Forstwirtschaft), especially Alfred Schubert for the access to the BZE and BDF data. We thank Dr. Karl-Heinz Häberle for sampling the needles in Kranzberger Forst. Special thanks go to Dr. Rasmus Ettl and Julian Fäth for proof reading and many fruitful discussions, as well as native speakers who work at Technical University of Munich (Coaching Center).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Agustín Merino.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manghabati, H., Weis, W. & Göttlein, A. Changes in phosphorus concentration in needles of adult Norway spruce - nutrient re-translocation or dilution effect?. Eur J Forest Res 138, 539–546 (2019). https://doi.org/10.1007/s10342-019-01188-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-019-01188-0