Abstract
The activity of Cinnamomum cassia essential oil (EO) and (E)-cinnamaldehyde was investigated on the phytoparasitic species Meloidogyne incognita, Globodera rostochiensis, and Xiphinema index. Juveniles (J2) or eggs of M. incognita and G. rostochiensis and mixed-age specimens of X. index were exposed to 12.5–100 µg mL−1 concentrations of the two products. The suppressiveness of soil treatments with 100–800 mg kg−1 soil rates of the C. cassia EO and (E)-cinnamaldehyde to M. incognita and G. rostochiensis was assessed on potted tomato and potato, respectively. A 24-h exposure to a 12.5 µg mL−1 solution of (E)-cinnamaldehyde resulted in more than 68% mortality of M. incognita J2, while a poor mortality occurred at the same concentration of the whole EO. The mortality of G. rostochiensis J2 ranged 39 and 42%, respectively, since after a 4-h exposure to a 12.5 µg mL−1 solution of both products. All the X. index specimens died after a 48- and 8-h exposure to a 100 µg mL−1 solution of the EO and (E)-cinnamaldehyde, respectively. Egg hatch was reduced by more than 90% after exposing the M incognita egg masses or the G. rostochiensis cysts to 800 µg mL−1 concentration of both EO and (E)-cinnamaldehyde for 24 and 96 h, respectively. The infestation of M. incognita and G. rostochiensis on tomato and potato, respectively, was significantly reduced by all soil treatments with both products, though (E)-cinnamaldehyde generally resulted more suppressive than the whole EO to both nematode species. According to these results, C. cassia EO and (E)-cinnamaldehyde could be suggested as a potential source of new environment-friendly nematicides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Key message
-
Study of the activity of C. cassia essential oil and cinnamaldehyde on different phytonematodes.
-
First assessment of the nematicidal activity of essential oil from C. cassia leaves and twigs.
-
Relationship between nematoxicity of C. cassia essential oil and its compositional profile.
-
Potential of C. cassia essential oil for the development of new sustainable nematicides.
Introduction
Phytoparasitic nematodes are among the most dangerous agricultural pests, as estimated to cause about 12–15% of annual world crop yield losses (Perry and Moens 2011). Root-knot species of genus Meloidogyne are unanimously stated to be the most harmful phytonematodes, as they worldwide spread on a wide range of agricultural crops and responsible of heavy yield reductions (Nicol et al. 2011). Severe economic losses can also derive from the attacks of the golden cyst nematode, Globodera rostochiensis Wollenweber, which represent a serious pest problem in the major potato-growing areas of the world (Price et al. 2021). The dagger nematode Xiphinema index Thorne et Allen has been also assuming an increasing economic relevance, mainly due to its role as vector of the grapevine (Vitis vinifera L.) fanleaf virus and to the derived threats to world grapevine yield and quality (Van Zyl et al. 2012).
The environmental policies of the European Union have focused since the early 2000s on the ban of the most hazardous synthetic nematicides, i.e., those on which phytoparasitic nematode control relied over the past decades. These measures aimed to a global 50% reduction of pesticide use within 2030 and to their replacement with more sustainable non-chemical control tools, also including the use of plant-derived nematicides (Marrone 2019; Bozzini 2017; D’Addabbo et al. 2014; EC Regulation 2009/1107).
Essential oils (EOs) from several aromatic and medicinal plants have been increasingly acknowledged as a huge source of new products suitable for a sustainable management of crop pathogens and pests, also including phytoparasitic nematodes (Pavela and Benelli 2016). The main points in favor of EOs-based nematicides are their limited persistence under field conditions and the absence of the resistance phenomena occurring for synthetic nematicides, due to the multisite activity of the complex mixtures of EOs’ bioactive constituents (Catani et al. 2023).
The genus Cinnamomum (Lauraceae family) includes 250 tree and shrub species distributed in Southeast Asia, China, and Australia, the most known of which are the camphor tree C. camphora (L.) J. Presl., the true cinnamon (C. verum J. Presl., syn. C. zeylanicum Blume), native of Sri Lanka and South India, and the Chinese cassia (C. cassia L., syn. C. aromaticum Nees), native of China and Vietnam (Sharifi-Rad et al. 2021). All these species have been largely documented for a wide spectrum of biological properties, such as anti-inflammatory, antibacterial, antifungal, antioxidant, immunomodulatory, and nematicidal activities (Pandey et al. 2020; Zhang et al. 2019). According to the literature data, the nematicidal activity of EOs from different Cinnamomum species is largely variable. The C. camphora EO was reported as moderately suppressive to the southern root-knot nematode M. incognita Kofoid et White on tomato, while the EO from C. verum was documented for a strong toxicity to M. incognita and M. graminicola Golden and Birchfield and to the pinewood nematode Bursaphelenchus xylophilus Nickle (D’Addabbo et al. 2020; Eloh et al. 2020; Faria et al. 2021). The information on the nematicidal properties of the C. cassia EO is limited to data from bioassays on B. xylophilus (Faria et al. 2021) and from a recent study of Jardim et al. (2018), reporting an in vitro activity of an EO from C. cassia bark on M. incognita J2 and eggs and its suppressiveness to M. incognita infestation on soybean (Glycine max (L.) Merr).
The compositional profile of the C. cassia EO is prevalently constituted by (E)-cinnamaldehyde (syn. trans-cinnamaldehyde or (2E)-3-phenylpropenal), which content can vary from 42 to 92% according to factors as pedoclimatic conditions, plant age, and method of EO extraction (Jugreet et al. 2020). (E)-cinnamaldehyde is the main agent of the nematicidal activity of the C. cassia EO, as proved for a strong in vitro activity on B. xylophilus, M. incognita, and M. javanica (Treub) Chitwood (Jardim et al. 2018; Faria et al. 2021; Oka et al. 2001) as well as for a strong suppressiveness to root-knot nematode infestation on soybean and tomato (Jardim et al. 2018; Oka et al. 2001). A poor information is available on the activity of the C. cassia EO, on other economically relevant phytonematode species such as G. rostochiensis and X. index. More generally, data on EOs’ effects on G. rostochiensis are limited to in vitro assays on the EOs from C. camphora, Cymbopogon martinii (Roxb.) Wats., Eucalyptus globulus Labill, Ocimum basilicum L., Salvia officinalis L., and Thymus vulgaris L. (Faria and Vicente 2021), while the only data on the activity of EOs on X. index are derived from laboratory assays on EOs from Moroccan ecotypes of Artemisia herba-alba, Citrus sinensis (L.) Osbeck, Rosmarinus officinalis L., and Thymus satureioides Cosson (Avato et al. 2017). Based on this lack of information, this study aimed to a comparative elucidation of the nematicidal activity of a C. cassia EO and cinnamaldehyde on nematode species with a different biology and feeding behavior, M. incognita, G. rostochiensis, and X. index, either through in vitro assays on infective stages viability and egg hatchability or experiments in soil infested by M. incognita and G. rostochiensis on tomato (Solanum lycopersicum Mill.) and potato (Solanum tuberosum L.), respectively.
Methods
Nematodes and chemicals
The population of M. incognita used in the experiments was originally recovered from infested tomato roots collected from a field at Castellaneta (Taranto, Apulia region) and then reared on tomato cv. Regina di Fasano in a glasshouse at 25 ± 2 °C. The population of G. rostochiensis (J2 and cysts) was recovered from an infested potato field at Polignano (Bari, Apulia region) and reared on potato cv. Desiree in a glasshouse at 20 ± 2 °C. The population of X. index was extracted from a naturally infested vineyard soil located at Ginosa (Taranto, Apulia region).
The tested C. cassia EO was a 100% pure organic product from Vietnam, extracted from plant leaves and twigs by steam distillation (Essenthya-Lab, Grugliasco, Italy). (E)-cinnamaldehyde (≥ 99%) was supplied by Sigma-Aldrich Co. (Milan, Italy). The nematicide oxamyl used in the experiments was a commercial formulation at 10% a.i. (Vydate® 10 L, Corteva Agriscience, Cernay, France).
Chemical analysis
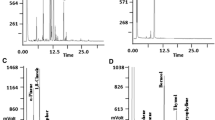
A Trace GC Ultra Thermo Finnigan gas chromatograph equipped with an FID detector was used for the compositional analysis of the C. cassia EO. A 1µL sample of the EO solubilized in hexane was injected in the cold on-column mode in a DB-5 (J&W Scientific) fused silica capillary column of 30 m × 0.25 mm and 0.25-μm film thickness. Chromatographic conditions were as follows: detector temperature 300 °C and column temperature programmed from 60 °C (5 min isothermal) to 280 °C (15 min isothermal) at 4 °C/min. Hydrogen was the carrier gas (35 kP; 2.0 mL/min). Data were processed using the Chrom-Card 32-bit computing software. Quantitative data of the EO constituents were expressed as a percentage composition from the total peak areas detected from GC–FID analyses without the use of correction factors.
GC–MS analyses were performed with a Hewlett-Packard 6890–5973 mass spectrometer interfaced with a HP ChemStation. The chromatographic conditions were as follows: column oven program from 40 (5 min isothermal) to 280 °C (30 min isothermal) at 4 °C/min and injector, 280 °C. Helium was the carrier gas (flow rate, 1 mL/min). A HP-5 MS capillary column (30 m × 0.25 mm; 0.25-µm film thickness) was utilized. MS operating parameters were: ion source, 70 eV; ion source temperature, 200 °C; electron current, 34.6 µA; and vacuum 10–5 torr. Mass spectra were acquired over the 40–800 amu range at 1 scan/s. The ion source was operating in the electron impact mode. Samples (1 µL) were injected using the splitless sampling technique.
Determination of the chemical composition of the analyzed EO was based on comparison of GC retention times of constituents with authentic reference compounds in combination with arithmetic indexes (AI) and by means of reference mass spectra from standard compounds and/or from library files (Adams 2007). AI index values were calculated using an n-alkane series (C6–C32) under the same GC conditions used for the EO sample.
Bioassays on nematode infective stages
The egg masses of M. incognita were handpicked from infested tomato roots and incubated in distilled water in a growth chamber maintained at 25 °C. The cysts of G. rostochiensis were extracted from the soil by a Fenwick funnel (Hallmann and Subbotin 2018) and incubated in a 0.6mM sodium metavanadate hatching solution (Russo and Greco 2006) in a growth chamber at 20 °C. The emerged J2 of both species were collected and stored at 5 °C until their use. Specimens of X. index at mixed developmental stages were extracted from soil by the Cobb’s decanting and sieving method (Hallmann and Subbotin 2018) and then handpicked and used immediately. Batches of 100 J2 of M. incognita and G. rostochiensis or of 50 specimens of X. index were suspended in 0.5 mL of distilled water and placed in 1.5mL Eppendorf tubes. Appropriate amounts of the C. cassia EO or of (E)-cinnamaldehyde were added to a 0.3% water solution of Tween 20, as to prepare 25, 50, 100, and 200 μg mL−1 solutions. A 0.5mL volume of each solution was poured into the Eppendorf tubes containing the nematode suspensions, as to reach 12.5, 25, 50, and 100 μg mL−1 final test concentrations. The nematodes were exposed to the test solutions for 4, 8, 24, or 48 h, providing four replicates for each concentration x exposure time combination and including distilled water, a 2 mL L−1 water solution of oxamyl, and a 0.3% Tween 20 water solution as controls. At the end of each exposure time, the nematodes from each replicate were ranked as motile or paralyzed under a light microscope and then transferred to distilled water for 72 h, after which the nematode mortality was confirmed by the persistence of immobility. Percentage mortality was calculated according to the Abbott’s formula m = 100 × (1 − nt/nc), in which m, percent mortality; nt, number of viable nematodes after the treatment; and nc, number of viable nematodes in the water control (Finney 1978). Probit analysis was applied to calculate the LC50 values of both the C. cassia EO and (E)-cinnamaldehyde on the three nematode species.
Bioassays on nematode eggs
Batches of 50 M. incognita egg masses (450 eggs per mass) or of 50 G. rostochiensis cysts (700 eggs per cyst) were placed in 1.5mL Eppendorf tubes containing 0.5 mL of distilled water and then added with the same volume of 200, 400, 800, and 1600 μg mL−1 solutions of C. cassia EO or (E)-cinnamaldehyde, as to reach 100, 200, 400, and 800 μg mL−1 final test concentrations. Four replicates were provided for each concentration × exposure time combination, including distilled water, a 2 mL L−1 oxamyl, and 0.3% Tween 20 water solutions as controls. The egg masses and the cysts were exposed to the test solutions and the controls for 24, 48, or 96 h, after which they were repeatedly rinsed in distilled water and placed in 2-cm-diameter sieves (215 μm aperture), each arranged within a 3.5-cm-diameter Petri dish. A 3mL volume of distilled water or 0.6mM sodium metavanadate hatching solution was added to submerge the M. incognita egg masses or the G. rostochiensis cysts, respectively. The Petri dishes were arranged in a growth chamber at 25 or 20 °C, respectively, over the whole hatching test duration. The emerged J2 of both species were removed and microscopically counted at weekly intervals, renewing the distilled water or the 0.6mM sodium metavanadate solution at the same time.
The hatching test on the M. incognita egg masses was discontinued after five weeks, while the assay on the G. rostochiensis cysts was prolonged for eight weeks. At the end of both experiments, the number of unhatched eggs was counted under a light microscope after dissolving the M. incognita egg masses by a 3-min shaking in a 1% sodium hypochlorite aqueous solution or crushing the G. rostochiensis cyst by the Bijloo’s modified method (Hallmann and Subbotin 2018). The total number of hatched J2 was expressed as cumulative percentages of the total egg content of egg masses or cysts.
Experiments in soil
Tomato roots infested by M. incognita were finely cut, and nematode density was determined by extracting eggs and J2 from six 10-g root samples by a 3-min shaking in a 1% sodium hypochlorite water solution (Hallmann and Subbotin 2018). The root inoculum was then thoroughly mixed with a steam-sterilized sandy soil (64.4% sand, 18.7% silt, 16.9% clay, pH 7.5, and 0.8% organic matter) as to reach an initial population density of 20 eggs and J2 mL−1 soil. A 1.2L volume of the infested soil was poured into 1.5-L clay pots and then treated with 100, 200, 400, or 800 mg kg−1 soil rates of the C. cassia EO or (E)-cinnamaldehyde vehiculated in a 400mL volume of a 0.3% Tween 20 water solution. A one-month-old tomato seedling (cv. Regina di Fasano) was transplanted in each pot three weeks after the treatments. Soil that is non-treated, either non-infested or infested by M. incognita, and infested soil treated with oxamyl (200 mg kg−1 soil rate applied three days before and 15 days after transplanting) or with a 0.3% Tween 20 solution (applied at the same time of treatments with the EO or (E)-cinnamaldehyde) were used as controls. Five replicates were provided for each treatment and controls, arranging the pots in a randomized block design on the benches of a greenhouse maintained at 25 ± 2 °C. The experiments were carried out throughout 60 days, after which the plants were uprooted and fresh weight of aerial parts and roots was recorded on each plant. Gall infestation index was evaluated on each tomato root according to the Taylor and Sasser’s 0–5 scale (0 = no galls, 1 = 1–2 galls, 2 = 3–10 galls, 3 = 11–30 galls, 4 = 31–100 galls, and 5 > 100 galls) (Hallmann and Subbotin 2018). The final population density of M. incognita was determined on each replicate by microscopically counting the eggs and the J2 extracted from each tomato root (3-min agitation in a 1% sodium hypochlorite solution) and from a 500-cm3 soil sample by Coolen’s flotation and centrifugation technique (Hallmann and Subbotin 2018).
In the experiment on potato, the same steam-sterilized sandy soil used for the experiment on M. incognita was added with cysts of G. rostochiensis extracted from infested soil by a Fenwick can (Hallmann and Subbotin 2018), as to reach a 20 eggs and juveniles mL−1 soil initial population density. As in the first experiment on M. incognita, a 1.2L volume of the artificially infested soil was poured into 1.5-L clay pots and treated with 100, 200, 400, or 800 mg kg−1 soil rates of the C. cassia EO. A potato tuber (cv. Desiree) was sown in each pot three weeks after the treatments. Soil non-treated, either non-infested and infested, or treated with oxamyl (200 mg kg−1 soil rate at potato emergence and 15 days later) or the 0.3% Tween 20 solution (at EO application) were included as controls. The pots were arranged in a randomized block design, five replicates per each treatment and controls, in a glasshouse at 20 ± 2 °C. The potato plants were cultivated for two months, after which the weight of aerial biomass was recorded on each plant. The final population of G. rostochiensis was determined by extracting cysts from a 100-g soil sample by the Fenwick can procedure (Hallmann and Subbotin 2018). The cysts were counted under a stereoscope and then crushed, as to count the number of eggs and J2.
Data analysis
Data from the two experimental runs of each in vitro assay and experiment in soil were pooled in the absence of experiment x treatment interactions, as emerging from a preliminary analysis of variance with experimental runs as factors. Pooled data from the in vitro experiments and nematode data from experiments in soil were arcsin- and Ln (x + 1)-transformed, respectively, as to remove heterogeneity of the error variance. The transformed experimental data were subjected to one-way analysis of variance (ANOVA) and means compared by Fisher’s Least Significant Difference Test (P ≤ 0.05) by using the statistical software PlotIT 3.2 (Scientific Programming Enterprises, Haslett, MI).
Results
Chemical composition of C. cassia EO
Components identified by GC–FID and GC–MS accounted for 100% of total EO composition (Table 1). The EO was constituted almost entirely by (E)-cinnamaldehyde (93.74%), with low amounts of (E)-cinnamyl acetate (2%), (E)-cinnamyl alcohol (1%), benzaldehyde (1%), and coumarin (0.79%) (Fig. 1). All the other components were present at percentages lower than 0.5%.
Effects on nematode infective stages
The mortality of M. incognita J2 ranged 12–13% at all the exposure times to the 12.5 µg mL−1 solution of the C. cassia EO, while peaked more than 68 and 73% after a 24- and 48-h exposure to the same concentration of (E)-cinnamaldehyde, respectively (Table 2). (E)-cinnamaldehyde was significantly more toxic than the whole EO also at most of the exposure times to the 25 and 50 µg mL−1 concentrations and after a 4-h treatment with 100 µg mL−1 solution. Adversely, no statistical difference occurred after 8-h or longer exposures to the 100 µg mL−1 concentration of both products. The toxicity of oxamyl to M. incognita J2 was significantly higher compared to almost all the treatments with the C. cassia EO but statistically not different from (E)-cinnamaldehyde or even lower at the 100 µg mL−1 concentration.
The G. rostochiensis J2 were largely more sensitive to both C. cassia EO and (E)-cinnamaldehyde compared to those of M. incognita, as even a 4-h exposure to a 12.5 µg mL−1 solution of the two products was able to cause about 39 and 42% mortality rates, respectively. A 24-h treatment with the 25 µg mL−1 solution of the EO and (E)-cinnamaldehyde was lethal to more than 80 and 76% of the treated J2, respectively. At the same concentration, an almost complete J2 mortality occurred after a 48-h exposure, without significant differences between the two products. The toxicity of both the whole EO and (E)-cinnamaldehyde to the G. rostochiensis J2 was significantly higher than that of oxamyl at the 4- and 8-h exposures but not statistically different at almost all the 24- and 48-h treatments with the 50 and 100 µg mL−1 solutions.
The specimens of X. index were not or poorly affected by the 12.5 and 25.0 µg mL−1 concentrations of both C. cassia EO and (E)-cinnamaldehyde up to a 24-h exposure, while 76.7 and 90% mortality occurred after a 48-h immersion in the 25 µg mL−1 solution, respectively. A 50 µg mL−1 concentration of the EO and (E)-cinnamaldehyde caused about 70 and 80% mortality within 8 h, respectively. A complete mortality occurred after a 48- and 8-h exposure of the X. index specimens to a 100 µg mL−1 solution of the EO and (E)-cinnamaldehyde, respectively.
The LC50 values of both the C. cassia EO and the (E)-cinnamaldehyde were always lower for G. rostochiensis compared to the other two nematode species, while treatment of M. incognita J2 with (E)-cinnamaldehyde always resulted in lower LC50 values compared to X. index. The LC50 of (E)-cinnamaldehyde was always lower than that of the whole EO for all the three nematode species.
Effects on egg hatchability
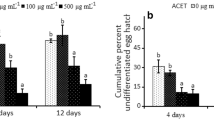
The hatchability of M. incognita eggs was not affected by the Tween 20 solution, while the exposure to the oxamyl solution resulted in a maximum 43.2% egg hatch reduction only after a 96-h exposure (Fig. 2). Adversely, the egg hatch was reduced to only 8.6 and 9.2% by a 24-h treatment of the egg masses with a 800 µg mL−1 solution of the C. cassia EO and (E)-cinnamaldehyde, respectively, vs. the 91.6% hatch in water. The number of hatched eggs was almost nil after a 96-h exposure to all the tested concentrations of the C. cassia EO or to the 400 and 800 µg mL−1 (E)-cinnamaldehyde solutions.
Percentage hatchability of eggs of Meloidogyne incognita and Globodera rostochiensis after 24-, 48-, or 96-h exposures of egg masses and cysts, respectively, to 100–800 μg mL−1 solutions of essential oil from Cinnamomum cassia and (E)-cinnamaldehyde (means ± SE of four replicates from two pooled experimental runs). Bars marked by the same letter are not significantly different according to the Least Significant Difference Test (P ≤ 0.05)
All treatments with the C. cassia EO and (E)-cinnamaldehyde significantly reduced the hatch of G. rostochiensis eggs compared to the Tween 20 and sodium metavanadate controls. The egg hatch reduction caused by C. cassia EO and (E)-cinnamaldehyde was statistically similar, ranging about 92% after a 96-h cyst treatment with 800 µg mL−1 solutions.
Effects on soil nematode infestation
Soil treatments with the C. cassia EO significantly reduced the number of M. incognita eggs and J2 and the gall formation on tomato roots, as well as the final nematode density in soil (Table 3). The suppressive effect of the EO on root-knot nematode infestation was not different from that of oxamyl or even statistically higher at doses ≥ 200 mg mL−1. Most of treatments with (E)-cinnamaldehyde resulted in a significantly lower M. incognita multiplication on tomato roots compared to the same dose of the C. cassia EO, as well as not statistically different or even more suppressive than oxamyl (Table 3). The tomato growth parameters were significantly improved by treatments with both the C. cassia EO and the (E)-cinnamaldehyde compared to the non-treated control, as well as were not statistically different or even higher compared to the treatment with oxamyl.
In the experiment on potato, the final number of G. rostochiensis cysts and eggs was always significantly lower in soil treated with both the C. cassia EO and the (E)-cinnamaldehyde than in the non-treated control or in soil applied with the Tween 20 solution, while the two highest rates of both products were also statistically more suppressive than the treatment with oxamyl (Fig. 3). The final population density of G. rostochiensis was always significantly lower in soil treated with (E)-cinnamaldehyde compared to the correspondent dose of the whole C. cassia EO. All the treatments with both the whole EO and the (E)-cinnamaldehyde provided a significant plant growth increase compared to the non-treated control and Tween 20 solution, without any statistical difference from the non-infested control or the treatment with oxamyl.
Effect of soil treatments with 100–1000 µg kg−1 soil rates of the essential oil from Cinnamomum cassia and (E)-cinnamaldehyde on the infestation of Globodera rostochiensis and growth potato plants cv. Desiree (means ± SE of five replicates from two pooled experimental runs). Bars marked by the same letter are not significantly different according to the Least Significant Difference Test (P ≤ 0.05)
Discussion
The C. cassia EO used in this study was almost entirely constituted by (E)-cinnamaldehyde, present at a higher percentage (93.7 vs. 83.3%) compared to the C. cassia EO investigated in a previous study of Jardim et al. (2018), which presented a higher percentage of methoxycinnamaldehyde (7.1% vs. the 0.4% of our EO). These differences can be attributable to the different source plant parts of the two EOs, i.e., leaves and twigs for our EO vs. bark for the C cassia EO used in the previous study. However, a large fluctuation of the (E)-cinnamaldehyde content in C. cassia EO was reported also by other studies, as ranging from 42 to 91% according to geographic region, climate, and agronomic factors (Kim et al. 2016; Geng et al. 2011).
The data from the in vitro assays indicated a strong effect of both the C. cassia EO and the (E)-cinnamaldehyde on J2 viability and egg hatchability of both the root-knot species M. incognita and the cyst nematode G. rostochiensis. The strong toxicity to M. incognita J2 and eggs was previously observed also for the EO from C. cassia bark tested by Jardim et al. (2018), as significantly reducing J2 viability and egg hatchability after a 48-h exposure to a 62 µg mL−1 EO concentration. Analogous effects on M. incognita J2 and eggs were also reported for an EO from C. verum (syn. C. zeylanicum Blume) prevalently (85%) constituted by (E)-cinnamaldehyde (D’Addabbo et al. 2020). In addition to the EO, also the water and methanolic extracts from C. cassia bark were found for a toxicity to root-knot nematodes, ranging from a temporary immobilization of M. javanica J2 to a 95.8% J2 mortality and 82% egg hatch inhibition observed on M. incognita (Seo et al. 2014). The activity of (E)-cinnamaldehyde on M. incognita J2 and eggs observed in this study confirmed the previous data of Jardim et al. (2018), as well as was also reported for the (E)-cinnamaldehyde from a C. zeylanicum EO () and also extended to M. javanica (Barros et al. 2021; Oka 2001).
This study is the first report of the activity of a C. cassia EO and (E)-cinnamaldehyde on the J2 and the eggs of the potato cyst nematode G. rostochiensis. A significant reduction of the hatchability of G. rostochiensis egg was previously documented only for a C. camphora EO (Renco et al., 2015), while the (E)-cinnamaldehyde was found toxic to the J2 and the eggs of other cyst nematode species, such as the cereal cyst nematode Heterodera avenae Wollenweber and the soybean cyst nematodes H. glycines Ichinohe (De Oliveira Barizon 2020; Li et al. 2017). In addition to EOs’ terpene components, G. rostochiensis J2 were found highly sensitive also to plant sesquiterpene compounds, such as caffeic and chlorogenic acid and artemisinin from annual wormwood, A. annua L. (D’Addabbo et al. 2013). Information on EOs’ activity on X. index is even poorer than on cyst nematodes, as limited to the report of a strong in vitro toxicity of the EOs from Moroccan ecotypes of A. herba-alba, C. sinensis, R. officinalis, and T. satureioides to an Italian population of X. index (Avato et al. 2017).
Based on the values of LC50, G. rostochiensis was the most sensitive of the three nematode species either to C. cassia EO or to (E)-cinnamaldehyde, while the sensitivity of M. incognita J2 to (E)-cinnamaldehyde was much higher compared to that of X. index, with a contrasting response of these two species to the C. cassia EO. The variability of the response to EOs and, more generally, to plant compounds among the nematode species was already documented by previous studies (Avato et al. 2017; D’Addabbo et al. 2013) and related to the different anatomy, biology, and feeding behavior of each species (Davies and Curtis 2011; Sánchez-Moreno and Ferris 2018).
In addition to the three species targeted by this study, the activity of C. cassia EO and (E)-cinnamaldehyde was also assessed on other phytoparasitic nematode. A strong toxicity of EOs from Cassia species was reported on adult specimens of B. xylophilus, as well as a complete mortality of the stem nematode Ditylenchus dipsaci (Kuehn) Filipjev was observed after a 4-h exposure to a 2,000ppm concentration of a C. cassia EO (Douda et al. 2010; Faria et al. 2021). (E)-cinnamaldehyde was documented for a strong toxicity to B. xylophilus and to the root-lesion nematodes of genus Pratylenchus (Barbosa et al. 2024; Faria et al. 2021).
Both the experiments in soil indicated a strong suppressiveness of soil treatments with both the whole C. cassia EO and the cinnamaldehyde to the infestation of M. incognita and G. rostochiensis on tomato and potato, respectively. There is no previous report on the effect of the C. cassia EO and, more generally, of EOs in soil infested by potato cyst nematodes, while the data from the experiment on M. incognita are in good agreement with the findings from soil experiments of Jardim et al. (2018), which described a significant reduction of gall formation and egg density on soybean roots following to soil treatments with an emulsion of the C. cassia EO. In addition to the EO, significant suppressive effects were also reported for treatments with C. cassia bark extracts in soil infested by M. incognita on coffee and cucumber, as well as following to soil amendments with 0.2% C. cassia stem bark (Luong et al. 2020; Nguyen et al. 2011; Kim et al. 2003a, b). In both the experiments on tomato and potato, the suppressive effect of soil treatments with (E)-cinnamaldehyde on the nematode infestation was significantly higher than that of the whole C. cassia EO, in good agreement with data from the previous studies in soil infested by root-knot nematodes on soybean or tomato (Jardim et al. 2018; Oka 2001).
In addition to nematicidal properties, C. cassia EO and (E)-cinnamaldehyde also showed a biocidal activity on other crop pests and pathogens. The insecticidal activity of the C. cassia EO and its components was documented on the spotted wing drosophila Drosophila suzukii Matsumura and the rice weevil Sitophilus oryzae L., as well as on the adult beetles of the storage pest Lasioderma serricorne Fabricius (Kim et al. 2016; Lee et al. 2008; Kim et al. 2003a, b). Analogously, (E)-cinnamaldehyde was reported for a fumigant toxicity to the kidney bean (Phaseolus vulgaris L.) bruchid Acanthoscelides obtectus Say and the oak nut weevil Mechoris ursulus Roelofs (Park et al. 2000; Regnault-Roger and Hamraoui 1995), as well as for an antifeedant activity on the fruit fly Ceratitis capitata Wiedemann (Moretti et al. 1998). The antifungal properties of the C. cassia EO and extracts were proved on various phytopathogenic fungi, such as Alternaria alternata (Fr.) Keissl, Rhizoctonia solani (Cooke) Wint., and Sclerotinia sclerotiorum (Lib.) de Bary (Ojaghian et al. 2014; Nguyen et al. 2009; Feng and Zheng 2007). (E)-cinnamaldehyde was also demonstrated for a fungicidal potential on phytopathogenic fungi such as Collectotrichum gloeosporioides (Penz. et Sacc., Fusarium solani (Mart.) Sacc. and R. solani (Lee et al. 2005).
The mechanisms of the nematicidal activity of EOs and their components on phytoparasitic nematodes are still unclear, though various mechanisms have been already hypothesized by analogy with insects. Kang et al. (2013) observed a more than 50% inhibition of acetylcholinesterases following the treatment of B. xylophilus with some monoterpene, phenylpropene, and sesquiterpene compounds. Other key enzymes, such as GABA and octopamine receptors or cytochrome P450, were also found to be affected by some EOs and their constituents (Regnault-Roger et al. 2012; Priestley et al. 2003; Enan 2001). In addition, Nguyen et al. (2011) described an increased activity of antioxidant enzymes, such as superoxide dismutase, catalase, and ascorbate peroxidase, in cucumber plants treated with the C. cassia extracts, hypothesizing an increased plant resistance against biotic stresses like a nematode attack. Helander et al. (1998) observed an effect of EO components as carvacrol on bacteria cell membranes, resulting in a leakage of cellular material and ATP. Changes in the internal pH and a leakage of potassium and phosphate ions were also found in bacterial cells exposed to an oregano EO or to thymol and carvacrol (Lambert et al. 2001). Data on the activity of the C. cassia EO, containing (E)-cinnamaldehyde as the main component, may be consistent with a mechanism of action determined by the structure of the molecule. (E)-cinnamaldehyde is a phenylpropanoid naturally deriving from the shikimate pathway and characterized by two reactive electrophilic sites, the α,β-unsaturated cinnamyl moiety and the aldehydic carbonyl group, which can act as pharmacophores. Due to this molecular feature, (E)-cinnamaldehyde can alkylate cellular nucleophiles through addition-type reactions and affect several biological events, such as inducing the inhibition of key enzymes or interacting with the parasite membranes.
Beside the nematicidal effect, both experiments in soil also evidenced a growth stimulation on both tomato and potato plants by the C. cassia EO, in agreement with the positive effects on soybean plants reported by Jardim et al. (2018) and with the results from our previous studies on other EOs (Laquale et al. 2018, 2015; Avato et al. 2017). A plant growth stimulation was also observed for the treatments with (E)-cinnamaldehyde, as confirming the increased tomato growth observed by Oka (2001) in soil treated with this compound.
The vehiculation in water by a drip irrigation system could be a suitable mode for distribution of the C. cassia EO or (E)-cinnamaldehyde in field or greenhouse, as the most technically feasible for the farmers. However, the high volatility of these products could limit their effectiveness on the target nematode species, as suggesting to avoid a direct dispersion. A preliminary stabilization of the products with biopolymer micro- or nanocapsules, chitosan, or other organic matrices could ensure their slower release in the soil and, therefore, could prolong their effects on phytoparasitic nematode populations. The doses of potential treatments with the two compounds could be referred to those of EOs-based nematicides currently present on the Italian market, ranging from the 35–50 L Ha−1 of a clove (Syzygium aromaticum L.) EO formulation to the 9 L Ha−1 of a geraniol–thymol mixture for treatments both before and after tomato transplanting.
Conclusion
Data from this study evidenced that the strong activity of C. cassia EO and (E)-cinnamaldehyde is extended not only to the root-knot nematodes of genus Meloidogyne but also to other phytonematode species with a different biology and feeding behavior, as the cyst nematode G. rostochiensis and the virus-vector nematode X. index. Therefore, these products could be suitable raw materials or simply model compounds for developing new wide-spectrum nematicides mainly addressed to organic crop systems, where few pest control tools are available, or to high-value greenhouse crops, as particularly affected by the current scarcity of effective synthetic nematicides.
Author contributions
TD conceived and designed the research, analyzed the data, and edited the manuscript; SL and PV carried out the experiments on nematodes; PA supervised the chemical analyses and drafted the manuscript; MPA carried out the chemical analyses. All the authors read and approved the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured Publishing Corporation, Carol Stream, IL, USA; ISBN 978-1-932633-21-4
Avato P, Laquale S, Argentieri MP, Lamiri A, Radicci V, D’Addabbo T (2017) Nematicidal activity of essential oils from aromatic plants of Morocco. J Pest Sci 90:711–722. https://doi.org/10.1007/s10340-016-0805-0
Barbosa P, Faria JMS, Cavaco T, Figueiredo AC, Mota M, Vicente CSL (2024) Nematicidal activity of phytochemicals against the root-lesion nematode pratylenchus penetrans. Plants 13:726. https://doi.org/10.3390/plants13050726
Barros AF, Campos VP, de Paula LL, Pedroso LA, Silva FJ, da Silva JCP, de Oliveira DF, Silva GH (2021) The role of Cinnamomum zeylanicum essential oil, (E)-cinnamaldehyde and (E)-cinnamaldehyde oxime in the control of meloidogyne incognita. J Phytopathol 169:229–238. https://doi.org/10.1111/jph.12979
Bozzini E (2017) Pesticide policy and politics in the European Union. Palgrave Macmillan, Springer, Cham, CH. https://doi.org/10.1007/978-3-319-52736-9
Catani L, Manachini B, Grassi E, Guidi L, Semprucci F (2023) Essential oils as nematicides in plant protection: a review. Plants 12(6):1418. https://doi.org/10.3390/plants12061418
D’Addabbo T, Carbonara T, Argentieri MP, Radicci V, Leonetti P, Villanova L, Avato P (2013) Nematicidal potential of Artemisia annua and its main metabolites. Eur J Plant Pathol 137:295–304. https://doi.org/10.1007/s10658-013-0240-5
D’Addabbo T, Laquale S, Lovelli S, Candido V, Avato P (2014) Biocide plants as a sustainable tool for the control of pests and pathogens in vegetable cropping systems. Ital J Agron 9:137–145. https://doi.org/10.4081/ija.2014.616
D’Addabbo T, Argentieri MP, Laquale S, Candido V, Avato P (2020) Relationship between chemical composition and nematicidal activity of different essential oils. Plants 9:1546. https://doi.org/10.3390/plants9111546
Davies KG, Curtis RHC (2011) Cuticle surface coat of plant–parasitic nematodes. Ann Rev Phytopathol 49:135–156. https://doi.org/10.1146/annurev-phyto-121310-111406
Douda O, Zouhar M, Mazáková J, Nováková E, Pavela R (2010) Using plant essences as alternative mean for northern root-knot nematode (Meloidogyne hapla) management. J Pest Sci 83:217–221. https://doi.org/10.1007/s10340-010-0287-4
Eloh K, Kpegb K, Sasanelli N, Koumaglo HK, Caboni P (2020) Nematicidal activity of some essential plant oils from tropical West Africa. Int J Pest Manag 66:131–141. https://doi.org/10.1080/09670874.2019.1576950
Enan E (2001) Insecticidal activity of essential oils: octopaminergic sites of action. Comp Biochem Physiol C: Toxicol Pharmacol 130:325–337. https://doi.org/10.1016/S1532-0456(01)00255-1
Faria JMS, Vicente C (2021) Essential oils and volatiles as nematocides against the cyst nematodes Globodera and Heterodera. Biol Life Sci Forum 3:1. https://doi.org/10.3390/IECAG2021-09689
Faria JMS, Barbosa P, Vieira P, Vicente CSL, Figueiredo AC, Mota M (2021) Phytochemicals as biopesticides against the pinewood nematode Bursaphelenchus xylophilus: a review on essential oils and their volatiles. Plants 10:2614. https://doi.org/10.3390/plants10122614
Feng W, Zheng X (2007) Essential oils to control Alternaria alternata in vitro and in vivo. Food Contr 18:1126–1130. https://doi.org/10.1016/j.foodcont.2006.05.017
Finney DJ (1978) Statistical Method in Biological Assay, 3rd edn. Charles Griffin & Company Ltd., High Wycombe, UK
Geng S, Cui Z, Huang X, Chen Y, Xu D, Xiong P (2011) Variations in essential oil yield and composition during Cinnamomum cassia bark growth. Ind Crops Prod 33:248–252. https://doi.org/10.1016/j.indcrop.2010.10.018
Hallmann J, Subbotin SA (2018) Methods for extraction, processing and detection of plant and soil nematodes. In: Sikora RA, Coyne D, Hallmann J, Timper P (eds) Plant parasitic nematodes in subtropical and tropical agriculture, 3rd edn. Cabi Publisher, Wallingford, UK, pp 87–119. https://doi.org/10.1079/9781786391247.0000
Helander IM, Alakomi H, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LGM, Wright A (1998) Characterisation of the action of selected essential oil components on gram-negative bacteria. J Agric Food Chem 46:3590–3595. https://doi.org/10.1021/jf980154m
Jardim IN, Oliveira DF, Silva GH, Campos VP, de Souza PE (2018) (E)-cinnamaldehyde from the essential oil of Cinnamomum cassia controls Meloidogyne incognita in soybean plants. J Pest Sci 91:479–487. https://doi.org/10.1007/s10340-017-0850-3
Jugreet BS, Suroowan S, Rengasamy RK, Mahomoodally MF (2020) Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci Technol 101:89–105. https://doi.org/10.1016/j.tifs.2020.04.025
Kang JS, Kim E, Lee SH, Park IK (2013) Inhibition of acetylcholinesterases of the pinewood nematode, Bursaphelenchus xylophilus, by phytochemicals from plant essential oils. Pestic Biochem Physiol 105:50–56. https://doi.org/10.1016/j.pestbp.2012.11.007
Kim YH, Hafeez UK, Kim JH, Jeon YH, Lee EJ, Chang SP (2003a) Efficacy of soil amendment with medicinal plant materials for the control of root-knot nematode (Meloidogyne incognita) in tomato. Plant Pathol J 19:138–142. https://doi.org/10.5423/PPJ.2003.19.3.138
Kim SI, Park C, Ohh MH, Cho HC, Ahn YJ (2003b) Contact and fumigant activities of aromatic plant extracts and essential oils against Lasioderma serricorne (Coleoptera: Anobiidae). J Stor Prod Res 39:11–19. https://doi.org/10.1016/S0022-474X(02)00013-9
Kim J, Jang M, Shin E, Kim J, Lee SH, Park CG (2016) Fumigant and contact toxicity of 22 wooden essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Pestic Biochem Physiol 133:35–43. https://doi.org/10.1016/j.pestbp.2016.03.007
Lambert RJW, Skandamis P, Coote PJ, Nychas GJE (2001) A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol 91:453–462. https://doi.org/10.1046/j.1365-2672.2001.01428.x
Laquale S, Candido V, Avato P, Argentieri MP, D’Addabbo T (2015) Essential oils as soil biofumigants for the control of the root-knot nematode Meloidogyne incognita on tomato. Ann Appl Biol 167:217–224. https://doi.org/10.1111/aab.12221
Laquale S, Avato P, Argentieri MP, Bellardi MG (2018) D’Addabbo T (2018) Nematotoxic activity of essential oils from Monarda species. J Pest Sci 91:1115–1125. https://doi.org/10.1007/s10340-018-0957-1
Lee HC, Cheng SS, Chang ST (2005) Antifungal property of the essential oils and their constituents from Cinnamomum osmophloeum leaf against tree pathogenic fungi. J Sci Food Agricu 85:2047–2053. https://doi.org/10.1002/jsfa.2216
Lee EJ, Kim JR, Choi DR, Ahn YJ (2008) Toxicity of cassia and cinnamon oil compounds and cinnamaldehyde-related compounds to Sitophilus oryzae (Coleoptera: Curculionidae). J Econ Entomol 101:1960–1966. https://doi.org/10.1603/0022-0493-101.6.1960
Li YC, Ji H, Li XH, Zhang HX, Li HT (2017) Isolation of nematicidal constituents from essential oil of Kaempferia galanga L rhizome and their activity against Heterodera avenae Wollenweber. Trop J Pharm Res 16:59–65. https://doi.org/10.4314/tjpr.v16i1.8
Luong TH, Nguyen TK, Jung WJ (2020) Nematicidal activity of cinnamon bark extracts and chitosan against Meloidogyne incognita and Pratylenchus coffeae. Nematology 23:655–666. https://doi.org/10.1163/15685411-bja10067
Marrone PG (2019) Pesticidal natural products: status and future potential. Pest Manag Sci 75:2325–2340. https://doi.org/10.1002/ps.5433
Moretti MD, Bazzoni E, Passino GS, Prota R (1998) Antifeedant effects of some essential oils on Ceratitis capitata Wied. (Diptera, Tephritidae). J Essent Oil Res 10:405–412. https://doi.org/10.1080/10412905.1998.9700930
Nguyen VN, Nguyen DMC, Seo DJ, Park RD, Jung WJ (2009) Antimycotic activities of cinnamon-derived compounds against Rhizoctonia solani in vitro. Biocontrol 54:697–707. https://doi.org/10.1007/s10526-009-9220-2
Nguyen DMC, Seo DJ, Park RD, Lee BR, Jung WJ (2011) Changes in antioxidative enzyme activities in cucumber plants with regard to biological control of root-knot nematode, Meloidogyne incognita with Cinnamomum cassia crude extracts. J Kor Soc Appl Biol Chem 54:507–514. https://doi.org/10.3839/jksabc.2011.078
Nicol, JM, Turner SJ, Coyne DL, Nijs L, Hockland S, Maafi ZT (2011) Current Nematode Threats to World Agriculture. In: Jones J, Gheysen G, Fenoll C, (Eds) Genomics and Molecular Genetics of Plant-Nematode Interactions. Springer, Dordrecht, The Netherlands. https://doi.org/10.1007/978-94-007-0434-3_2
Ojaghian MR, Chen Y, Chen S, Cui ZQ, Xie GL, Zhang J (2014) Antifungal and enzymatic evaluation of plant crude extracts derived from cinnamon and rosemary against Sclerotinia carrot rot. Ann Appl Biol 164:415–429. https://doi.org/10.1111/aab.12111
Oka Y (2001) Nematicidal activity of essential oil components against the root-knot nematode Meloidogyne javanica. Nematology 3:159–164. https://doi.org/10.1163/156854101750236286
de Oliveira Barizon J (2020) Evaluating analogs and derivatives of constituents of plant essential oils for management of plant-parasitic nematodes. Dissertation, Iowa State University.
Pandey DK, Chaudhary R, Dey A, Nandy S, Banik RM, Malik T, Dwivedi P (2020) Current Knowledge of Cinnamomum Species: A Review on the Bioactive Components, Pharmacological Properties, Analytical and Biotechnological Studies. In: Singh J, Meshram V, Gupta M (eds) Bioactive natural products in drug discovery. Springer, Singapore. https://doi.org/10.1007/978-981-15-1394-7_3
Park IK, Lee HS, Lee SG, Park JD, Ahn YJ (2000) Insecticidal and fumigant activities of Cinnamomum cassia bark-derived materials against Mechoris ursulus (Coleoptera: Attelabidae). J Agric Food Chem 48:2528–2531. https://doi.org/10.1021/jf9904160
Pavela R, Benelli G (2016) Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci 21:1000–1007. https://doi.org/10.1016/j.tplants.2016.10.005
Perry RN, Moens M (2011) Introduction to plant-parasitic nematodes; modes of parasitism. In: Jones J, Gheysen G, Fenoll C (eds) Genomics and molecular genetics of plant-nematode interactions. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0434-3_1
Price JA, Coyne D, Blok VC, Jones JT (2021) Potato cyst nematodes Globodera rostochiensis and G. pallida. Mol Plant Pathol 22:495–507. https://doi.org/10.1111/mpp.13047
Priestley CM, Williamson EM, Wafford KA, Sattelle DB (2003) Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABA(A) receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br J Pharmacol 140:1363–1372. https://doi.org/10.1038/sj.bjp.0705542
Regnault-Roger C, Hamraoui A (1995) Fumigant toxic activity and reproductive inhibition induced by monoterpenes on Acanthoscelides obtectus (Say)(Coleoptera), a bruchid of kidney bean (Phaseolus vulgaris L.). J Stor Prod Res 31:291–299. https://doi.org/10.1016/0022-474X(95)00025-3
Regnault-Roger C, Vincent C, Arnason JT (2012) Essential oils in insect control: low risk products in a high-stakes world. Annu Rev Entomol 57:405–424. https://doi.org/10.1146/annurev-ento-120710-100554
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of Europe. http://eur-lex.europa.eu/.
Renčo M, Andrea Č, Sasanelli N, Toderas I (2015) Nematicidal activity of essential oils against the potato cyst nematode Globodera rostochiensis. Nat Vol Essent Oil 2:122
Russo G, Greco N (2006) Responses of Italian populations of Globodera rostochiensis and G. pallida to hatching agents. Nematol Medit 34:93–94
Sánchez-Moreno S, Ferris H (2018) (2018) Nematode ecology and soil health. In: Sikora R, Coyne D, Hallmann J, Timper P (eds) Plant parasitic nematodes in subtropical and tropical agriculture. CAB International, Wallingford, pp 62–86
Seo DJ, Park RD, Jung WJ (2014) Chitosan-cinnamon beads enhance suppressive activity against Rhizoctonia solani and Meloidogyne incognita in vitro. Microb Pathog 66:44–47. https://doi.org/10.1016/j.micpath.2013.12.007
Sharifi-Rad J, Dey A, Koirala N, Shaheen S, El Omari N, Salehi B, Cirone GT, Silva NC, Bouyahya A, Vitalini S, Varoni EM, Martorell M, Abdolshahi A, Docea AO, Iriti M, Calina D, Les F, López V, Caruntu C (2021) Cinnamomum species: bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Front Pharmacol 12:600139. https://doi.org/10.3389/fphar.2021.600139
Van Zyl S, Vivier MA, Walker MA (2012) Xiphinema index and its relationship to grapevines: a review. South Afr J Enol Vitic 33:21–32. https://doi.org/10.21548/33-1-1302
Zhang C, Fan L, Fan S, Wang J, Luo T, Tang Y, Chen Z, Yu L (2019) Cinnamomum cassia Presl: a review of its traditional uses, phytochemistry, pharmacology and toxicology. Molecules 24:3473. https://doi.org/10.3390/molecules24193473
Acknowledgments
We acknowledge technical assistance of Fabio Catalano for the experiments on nematodes.
Funding
Open access funding provided by Consiglio Nazionale Delle Ricerche (CNR) within the CRUI-CARE Agreement. This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)–MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4–D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions; neither the European Union nor the European Commission can be considered responsible for them.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I declare that the authors have no competing interests as defined by Springer or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Murray Isman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
D’Addabbo, T., Laquale, S., Veronico, P. et al. Nematicidal activity of the essential oil from Cinnamomum cassia and (E)-cinnamaldehyde against phytoparasitic nematodes. J Pest Sci (2024). https://doi.org/10.1007/s10340-024-01816-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10340-024-01816-8