Abstract
Northern red oak (Quercus rubra) was introduced to Europe in the late seventeenth century and has since become the most important deciduous non-native tree species in Germany. Despite its importance, little is known about the origin and patterns of genetic variation in German red oak stands. To be able to make recommendations regarding the adaptive potential of red oak stands, which might be related to their origin and the selection of provenances, with respect to climate change, a better understanding of the genetic diversity and structure of German red oak stands is needed. Individuals from 62 populations in Germany and North America were genotyped at five chloroplast microsatellite loci to characterize chloroplast haplotype diversity and geographic structure. Compared to reference populations from the natural distribution range, German red oak stands demonstrated a relatively low genetic differentiation among populations and represented only a fraction of the haplotype diversity found in North America. For several stands located in southern Germany, considerably higher haplotype diversity than in other German stands was found. While most German stands showed signatures of founder effects, the diversity of stands in southern Germany might have been increased due to admixture and multiple introductions of different North American provenances. Overall, we conclude that German stands originated from a limited geographic area, possibly located in the northern part of the native distribution range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the rediscovery of the Americas in the end of the fifteenth century, thriving trade routes connecting the ports of the old and new worlds also transported alien plants and animals. While numbers of established alien species were relatively low until 1800, introductions increased significantly at the turn of the century (Hulme 2009). Introduced populations of non-native species often contained only a fraction of the genetic information compared to the natural range they originated from (Nei et al. 1975; Barrett and Husband 1990). This founder effect, thus, could result in the establishment of new populations with their own specific gene pool. Founder populations are usually small and hence more affected by random drift (Graw 2005). As a consequence, the adaptive potential of founder populations could be limited. However, multiple introductions seem to increase diversity over a longer period (Dlugosch and Parker 2008). Suarez and Tsutsui (2008) even suggested that multiple introductions can enhance the adaptive potential by providing a source of variation important for adaptation, which can be critical for successful establishment and spreading. A different situation arises when seeding material originates from a limited geographic area within a species’ natural range. Limited sampling induces a genetic bottleneck, whereby the newly founded population does not represent the full range of the species’ variation.
Northern red oak was first introduced to Europe at the end of the seventeenth century, planted for ornamental reasons in parks and botanical gardens until the middle of the eighteenth century (Bauer 1951; Nagel 2015). Today, it is the most important foreign deciduous tree species for wood production in Germany (Bundesministerium für Ernährung und Landwirtschaft (BMEL), 2014). Mainly, this is due to a shorter rotation period of only 80–120 years compared to more than 140 years in the native oak species, combined with lower water and nutrient availability (Nagel 2015). In some European countries, Q. rubra is considered invasive (Möllerová 2005; Riepšas and Straigytė 2008; Chmura 2013). However, this is not the case in Germany, because it is less shade tolerant than the main tree species Fagus sylvatica and only little more shade tolerant than the native Q. robur (Vor and Lüpke 2004; Niinemets and Valladares 2006; Nagel 2015). Furthermore, it is subject to heavy browsing and can be easily controlled by tending measures (Vor 2005; Nagel 2015). Natural regeneration is not the most important way of establishing red oak in Germany. Most stands are founded by planting or seeding, formerly in pure stands, now as groups in mixed stands (Nagel 2015). Q. rubra belongs to section Lobatae, which is restricted to North America, and it does not hybridize with native white oak species of the section Quercus (Magni Diaz 2004; Nagel 2015).
Nagel (2015) characterized Q. rubra as a species growing in a wide range of climatic (annual precipitation between 600 and 2000 mm, average temperature between 4 and 15 °C) and soil conditions. Even outside its natural range, Q. rubra shows good to satisfactory growth despite a level of annual precipitation that, in some cases in Germany, does not reach the minimum requirement for red oak (Dreßel and Jäger 2002; Magni Diaz 2004). Thus, under climate change conditions it can be expected that Q. rubra will be able to increase forest adaptability and productivity in particular sites in Germany, especially in mixed forest communities, if suitable provenances are chosen. In order to evaluate the adaptive potential of German red oak stands, a detailed understanding of the geographic origin of the provenances used for the establishment of German red oak stands is required. This is especially important for stands that will be part of tree improvement strategies and serve as seed orchards.
In this study, chloroplast microsatellite (simple sequence repeat or cpSSR) markers were used to analyse haplotype diversity. Due to maternal inheritance of the chloroplast organelle in oaks and lack of recombination, cpDNA haplotypes usually have relatively low variation within oak populations (Zhang et al. 2015) and are more affected than nuclear DNA by stochastic processes, such as genetic drift and founder events (Alexander and Woeste 2014). Haplotype differentiation within one species can be high across both populations and regions, and significant geographic variation can be found even within mixed populations of hybridizing species (Petit et al. 1993; Grivet et al. 2006; López de Heredia et al. 2007; Zhang et al. 2015). Chloroplast DNA markers have been successfully used to trace post-glacial recolonization routes of white oak and other species in Europe (Petit et al. 2002; Palmé et al. 2003; Heuertz et al. 2004) and to reveal the haplotype composition of autochthonous populations (Gailing et al. 2009). Identified haplotypes and haplotype variation can be compared with reference populations within the natural range to infer their origin.
While the analysis of genetic variation patterns in northern red oak populations within their natural range was subject of several past studies (e.g. Daubree and Kremer 1993; Romero-Severson et al. 2003; Magni et al. 2005; Zhang et al. 2015; Borkowski et al. 2017), it has rarely been studied in Europe (Magni Diaz 2004; Merceron et al. 2017). It was found that within the natural range of red oak, genetic differentiation increased northwards (Borkowski et al. 2017). In contrast, no geographic pattern was found in Europe (Magni Diaz 2004), where most of the populations were established in France and Germany and a few others also in the Netherlands, Belgium, Spain, Italy and Romania. This observation was explained by multiple introductions and admixture of material within Europe (Magni Diaz 2004), a suggestion also partly shared by Daubree and Kremer (1993). While identifying the region of origin of the introduced populations was one of the objectives in the Magni Diaz’s (2004) study, it was not succeeded due to the absence of a clear phylogeographic structure within the natural range at a smaller scale. Merceron et al. (2017) studied SNP markers in both North American and European red oak populations with focus on France. Samples from Germany, the Netherlands, Belgium, Spain, Italy and Romania were also included in this study, but in smaller quantities. Merceron et al. (2017) found three main genetic clusters in the red oaks’ natural range, with only two of these clusters observed in Europe. This likely suggests that European populations originated from the northern parts of the natural range. These findings are in line with other studies on this topic (Bauer 1954; Magni Diaz 2004; Nagel 2015). Furthermore, Merceron et al. (2017) reported a continuous, predominantly latitudinal gradient and only a weak phylogeographic structure in the natural range. One explanation could be that, unlike the situation of European white oaks during post-glacial recolonization, northern red oak did not survive the glacial period in separated refugia, but instead in a wide longitudinal range in the south of its natural range (Petit et al. 2002; Magni Diaz 2004).
This study aims to assess how the genetic variation in German red oak stands differs from variation found within the species’ natural range. CpSSR markers have been used in our study for the first time to analyse and compare chloroplast haplotype diversity and structure in Q. rubra plantations across both German stands and North American reference populations. Our objectives were to assess (i) to what extent the haplotype diversity found in the natural range is represented in Germany and (ii) to what extent haplotype diversity varies between the different regions within Germany.
We hypothesized that (1) the introduction of Q. rubra to Germany resulted in reduced overall haplotype diversity due to bottleneck effects and (2) the introduction of seed material from unknown sources led to an artificial differentiation of haplotypes across Germany.
Materials and methods
Plant material
Buds or green leaves were collected from 432 trees in total: 385 trees representing 39 stands in Germany of unknown origin (8–10 samples per stand, Supplementary Table 1S and Fig. 1S) plus material from 47 trees of 8 populations of known North American origin (5–7 samples per population, Supplementary Fig. 2S) from a provenance trial in Northern Germany (Supplementary Table 2S) (Liesebach and Schneck 2011). In addition, published data from 8 natural populations (8 samples per population) as well as unpublished data from 3 natural populations from the northern distribution range of the species were included (Supplementary Table 3S and Fig. 2S) (Lind and Gailing 2013; Lind-Riehl et al. 2014). Samples were taken randomly within each population. All North American populations served as reference having known origin, unlike German stands.
To cover a wide geographic range, sample stands were selected in 5 different federal states of Germany: 10 stands in Lower Saxony, 10 in North-Rhine Westphalia, 6 in Brandenburg, 7 in Thuringia and 6 in Baden-Wuerttemberg. Sample stands were chosen to match the following criteria: (1) they should be pure Q. rubra stands; (2) 50–80 year old; (3) feature a rectangular shape for easier data acquisition; and (4) should be in locations, where they will be cultivated in the future as well (according to the forest administration), as not only the present, but the expected future climatic conditions would match their autecological properties.
DNA isolation
DNA was extracted from about 1-cm2 leaf tissue per tree with the DNeasy™ 96 Plant Kit from Qiagen (Hilden, Germany). Depending on the availability, we used either a small piece of the fresh leaf or 1–2 whole buds from a fresh twig.
Chloroplast microsatellites
Eight different universal cpSSR markers for angiosperms (ccmp1, ccmp2, ccmp3, ccmp4, ccmp5, ccmp6, ccmp7 and ccmp10) (Weising and Gardner 1999) and three cpSSRs developed for oaks (ucd4, udt1 and udt4) (Deguilloux et al. 2003) were tested for amplification and variation in 43 red oak samples from 11 different populations. Five of these cpSSR markers showed variation and were thus used in the study: two universal (ccmp2 and ccmp4) and three oak-specific markers (udt1, udt4 and ucd4, see Supplementary Table 4S for further information).
We used a touchdown PCR program for all markers in a Biometra TProfessional thermocycler (Jena, Germany). The PCR protocol started with 15 min for initial denaturation at 95 °C, followed by 10 cycles of 1 min denaturation at 94 °C, 1 min annealing at 60 °C (− 1 °C per cycle) and 1 min extension at 72 °C. This first set of cycles was then followed by another 25 cycles of 1 min denaturation at 94 °C, 1 min annealing at 50 °C and 1 min extension at 72 °C. The PCR ended with a final 20-min extension step.
For each single primer pair, PCRs were conducted in a 14 µl volume containing 1 µl of genomic DNA (about 0.6 ng/µl), 6.8 µl ddH2O, 1.5 µl PCR buffer (containing 0.8 M Tris–HCl and 0.2 M (NH4)2SO4), 1.5 µl MgCl2 (25 mM), 1 µl of each dNTP (2.5 mM), 1 µl primer (forward, 5 pM/µl), 1 µl primer (reverse, 5 pM/µl) and 1 U HOT FIREPol® Taq Polymerase from Solis BioDyne (Tartu, Estonia). For multiplexing of markers ccmp2 and ccmp4, we used the following PCR mix: 1 µl of genomic DNA (about 0.6 ng/µl), 4.8 µl ddH2O, 1.5 µl PCR buffer (containing 0.8 M Tris–HCl and 0.2 M (NH4)2SO4), 1.5 µl MgCl2 (25 mM), 1 µl of each dNTP (2.5 mM), 2 × 1 µl primer (forward, 5 pM/µl), 2 × 1 µl primer (reverse, 5 pM/µl) and 1 U HOT FIREPol® Taq Polymerase (Solis BioDyne; Tartu, Estonia).
The PCR products were tested in an agarose gel electrophoresis to determine the ideal dilution ratio for the capillary electrophoresis. The gel electrophoresis was carried out at 90 v for 20 min in a 1.5% agarose gel with TAE as a running buffer (1X working solution). The DNA was stained with Roti-Safe GelStain from Roth (Karlsruhe, Germany). Samples were diluted according to the intensity of amplification products on the gel.
For the separation of the cpDNA fragments, we performed a capillary electrophoresis on an ABI Prism Genetic Analyzer 3130xl (Applied Biosystems, Foster City, USA). The fragment sizes were scored using the GeneMapper software version 3.7 (Applied Biosystems, Foster City, USA).
Data analyses
Chloroplast DNA haplotypes were based on all genotyped cpSSR markers. The software PermutCpSSR (https://www6.bordeaux-aquitaine.inra.fr/biogeco/Production-scientifique/Logiciels/Contrib-Permut/Permut; Pons and Petit 1996; Burban et al. 1999) was used to determine the total haplotypic diversity HT, the average expected within-population haplotypic diversity HS and the genetic differentiation among populations GST. Assuming a stepwise mutational model, the software was also used to compute RST that takes into account allele size and is computed as the ratio of the variance between populations and the total variance of allele size in terms of number of repetitive motifs (Slatkin 1995). In contrast to GST, RST accounts better for the relatively low mutation rates occurring at chloroplast SSR markers. The haplotype network was generated using Arlequin version 3.5 (Excoffier and Lischer 2010). The software computes a matrix of pairwise distances between all haplotypes, using the sum of squared size differences.
The software package GENECLASS2 (Piry et al. 2004) was used to tentatively assign German red oak stands to North American reference populations. It uses a Bayesian method introduced by Rannala and Mountain (1997) to assign German stands to their three most probable reference populations.
To analyse a possible correlation between genetic and geographic distances, Mantel tests were performed separately for German stands and North American reference populations. The correlation value RXY ranges from − 1 to 1. A positive correlation would imply that two populations become increasingly genetically distant the further away they are located from each other. A negative correlation would imply the contrary. First, pairwise genetic distances were calculated between all populations (Bruvo et al. 2004). To weigh them by the number of individuals in each population, the pairwise genetic distances between individual trees in each population pair were summed, and the sum was divided by the number of pairwise distances between the two populations. Secondly, a matrix of the geographic distance (in km) was computed. Lastly, the software package GenAlEx 6.5 (Peakall and Smouse 2006, 2012) was used to perform both a Mantel test (9999 permutations) and a Principle Coordinates Analysis (PCoA). The PCoA is a cluster analysis, which can visualize individual and/or population differences based on the genetic distance between them by assigning for each individual or population a location in a multidimensional space. In this study, the Bruvo distance between populations was used. The PCoA tries to find the main axes through a distance matrix that explain most of the genetic differentiations between individuals and/or populations. Usually, these are the first two or three main axes in a multidimensional space (Peakall and Smouse 2012). In our case, the first axis explained 68.10% and the second axis 8.77% (together 76.87%) of the differentiation.
Results
Chloroplast haplotype distribution
In total, 13 different chloroplast haplotypes were found among all studied populations. Five of them (D, E, F, M and O) were found only in Germany, while 5 others (G, H, I, K and L) were found solely in North America (Table 1). Most of these haplotypes were rare. Haplotype K (8.9%) was found in the upper Midwest and southern Canada, and haplotype L (5.2%) was found only in the Great Lakes region of North America. Haplotypes G and H were identified only in samples representing southern regions of the natural range. Unlike private haplotypes in North America, private haplotypes in German stands were found rarely. While haplotypes D and E are more similar to haplotype A, haplotypes F, M and O appear to be closely related to haplotype B.
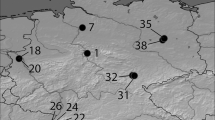
Among the common types, haplotype A was dominant in both North America and Germany, although a much higher relative frequency was found in Germany (80.3%) compared to North America (54.8%). Haplotype B was the next most frequent haplotype in Germany (12.0%) with a similar frequency in North American samples (13.3%). Interestingly, this haplotype is different from haplotype A in each of the five loci (Table 1). While haplotype B was found in Germany in each of the five federal states, it was predominantly located in the centre and northern regions of the natural distribution range in North American samples (Fig. 1). Haplotype C, which is closely related to haplotype A, was found only in the northern regions of the natural range (on the tip of the Keweenaw Peninsula and in Ontario). In German stands, haplotype C was usually found at low frequency, only in single samples, except Baden-Wuerttemberg, where it was found at relatively high frequencies (57%), although only 6 stands were sampled there (Fig. 2).
a Chloroplast haplotype distribution south of the Great Lakes. Populations were sampled and genotyped in earlier studies at gSSRs (Lind and Gailing 2013; Lind-Riehl et al. 2014; Google Maps 2017b). Data from populations Keweenaw, Mine and Porcupine have not been published before. b Distribution of Quercus rubra chloroplast haplotypes in North America. Samples were partly obtained from a provenance trial in Lübeck, Germany (Liesebach and Schneck 2011; Google Maps 2017c)
Haplotype diversity and structure
The minimum spanning tree in Fig. 3 shows the presence of three main lineages or clusters and their related haplotypes (A, B and H). In our samples representing North America, lineage A was found in all regions, but predominantly in the north and north-east; B was found in one population in the south, but also in the north-central and the north-west of the species’ natural distribution; H was mainly found in the south. Haplotype A is different from B and H in all the five loci, whereas B and H have one locus in common.
Minimum spanning tree representing the chloroplast haplotype network (Excoffier and Lischer 2010) of Quercus rubra populations. Numbers next to the lines indicate the number of markers which differ between two haplotypes
Due to the fixation of the same one haplotype per site, haplotype diversity within North American populations was low (HS = 0.177), despite total haplotype diversity being high (HT = 0.652) accounting for a relatively high number of different haplotypes (Table 2). High genetic differentiation was found among all North American populations (GST = 0.729, RST = 0.772). In Germany, the average haplotype diversity within populations was higher (HS = 0.291), especially in stands from Southwest Germany (HS = 0.537) (Fig. 2). Oppositely, total haplotype diversity was moderate with average values considerably lower than in North America (HT = 0.337) with the exception of the Baden-Wuerttemberg region (HT = 0.655). Apart from the high haplotype diversity, Baden-Wuerttemberg featured two of the five private haplotypes in Germany and the greatest share of haplotype C among all regions analysed. As a consequence of the frequent occurrence of haplotype A in most German stands, genetic differentiation among all stands was relatively low (GST = 0.137, RST = 0.047). This higher difference between the two values of GST and RST can be explained by the different mutation models behind these parameters. RST assumes a stepwise mutation model that takes into account to what extent alleles differ in their size in terms of number of repetitive motifs. Thus, two alleles of one marker differentiate stronger, when more mutational steps lie between them. In contrast to North American populations, markers differ mostly by only a single nucleotide motif among German stands. As a result, the RST value is smaller (i.e. demonstrating less differentiation) than the calculated GST.
Relationship between German and North American populations
The PCoA showed that all German stands densely cluster with reference populations from most areas of North America (Fig. 4). Within this large group, there is further grouping for stands from North-Rhine Westphalia, Lower Saxony and Brandenburg. Stands from Thuringia and Baden-Wuerttemberg were linearly distributed along Coord. 1 (see detailed representation in Supplementary Fig. 3S). Overall, the North American reference populations were more differentiated than the German ones (Fig. 4). NQ-E, NQ-R and Nantahala (USA-4) of the upper Midwest and the south, which all contained rare haplotypes of lineage H, clustered together. MTU and Mine consisted mainly of haplotype B, while BR1 and Keweenaw on the upper end of the distribution mainly contained haplotype C. These four populations are located on the Keweenaw Peninsula at Lake Superior. The PCoA analyses showed that some German stands were similar in haplotype composition to North American populations and were consequently assigned to these reference populations with the GENECLASS2 analyses (Supplementary Table 5S). This is particularly the case for the German stands 23, 24, 30, 31 and 38, which all were assigned to the North American population Anderson in the north-central part of the natural range. In general, most German stands could be assigned to populations from the northern part of the natural range. However, most assignments were apparently inaccurate, because only a low score for each assigned population was calculated.
Relationship between genetic and geographic distances
While the Mantel test showed no significant correlation (RXY = 0.148, p = 0.180) between genetic and geographic distances for North American reference populations (Supplementary Fig. 4S), it revealed a slightly positive significant correlation (RXY = 0.284, p = 0.001) for the stands in Germany (Supplementary Fig. 5S). In addition, while still very low, the R2 value in Germany was four times higher (R2 = 0.08) than for North American reference populations (R2 = 0.02).
Discussion
German plantations originated from a limited geographic range in North America
Northern red oak stands in Germany seem to originate from a geographically restricted region in the northern part of North America, covering two lineages, one of which is found all over North America, but mainly in the north and north-east (lineage A) and one that was found mainly in the north-central and north-western parts (lineage B, see Figs. 1, 3). Both lower haplotype diversity and lower differentiation among German stands point to founder effects, suggesting that German stands represent only a fraction of the diversity found in North America. This is supported by the multivariate PCoA analysis where populations from North America are widely scattered while German stands form a compact group.
Likewise, the lower differentiation among populations (GST = 0.137) also suggests that German stands originate from a restricted geographic range. In addition, the absence of haplotypes G, K and L (27.0% in North America) supports the conclusion that German Q. rubra stands originated from a limited geographic range in North America. In accordance with our data, genetic differentiation at cpDNA markers among Q. rubra populations from a restricted region in the Great Lakes region was considerably lower (GST = 0.206, Zhang et al. 2015) as compared to differentiation described in studies that covered a range exceeding the Great Lakes region (GST = 0.73; this study; GST = 0.58, populations in the north-western part of the natural range, Birchenko et al. 2009; GST = 0.46, populations covering the whole natural range, Magni et al. 2005). In agreement with previous findings by Magni et al. (2005), no clear geographic genetic structure of haplotypes was found within the natural distribution area of Q. rubra in this study. The absence of geographic genetic structures within the natural range, as indicated also by the Mantel test, impedes the detailed identification of the geographic origin of German plantations (e.g. with GENECLASS2). Although their exact origin cannot be determined, the presence of haplotype B with restricted distribution in North America (two populations on the Upper Peninsula of Michigan, one population in Indiana) would also point to the origin of German stands from the northern part of the natural distribution range. A range-wide high-density characterization of haplotypes in North American Q. rubra populations, as it was done for European white oak species (Petit et al. 2002), might allow to narrow down the origin of German red oak stands. The fully sequenced chloroplast genome of Q. rubra (Alexander and Woeste 2014), including locations for intraspecific polymorphisms, can serve as a reference for the assembly of chloroplast genomes of red oak samples from all over the natural range.
Our findings conform with a recent study analysing SNP markers in North American red oak populations as well as in European populations that are located mainly in France: Merceron et al. (2017) found that from three identified genetic clusters (G1, G2, G3) within the natural range, only two were observed in Europe. From these three clusters, one occurs mainly in the south (G2), one mainly in the north-east (G1), and the third cluster (G3), which supposedly diverged from the first two clusters, is mainly located in the north-central and north-west of the natural distribution (Merceron et al. 2017). Furthermore, G3 is reported to occur more evenly distributed over all regions. In their study, the genetic cluster mainly found in the south of the natural distribution could have been largely extirpated in or have never been introduced to Europe in the first place. Merceron et al. (2017) stated that this could be due to the source populations for the European gene pool being located in the Northern part of the range. Likewise, an early study addressing the taxonomy of Q. rubra variants by means of phenotypic traits came to the conclusion that German red oak stands comprise mainly Q. rubra var. rubra, a variety that is characterized by a shallower cupule and bigger acorns and is predominant in the North of the natural distribution area (Bauer 1954).
Although our sampling in North America was limited and not designed to analyse haplotype diversity across the whole natural distribution area, the geographic distribution of the three cpDNA lineages found in this study seems to match the distribution of the three genetic clusters derived from SNP markers found by Merceron et al. (2017). According to both studies, only the two northern lineages or clusters were introduced to Europe, one that occurs mainly in north-central and north-west regions (G3 or lineage B) and one that occurs mainly in the north and north-east regions (G1 or lineage A) of the natural distribution area. In addition, haplotype B from lineage B was found in all regions in Germany, but was present only in the north and central part of the natural distribution range in North America.
When a species migrates to an unoccupied geographic area (e.g. an island or another continent), its gene pool hardly represents its full range of varieties. Merely a fraction of the species’ variation provides the foundation for the future population, being challenged not only by new, but also constantly changing environmental conditions (Mayr 1954; Meimberg et al. 2006). Northern red oak seems to find itself in exactly this position in Germany: sampling from a limited geographic range has limited the genetic diversity at chloroplast markers in most German stands, reflecting founder events far away from the original distribution area. Although Q. rubra is currently well adapted to conditions in Germany (Roloff and Grundmann 2008), putative limited diversity at nuclear genes might lower the capacity to react to changing environmental conditions in the future. To describe the adaptive potential of red oak stands in Germany, future studies should focus on the assessment of genetic diversity at nuclear SSRs and genic EST-SSRs and at candidate genes in representative samples of selected German and North American reference populations.
Admixed material at least for plantations from Southwest Germany
While stands in Germany generally show low overall haplotype diversity and low genetic differentiation among populations, a different pattern is found for the stands probed in Baden-Wuerttemberg. Here, higher haplotype diversity and numbers of private haplotypes were found compared to all other examined regions of Germany (e.g. the more frequent occurrence of haplotype C in Baden-Wuerttemberg, while this haplotype is rare in other federal states). This suggests that imported seeding material for the establishment of the stands in Baden-Wuerttemberg was admixed to consist of material from different regions within the natural distribution range (e.g. due to multiple introductions). In fact, northern red oak went through several periods of cultivation since its first introduction at the end of the seventeenth century. After first unsuccessful trials in the middle of the eighteenth century, there were intensified efforts to establish Q. rubra in the second half of the nineteenth century and in the middle of the twentieth century (Nagel 2015). The material for the establishment of the new stands could partly have been collected from red oak stands of the first two generations, but as France and the Netherlands were also making efforts to establish northern red oak (Nagel 2015), the material could also have been obtained from there. Furthermore, additional material could have been brought directly from populations within the natural distribution area. In either case, the positive correlation between the genetic and geographic distances for German red oak stands found in this study (Supplementary Fig. 5S) is in accordance with the introduction of different gene pools at different regions in Germany.
Future perspective
Our study revealed that although chloroplast haplotype variation in red oak in Germany is relatively low, some evidence for admixture and multiple introductions of this species are reflected in higher haplotypic diversity and genetic differentiation in stands in Baden-Wuerttemberg. Unlike North America where it has a limited geographic distribution (mainly in the northern part of the natural distribution range), the presence of haplotype B in all regions in Germany suggests an origin of German stands from the northern part of the natural range. These results support the similar conclusion drawn by Merceron et al. (2017) that red oak populations in Europe originated from the north of the natural distribution area. Further analyses at microsatellite and SNP markers in adaptive genes should be performed to confirm the genetic divergence in two lineages within Europe. If the number of sampled populations in both North America and Germany and the number of cpDNA and other markers are increased, the origin of German red oak stands in the north of the natural distribution area could potentially be narrowed down further based on the occurrence of rare and geographically restricted variants. The level of genetic variation and adaptive potential of the species should also be characterized at nuclear markers including candidate genes with potential role in local adaptation.
References
Alexander LW, Woeste KE (2014) Pyrosequencing of the northern red oak (Quercus rubra L.) chloroplast genome reveals high quality polymorphisms for population management. Tree Genet Genomes 10:803–812
Barrett SC, Husband BC (1990) The genetics of plant migration and colonization. In: Brown HD, Clegg MT, Kahler AL et al (eds) Plant population genetics, breeding, and genetic resources. Sinauer Associates Inc., Massachusetts, pp 254–277
Bauer F (1951) Die Roteiche 1950. Eine ertragskundlich-biologische und holzkundliche Untersuchung. Dissertation, Universität Göttingen
Bauer F (1954) Zur Rassenfrage der Roteiche. Allgemeine Forstzeitschrift 9:470–474
Birchenko I, Feng Y, Romero-Severson J (2009) Biogeographical distribution of chloroplast diversity in northern red oak (Quercus rubra L.). Am Midl Nat 161:134–145
Borkowski DS, Hoban SM, Chatwin W, Romero-Severson J (2017) Rangewide population differentiation and population substructure in Quercus rubra L. Tree Genet Genomes 13:472
Bruvo R, Michiels NK, D’Souza TG, Schulenburg H (2004) A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Mol Ecol 13:2101–2106
Bundesministerium für Ernährung und Landwirtschaft (BMEL) (2014) Der Wald in Deutschland. Ausgewählte Ergebnisse der dritten Bundeswaldinventur. https://www.bundeswaldinventur.de/fileadmin/SITE_MASTER/content/Dokumente/Downloads/BMEL_Wald_Broschuere.pdf. Accessed 20 Mar 2018
Burban C, Petit RJ, Carcreff E, Jactel H (1999) Rangewide variation of the maritime pine bast scale Matsucoccus feytaudi Duc. (Homoptera: Matsucoccidae) in relation to the genetic structure of its host. Mol Ecol 8:1593–1602
Chmura D (2013) Impact of alien tree species Quercus rubra L. on understorey environment and flora: A study of the silesian upland (southern Poland). Polish J Ecol 61:431–442
Daubree JB, Kremer A (1993) Genetic and phenological differentiation between introduced and natural populations of Quercus rubra L. Ann For Sci 50:271s–280s
Deguilloux M-F, Dumolin-Lapègue S, Gielly D, Grivet D, Petit RJ (2003) A set of primers for the amplification of chloroplast microsatellites in Quercus. Mol Ecol Notes 3:24–27
Dlugosch KM, Parker IM (2008) Founding events in species invasions. Genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Dreßel R, Jäger EJ (2002) Beiträge zur Biologie der Gefäßpflanzen des herzynischen Raumes. 5. Quercus rubra L. (Roteiche): Lebensgeschichte und agriophytische Ausbreitung im Nationalpark Sächsische Schweiz. Hercynia 35:37–64
Excoffier L, Lischer HLE (2010) Arlequin suite ver 3.5. A new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567
Gailing O, Wachter H, Heyder J, Rogge M, Finkeldey R (2009) Chloroplast DNA analyses of very old, presumably autochthonous Quercus robur L. stands in North Rhine-Westphalia. Allgemeine Forst- und Jagdzeitung 180:221–227
Google Maps (2017a) Map. Populations in Germany. https://www.google.de/maps/@50.8871706,7.9740269,7z/data=!3m1!4b1!4m2!6m1!1s18cXo5V8opHdmODvdDhOnbGxKt3Q1t6S0?hl=de. Accessed 15 Dec 2017
Google Maps (2017b) Map. Populations south of the great lakes. https://www.google.de/maps/@42.6170767,-82.2325253,7z/data=!4m2!6m1!1s1CaBHN6sHvvBYSIyeuU2vk4sRkzs?hl=de. Accessed 12 Dec 2017
Google Maps (2017c) Map. USA & Canada (East). https://www.google.de/maps/@42.6170767,-82.2325253,7z/data=!4m2!6m1!1s1CaBHN6sHvvBYSIyeuU2vk4sRkzs?hl=de. Accessed 12 Dec 2017
Graw J (2005) Genetik, 4th edn. Springer, Berlin
Grivet D, Deguilloux M-F, Petit RJ, Sork VL (2006) Contrasting patterns of historical colonization in white oaks (Quercus spp.) in California and Europe. Mol Ecol 15:4085–4093
Heuertz M, Fineschi S, Anzidei M, Pastorelli R, Salvini D, Paule L, Frascaria-Lacoste N, Hardy OJ, Vekemans X, Vendramin GG (2004) Chloroplast DNA variation and postglacial recolonization of common ash (Fraxinus excelsior L.) in Europe. Mol Ecol 13:3437–3452
Hulme PE (2009) Trade, transport and trouble. Managing invasive species pathways in an era of globalization. J Appl Ecol 46:10–18
Liesebach M, Schneck V (2011) Entwicklung von amerikanischen und europäischen Herkünften der Roteiche in Deutschland. Forstarchiv 82:125–133
Lind JF, Gailing O (2013) Genetic structure of Quercus rubra L. and Quercus ellipsoidalis E. J. Hill populations at gene-based EST-SSR and nuclear SSR markers. Tree Genet Genomes 9:707–722
Lind-Riehl JF, Sullivan AR, Gailing O (2014) Evidence for selection on a CONSTANS-like gene between two red oak species. Ann Bot 113:967–975
Little EL (1999) Range of Quercus rubra. U.S. Geological Survey
López de Heredia U, Carrión JS, Jiménez P, Collada C, Gil L (2007) Molecular and palaeoecological evidence for multiple glacial refugia for evergreen oaks on the Iberian Peninsula. J Biogeography 34:1505–1517
Magni Diaz CR (2004) Reconstitution de l’introduction de Quercus rubra L. en Europe et conséquences génétiques dans les populations allochtones. Dissertation, Ècole Nationale du Génie Rural, des Eaux et des Forêts (in French)
Magni CR, Ducousso A, Caron H, Petit RJ, Kremer A (2005) Chloroplast DNA variation of Quercus rubra L. in North America and comparison with other Fagaceae. Mol Ecol 14:513–524
Mayr E (1954) Change of the genetic environment and evolution. In: Huxley J, Hardy AC, Ford EB (eds) Evolution as a process. Allen & Unwin, London, pp 157–180
Meimberg H, Hammond JI, Jorgensen CM, Park TW, Gerlach JD, Rice KJ, McKay JK (2006) Molecular evidence for an extreme genetic bottleneck during introduction of an invading grass to California. Biol Invasions 8:1355–1366
Merceron NR, Leroy T, Chancerel E, Romero-Severson J, Borkowski DS, Ducousso A, Monty A, Porté AJ, Kremer A (2017) Back to America. Tracking the origin of European introduced populations of Quercus rubra L. Genome 60:778–790
Möllerová J (2005) Notes on invasive and expansive trees and shrubs. Journal of Forest Science 51:19–23
Nagel R-V (2015) Roteiche (Quercus rubra L.). In: Vor T, Spellmann H, Bolte A et al (eds) Potenziale und Risiken eingeführter Baumarten. Baumartenportraits mit naturschtuzfachlicher Bewertung. Univ.-Verl. Göttingen, Göttingen, pp 219–267
Nei M, Maruyama T, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution 29:1–10
Niinemets Ü, Valladares F (2006) Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol Monogr 76:521–547
Palmé AE, Su Q, Rautenberg A, Manni F, Lascoux M (2003) Postglacial recolonization and cpDNA variation of silver birch, Betula pendula. Mol Ecol 12:201–212
Peakall R, Smouse PE (2006) GenAlEx 6. Genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Peakall R, Smouse PE (2012) GenAlEx 6.5. Genetic analysis in Excel. Population genetic software for teaching and research–an update. Bioinformatics (Oxford, England) 28:2537–2539
Petit RJ, Kremer A, Wagner DB (1993) Geographic structure of chloroplast DNA polymorphisms in European oaks. TAG. Theoretical and applied genetics. Theoretische und angewandte Genetik 87:122–128
Petit RJ, Brewer S, Bordács S, Burg K, Cheddadi R, Coart E, Cottrell J, Csaikl UM, van Dam B, Deans JD, Espinel S, Fineschi S, Finkeldey R, Glaz I, Goicoechea PG, Jensen JS, König AO, Lowe AJ, Madsen SF, Mátyás G, Munro RC, Popescu F, Slade D, Tabbener H, de Vries SGM, Ziegenhagen B, de Beaulieu J-L, Kremer A (2002) Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. For Ecol Manage 156:49–74
Piry S, Alapetite A, Cornuet J-M, Paetkau D, Baudouin L, Estoup A (2004) GENECLASS2. A software for genetic assignment and first-generation migrant detection. J Heredity 95:536–539
Pons O, Petit RJ (1996) Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144:1237–1245
Rannala B, Mountain JL (1997) Detecting immigration by using multilocus genotypes. Proc Natl Acad Sci 94:9197–9201
Riepšas E, Straigytė L (2008) Invasiveness and ecological effects of red oak (Quercus rubra L.) in Lithuanian Forests. Baltic Forestry 14:122–130
Roloff A, Grundmann B (2008) Klimawandel und Baumarten-Verwendung für Waldökosysteme. Technische Universität Dresden. http://waldundklima.de/klima/klima_docs/swin_roloff_klam_2008.pdf. Accessed 26 Feb 2018
Romero-Severson J, Aldrich P, Feng Y, Sun W, Michler C (2003) Chloroplast DNA variation of northern red oak (Quercus rubra L.) in Indiana. New Forest 26:43–49
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics 139:457–462
Suarez AV, Tsutsui ND (2008) The evolutionary consequences of biological invasions. Mol Ecol 17:351–360
Vor T (2005) Natural regeneration of Quercus rubra L. (Red Oak) in Germany. In: Nentwig W, Bacher S, Cock MJW et al (eds) Biological invasions—From ecology to control, pp 111–123
Vor T, Lüpke Bv (2004) Das Wachstum von Roteiche, Traubeneiche und Rotbuche unter verschiedenen Lichtbedingungen in den ersten beiden Jahren nach der Pflanzung. Forstarchiv 75:13–19
Weising K, Gardner RC (1999) A set of conserved PCR Primers for the analysis of simple sequence repeat polymorphisms in chloroplast genomes of dicotyledonous angiosperms. Genome 42:9–19
Zhang R, Hipp AL, Gailing O (2015) Sharing of chloroplast haplotypes among red oak species suggests interspecific gene flow between neighboring populations. Botany 93:691–700
Acknowledgments
We thank Alexandra Dolynska, Andreas Parth, Oliver Caré, Katrin Burger and Maximilian Boersch for their technical assistance. We also thank two anonymous reviewers for their helpful comments on earlier drafts and Edward Jones for attentive English editing. The study was supported by the German Federal Ministry of Food and Agriculture (Funding code 22023314).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Lluís Coll.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pettenkofer, T., Burkardt, K., Ammer, C. et al. Genetic diversity and differentiation of introduced red oak (Quercus rubra) in Germany in comparison with reference native North American populations. Eur J Forest Res 138, 275–285 (2019). https://doi.org/10.1007/s10342-019-01167-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-019-01167-5