Abstract
This study investigated the effects of vermicompost, growth-promoting bacteria (Bacillus subtilis OSU-142), and algae extract combinations on total phenolics, flavonoid, anthocyanin, glucose, fructose, some phenolic compounds, and leaf chlorophyll content in ‘0900 Ziraat’ sweet cherry cultivar. For this purpose, vermicompost, bacteria, and algae extracts were applied using three different methods to tree canopy, soil, and both tree canopy and soil. Soil applications were made once during bud swelling, and tree canopy applications were made twice, at full bloom and 15 days after full bloom. All applications increased the total phenolics and flavonoid content. While the “algae application to tree canopy” increased the total flavonoid to 15.76 mg CE 100 g−1 from 7.23 mg CE 100 g−1, “the application of algae to soil and tree canopy” increased the total phenolics to 85.80 mg GAE 100 g−1 from 71.05 mg GAE 100 g−1. Except for “bacterial applications to tree canopy alone”, all applications significantly increased the total chlorophyll. The highest total chlorophyll (74.94 mg g−1) was obtained from “the algae application to tree canopy”. However, it was determined that algae, bacteria, and vermicompost did not have a positive significant effect on glucose, fructose, campherol, catechin, ferulic acid, and anthocyanin content. In addition, the application of “bacteria to both tree canopy and soil” increased the chlorogenic acid content of fruits by approximately 50% compared to the control. Applications of “vermicompost to soil + bacteria to both soil and tree canopy”, “algae extract to the tree canopy”, and “bacteria to tree canopy” (28.75, 28.30 and 25.20 µg g−1, respectively) increased the caffeic acid content of fruit compared to control. It was observed that only the application of “vermicompost to soil + bacterial to soil and tree canopy” had a positive effect on the quercetin content of fruits based on control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sweet cherry (Prunus avium L.) is a fruit species belonging to the genus Prunus, member of the Rosaceae family. The south Caucasus, Caspian Sea coasts, and northeast Anatolia are considered the center of origin of sweet cherry. Sweet cherries have a wide distribution around the world. An area of 454,664 ha in the world produces 2,765,827 tons of sweet cherries. In terms of sweet cherry production, Türkiye ranks first in the world with 656.041 tons, followed by Chile, Uzbekistan, the USA, and Spain (FAO 2022).
Besides being consumed as fresh or dried fruits, sweet cherry fruits are used to make vinegar, jam, canned, or frozen food. Sweet cherry fruit provides antimicrobial and antioxidant effects thanks to the phenolic compounds (Hojjati et al. 2024). Therefore, it is an important fruit for human health. Recently, increasing interest in nutraceuticals and functional foods has led plant breeders to choose fruits with higher than normal phenolics content and antioxidant capacity (Asadollahi et al. 2022; Milosevic et al. 2022; Jalali et al. 2022). Antioxidant compounds play key roles in protecting cells against oxidative damage caused by free radicals, associated with cancer, and prevent diseases, such as cardiovascular diseases and Alzheimer’s disease. Anthocyanin in fruits is known to have a beneficial effect on nerve cells (Michalak 2022; Çelik and Şan 2023). In addition to products with high phenolics content, cultural practices that increase phenolics content have emerged in recent years. To increase fruit yield and quality in conventional agriculture, producers use synthetic and chemical inputs. While the use of such chemicals in agricultural production causes the deterioration of the natural balance, it also reaches all living things in the food chain through environmental pollution (Asadollahi et al. 2022; Milosevic et al. 2022). Due to excessive chemical fertilization, nitrate accumulation in the soil increases and reaches the lakes by mixing with ground water. The increase in algae population in the lakes covers the lake surface, reducing the oxygen levels in the water, leading to the death of organisms living in lakes. The use of different organic materials that do not harm the environment, and human health in agricultural production has become obligatory to protect environment and human health. Use of organic fertilizers and plant growth-promoting microorganisms has gained importance to obtain healthy food without harming environment, to increase yield and quality, and to produce secondary metabolites (Asadollahi et al. 2022). Hence, it is necessary to investigate the possible usage of organic materials in fruit growing, which can be an alternative to chemical fertilizers. Organic material applications, such as seaweed, plant growth-promoting microorganisms, and vermicompost increased fruit yield and quality (Frioni et al. 2018; Thakur et al. 2021; Jalali et al. 2022). Growth-promoting microorganisms promote plant development through phosphorus acquisition, nitrogen fixation and iron uptake, production of plant hormones and reduction of ethylene levels in the plant (Esitken et al. 2006). Vermicompost applications enhance soil structure and contain microorganisms that promote plant growth (Torun Kayabası and Yılmaz 2021), algae extract applications transform some elements that the plant cannot absorb from the soil into chelate form, thereby facilitating their uptake (Correia et al. 2015). Although extracts obtained from various algae species are used in agricultural production, the use of algae extracts as biofertilizer in fruit species, especially use of Chara sp. algae species, is quite limited. Therefore, the effects of Bacillus subtilis OSU-142 bacterial strain, vermicompost, and algae extract (Chara sp.) applications as biofertilizer on the biochemical content of the ‘0900 Ziraat’ sweet cherry cultivar were investigated in the current study. The combinations of bacteria, vermicompost and algae that promote the biochemical responses in terms of total phenolics, total flavonoids, and total chlorophyll contents was determined.
Materials and Methods
The research was conducted on the ‘0900 Ziraat’ cultivar grafted on ‘Gisela 6’ clonal rootstock in the orchard, established in 2002, of the Fruit Research Institute, (Egirdir, Isparta) in 2020 and 2021. Vermicompost, Bacillus OSU-142 bacterial strain, and algae extract were used in the study. Algae extraction was conducted according to the method of Koç (2013). Algae (Chara sp.) was harvested from Eğirdir Lake (Isparta, Türkiye), washed and cleaned, mixed with water at a ratio of 1:1, and left for fermentation for 80 days in 20‑L closed drums at room temperature. At the end of the fermentation period, the algae were filtered and 100% algae extract was obtained. Bacillus OSU-142 bacterial strain was obtained from the Plant Biotechnology Laboratory at Yeditepe University. Vermicompost was purchased from a commercial supplier in Isparta, Türkiye. The bacterial solution had a density of 109 CFU. The Bacillus OSU-142 takes part in nitrogen fixation, phosphate solubilization, and plant hormone synthesis (Esitken et al. 2006). The analysis results of the vermicompost and algae extract used in the experiment are given in Tables 1 and 2, respectively.
The treatments in the research are as follows:
-
1.
Control (C): Tap water containing 2% Tween 20 (spreader-adhesive) was applied to the trees as a control in the study.
-
2.
Application of vermicompost to the soil (VS): Vermicompost was applied once during the bud swelling period, at a rate of 10 kg per tree, into pits measuring approximately 15–20 cm in the root zone of the sweet cherry trees.
-
3.
Application of algae extract to the soil (AS): A volume of 1 L of the algae extract was applied to 15–20 cm of pits during the bud swelling period.
-
4.
Application of algae extract to the tree canopy (AT): The algae extract was prepared with 15% solutions and sprayed on the tree canopy with a back sprayer at a rate of 3 L per tree. Algae extract was applied twice: once on the full bloom and once at 15 days after full bloom.
-
5.
Application of algae extract to both soil and tree canopy (AST): A volume of 1 L of algae extract per tree was given to the pits opened to the root zone during the bud swelling period. Algae extract solution (15%) was applied to the tree canopy at 3 L per tree, twice at the time of full bloom and 15 days after full bloom.
-
6.
Application of Bacillus OSU-142 bacterial strain to soil (BS): A volume of 1 L per tree from the 3% solution was applied to 15–20 cm pits dug in the root zone once at the bud swelling period.
-
7.
Application of Bacillus OSU-142 bacterial strain to tree canopy (BT): A volume of 3 L of the bacterial solution prepared at a rate of 3% were sprayed onto the trees with a back sprayer. Bacteria solution was applied twice: once at the full bloom and 15 days after full bloom.
-
8.
Application of Bacillus OSU-142 bacterial strain to both soil and tree canopy (BST): A volume of 1 L per tree from the 3% solution was applied to 15–20 cm pits dug in the root zone once during the bud swelling period. In addition, 3 L of the bacterial solution prepared at a rate of 3% were sprayed onto the trees twice, at full bloom and 15 days after the full bloom.
-
9.
Application of vermicompost to soil + algae extract to both soil and tree canopy (VS + AST): Vermicompost was applied once during the bud swelling period, at a rate of 10 kg per tree, into pits measuring 15–20 cm in the root zone of the sweet cherry trees. Algae extract was given 1 L per tree to the pits opened to the root zone during the bud swelling period. Algae extract solution (15%) was applied to the tree canopy, at 3 L per tree, twice at the time of full bloom and 15 days after full bloom.
-
10.
Applications of vermicompost to soil + Bacillus OSU-142 bacterial strain to both soil and tree canopy (VS + BST): Vermicompost was applied once during the bud swelling period, at a rate of 10 kg per tree, into pits measuring 15–20 cm in the root zone of the sweet cherry trees. In addition, 1 L per tree of the 3% bacteria solution was applied to 15–20 cm pits dug in the root zone once during the bud swelling period. A volume of 3 L of the bacteria solution prepared at a rate of 3% were sprayed onto the trees twice, on the full bloom date and 15 days after the full bloom.
-
11.
Application of bacteria + algae extract to both soil and tree canopy (BST + AST): A volume of 1 L of algae extract was given per tree to the pits opened to the root zone during the bud swelling period. On the other hand, 15% algae extract solution was applied to the tree canopy, at 3 L per tree, twice at the time of full bloom and 15 days after full bloom. Additionally, 1 L of 3% bacteria solution was applied to 15–20 cm pits dug in the root zone once during the bud swelling period for per tree. A volume of 3 L of the bacteria solution prepared at a rate of 3% were sprayed onto the trees twice, on the full bloom date and 15 days after the full bloom.
On June 17, when the fruits were ripe, approximately 1 kg of fruit samples from different sides of each tree was harvested and brought to the laboratory and the samples were stored at −20 °C until analysis.
Biochemical Analysis
Analysis of Chlorophyll
The determination of chlorophyll content was made according to the method specified by Zhang and Huang (2013). Leaf samples were taken from the middle part of the shoots selected from different sides of the tree on July 15, 2020, and 2021. The leaf samples (0.1 g) crushed in 1 mL of 100% dimethylformamide with a homogenizer. The mixture was then centrifuged at 10,000 g for 10 min. The color intensity of the collected supernatant was determined with a spectrophotometer at 664 and 647 nm wavelengths. With the obtained absorbance values, chlorophyll a, chlorophyll b, and the total chlorophyll amount were calculated.
Analysis of Total Phenolics
Total phenolics analysis was performed according to the method specified by Cemeroğlu (2007). Approximately 100 g of fruit samples were separated from their seeds and homogenized. A volume of 1 mL of 80% methanol solution was added to 1 g of homogenized fruit flesh. After shaking with a vortex for 1 min, it was centrifuged for 20 min at 4000 rpm at 4 °C. Upper phase (0.5 mL) was taken and 0.5 mL of Folin–Ciocalteu reagent, then 15 mL of distilled water was added and the slurry were vortexed for 1 min. After waiting for 10 min, 0.5 mL of a 20% sodium carbonate (Na2CO3) solution was added. Samples were incubated for 2 h in dark. Then, absorbance values were measured at 720 nm with a spectrophotometer. Using the standard calibration curve, total phenolics content was calculated and reported as mg gallic acid equivalent (GAE) 100 g−1 FW.
Analysis of Total Flavonoids
Total flavonoid analysis was performed according to the method specified by Zhishen et al. (1999). Approximately 100 g of fruit samples were separated from their seeds and homogenized. After 0.25 mL of methanolic extract was taken into the analysis tube, 1.5 mL of distilled water and 75 µL of a 5% sodium nitrite solution were added and incubated for 6 min at room temperature. After incubation, 0.15 mL of 10% aluminum chloride was added to the mixture, vortexed and allowed to incubate again for 5 min. After incubation, 0.5 mL of 1 M sodium hydroxide was added to the mixture, and absorbance values were read at 510 nm wavelength with a spectrophotometer. The results were calculated using catechin standard and expressed as mg catechin equivalent (CE) 100 g−1FW.
Analysis of Total Anthocyanin
The analysis of total anthocyanin was carried out according to Giusti and Wrolstad (2001). Approximately 100 g of fruit samples were homogenized. A volume of 5 g of fruit samples were mixed with 10 mL of methanol solution containing 1% HCl overnight, and then filtered with Whatman No: 2 filter paper. The filtrate was kept in pH 1.0 and pH 4.5 buffer for 15 min, and the absorbance values were measured spectrophotometrically at 530 and 700 nm wavelengths. The total anthocyanin content was calculated using the molar absorptivity coefficient of cyanidin 3‑glycoside using the formulas below.
- A::
-
Absorbance,
- MW::
-
Molecular weight, 449.2 g.mol−1 for cyanidin-3-glucoside,
- DF::
-
Dilution factor,
- e::
-
Molar absorption coefficient,
- L::
-
Layer thickness of the absorbance measuring cuvette (cm),
- TA::
-
Total anthocyanin
High Pressure Liquid Chromatography Analysis of Glucose and Fructose
Analyses were performed using the method reported by Veberic and Stampar (2005). The glucose and fructose contents of sweet cherry fruits were determined by a Shimadzu high pressure liquid chromatography (HPLC) device (Shimadzu Inc., Kyoto, Japan). Fruit flesh (25–30 g) was shredded, and 5 g was taken into a tube, homogenized for 5 min by adding 20 mL of distilled water. The slurry was filtered through filter paper. After passing the filtrate through a 0.45 μm filter, 20 μL of it was injected into the HPLC. Results are given as mg g−1 FW.
HPLC conditions were as follows:
-
Detector: RID 10A
-
Pump: LC-10ADvp
-
Degasser: DGU-14A
-
Column oven: CTO-10ACvp
-
Column: Benson polymeric BP-800 Ca (300*7.8 mm), Serial No. 30528
-
Mobile phase: Pure water
-
Flow Rate: 0.6 mL min−1
-
Column temperature: 800 °C
-
Injection volume: 20 µL
High Pressure Liquid Chromatography Analysis of Phenolic Compounds
The phenolic compounds of sweet cherry fruits were determined by a Shimadzu HPLC device (Shimadzu Inc., Kyoto, Japan). The extraction solution was prepared by adding 20% water and 1% HCl to 80% methanol. Fruits are separated from their seeds and shredded. After weighing 10 g of the samples, 20 mL of extraction solution and 0.1 g of BHT were added and homogenized. The mixture was filtered into a flask. After the filtrate was evaporated at 40 °C in an evaporator, the flask was washed with 5 mL of methanol and 20 µL of this was injected into the HPLC device. Quercetin, campherol, catechin, chlorogenic acid, caffeic acid and ferulic acid amounts in fruits were determined and the results are given as µg g−1 FW.
HPLC conditions were as follows:
-
Detector: SPD‑M 10A vp DAD detector (λmax = 278 nm)
-
Auto sampler: SIL-10AD vp
-
System controller: SCL-10Avp
-
Pump: LC-10ADvp
-
Degasser: DGU-14A
-
Column oven: CTO-10Avp
-
Column: Agilent Eclipse XDB-C18 (250 × 4.60 mm) 5 µm
-
Mobile phase: A (3% acetic acid), B (Methanol)
-
Flow Rate: 0.8 mL min−1
-
Column temperature: 300 anthocyanin content of the fruits was not significantly affected by the applications °C
-
Injection volume: 20 µL
Experimental Design and Data Analysis
The experiment was set up as a randomized plot design with three replications and one tree per replication. All analyses were performed in triplicate. The experiment was repeated for 2 years, and the analysis results of the 2 years were averaged for each replication. The data were subjected to variance analysis using the MINITAB package (Minitab 17 Inc.) program. The difference between the significant means was determined according to the Tukey multiple comparison test (p < 0.05) and shown with different letters. In order to determine the relationships between biochemical properties, correlation analysis were performed using the Origin pro software.
Results
Biochemical Contents
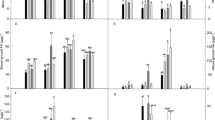
Table 3 presents the effects of the treatments on the chlorophyll content of the ‘0900 Ziraat’ sweet cherry cultivar. The study found a significant effect of applications on chlorophyll content. All treatments significantly increased the chlorophyll a content compared to the control. Chlorophyll b content varied between 42.55 mg g−1 and 48.80 mg g−1 based on the applications. All applications, with the exception of BT, increased the total chlorophyll content in comparison to the control.
The applications did not produce statistically important effect on the glucose and fructose content of sweet cherry fruits compared to the control (Table 4). Glucose content of the fruits varied between 66.47 mg g−1 and 75.84 mg g−1, and the fructose content varied between 50.11 mg g−1 and 57.12 mg g−1 among the applications.
Applications had significant effects on the total phenolics and total flavonoid content of sweet cherry fruits compared to the control. However, anthocyanin content of the fruits was not significantly affected by the applications (Table 4). The fruits’ total phenolics content ranged from 71.05 mg GAE 100 g−1 to 85.80 mg GAE 100 g−1. The AST application increased the total phenolic substances by 20.76% compared to the control. The AT and AST applications yielded the highest total flavonoid content (15.76 and 14.97 mg CE 100 g−1, respectively), while the control had the lowest value (7.23 mg CE 100 g−1). The AST application provided an increase of 117.9% in flavonoid content compared to the control (Table 4).
The effects of the applications on the chlorogenic, caffeic, ferulic acids, quercetin, and campherol content of the fruits were statistically significant compared to the control, while the effect on the catechin content was not significant (Table 5). Even though applications affected the ferulic acid and campherol content, their effects did not outperform the control. The application of BST increased the chlorogenic acid content by approximately 50% compared to the control. AT, BT, and VS + BST applications significantly increased the caffeic acid content. It was observed that the quercetin content in sweet cherry fruits ranged from 0.57 µg g−1 to 1.55 µg g−1. Only VS + BST application provided a significant increase for quercetin content compared to the control. The effects of the other applications were not statistically significant (Table 5).
The Correlations Between Biochemical Properties
Correlation coefficients between the biochemical properties of the sweet cherry cultivar ‘0900 Ziraat’ were calculated. There was a highly positive relationship between total flavonoid, phenolics, anthocyanin, and caffeic acid (Fig. 1). Similarly, a significant positive relationship between total anthocyanin and fructose and glucose, and between glucose and fructose was detected. Total chlorophyll content of the leaves and chlorophyll b content also showed significant positive correlation. Catechin content of fruits is highly related to the chlorogenic acid content. A moderate negative correlation was detected between the catechin content of fruits and the chlorophyll a and total chlorophyll contents of the leaves. Similarly, a moderate negative relationship was found between chlorophyll a and ferulic acid and quercetin. A moderate negative relationship was also detected between the total phenolics of the fruits and the ferulic acid content.
Pearson correlation matrix between biochemical traits in sweet cherry. (Correlations between biochemical properties are shown in different shades of blue and red. 1 The dark red large circle indicates a completely positive relationship between the two; −1 the dark blue large circle indicates a completely negative relationship between the two features)
Discussion
In the present study, AT, AS, and AST applications increased total chlorophyll content by 11% on average. Similar to our results, foliar applications of algae extracts increased the leaf chlorophyll content in peach (Rawi et al. 2016), lettuce and pepper (Quelal et al. 2022; Seğmen and Özdamar Ünlü 2023), parsley plants (Abd El-Aleem et al. 2021). The increase in chlorophyll content is attributed to the presence of high levels of minerals, carotene-xanthophyll pigments, free amino acids, and various plant hormones in algae species. Moreover, it has been reported that humic acid in the algae promote root development in plants and have positive effects on the adaptation of plants to different stress conditions (Battacharyya et al. 2015).
Bacillus subtilis OSU-142 bacterial strain, vermicompost, and algae extract applications did not increase the glucose and fructose content of sweet cherry fruit compared to the control. Sugars are water-soluble carbohydrates found in small or large quantities in fruits in different forms. Glucose and fructose constitute approximately 80% of the total sugar content in sweet cherry fruits (Usenik et al. 2010). Ballistreri et al. (2013) determined the glucose and fructose contents of sweet cherry cultivar ‘Sweetheart’ as 8.77 and 6.65 mg 100 g−1, respectively, in Italy. Some studies reported that bacteria, algae, and vermicompost applications increased sugar content of plants (Karabıyık 2013; Battacharyya et al. 2015), while others reported no meaningful changes in sugar content (Göksu and Kuzucu 2017), as was observed in the present study, suggesting the effects of organic material applications on sugar content can vary depending on species, ecological conditions, harvest times, application times, methods and doses.
In general, all treatments had a significant effect on the total phenolics and total flavonoid content of sweet cherry fruits. However, applications did not affect the anthocyanin content of the fruits compared to the control. Total phenolics content of sweet cherry fruit varies between 67.89 mg GAE 100 g−1 and 265.4 mg GAE 100 g−1 depending on the cultivars and ecology (Faniadis et al. 2010; İlhan and Artık 2021). The results obtained in our study are similar to previous reports. AST application increased the total phenolics content compared to the control. Similarly, application of algae extract increased total phenolics and total flavonoid content in apples (Çakır et al. 2021), and spinach (Fan et al. 2011). In addition, Siringi et al. (2022) reported that spirulina application increased total phenolics content to 852.9 mg GAE/100g from 345.8 mg GAE/100g in lettuce. There is a positive relationship between total phenolic content and antioxidant capacity. With the increase in the total phenolics content of herbal products, the antioxidant content also increases. Algae applications increased antioxidant compounds, such as phenolics, which also increased antioxidant activity (Siringi et al. 2022). It has been reported that the positive effect of algae applications on the total phenolics content may be due to the increase in soil bacteria and uptake of nutrients by plants (Siringi et al. 2022). A study on the applications of algae extract revealed a significant increase in the activity of chalcone isomerase, an enzyme essential for the biosynthesis of flavanone precursors and plant defense compounds like phenylpropanoid. Chalcone isomerase-mediated biosynthesis may be primarily responsible for the increase in total flavonoids (Fan et al. 2011). Plant growth-promoting microorganisms, such as Glomus, Pseudomonas, Azopirillum, Thiobacillus and Basillus species had a positive effect on the total phenolics and total flavonoid amounts, although effects varied according to species and varieties (Hashempoor et al. 2022; Yaman et al. 2023). In research conducted to date, it has been stated that the growth-promoting microorganisms are effective on nitrogen fixation, phosphate and calcium solubility, amino acids, organic acids and hormone synthesis (Kotan et al. 2021; Hashempoor et al. 2022; Yaman et al. 2023). It has also been reported that vermicompost applications increase the total phenolic content of carrot (Karabıyık 2013), cone flower (Hashempoor et al. 2022), and Satureja mutica (Asadollahi et al. 2022). The positive effect of algae, bacteria, and vermicompost applied in our study on total phenolics and total flavonoid content is similar to the reported literature, but the applications had no effect on the anthocyanin content of cherry fruits. In contrast to our findings, reports suggest that seaweed applications enhance the anthocyanin content in grapes (Frioni et al. 2018; Salvi et al. 2019).
Due to the strong antioxidant properties of phenolic components, their antiviral, anticancer, antibacterial, and anti-inflammatory effects are very significant (Khanam et al. 2015). In our study, we found that the fruits’ caffeic acid and quercetin contents increased with VS + BST application, caffeic acid content increased with AT, and chlorogenic acid content increased with BST applications compared to the control. It has been reported that the content of phenolic substances in sweet cherry fruits can differ significantly according to cultivar, maturity, and ecological factors (Usenik et al. 2010; Chockchaisawasdee et al. 2016; Ballistreri et al. 2013). Vermicompost generally had a positive effect on the content of chlorogenic acid, caffeic acid, campherol, and quercetin, and similar to our results, vermicompost application increased the caffeic acid content of the chicory plants (Gholami et al. 2018).
Conclusions
In this study, it was determined that all applications increased the total phenolics, flavonoid, and chlorophyll content (except “bacteria to tree canopy” [BT] application) compared to the control. However, it was determined that algae, bacteria, and vermicompost applications did not have significant effects on the amount of sugar, catechin, and anthocyanin. In addition, it was observed that “bacteria to both tree canopy and soil” (BST) and “vermicompost to soil + bacteria to both soil and tree canopy” (VS + BST) applications had a positive effect on some phenolic compounds, i.e., chlorogenic acid, caffeic acid, and quercetin. Use of environmentally friendly algae, bacteria, and vermicompost applications alone or in combination has a positive effect on the biochemical content of sweet cherry fruits.
References
Abd El-Aleem WH, Hamed ES, Toaima WIM (2021) Effect of blue green algae extract on three different curly parsley varieties under Sinai conditions. Bulg J Agric Sci 27(5):887–895
Asadollahi A, Abbaszadeh B, Torkashvand AM, Jahromi MG (2022) Effect of levels and types of organic, biological, and chemical fertilizers on morphological traits, yield, and uptake rate of elements in Satureja mutica. Ind Crop Prod 181:114763. https://doi.org/10.1016/j.indcrop.2022.114763
Ballistreri G, Continella A, Gentile A, Amenta M, Fabroni S, Rapisarda P (2013) Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem 140:630–638. https://doi.org/10.1016/j.foodchem.2012.11.024
Battacharyya D, Babgohari MZ, Rathor P, Prithiviraj B (2015) Seaweed extracts as biostimulants in horticulture. Sci Hortic 196:39–48. https://doi.org/10.1016/j.scienta.2015.09.012
Çakır M, Yıldırım A, Çelik C, Esen M (2021) The effect of different plant growth regulators on the quality and biochemical content of ‘Jeromine’ apple cultivar. Anadolu J Agric Sci 36(3):478–487. https://doi.org/10.7161/omuanajas.936081
Çelik B, Şan B (2023) Determination of biochemical contents of myrtle (Myrtus communis L.) fruits at different maturity levels. Erwerbs-Obstbau 65(6):2501–2507. https://doi.org/10.1007/s10341-023-00938-0
Cemeroğlu B (2007) Meyve ve sebze işleme endüstrisinde temel analiz metotları. Biltav Yayınları, Ankara
Chockchaisawasdee S, Golding JB, Vuong QV, Papoutsis K, Stathopoulos CE (2016) Sweet cherry: composition, postharvest preservation, processing and trends for its future use. Trends Food Sci Technol 55:72–83. https://doi.org/10.1016/j.tifs.2016.07.002
Correia S, Oliveir I, Queiros F, Ribeir C, Ferreira L, Luzio A, Gonçalves B (2015) Preharvest application of seaweed based biostimulant reduced cherry (Prunus avium L.) cracking. Procedia Environ Sci 29:251–252. https://doi.org/10.1016/j.proenv.2015.07.187
Esitken A, Pirlak L, Turan M, Sahin F (2006) Effects of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Sci Hortic 110(4):324–327. https://doi.org/10.1016/j.scienta.2006.07.023
Fan D, Hodges DM, Zhang J, Kirby CW, Ji X, Locke SJ, Critchley AT, Prithiviraj B (2011) Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinacia oleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem 124(1):195–202. https://doi.org/10.1016/j.foodchem.2010.06.008
Faniadis D, Drogoudi PD, Vasilakakis M (2010) Effects of cultivar, orchard elevation, and storage on fruit quality characters of sweet cherry (Prunus avium L.). Sci Hortic 125(3):301–304. https://doi.org/10.1016/j.scienta.2010.04.013
FAO (2022) Food and agriculture organization of the united nations, statistical database
Frioni T, Sabbatini P, Tombesi S, Norrie J, Poni S, Gatti M, Palliotti A (2018) Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci Hortic 232:97–106. https://doi.org/10.1016/j.scienta.2017.12.054
Gholami H, Saharkhiz MJ, Fard FR, Ghani A, Nadaf F (2018) Humic acid and vermicompost increased bioactive components, antioxidant activity and herb yield of chicory (Cichorium intybus L.). Biocatal Agric Biotechnol 14:286–292. https://doi.org/10.1016/j.bcab.2018.03.021
Giusti MM, Wrolstad RE (2001) Anthocyanins, characterization and measurement with UV visible spectroscopy. In: Wrolstad RE (ed) Current protocols in food analytical chemistry. Wiley, New York
Göksu GA, Kuzucu CÖ (2017) Effects of different doses of vermicompost applications on fruit yield and some quality parameters of watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai). Çanakkale Onsekiz Mart University. J Graduate Sch Nat Appl Sci 3(2):48–58. https://doi.org/10.28979/comufbed.344546
Hashempoor J, Asadi-Sanam S, Mirza M, Jahromi MG (2022) The effect of different fertilizer sources on soil nutritional status and physiological and biochemical parameters of cone flower (Echinacea purpurea L.). commun soil sci plant anal 53(10):1246–1260. https://doi.org/10.1080/00103624.2022.2046025
Hojjati M, Jahromi MG, Abdossi V, Torkashvand AM (2024) Exogenous melatonin modulated drought stress by regulating physio-biochemical attributes and fatty acid profile of sweet cherry (Prunus avium L.). J Plant Growth Regul 43:299–313. https://doi.org/10.1007/s00344-023-11085-x
İlhan Ö, Artık N (2021) Determination of some physicochemical properties and aroma components of sweet cherries (Prunus avium) grown in different locations. Eur J Sci Technol 21:437–443. https://doi.org/10.31590/ejosat.818911
Jalali P, Roosta HR, Khodadadi M, Torkashvand AM, Jahromi MG (2022) Effects of brown seaweed extract, silicon, and selenium on fruit quality and yield of tomato under different substrates. PLoS ONE 17(12):e277923. https://doi.org/10.1371/journal.pone.0277923
Karabıyık T (2013) Carotenoid content and antioksidant capacities of carrots treated with vermicompost and biofarm applications under organic production systems. Gaziosmanpaşa University, Graduate School of Natural and Applied Sciences, Horticulture Department, Tokat (master’s thesis)
Khanam Z, Wen CS, Bhat IUH (2015) Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat Ali). J King Saud Univ Sci 27(1):23–30. https://doi.org/10.1016/j.jksus.2014.04.006
Koç H (2013) Production processes of organic fertilizer from some marine algae (Ulva sp., Cystoseira sp. and Corallina sp.) collected along Giresun coastals and determination of their plant nutrient elements. Giresun University, Graduate School of Natural and Applied Sciences, Biology Department, Giresun (master’s thesis)
Kotan R, Tozlu E, Güneş A, Dadaşoğlu F (2021) Investigation of possibilities of using Bacillus subtilis microbial fertilizer in apple sapling growing. Atatürk Univ J Agric Fac 52(1):46–55. https://doi.org/10.17097/ataunizfd.762325
Michalak M (2022) Plant-derived antioxidants: Significance in skin health and the ageing process. Int J Mol Sci 23:585. https://doi.org/10.3390/ijms23020585
Milosevic T, Milosevic N, Mladenovic J (2022) The influence of organic, organo-mineral and mineral fertilizers on tree growth, yielding, fruit quality and leaf nutrient composition of apple cv. ‘Golden delicious Reinders. Sci Hortic 297:110978. https://doi.org/10.1016/j.scienta.2022.110978
Quelal C, Nicolas A, Salazar U (2022) Comparison of the effect of three biofertilizers: Biol, seaweed and spirulina (Arthrospira platensis), on the organic production of lettuce (Lactuca sativa). Universidad de las Fuerzas Armadas, Department of Life Sciences and Agriculture, Degree Work of Agricultural Engineering
Rawi WAA, Al-Hadethi MEA, Abdul-Kareem AA (2016) Effect of foliar application of gibberellic acid and seaweed extract spray on growth and leaf mineral content on peach trees. Iraqi J Agric Sci 47(7):98–105
Salvi L, Brunetti C, Cataldo E, Niccolai A, Centritto M, Ferrini F, Mattii GB (2019) Effects of Ascophyllum nodosum extract on Vitis vinifera: consequences on plant physiology, grape quality and secondary metabolism. Plant Physiol Biochem 139:21–32. https://doi.org/10.1016/j.plaphy.2019.03.002
Seğmen E, Özdamar Ünlü H (2023) Effects of foliar applications of commercial seaweed and spirulina platensis extracts on yield and fruit quality in pepper (Capsicum annuum L.). Cogent Food Agric 9(1):2233733. https://doi.org/10.1080/23311932.2023.2233733
Siringi JO, Turoop L, Njonge F (2022) Biostimulant effect of spirulina (Arthrospira platensis) on lettuce (Lactuca sativa) cultivated under aquaponic system. SCIREA J Biol 7(1):23–40. https://doi.org/10.54647/biology18204
Thakur A, Kumar A, Kumar CV, Kıran BS, Kumar S, Athokpam V (2021) A review on vermicomposting: by-products and its importance. Plant Cell Biotechnol Mol Biol 22(11, 12):156–164
Torun Kayabası E, Yılmaz O (2021) The importance of vermicompost in agricultural production and economy. Eurasian J Agric Res 5(2):146–159
Usenik V, Fajt N, Mikulic-Petkovsek M, Slatnar A, Stampar F, Veberic R (2010) Sweet cherry pomological and biochemical characteristics influenced by rootstock. J Agric Food Chem 58:4928–4933. https://doi.org/10.1021/jf903755b
Veberic R, Stampar F (2005) Quality of apple fruits (Malus domestica) from organic versus integrated production. Inf Technol Sustain Fruit Veg Prod Frutic 5:12–16
Yaman M, Yildiz E, Sumbul A, Ercisli S, Sonmez O, Gunes A, Say A, Kece YM, Unsal HT (2023) The Effect of PGPR applications on bioactive content and fruit characteristics of different apple scion-rootstock combinations. Erwerbs-Obstbau 65:1267–1273. https://doi.org/10.1007/s10341-023-00918-4
Zhang Z, Huang R (2013) Analysis of malondialdehyde, chlorophyll proline, soluble sugar, and glutathione content in Arabidopsis seedling. Bio Protocol 3(14):e817–e817. https://doi.org/10.21769/BioProtoc.817
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4):555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Acknowledgements
This work was supported by the Scientific Research Projects Coordination Unit in Isparta University of Applied Sciences under the project number: 2020-BTAP1-0068. We thank Dr. Muhammet Tonguç for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B. Çelik and B. Şan declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors contributed equally to the manuscript.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Çelik, B., Şan, B. The Effects of Organic Material Applications on the Biochemical Content of ‘0900 Ziraat’ Sweet Cherry Cultivar. Applied Fruit Science (2024). https://doi.org/10.1007/s10341-024-01184-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10341-024-01184-8