Abstract
Creation of canopy gaps can be used as a restoration measure to aid in the diversification of structural variability and tree species composition in forests. However, it is not generally known how the specific properties of gaps influence restoration success. In this study, we examined tree seedling regeneration in 94 small canopy gaps that were established for restoration purposes in two protected Pinus sylvestris (Scots pine)-dominated forests in eastern Finland 10 years earlier. In particular, we assessed the effect of gap size, within-gap position, and soil preparation (exposure of the mineral soil) within the gaps (soil disturbance) on seedling establishment. We found that tree species composition in the sample plots consisted mainly of P. sylvestris (73 %) and Betula spp. (16 %) seedlings. Most Pinus and Betula seedlings were found in the disturbed plots, and only 6 % of the seedlings were found in the undisturbed plots (no soil preparation applied). The mean number of pooled Pinus and Betula seedlings per disturbed plot (40 × 60 cm) was 0.5 and 0.1, respectively. Mean height of the Pinus and Betula seedlings was 9.2 cm and 51.5 cm, respectively, which suggests that seedling development in the disturbed plots was very slow and is probably hindered by an insufficient amount of solar radiation reaching the forest floor, as well as by root competition with the trees that surround the gap. Both factors are affected by gap size, which was probably not large enough (mean diameter ≤23 m) in our study forests to reach a threshold value that meets the requirements of Pinus and Betula seedlings. We conclude that the creation of small canopy gaps does not appear to be an effective method to restore the age class structure and tree species composition in boreal pine-dominated forests. Further studies that manipulate the gap size more widely than this current study are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past decades, increasing attention has been given to the concept of emulating natural disturbance dynamics in order to promote more sustainable forest management (Kuuluvainen 2002; Perera et al. 2004). The concept has produced a number of practical applications that have been employed in both the management of production forests and in the restoration of nature reserves (Long 2009; Kuuluvainen and Grenfell 2012; Lindenmayer et al. 2012). One such application is the creation of canopy gaps that seek to emulate the small-scale disturbances that often characterize the natural forests of boreal Fennoscandia (Kuuluvainen and Aakala 2011; Halme et al. 2013). While canopy gaps can be created in forest reserves to increase structural variability and to move tree species composition closer to their natural state, they can also be used in commercial forestry as an alternative to more conventional even-aged management methods (Similä and Junninen 2012; Puettmann et al. 2015).
Irrespective of whether the canopy opening has been artificially created or has developed from natural disturbance, tree mortality in the gaps mainly facilitates tree seedling recruitment by increasing the availability of above and belowground resources (Canham et al. 1990; McCarthy 2001). In contrast to artificially created canopy gaps that are made by felling the trees, gaps in natural forests often provide suitable microsites for regeneration if the trees are uprooted by wind (Kuuluvainen 1994; Ulanova 2000). Due to various stochastic factors, such as plant–herbivore interactions and the proximity of seed sources, post-disturbance succession within gaps in a given forest type is ultimately dictated simultaneously by multiple factors (Chapin et al. 1987; Kuuluvainen and Juntunen 1998; McCarthy 2001).
Depending on the gap size, the canopy architecture of the surrounding forest, and the location of the gap (latitude and topography), the amount of solar radiation increases within gaps and enhances the growth of advance regeneration, as well as permitting the establishment of shade-intolerant tree species (Canham et al. 1990; Kuuluvainen 1994; Lieffers et al. 1999; McCarthy 2001). Although the most prominent change in canopy gaps following a disturbance is the increase in the availability of solar radiation, the concurrent mortality of the dominant trees results in decreased root competition below the gap (Casper and Jackson 1997). Hence, it is often difficult to disentangle the relative importance of light availability from root competition. Several studies (e.g. Björkman and Lundeberg 1971; Kuuluvainen and Ylläsjärvi 2011; Axelsson et al. 2014) from northern boreal Europe suggest that competition for belowground resources is a more important component limiting seedling emergence and growth in drier sites deficient in nutrients. The role of root competition may become less important as the moisture and nutrient content of the soil increases (Casper and Jackson 1997; Coomes and Grubb 2000; McCarthy 2001).
The type of disturbance largely determines if the trees in the gap die standing up, snapped off, or uprooted. For instance, windthrow often results in the uprooting of trees and the creation of pit-and-mound microtopography that has been shown to be important for the early establishment of seedlings in both pine- and spruce-dominated boreal forests (Kuuluvainen and Juntunen 1998; Ulanova 2000; Kuuluvainen and Kalmari 2003; Mitchell 2012). In artificial canopy gaps, soil preparation treatments, such as exposing the mineral soil in small patches, can be used to compensate for the missing variation in soil microtopography and to enhance the germination of seeds and to facilitate the early development of seedlings. Studies on the response of tree recruitment to canopy gap disturbance are extensive in tropical and temperate forests (McCarthy 2001). In boreal Fennoscandia, however, research on the regeneration dynamics of trees following a gap disturbance has traditionally drawn less interest and most of the published studies are conducted in Picea abies (Norway spruce)-dominated forests (e.g. Liu and Hytteborn 1991; Gray and Spies 1996; Hökkä et al. 2011, 2012). In Pinus sylvestris (Scots pine)-dominated forests, only the short-term results of canopy gap characteristics on tree seedling establishment are available (Kuuluvainen and Juntunen 1998; Rouvinen and Kouki 2011).
The objective of this study was to assess tree seedlings in small canopy openings, which were established for restoration purposes in two P. sylvestris-dominated forests, approximately 10 years after their creation. Thus, the current study adds significantly to the previous short-term results. We expect that the long-term and the short-term responses may differ widely, because both the forest surrounding a gap and the soil properties (such as mineral soil exposure) can change a lot during the first 10 years after the small-scale disturbance. These are likely to have an effect on competitive abilities of young seedlings in small gaps. The specific study questions are: (1) Do small artificial canopy gaps facilitate the recruitment of tree seedlings in P. sylvestris-dominated forests? (2) Does successful tree regeneration in these gaps require soil preparation? (3) What is the influence of canopy gap size on tree seedling regeneration in these forests? (4) Does the bottom and field-layer vegetation in the disturbed plots in gaps influence the number of seedlings?
Materials and methods
Study areas
The two study areas, Koitajoki (62°57′N, 31°27′E; 170 m a.s.l.) and Reposuo (63°20′N, 30°13′E; 130 m a.s.l.), are situated in eastern Finland, in the middle boreal vegetation zone. The mean annual temperature in these areas is 2–3 °C, and the mean annual precipitation is between 550 and 650 mm (Pirinen and Kersalo 2009). The growing season is, on average, 140–160 days and the effective temperature sum (>5 °C) is 1000–1200 degree days (Pirinen and Kersalo 2009).
Both study areas are located on dry and nutrient-poor mineral soils that represent a typical forest type in the region. The forest stands were classified as Vaccinium-type forests based on the ground vegetation (sensu Cajander 1949), although within-stand variation existed (see Table 3). In the Reposuo area, the mean height of the dominant trees was 10.5 m and the mean diameter of the trees was 10.1 cm. In Koitajoki, the respective values were 14.5 m and 16.5 cm. The basal area of the trees was 15.1 m2 in Koitajoki and 14.8 m2 in Reposuo. The canopy layer in both study areas was dominated by P. sylvestris with scattered birch (Betula pendula and Betula pubescens) and P. abies also present.
Restoration treatments and experimental design
Canopy gaps were created in 2003 in the Reposuo area, and 1 year later (2004) in the Koitajoki area. A total of 60 gaps were created in both areas and were treated in a similar manner. The gaps were located at least 30 m from each other. Since this minimum distance was greater than the tree heights in the study area, this distance was assumed to prevent any direct dependencies between the experimental gaps. All live trees within the gaps that exceeded a height of 1.3 m were either felled or girdled using a chainsaw and were left in the gaps. The gaps were circular in shape, and their size was determined by the gap diameter/dominant height of the surrounding trees ratio. Four different ratios were used: 0.5, 1, 1.5, and 2.
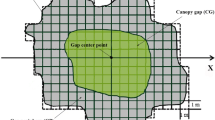
Rectangular sample plots (40 × 60 cm) were used to assess the regeneration of tree seedlings within the gaps. In the smaller gaps (size classes 0.5 and 1), four sample plots were established around the gap centre. One of the plots was seeded with 60 B. pendula (silver birch) seeds, and the humus, vegetation, and litter layers were left intact. In two plots, the mineral soil was exposed by the removal of the humus, vegetation, and litter layers, of which the other was also seeded with 60 B. pendula seeds. The fourth plot was left untreated (Fig. 1). The order of the plots was randomized for each gap. In the larger gaps (size classes 1.5 and 2), 12 sample plots were divided between three transects: one in the northern, one in the centre, and one in the southern part of the gap. Each transect included the same treatments as the smaller gaps and their order was randomized for each transect. Additional information on the study design is provided in Rouvinen and Kouki (2011).
Illustration of the experimental design within canopy gaps. X marks the gap centre, and the dashed circles denote the gap edge. Plot treatments were randomized for each small canopy gap and for each transect (north, centre, and south) in large canopy gaps. The distance between gaps was at least 30 m
Data collection
Data were collected between June and August 2014. Initially, 60 canopy gaps were created in each study area. In the present study, 16 gaps in the Koitajoki area and 10 gaps in the Reposuo area were excluded as not all of the within-gap sample plots (initially marked with white plastic tubes) were unambiguously identifiable in the field 10 years after their establishment. Hence, a total of 94 gaps were included in the study.
To determine the current gap size, the longest and shortest diameter was measured from each gap using the trunks of the bordering trees as gap edges. The diameter for each gap was then calculated as a mean value of the two measurements. The mean height of the dominant trees surrounding each gap was measured using a Suunto PM5-1520 PC hypsometer (Suunto Ltd., Vantaa, Finland).
The number of live tree seedlings, seedling height, and species type within each 40 × 60 cm sample plot were recorded. All tree seedlings were also inspected for signs of herbivory using a dichotomous scale (damaged vs. not damaged). Litter, mineral soil, coarse woody debris (>5 cm in diameter), and vegetation cover were estimated visually when they exceeded 5 % of the plot area. Vegetation on the plots was examined for three reasons: (1) to compare the two study areas for possible differences in site conditions in terms of soil fertility, (2) to determine differences between disturbed and undisturbed plots, and (3) to assess the effect of vegetation and other plot characteristics on seedling establishment in the disturbed plots. A threshold value of 5 % was used as individual species were not of primary interest in the study. Due to vertical overlapping of species, the vegetation cover was recorded separately for ground-layer and field-layer species. Hence, the vegetation cover for an individual sample plot may not add up to 100 %.
Data analyses
Only the seedlings of Pinus and Betula were included in the statistical analyses due to the very low number of other tree species found in the sample plots (Table 1). As the number of Betula seedlings was also low, they were not analysed separately but were combined with the Pinus seedlings.
Spearman’s rank correlation was used to examine the association between gap size and the mean number of pooled Pinus and Betula seedlings in the disturbed sample plots. Only the disturbed plots were chosen for the analysis as the majority of seedlings were found on those plots. Mean seedling number per disturbed plot was used to account for the different number of sample plots between the small and large gaps. Spearman’s rank correlation was used rather than Pearson’s correlation, as it is more robust against outliers and because the seedling data were not normally distributed (count data with many zero observations).
The influence of within-gap position on tree seedling number in the large gaps was analysed using generalized linear mixed models (GLMM) in R (R Core Team 2014). The number of pooled Pinus and Betula seedlings was used as a response variable and within-gap position (north, centre, and south) as a categorical predictor variable. Each gap was used as a random effect. Because of over dispersion in the initial Poisson GLMM, a negative binomial GLMM was used instead (function glmer.nb) in package lme4 (Bates et al. 2014). Pairwise comparisons among the predictor variables were conducted using function glht in multcomp package (Hothorn et al. 2008). Negative binomial GLMM (due to overdispersion in the Reposuo data) and Poisson GLMM (in Koitajoki) were also used to assess the effect of vegetation and coarse woody debris on pooled Pinus and Betula seedling numbers in the disturbed sample plots. The model included coarse woody debris, moss, lichen, and shrub cover as predictor variables and each gap as a random effect. Percentage cover in all predictor variables in the model was rescaled from 0–100 to 0–1. Other plot characteristics were not included in the model because exposed mineral soil was found in only 17 (out of 776) sample plots, and litter cover was strongly correlated with moss cover. The two-sample Kolmogorov–Smirnov test was used to assess whether the height distributions of tree seedlings between the two study areas were statistically different.
Differences in the cover of coarse woody debris, mineral soil, litter, and vegetation between within-gap plots were tested using an independent samples t test in IBM SPSS version 21.0 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.). A t test was used for two reasons even though the compared variables did not always display normal distribution: (1) with large sample sizes, the t test is rather robust for violations of the assumption of normal distribution, and (2) as the measured variables did not follow any specific distribution, transformation of the variables to reach the assumptions of normality would have been difficult. When the assumption of homogeneity of variances was violated, Welch’s t test was used instead of Student’s t test (Ruxton 2006). Standard error of the mean (SE) was used as a measure of reliability and variability throughout the results section.
Results
A total of 269 tree seedlings were found in the 776 within-gap sample plots (Table 1). P. sylvestris was the most numerous tree species (196 seedlings) and together with Betula spp. (6 B. pendula and 36 B. pubescens seedlings) accounted for 88 % of all tree seedlings recorded. No B. pendula seedlings were recorded on plots that were seeded in 2003 and 2004. The majority of Pinus and Betula seedlings in both study areas (91 % in Koitajoki and 95 % in Reposuo) were found in the disturbed plots. The mean number of pooled Pinus and Betula seedlings per disturbed plot (disturbed and disturbed + seeded plots) was approximately two and a half times higher in Reposuo (0.81 ± 0.10) compared to Koitajoki (0.32 ± 0.06). Both study areas had a similar trend in the mean number of Pinus seedlings in the disturbed plots: seedling numbers were highest 4–5 years after the restoration treatments, following a clear decline 10 years after the treatments (Fig. 2, based partly on Rouvinen and Kouki 2011 and unpublished data). The number of Betula seedlings was rather low throughout the monitoring period and showed a decreasing trend in the initial years following the creation of the gaps.
Mean seedling number for Pinus sylvestris and Betula spp. seedlings on disturbed plots in the two study areas. The year of measurement is marked with a triangle (Betula spp.) and a filled circle (Pinus) ±standard error of the mean. Dashed line between the measurement points is drawn to illustrate temporal trend between the years
Weak positive correlations were found between the mean number of pooled Pinus and Betula seedlings in the disturbed plots and the gap diameter/height of the gap edge trees ratio, although the correlations were significant (P > 0.05) only in Reposuo and only marginally significant in Koitajoki (Fig. 3). Both study areas showed a similar trend; a higher mean number of pooled Pinus and Betula seedlings growing in the centre of the gaps (1.13 ± 0.27 in Koitajoki and 2.33 ± 0.47 in Reposuo) compared to the northern (0.35 ± 0.10 in Koitajoki and 1.33 ± 0.29 in Reposuo) and southern parts (0.57 ± 0.23 in Koitajoki and 1.66 ± 0.56 in Reposuo) (Fig. 4). According to the negative binomial GLMM, this difference was statistically significant only in Koitajoki where seedling numbers were significantly higher in the centre compared to the northern part of the gap (Table 2).
Mean number of pooled Pinus sylvestris and Betula spp. seedlings per disturbed sample plot in a gap versus gap diameter/height of the gap edge trees ratio in the two study areas. Each circle denotes a canopy gap, n is number of gaps, r s is the Spearman’s rank correlation coefficient, P denotes the statistical significance of a two-sided test. Note the different scale on y-axis
Number of pooled Pinus sylvestris and Betula spp. seedlings in the northern, centre, and southern transects within the large gaps (23 gaps in Koitajoki and 27 gaps in Reposuo). Box hinges represent 25th and 75th percentiles, the vertical T-bars mark the range (if no outliers), the horizontal line inside the boxes is the median, and filled grey circles mark the mean. Outliers are marked with open circles. Brackets on top of boxes denote statistical differences (P < 0.05) between the groups. Note the different scale on y-axis
The mean height of Pinus seedlings was 9.9 ± 1.4 cm in Koitajoki and 9.0 ± 1.0 cm in Reposuo. In Koitajoki, the mean height of Betula seedlings was 56.7 ± 16.2 cm and in Reposuo 42.3 ± 7.3 cm. The majority of the Pinus and Betula seedlings (84 % in Reposuo and 74 % in Koitajoki) were less than 20 cm in height (Fig. 5). Compared to Pinus, the seedlings of Betula were scattered more evenly across the height classes, although their overall number was generally low. According to the two-sample Kolmogorov–Smirnov test, there was a statistically significant difference between the height distributions of the two study areas (Pinus and Betula seedlings pooled, D = 0.35, P < 0.001).
The majority of the seedlings (89 %) in both study areas did not display signs of herbivory. In Koitajoki, 11 % of Betula and 6 % of Pinus seedlings were affected by herbivory, and in Reposuo, none of the Betula and 3 % of Pinus seedlings displayed signs of herbivory.
Vegetation in the undisturbed plots was characterised by rather similar species assemblages in both study areas; Vaccinium myrtillus and V. vitis-idaea were observed to have the highest coverage in the field layer and Pleurozium schreberi dominated the ground layer (Table 3). The main differences in the field layer between study areas were a higher percentage cover of Calluna vulgaris and Deschampsia flexuosa (not found in Reposuo) in Koitajoki. Also, Empetrum nigrum was clearly more common in the field layer in Reposuo than in Koitajoki. The most distinct difference in the ground layer was the higher coverage of pooled lichens (Cetraria islandica, Cladonia spp., and Peltigera aphthosa) in Reposuo compared to Koitajoki. In addition, a clearly higher coverage of Polytrichum spp. mosses was found in Koitajoki compared to Reposuo.
The coverage of V. myrtillus and V. vitis-idaea was lower in both study areas in the disturbed plots and had not yet reached the same levels as in the undisturbed plots. Moreover, P. schreberi was more abundant in the ground layer in the undisturbed plots compared to the disturbed plots in both study areas. Conversely, Polytrichum spp. mosses were more frequent in the disturbed plots, especially in Koitajoki but also in Reposuo. Rhododendron tomentosum, V. uliginosum, and Sphagnum spp. mosses (i.e. species that indicate moist site conditions) were more frequent in Koitajoki (found in 30 gaps) than in Reposuo (found in 6 gaps).
According to the GLMM, lichen cover had a significant positive effect on the number of pooled Pinus and Betula seedlings in Koitajoki but not in Reposuo (Table 4). In Reposuo, the moss cover had a significant positive effect on the number of pooled Pinus and Betula seedlings.
Discussion
The results of our study show that tree seedlings, mainly Pinus and Betula, do emerge in the created canopy gaps 10 years after gap formation but occur mostly on plots that were disturbed by exposing the mineral soil. Emergent seedlings in the gaps, however, are short-lived or grow very slowly and their development appears to be hindered. Because the majority of tree seedlings in the plots did not show signs of browsing by herbivores, the main reasons for their weak establishment are likely connected with the competitive environment within the gaps.
Our finding that only a low proportion of Pinus and Betula seedlings occurred on the undisturbed plots is in accordance with earlier studies that emphasized the importance of disturbed microhabitats for the emergence of tree seedlings in pine-dominated boreal forests (e.g. Yli-Vakkuri 1962; Kuuluvainen and Juntunen 1998; Rouvinen and Kouki 2011; Hekkala et al. 2014). Factors that hinder tree seedling emergence on undisturbed forest floors mainly include vegetation competition, particularly by mosses, unstable moisture conditions, and the presence of a humus layer that prevents the roots of small seedlings from reaching the mineral soil after germination (Steijlen et al. 1995; Zackrisson et al. 1995; Hille and den Ouden 2004; Nilsson and Wardle 2005). On plots that were disturbed by exposing the mineral soil, the effect of the abovementioned factors was reduced and this effect was still clearly visible 10 years after the treatments. This implies that the creation of artificial canopy gaps should be accompanied by “soil gaps” in the vegetation to enhance germination and early-phase development of tree seedlings in Vaccinium-type forests.
As both study areas showed a decreasing temporal trend in the mean number of tree seedlings on the disturbed plots, especially after 2008, the effect of soil preparation appears to decline over time. As shrubs covered only 32 % of the disturbed plots in Koitajoki and 19 % in Reposuo and were non-significant predictors in the GLMM analysis, their effect on seedling numbers in the disturbed plots appears to be of minor importance 10 years after the treatments. The increase in moss cover, however, had a significant negative influence on the number of Pinus and Betula seedlings in Reposuo. In the northern Swedish boreal zone, mosses, particularly P. schreberi, have also been documented to have a similar influence on Pinus seedlings due to chemical interference and on their capacity to efficiently absorb available nutrients (Steijlen et al. 1995; Nilsson and Wardle 2005). A similar effect, however, was not found in Koitajoki where lichen cover was the only significant predictor in the GLMM and had a positive effect on the pooled number of Pinus and Betula seedlings. Analogous results have also been reported from northern boreal forests in Sweden where the growth of seedlings, which were planted in lichen-dominated ground was better compared to those planted in other ground vegetation (Steijlen et al. 1995; Nilsson and Wardle 2005).
Although the exposure of the mineral soil clearly enhanced the emergence of Pinus and Betula seedlings, the development of these seedlings in the gaps appears to have been hindered. P. sylvestris, B. pendula, and B. pubescens are light-demanding tree species that are adapted to establish in open areas after allogenic disturbances (Kellomäki 2005; Hynynen et al. 2010), and it is very likely that their development in the disturbed plots, especially in the smaller gaps, was partly hindered because of an insufficient amount of radiation reaching the forest floor. This is particularly important in high-latitude boreal forests where the low sun angle prevents a significant increase in radiation on the forest floor in small canopy gaps (Canham et al. 1990; Kuuluvainen 1994). Moreover, as the gaps in this study were located on rather dry and infertile soils (Vaccinium-type), root competition by the edge trees may be another factor impeding the further development of tree seedlings in the gaps (Kuuluvainen 1994; Axelsson et al. 2014). Depending on the height of the dominant trees surrounding the gap, root competition may extend up to 10 m from the edge trees (Björkman and Lundeberg 1971; Kalliokoski et al. 2008; Axelsson et al. 2014). Therefore, it could be expected that within this area, it is difficult for small tree seedlings to compete for nutrients and water against the dominant edge trees that surround the gap.
Interestingly, no significant differences in the number of pooled Pinus and Betula seedlings were found between the northern and southern edges of the gaps in both study areas, even though it could be expected that more radiation would be available in the northern part of the gap in boreal forests, especially in the larger gaps (Canham et al. 1990). This may imply that root competition (with the dominant trees) for water and nutrients has overruled the effect of increased radiation in the northern edge (for similar results, see also Coates (2000)). The effect of root competition may be a likely explanation for the presence of more abundant Pinus and Betula seedlings in the centre of the gap compared to northern part, especially in Koitajoki study area.
Because the availability of light and the area affected by root competition are to a large extent dictated by gap size (McCarthy 2001), increasing the gap diameter would most likely result in more successful establishment of Pinus and Betula seedlings provided that sufficient regeneration sites allow seeds to germinate and escape from the competition by ground vegetation. Although a weakly significant association was found between relative gap size and the mean number of Pinus and Betula seedlings in the disturbed plots in Reposuo, it is not possible to provide a threshold gap diameter that would meet the requirements of Pinus and Betula seedlings based on the results of this study. Obviously, such a threshold value would be limited as it would only cover the inner area of the gap as edge effects, particularly root competition, would presumably hinder the establishment of tree seedlings at the edges of the gap (Ruuska et al. 2008; Axelsson et al. 2014).
As the influence of belowground competition becomes less important as the moisture and nutrient content in the soil increases (Casper and Jackson 1997; Coomes and Grubb 2000; McCarthy 2001), the results from this study are not necessarily applicable to forests that are located on more fertile and moist sites. For instance, regeneration of P. abies seedlings in small canopy gaps in drained spruce mire stands appears to be successful and does not require soil preparation (Hökkä et al. 2011, 2012). In addition, canopy cover decreases in older forests (due to self-thinning), thereby allowing more light to pass through the canopy (Hynynen 1993), and possibly results in a different response by light-demanding tree species if canopy gaps are created in more mature forests.
Further studies that assess tree seedling regeneration in larger gaps are needed to provide robust recommendations that will guide both restoration activities but will also assist in the management of production forests. Future research should also be conducted to assess whether uprooted pits and mounds would result in a different outcome in seedling regeneration dynamics compared to the method used in this study (i.e. removal of humus and litter in small patches). The uprooting of trees would most likely replace the need to expose the mineral soil, and it has been previously shown to enhance tree seedling regeneration in the restoration of pine-dominated boreal forests (Hekkala et al. 2014). Although, pit-and-mound microtopography may be a more unstable regeneration site for tree seedlings in the long term, it can have an effect on tree species composition as Betula seedlings might gain a competitive advantage over Pinus seedlings in uprooted areas (Kuuluvainen and Juntunen 1998).
Conclusions
We conclude that small canopy gaps, i.e., diameter up to twice the height of the dominant trees, do not successfully facilitate the establishment of Pinus and Betula seedlings in dry and infertile pine-dominated boreal forests. Hence, the creation of small gaps does not appear to be a proven method to restore age class structure and tree diversity in pine-dominated protected forests. As the small seedlings were predominantly found in the disturbed plots, the early development of seedlings in the gaps seems to require soil preparation. Increasing the gap diameter would presumably lead to better establishment and subsequent development of tree seedlings in the gaps. Further studies that manipulate the gap size more widely than this current study are needed.
References
Axelsson EP, Lundmark T, Högberg P, Nordin A (2014) Belowground competition directs spatial patterns of seedling growth in Boreal forests in Fennoscandia. Forests 5:2106–2121
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-7. http://CRAN.Rproject.org/package=lme4
Björkman E, Lundeberg G (1971) Studies on root competition in a poor pine forest by supply of labeled nitrogen and phosphorus. Stud For Suec 94:1–16
Cajander AK (1949) Forest types and their significance. Acta For Fenn 56:1–71
Canham CD, Denslow JS, Platt WJ, Runkle JR, Spies TA, White PS (1990) Light regimes beneath closed canopies and tree-fall gaps in temperate and tropical forests. Can J For Res 20:620–631
Casper BB, Jackson RB (1997) Plant competition underground. Annu Rev Ecol Syst 28:545–570
Chapin FS, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. Bioscience 37:49–57
Coates KD (2000) Conifer seedling response to northern temperate canopy gaps. For Ecol Manage 127:249–269
Coomes DA, Grubb PJ (2000) Impacts of root competition in forests and woodlands: a theoretical framework and review of experiments. Ecol Monogr 70(2):171–207
Gray AN, Spies TA (1996) Gap size, within-gap position and canopy structure effects on conifer seedling establishment. J Ecol 84:635–645
Halme P, Allen KA, Auniņš A, Bradshaw RHW, Brūmelis G, Čada V, Clear JL, Eriksson A-M, Hannon G, Hyvärinen E, Ikauniece S, Iršėnaitė R, Jonsson BG, Junninen K, Kareksela S, Komonen A, Kotiaho JS, Kouki J, Kuuluvainen T, Mazziotta A, Mönkkönen M, Nyholm K, Oldén A, Shorohova E, Strange N, Toivanen T, Vanha-Majamaa I, Wallenius T, Ylisirniö A-L, Zin E (2013) Challenges of ecological restoration: lessons from forests in northern Europe. Biol Conserv 167:248–256
Hekkala A-M, Tarvainen O, Tolvanen A (2014) Dynamics of understory vegetation after restoration of natural characteristics in the boreal forests in Finland. For Ecol Manage 330:55–66
Hille M, den Ouden J (2004) Improved recruitment and early growth of Scots pine (Pinus sylvestris L.) seedlings after fire and soil scarification. Eur J Forest Res 123:213–218
Hökkä H, Repola J, Moilanen M, Saarinen M (2011) Seedling survival and establishment in small canopy openings in drained spruce mires in northern Finland. Silva Fenn 45(4):633–645
Hökkä H, Repola J, Moilanen M, Saarinen M (2012) Seedling establishment on small cutting areas with or without site preparation in a drained spruce mire—a case study in northern Finland. Silva Fenn 46(5):695–705
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50(3):346–363
Hynynen J (1993) Self-thinning models for even-aged stands of Pinus sylvestris, Picea abies and Betula pendula. Scand J For Res 8(3):326–336
Hynynen J, Niemistö P, Viherä-Aarnio A, Brunner A, Hein S, Velling P (2010) Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in northern Europe. Forestry 83:103–119
Kalliokoski T, Nygren P, Sievänen R (2008) Coarse root architecture of three boreal tree species growing in mixed stands. Silva Fenn 42(2):189–210
Kellomäki S (2005) Metsäekologia. Silva Carelica 7. Joensuun yliopisto
Kuuluvainen T (1994) Gap disturbance, ground microtopography, and the regeneration dynamics of boreal coniferous forests in Finland: a review. Ann Zool Fenn 31(1):35–51
Kuuluvainen T (2002) Natural variability of forests as a reference for restoring and managing biological diversity in boreal Fennoscandia. Silva Fenn 36(1):97–125
Kuuluvainen T, Aakala T (2011) Natural forest dynamics in boreal Fennoscandia: a review and classification. Silva Fenn 45(5):823–841
Kuuluvainen T, Grenfell R (2012) Natural disturbance emulation in boreal forest ecosystem management—theories, strategies, and a comparison with conventional even-aged management. Can J For Res 42(7):1185–1203
Kuuluvainen T, Juntunen P (1998) Seedling establishment in relation to microhabitat variation in a windthrow gap in a boreal Pinus sylvestris forest. J Veg Sci 9(4):551–562
Kuuluvainen T, Kalmari R (2003) Regeneration microsites of Picea abies seedlings in a windthrow area of a boreal old-growth forest in southern Finland. Ann Bot Fenn 40:401–413
Kuuluvainen T, Ylläsjärvi I (2011) On the natural regeneration of dry heath forests in Finnish Lapland: a review of V. T. Aaltonen (1919). J For Res 26(S10):34–44
Lieffers VJ, Messier KJ, Stadt F, Gendron F, Comeau PG (1999) Predicting and managing light in the understory of Boreal forests. Can J For Res 29:796–811
Lindenmayer DB, Franklin JF, Lõhmus A, Baker SC, Bauhus J, Beese W, Brodie A, Kiehl B, Kouki J, Martinez Pastur G, Messier C, Neyland M, Palik B, Sverdrup-Thygeson A, Volney J, Wayne A, Gustafsson L (2012) A major shift to the retention approach for forestry can help resolve some global forest sustainability issues. Conserv Lett 5:421–431
Liu Q-H, Hytteborn H (1991) Gap structure, disturbance and regeneration in a primeval Picea abies forest. J Veg Sci 2:391–402
Long JN (2009) Emulating natural disturbance regimes as a basis for forest management: a North American view. For Ecol Manag 257:1868–1873
McCarthy J (2001) Gap dynamics of forest trees: a review with particular attention to boreal forests. Environ Rev 9:1–59
Mitchell SJ (2012) Wind as a natural disturbance agent in forests: a synthesis. Forestry 86:147–157
Nilsson M-C, Wardle DA (2005) Understory vegetation as a forest ecosystem driver: evidence from the northern Swedish boreal forest. Front Ecol Environ 3(8):421–428
Perera AH, Buse LJ, Weber MG (eds) (2004) Emulating natural forest landscape disturbances: concepts and applications. Columbia University Press, New York
Pirinen J, Kersalo J (eds) (2009) Suomen maakuntien ilmasto. Yliopistopaino, Helsinki
Puettmann KJ, Wilson SMG, Baker SC, Donoso PJ, Drössler L, Amente G, Harvey BD, Knoke T, Yuanchang L, Nocentini S, Putz FE, Yoshida T, Bauhus J (2015) Silvicultural alternatives to conventional even-aged forest management-what limits global adoption? For Ecosyst 2:8
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rouvinen S, Kouki J (2011) Tree regeneration in artificial canopy gaps established for restoring natural structural variability in a Scots pine stand. Silva Fenn 45(5):1079–1091
Ruuska J, Siipilehto J, Valkonen S (2008) Effect of edge stands on the development of young Pinus sylvestris stands in southern Finland. Scand J For Res 23:214–226
Ruxton GD (2006) The unequal variance t-test is an underused alternative to Student’s t-test and the Mann-Whitney U test. Behav Ecol 17:688–690
Similä M, Junninen K (eds) (2012) Ecological restoration and management in Boreal forests: best practices from Finland. Metsähallitus, Natural Heritage Services, Vantaa
Steijlen I, Nilsson M-C, Zackrisson O (1995) Seed regeneration of Scots pine in boreal forest stands dominated by lichen and feather moss. Can J For Res 25:713–723
Ulanova NG (2000) The effects of windthrow on forests at different spatial scales: a review. For Ecol Manag 135(1):155–167
Yli-Vakkuri P (1962) Emergence and initial development of tree seedlings on burnt-over forest land. Acta For Fenn 74(1):1–50
Zackrisson O, Nilsson M-C, Steijlen I, Hörnberg G (1995) Regeneration pulses and climate vegetation interactions in nonpyrogenic boreal Scots pine stands. J Ecol 83:469–483
Acknowledgments
We thank Metsähallitus for creating the canopy gaps in both study areas and Lauri Mehtätalo for assistance in statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dr. Gediminas Brazaitis.
Rights and permissions
About this article
Cite this article
Pasanen, H., Rouvinen, S. & Kouki, J. Artificial canopy gaps in the restoration of boreal conservation areas: long-term effects on tree seedling establishment in pine-dominated forests. Eur J Forest Res 135, 697–706 (2016). https://doi.org/10.1007/s10342-016-0965-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-016-0965-8