Abstract
Root-knot nematodes (Meloidogyne spp.) are notorious plant-parasitic nematodes that affect agricultural crops. These obligate soil-dwelling parasites typically maneuver the host plant physiology by forming specialized feeding cells resulting in heavy yield losses. Scant management tools are available to effectively combat this pest. In an exploratory attempt of identifying new fungal biocontrol agent(s) for M. incognita from India, a Paecilomyces tenuis isolate from rhizosphere soil was found to incur > 90% mortality of the infective second-stage juveniles (J2s) at 24 h post-exposure to the fungal filtrate with about 87% parasitization. The fungal filtrate also significantly reduced the egg hatching and host-root penetration of M. incognita under in vitro and in vivo conditions revealing its effectiveness in curbing nematode pathogenicity with positive effects on plant growth. Chromatographic analyses revealed the presence of Huperzine A (433.56 mg L−1) in the P. tenuis isolate. Besides, the isolate possessed acetylcholinesterase inhibition attribute with an IC50 of 2.85 ± 0.12 mg mL−1 of the fungal filtrate. Further, GC-MS analysis revealed the production of other nematicidal compounds by the fungus including acetic acid. To conceptualize the mode of nematicidal action, RNA-Seq was done post-treatment of the M. incognita J2s and model worm Caenorhabditis elegans with fungal filtrate and pure Huperzine A. The transcriptomic profile unraveled the molecular intricacies underlying the nematicidal action affecting several biological pathways and developmental checkpoints of the nematode. Thus, the P. tenuis isolate offers significant potential to be used as a biocontrol agent against M. incognita along with its commercial use for Huperzine A production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Root-knot nematodes (RKNs) belonging to the genus Meloidogyne are considered to be the most destructive and widespread worm pathogens infecting a wide range of agricultural and horticultural crops under open-field and protected conditions (Jones et al. 2013; Phani et al. 2021). Being typical soil-dwelling obligate root parasites, they sustainably reduce the crop yield by producing some dramatic symptoms, such as root galls, stunting, yellowing and wilting of plants, and also predispose the roots to infection by other soil microorganisms (Mitkowski and Abawi 2003). In spite of the extensive damage caused by RKN species, any effective management option(s) is still scant as compared to control measures of other pests and pathogens of agricultural importance. To date, only few acclaimed target-specific nematicides are available, such as fluensulfone, fluazaindolizine, tioxazafen, fluopyram and ethanedinitrile (Kearn et al. 2014; Slomczynska et al. 2014; Faske and Hurd 2015; Lahm et al. 2017; Douda et al. 2021), but their use on all types of crops is yet to be recommended due to the label claim restrictions. Furthermore, the old-school fumigant and non-fumigant nematicides have largely been withdrawn in view of their harsh impact on non-targets and environments. Under the prevalence of such therapeutic bottleneck, there has been a profound demand for novel and target-specific nematode management option(s) for sustainable agri-horticultural production.
Under the circumstances of existing nematode management inadequacies, biological control using microbes and/or their secondary metabolites can serve as a reliable, safe, effective and practicable alternative to combat the plant-parasitic nematodes (PPNs) including the RKNs. Additionally, the richness of organisms present in nature provides a continuous opportunity for their exploitation. Numerous fungal antagonists have been identified for PPN management, and based on their action, they are divided into four groups- (i) endophytes that can trap and kill nematodes at different life stages, (ii) antagonists against rhizosphere microorganisms for food resources, (iii) fungi that secrete secondary metabolites having nematicidal activity, and (iv) fungi having repellent action due to colonization on roots resulting in reduced nematode juvenile attraction (Li et al. 2015). These unique attributes have drawn considerable attention of the researchers to study and use them as biocontrol agents against the PPNs under open-field conditions. In this regard, some of the most commonly used fungi for RKN management include Actylellina spp., Arthrobotrys spp., Aspergillus spp., Catenaria spp., Dactylellina spp., Hirsutella spp., Pochonia spp., Paecilomyces spp. and Trichoderma spp. (Abd-Elgawad and Askary 2018; Forghani and Hajihassani 2020).
The species belonging to the genus Paecilomyces (Eurotiales: Trichocomaceae) play important role as endophytes in several plants by producing phytohormones that promote and support plant growth and development (Baron et al. 2020). Several Paecilomyces species are also regarded as potential biocontrol agents for the PPNs and insects of different Orders (Moreno-Gavíra et al. 2020; Li et al. 2020). Notably, Perveen and Shahzad (2013) and Dahlin et al. (2019) showed P. variotii and P. lilacinus (= Purpureocillium lilacinum) to possess nematode egg hatching inhibition properties. Sornakili et al. (2020) reported Paecilomyces tenuis to produce enzymes such as cellulases, lipases and xylases that inhibit the hyphal growth of phytopathogenic fungi such as Macrophomina, Pythium, Fusarium, Colletotrichum and Rhizoctonia. Undoubtedly, the genus Paecilomyces is an excellent natural repository of novel compounds having ability to produce many bioactive substances (Li et al. 2020). Besides, the fungal genus has also received attention in medical and pharmaceutical industries for its ability to produce Huperzine A (Hup A). Su and Yang (2015), for the first time, showed the presence of Hup A in P. tenuis YS-13 by chromatography and mass spectrometry. Hup A is a sesquiterpene alkaloid that inhibits the neurotransmitter acetylcholinesterase (AChE), and has been largely studied for its possible therapeutic application against neurodegenerative diseases such as Alzheimer’s and myasthenia gravis due to its unique pharmacological activities (Wang and Tang 2005; Yang et al. 2013). Apart from Paecilomyces, species of Acremonium, Aspergillus, Blastomyces, Botrytis, Ceriporia, Colletotrichum, Cladosporium, Hypoxylon, Leptosphaeria, Mycoleptodiscus, Penicillium, Phlegmariurus, Shiraia and Trichoderma are also known to produce Hup A (Zhu et al. 2010; Shu et al. 2014; Dong et al. 2014; Wang et al. 2015; Su and Yang 2015; Zhang et al. 2015; Le et al. 2020; Wen-Xia et al. 2020). Furthermore, Hup A extracted from New Zealand Clubmoss (Lycopodium varium) was demonstrated to have both insecticidal and antifeedant effects against carpet beetle (Anthrenocerus australis), sheep blowfly (Lucilia cuprina) and webbing clothes moth (Tineola bisselliella) (Ainge et al. 2002). Hence, Hup A is no doubt a promising molecule to be used in agriculture for targeted pest control purpose. On account of this information about fungi producing Hup A, we specifically designed this study in search of a native Paecilomyces species that can be effectively used for managing the RKN species M. incognita and further exploring its ability to produce Hup A as a soil-inhabiting fungus. Hence, we hypothesized that isolation and characterization of native soil fungal strains would be beneficial to screen for their potential under the prevalent agroecosystem. Additionally, understanding the fungal ecology, biology and mode of action could contribute its successful deployment under field conditions.
In the recent years, advances in omics technologies and next-generation sequencing (NGS) have opened up new horizons to comprehend the molecular mechanisms of how the biocontrol agents act on their targets which could eventually help in improving their potency (Morton et al. 2004; Davies et al. 2005). For example, transcriptomic analyses deciphered the divergent lifestyle features of the nematode endoparasitic fungus Hirsutella minnesotensis (Lai et al. 2014). Likewise, comparative transcriptomics revealed different strategies of parasitism of Trichoderma sp. (Atanasova et al. 2013). Besides, Pandit et al. (2017) demonstrated the core reason for predatory activity of Arthrobotrys conoides based on differentially expressed gene (DEG) profiling in the presence and absence of nematode extracts. All these studies suggest that modern omics approaches can serve as excellent platforms to explore the mechanisms underlying such soil fungi stasis. Here, aiming to isolate, identify and evaluate novel and potential Paecilomyces sp. capable of parasitizing M. incognita, (1) we collected rhizosphere soils of different crops from various geographical regimes of India, (2) the soil samples were screened for presence of the desired fungus, and the effects of this fungus against RKN species M. incognita were evaluated under in vitro and in vivo conditions, (3) the fungal isolate was then studied for Hup A production with quantification of the metabolite titer, and (4) the genome-wide transcriptional changes induced by soaking the worms in the Hup A containing fungus culture filtrate were investigated using M. incognita and the model nematode Caenorhabditis elegans.
Materials and methods
Nematode population
An Indian isolate of M. incognita was maintained and multiplied on tomato plant (Solanum lycopersicum cv. Pusa Ruby) in a glasshouse at ICAR-Indian Agricultural Research Institute, New Delhi, India. Nematode-infested roots were washed, eggmasses were hand-picked and second-stage juveniles (J2s) were hatched via ‘modified Baermann’s funnel assembly’ (Whitehead and Hemming 1965). Freshly hatched J2s were used for all the experiments.
Caenorhabditis elegans (strain N95) was multiplied on nematode growth medium (NGM) using Escherichia coli strain OP50 as food source. NGM plates were seeded with overnight grown E. coli OP50 culture and incubated for 16 h. Gravid worms were randomly selected from the stock population and placed on NGM plates. Plates were incubated at 25 °C for 3 days for nematode multiplication (Flatt and Heyland 2011). Freshly obtained L3s were used in all the experiments.
Isolation of fungi and their morphological and molecular characterization
Rhizosphere soil samples were collected from different crops from 10 different states of India (Supplementary Doc 1). Fungal isolates were obtained following soil sprinkling method (Bailey and Gray 1989) and purified using potato dextrose agar (PDA) medium. Among the various fungi isolated, we specifically isolated Paecilomyces species based on morphological characterization. Various microscopic features were recorded under Olympus BX50 light microscope and Tescan VEGA3 Make scanning electron microscope to identify the isolate. Fixation and sample preparation for SEM studies were done as described by Belder et al. (1993). Further, growth response of the selected fungal isolate was evaluated using three different media, viz. PDA, corn meal agar (CMA) and Czapek's agar (CZA).
Thereafter, the fungal isolate was characterized by amplifying and sequencing the ribosomal internal transcribed spacer (ITS rDNA) for species confirmation. For this, genomic DNA (gDNA) was extracted from 1-week-old mycelial mat using CTAB (N-cetyl-N,N,N-trimethyl ammonium bromide) method as previously described (Wang et al. 2015). ITS rDNA sequence was amplified using ITS-1 forward (5′TCCGTAGGTGAACCTGCGG3′) and ITS-4 reverse (5′TCCTCCGCTTATTGATATGC3′) primer pairs. PCR was carried out as outlined in Glass and Donaldson (1995) and sequenced (Eurofins Scientific India Pvt. Ltd., Bengaluru, India). The sequence was subjected to BLAST (blast.ncbi.nlm.nih.gov/) search against NCBI database to confirm its identity and identify the homologs. The sequence was submitted to NCBI GenBank. Additionally, fungus-specific ITS sequences were retrieved from GenBank database, and phylogenetic analysis was carried out using MEGA 6. Sequence of Metarhizium marquandii (accession no. MH854923) was used as outgroup. The identified fungal isolate was submitted to Indian Type Culture Collection (ITCC), ICAR-Indian Agricultural Research Institute, New Delhi, India, an affiliated center to World Federation for Culture Collections (WFCC) registered with World Data Centre for Microorganisms (WDCM, registration number 430).
In vitro evaluation of fungal isolate on M. incognita
The selected fungal isolate was initially evaluated against M. incognita J2s under in vitro condition. For this, cell-free fungal filtrate (FF) was prepared by growing the fungus on PDA medium for 6 days at 25 °C, then a 5 mm diameter disk was cut, transferred to 100 ml of potato dextrose broth (PDB) in a 250 ml flask and incubated at 25 °C for 10 days with continuous rotation at 180 rpm in dark. Later, 1 ml of FF was poured into a microcentrifuge tube, and 100 surface-sterilized J2s (Du et al. 2020) were added into it. The J2s were then incubated on a rotator (15 rpm) at 25 °C for different time intervals such as 6, 12, 24 and 48 h. The incubated J2s were harvested at different time intervals, washed thrice with sterile spring water (SSW), and re-incubated in SSW for 24 h for any possible revival. Thereafter, the number of dead J2s was counted and percentage mortality was calculated as [(Number of dead nematodes/Total number of nematodes) × 100]. The ‘dead’ J2s were discriminated by touching the posterior and anterior body portion with a feather pick, where they did not show any sign of movement or mobility. Five replicates were maintained for each treatment, and J2s soaked in PDB served as negative control. Simultaneously, worms soaked in 100 ppm of Hup A (Sigma-Aldrich, USA) were used as positive control.
Additionally, to evaluate the hatching inhibition activity of the tested fungal isolate, 1 ml of FF was poured into a 24 well plate, followed by dispensing of 100 M. incognita eggs, and incubated at 25 °C for 72 h. For this, the eggmasses were first subjected to NaOCl treatment to dissolve the gelatinous matrix and viable eggs collected for experimental purpose (Ehwaeti et al. 1998). The eggs were thoroughly washed with sterile double-distilled water to get rid of any traces of NaOCl. After 72 h, the hatched J2s were counted under a stereoscopic binocular microscope (Carl Zeiss, Germany), and percentage of hatching inhibition was calculated as [{(Total number of eggs − Number of hatched eggs)/Total number of eggs} × 100]. Five replicates were maintained for each treatment, and eggs soaked in PDB and 100 ppm Hup A served as controls.
Further, to assess the effect of FF on behavior of M. incognita J2s (if any), we observed the movement and root penetration ability of the FF-soaked J2s in susceptible tomato roots on a soil less Pluronic PF-127 medium (Wang et al. 2009). For this, initially the soaking time of J2s in FF was optimized to be 12 h. Then, J2s were soaked in FF for 12 h followed by washing in SSW thrice. Meanwhile, a 4-day-old tomato seedling was placed on Pluronic gel in a Petri plate and inoculated with 20 FF-treated J2s at a distance of 1.5 cm from the root tip (Phani et al. 2017). Worms soaked in PDB and 100 ppm Hup A served as controls. Ten replications were maintained for each treatment, and the number of J2s penetrated at 24 h post-inoculation was observed by staining the roots by acid fuchsin method (Byrd et al. 1983). Besides, pattern of the worm tracks was also recorded to observe any aberration in the mobility of worms under a ZEISS SteREO Discovery V20 microscope and documented using Canon PowerShot G5 imaging system.
The fungal isolate was also evaluated for its ability to parasitize M. incognita J2s. For this, the fungus was grown on PDA medium at 25 °C for 10 days, and spores with mycelia were scraped from the culture using a spatula and suspended in a mixture of double-distilled water and Tween 80 (0.05% v/v). The suspension was then filtered through a muslin cloth to remove any mycelial fragment. The number of conidia in the suspension was counted using hemocytometer and adjusted to a final concentration of 1 × 106 spores mL−1. Thereafter, 3 mL of water agar (1.5% v/v with 1% ampicillin 100 mg mL−1) and 100 µl of spore suspension were added to 4 cm diameter Petri plates and incubated for 18 h. Following incubation, 100 surface-sterilized M. incognita J2s were added to the Petri plate and again incubated at 25 ± 2 °C in dark for 4 days (Contina et al. 2017; Silva et al. 2017). The number of J2s parasitized by the fungus was visualized and counted using a ZEISS SteREO Discovery V20 microscope, and percent parasitization was calculated as [(Number of parasitized nematodes/Total number of nematodes) × 100] (Siddiqui and Shaukat 2004). The parasitized J2s were also observed under a scanning electron microscope following the methodologies described earlier (Belder et al. 1993), and photomicrographs were documented. The bioassay was performed in five replicates, and mixture of double-distilled water and Tween 80 served as a control.
In vivo evaluation of fungal isolate on M. incognita
One-month-old tomato seedlings (S. lycopersicum cv. Pusa Ruby) were transplanted into 4-inch-diameter pots filled with 400 g of autoclaved mixture of soil, sand and FYM (50:25:25) and kept under greenhouse condition. Simultaneously, fungal suspensions were prepared by culturing the isolate on PDB medium as mentioned above. The number of spores was adjusted to obtain 1 × 106 spores mL−1. Three different treatments were used in terms of time of fungus inoculation to the plants - (i) application of spore suspension at time of transplantation followed by nematode inoculation after 1 week, (ii) nematode inoculation immediately after transplantation followed by application of spore suspension after 1 week and (iii) simultaneous application of nematode and fungal spores immediately after transplantation. All nematode inoculations were done with 800 J2s per plant so as to obtain 2 nematodes g−1 of soil. Three different controls were used: (i) plants inoculated only with nematodes, (ii) plants inoculated only with fungal spores and (iii) healthy plants. Five replicates were maintained for each treatment arranged in randomized complete block design (RCBD), and observations were recorded for all the replicates individually. At 40 days post-inoculation (dpi), plants were carefully uprooted and roots were washed to remove any adhering soil. Plant growth parameters were recorded in terms of root and shoot lengths and their dry weights. Nematode disease burden per plant was scored in terms of total number of galls, number of endoparasites, females, eggmasses and eggs per eggmass. Nematode multiplication factor (MF) was derived as described previously (Hada et al. 2020).
Chromatographic analyses
Selected fungus was grown in 100 ml of PDB for 15 days and kept in − 20 °C for 16 h followed by lyophilization. The lyophilized product was used to extract Hup A from the fungal mycelia. For this, 1 g of lyophilized product was extracted with 0.5% hydrochloric acid overnight, followed by ultrasonication in a water bath at 40 °C for 1 h. Subsequently, extracts were filtered and concentrated with ammonia solution. After 1 h, the aqueous phase was extracted thrice with chloroform and then evaporated under reduced pressure. The dried residue was dissolved in 1 ml methanol and passed through a 0.45 mm syringe filter. The filtered extract was analyzed using thin layer chromatography (TLC) and high-performance liquid chromatography (HPLC) for detection of Hup A in the fungal isolate (Wu and Gu 2006).
TLC was conducted on a TLC 60 F254 silica plate (20 cm × 20 cm) (Merck, Germany). Precise spots of the sample along with standard Hup A were applied on the plates and developed using chloroform–methanol (98:2 v/v) as mobile phase. After development, plates were dried in a stream of cold air for 10 min, and chromatograms were viewed under UV light at a wavelength of 254 nm in a Camag UV viewing cabinet. The plate was visualized for the detection of compound of interest (Hup A) by adding iodine solution, and Rf value was estimated for Hup A determination. It was them exhaustively eluted using methanol and was further confirmed and quantified by HPLC assay (Su and Yang 2015; Mishra et al. 2018).
Reverse-phase HPLC was performed using a Shim-pack C18 reverse phase-column (5 µm, 4.6 × 250 mm) (Shimadzu, Japan). The mobile phase used for detection of Hup A was optimized to be methanol and water containing 0.1% formic acid in a ratio of 70:30. For analysis purpose, 10 µl of methanolic extract was injected, a uniform flow rate of 0.5 mL min−1 was maintained over a run time of 40 min, and the compound was detected at 310 nm. Further, quantification was done based on the standard curve prepared using pure Hup A (Sigma–Aldrich, Germany) over a concentration range of 0–200 ppm at which the peak area showed a linear relationship with the concentration (R2 = 0.997) (Zhang et al. 2011).
AChE inhibition assay
AChE (Sigma-Aldrich, Germany) was dissolved in phosphate buffer (pH 8.0) to prepare 20 U mL−1 stock solution and adjusted to 0.4 U mL−1 for assay purpose. Acetylthiocholine iodide (ATCI; Sigma–Aldrich, Germany) 6.2 mM solution and 5, 50-dithiobis 2-nitrobenzoic acid (DTNB; Sigma–Aldrich, Germany) 7.6 mM solution were prepared in the same phosphate buffer. All solutions were prepared afresh before use (Zelík et al. 2009). AChE inhibitory activity of the FF was conducted using Ellman’s method (Ellman et al. 1961) in 96 well microplates. For this, 125 µL of 0.1 M phosphate buffer (pH 8.0), 50 µL of 0.4 U mL−1 AChE, 25 µL of 7.6 mM DTNB and 20 µL of FF were mixed in a microcentrifuge tube, incubated for 30 min at 30 °C and then added with 30 µL of 6.2 mM ATCI. The color development was measured using an absorbance microplate reader (Tecan Sunrise absorbance reader, Switzerland) at 412 nm at every 10 min interval for 30 min. AChE activity was standardized with control measurements, and all analyses were performed in triplicate. Percentage inhibition (I%) was calculated as: [{(Absorbance of control − Absorbance of sample)/Absorbance of control} × 100]. The IC50 values were obtained from logistic regression analysis of three independent replicates.
Gas chromatography–mass spectrometry (GC–MS) analysis
GC-MS analysis was carried out in a 7890A GC (Agilent Technologies, USA) using a HP-5MS column (30 m × 0.25 mm; 0.25 µm i.d., Agilent Co., USA) which was directly connected to a triple axis mass spectrometer (Agilent Technologies, USA). For GC-MS analyses, aqueous phase of the lyophilized fungal culture filtrate was extracted overnight with 0.5% hydrochloric acid, partitioned with ethyl acetate thrice, then evaporated under reduced pressure below 35 °C and re-dissolved in GC-MS grade ethyl acetate (2 mL) for analysis. Helium (high purity > 99%, New Delhi, India) was used with the head pressure of 10 psi and flow of 0.75 mL min−1. GC-MS condition was maintained with the oven temperature ramping initiated at 40 °C and increased 4 °C min−1 to reach 120 °C. Then, the temperature increased again at the rate of 5 °C min−1 up to 200 °C with the hold time of 5 min. Finally, temperature was raised up to 300 °C with increment of 10 °C min−1. Other data acquisition conditions were standardized with the ion source temperature 250 °C, electron ionization 60 eV under full-scan mode (50–550 AMU) with the transfer line temperature 280 °C and E.M voltage 1222. Compounds were identified by matching their mass spectra with the library, retention indices and mass fragmentation pattern using NIST (National Institute of Standards and Technologies) Mass Spectra Library.
Extraction of total RNA, cDNA synthesis, library preparation and RNA sequencing
About 20,000 M. incognita J2s and C. elegans L3s were soaked in FF and Hup A (100 ppm) separately for 8 h in dark on a slowly moving rotator (15 rpm) at room temperature (~ 28 °C). To protect the integrity of RNA extracted from the soaked worms, concentration and exposure time was combined optimally so that no worm dies, but nematode behavior gets affected. The worms were microscopically assessed for any behavioral changes, immobility and mortality. Three biological replicates were maintained for each treatment and worms soaked in SDDW served as control. Post-soaking, total RNA was extracted from the M. incognita J2s and C. elegans L3s using NucleoSpin RNA kit (Macherey–Nagel, Germany) following manufacturer’s instructions and previously mentioned protocol (Phani et al. 2018). The quality of RNA was analyzed by electrophoresis and a NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific, USA). Thereafter, quality assessment, mRNA purification and cDNA synthesis were performed as described previously (Phani et al. 2018). In total, 16 libraries were prepared: 9 for C. elegans (3 replicates of FF treatment, 3 replicates of Hup A treatment, 3 replicates of water control) and 7 for M. incognita (2 replicates of FF treatment, 3 replicates of Hup A treatment, 2 replicates of water control). However, the analyses were performed with 15 libraries excluding one C. elegans water control having lower correlation value. The cDNA libraries were then sequenced on the Illumina MiSeq platform by outsourcing to Bionivid Technologies Pvt. Ltd., Bengaluru, India.
Transcriptome analyses and identification of differentially expressed transcripts
Quality filtering of raw reads was done using Fastp v. 0.20.0 (Chen et al. 2018) with > 20 Phred scores and > 95% high-quality (HQ) per sample. HISAT 2.2.1 (release 7/24/2020) (Kim et al. 2019) was used for aligning the raw reads against the reference genome. The transcripts were assembled, and the expression level was estimated using FPKM and TPM by StringTie v2.2.0 (Pertea et al. 2016). The DESeq 2 (Love et al. 2014) package was used to find the differentially expressed transcripts between the treatments (FF and Hup A) and control (water soaked worms) in R software v. 3.2.5. The threshold of DEGs was set as |log2fold|≥ 2.0 and P-value ≤ 0.05. The unique and overlapping transcripts were analyzed by a Venn diagram for both the treated nematode species; and gene ontology and pathway mapping were performed using BLAST2GO (Conesa and Götz 2008) and KAAS (Moriya et al. 2007).
Validation of RNA-Seq gene expression data by quantitative real-time PCR (qRT PCR)
For confirmation of the expression pattern of transcripts as obtained in RNA-Seq experiment, 22 differentially expressed transcripts were selected for qRT PCR validation for both the nematodes M. incognita and C. elegans, respectively. The selected transcripts were randomly selected and contained few of the top up- and down-regulated transcripts common to both FF and HupA treatments, whereas some were involved in diverse functions of growth, development and nervous system in the nematode. The selected sequences were retrieved from the WormBase database (v. WS260) and were BLAST searched in WormBase Parasite (http://parasite.wormbase.org/Tools/Blast) and INRA database (http://www6.inra.fr/meloidogyne_incognita/Genomic-resources2/Blast) to fetch the nucleotide sequences. The sequences were then checked for the presence of corresponding conserved domains, and specific primers were designed. The transcripts included 11 down-regulated and 11 up-regulated transcripts for both the nematode species. qRT PCR was carried out in a Realplex2 thermal cycler (Eppendorf, Germany) using SYBR Green Supermix Kit (Eurogentec, Belgium). The 18S rDNA of M. incognita (accession no. HE667742) and C. elegans (accession no. X03680) were used as internal controls for gene expression normalization. Three biological and three technical replicates were maintained, fold change in gene expression was calculated using 2−ΔΔCT (Livak and Schmittgen 2001), and results were expressed as log2-transformed fold change values. One tailed t-test was performed to calculate the mean of Ct values, and Duncan’s multiple-comparison test at P < 0.05 was performed to determine the statistical significance. The primer details are provided in Supplementary Doc 2.
Statistical Analyses
All experiments were conducted in completely randomized design (CRD) and randomized complete block design (RCBD), and data were subjected to analysis of variance (ANOVA). Duncan’s multiple range test (DMRT) at 1–5% level of significance was done using SPSS software package v. 160 (IBM Corp., USA).
Results
Morphological and molecular identification of fungal isolate
Here, we could isolate 42 rhizosphere fungal isolates from 10 states of India. Upon screening for Paecilomyces sp. in particular, it was found to be present in neem, eggplant, pearl millet, bamboo and eggplant rhizosphere soils collected from Assam, Haryana, Nagaland, Manipur and Rajasthan states, respectively. Morphologically, the fungal colonies were round, powdery, white-colored with bulged centre and densely floccose surface, on PDA. Microscopically, vegetative hyphae were smooth-walled, septate, swollen at the basal portion. Conidiophores were 35–82 μm in length, 2.5–3.5 μm wide, arising from aerial hyphae and had verticillate branches terminating with 2–5 phialides. Conidia were produced from the phialide tip in chains, sometimes remained scattered, fusiform, one-celled, smooth-walled with dimension of 1.9–2.3 μm × 2.5–3.6 μm (Fig. 1). The characteristics of the isolate matched with the description of Paecilomyces tenuis (Han et al. 2007; Adhikari et al. 2016). Further, the isolate, cultured on PDA, CMA and CZA, showed clear variations in the growth rate at 25 °C. After 7 days of incubation, the isolate exhibited faster growth on PDA with about 40 mm diameter, while on CMA and CZA it was 26 and 30 mm, respectively. Based on the preliminary laboratory experiment with the P. tenuis isolates obtained from five states, we found the isolate from Rajasthan was best performing among all (data not shown). Hence, we carried out all the downstream experiments with this isolate only. After confirming the specific identity based on morphological and morphometric studies, we designated it as isolate Pt_RK while being deposited at ITCC (accession no. ITCC 8971).
Fungal morphology of Paecilomyces tenuis Pt_RK and growth characteristics on culture plates. Scanning electron and light compound photomicrographs show conidia produced at phialide tip in chains (a), and fusiform, one-celled, smooth-walled conidia (b, c) produced by the fungus. Fungal growth characteristics were observed on PDA (d), CMA (e) and CZA (f) media 4 days post-inoculation from front surface of Petri plates. [scale bars: (a, c) 10 µm, (b) 5 µm]
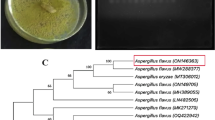
In order to confirm the species identity, P. tenuis Pt_RK was characterized by amplification and sequencing of the ITS rDNA region. A 580 bp ITS rDNA amplified sequence showing similarity to other P. tenuis strains was submitted to NCBI GenBank under the accession no. MW301362. The ML phylogenetic analysis (Fig. 2) showed that P. tenuis Pt_RK was closest to P. tenuis strain GZUIFR-C43-1 (accession no. EU004812; from China) and isolate P-I (accession no. MT337558; from India). Multiple Sequence Alignment with Clustal Omega revealed sequence similarity of P. tenuis PT_RK with GZUIFR-C43-1 and P-I isolates to be 98.22 and 98.33, respectively.
Evolutionary phylogenetic analysis of Paecilomyces tenuis Pt_RK. The evolutionary history was inferred by maximum likelihood method based on Tamura 3-parameter model. Bootstrap consensus was inferred from 1000 replicates, and branches corresponding to less than 60% replicates were collapsed. A discrete gamma distribution was used to model evolutionary rate differences among sites [5 categories (+ G, parameter = 1.1989)]. The analysis involved 18 sequences, all gaps and missing data positions were eliminated, and a total of 353 positions remained in the final dataset. Accession numbers of candidate genes are provided within parentheses. The corresponding sequence from Metarhizium marquandii was used as outgroup (in normal font), and entry for present isolate kept in bold font. Evolutionary analyses were conducted in MEGA 6 software
In vitro evaluation of P. tenuis Pt_RK against M. incognita
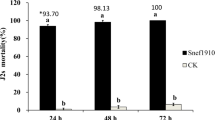
The P. tenuis Pt_RK fungal filtrate (FF) was evaluated against M. incognita J2s for its effect on nematode mortality and egg hatching at different time intervals. The FF was effective and resulted in significant J2s mortality of 0.25 ± 0.5, 11.25 ± 1.3, 92.4 ± 3.1 and 100 ± 0.1% at 6, 12, 24 and 48 h, respectively, compared with PDB control (Table 1). In case of 100 ppm Hup A treatment, J2 mortality was about 34.34% even at 12 h and 100% at 24 h itself. Further, P. tenuis Pt_RK FF significantly inhibited the egg hatching by 75.5 ± 5.1% as compared to PDB control (15.75 ± 7.8) at 72 h post-exposure, while 100 ppm Hup A gave a higher inhibition of 93.6 ± 0.5%. All worms displayed normal behavior in the PDB control without any behavioral anomalies.
Effect of soaking of M. incognita J2s in FF and 100 ppm of Hup A for 6 h was evaluated on nematode movement and penetration in tomato root in Pluronic gel. In general, soaking in P. tenuis Pt_RK FF reduced penetration of the J2s into tomato roots, compared with PDB control. The number of J2s penetrated at 24 h in FF and Hup A treatments was 2.3 ± 0.5 and 0.4 ± 0.2, respectively, which was significantly less than PDB control (17.6 ± 1.5) (Table 1, Fig. 3a,b). The J2s in PDB control showed normal sinusoidal tracks on Pluronic gel while migrating toward tomato roots, while FF- and Hup A-treated J2s exhibited random circled tracks (Fig. 3c-e). Further, the tested isolate was also found to directly parasitize about 87% of the M. incognita J2s (Table 1, Fig. 3f).
Effect of Paecilomyces tenuis Pt_RK fungal filtrate and Huperzine A treatment on nematode penetration, migration and parasitization. The treatment with fungal filtrate reduced the root penetration of M. incognita J2s (a), as compared to normal penetration for non-treated J2s (b), as observed after acid fuchsin staining. Sinusoidal locomotory behavior (c) was observed by non-treated worms, whereas treatment with fungal filtrate (d) and Huperzine A (e) caused locomotory defects in the J2s showing circular tracks. The fungus also directly parasitized the J2s (f). [arrows point stained J2s (a, b), locomotory tracks (c–e), nematode J2 (f)]
In vivo evaluation of P. tenuis Pt_RK against M. incognita
Established tomato plants grown in 4-inch-diameter pots kept in greenhouse were used to evaluate in vivo biocontrol efficiency of P. tenuis Pt_RK against M. incognita. The results revealed that all the three treatments caused a significant reduction in nematode parasitism as compared to control (Table 2). The first treatment involving application of fungal suspension at the time of transplantation followed by nematode inoculation after 1 week showed the highest reduction in the nematode infection with less number of galls (43.33 ± 6.1) as compared to control plants (175.2 ± 9.14). As a result, 75% reduction in nematode infection occurred due to the fungal treatment. This eventually resulted in significant lowering in total endoparasites (17.6 ± 4.6) as compared to the control (63 ± 2.7). Corroborating this, the average number of females was found to be less (36.3 ± 5) in the treated plants, whereas in control plants it was 131.3 ± 8.2. Likewise, the average number of eggmasses and eggs per eggmass was 33.4 ± 5.3 and 216.2 ± 4.3, respectively, in the treated plants as compared to the control plants (112.3 ± 7.1 and 1029 ± 11.9, respectively). The derived nematode MF was 9.02 in the treated samples, while control plants showed 144.4 that led to 93.75% reduction due to fungus treatment. Similarly, application of fungus simultaneously along with nematode and 1 week prior to nematode inoculation also showed significant reduction in the infection parameters that ultimately reduced the nematode MF by 75.85% and 63.61%, respectively, compared with the control plants (Table 2).
Further, the plants treated with P. tenuis Pt_RK before nematode inoculations were found to be healthier in terms of the growth parameters measured, as compared to only nematode infected plants and healthy control (Table 2). Plants treated with P. tenuis Pt_RK showed an average root and shoot length of 22.67 ± 3.8 and 25.67 ± 0.6 cm, while only nematode-inoculated plants exhibited 8.43 ± 0.7 and 13.3 ± 0.5 cm, respectively. Similarly, average root and shoot weight was 2.3 ± 0.1 and 3.3 ± 0.5 g, respectively, in the fungus-treated plants, while only nematode-inoculated plants showed 1.0 ± 0.5 and 2.64 ± 0.3 g, respectively. The percentage growth reduction in the nematode-treated plants as compared to healthy plants in terms of root length, shoot length, root weight and shoot weight was 73.73%, 58.82%, 66.22% and 48.84%, respectively (Table 2).
Chromatographic analyses of P. tenuis Pt_RK for Hup A production
The P. tenuis Pt_RK was scanned by TLC and HPLC for the presence of Hup A. Results displayed prominent band with Rf value of 0.72 (Supplementary Doc 3), same as standard Hup A used for comparison. The HPLC analysis for confirmation and quantification of Hup A in P. tenuis Pt_RK generated a UV absorption spectrum similar to standard Hup A with distinctive peak at 310 nm. The retention time of Hup A in sample was established as 5.8 min, which coincided with that of standard Hup A (= 5.7 min). Hup A produced by our fungal isolate quantified by ultra-fast liquid chromatography (UFLC) was found to be 433.56 mg L−1 (Supplementary Doc 3).
AChE inhibition by P. tenuis Pt_RK fungal filtrate
AChE inhibition by the HupA standard and the crude extracts of P. tenuis Pt_RK are summarized in Supplementary Doc 4. The IC50 for the Hup A standard was 194.89 ± 12.30 ng mL−1, which was less than the IC50 of our P. tenuis Pt_RK (2.85 ± 0.12 mg mL−1).
GC–MS analysis of P. tenuis Pt_RK fungal filtrate
Total ion chromatogram (TIC) of GC–MS analysis of the fungal filtrate displayed identification of various metabolites (Supplementary Doc 5). A total 16 secondary metabolites were identified in GC–MS and listed based on their order of elution from the HP-5MS column (Table 3). Among the identified metabolites, 1-nonadecene (23.17%) was most abundant followed by octadecane (7.82%) and 1,3-butanediol (3.35%). Other notable predominant metabolites were acetic acid (2.98%), 9-octadecenoic acid methyl ester (2.95%), phenyl ethyl alcohol (2.72%), heptacosane (2.09%), tetracosane (1.56%), hexadecanoic acid methyl ester (1.21%), 15-heptadecenal (1.01%) etc.
RNA-seq data statistics
The paired-end reads of 150 base pair size were generated via Illumina MiSeq platform for the FF- and Hup A-treated and untreated samples of C. elegans and M. incognita. The raw reads generated for C. elegans and M. incognita were approximately 26–35 million and 16–36 million per sample, respectively (Supplementary Doc 6, 7). Quality filtering of C. elegans and M. incognita raw reads yielded approximately 22–32 and 14–33 million HQ reads, respectively. The percentage of HQ reads generated after quality filtering was > 90%. Further, the HQ reads were mapped onto respective reference genomes using splice-aware read aligner. Approximately 95–98% C. elegans reads were aligned to the Ensemble-Wbcel235 (https://metazoa.ensembl.org/Caenorhabditis_elegans/Info/Index) reference genome, and 56–78% M. incognita reads got aligned to the reference genome Meloidogyne_incognita_V3 PRJEB8714 (https://parasite.wormbase.org/Meloidogyne_incognita_prjeb8714/Info/Index/) (Supplementary Doc 8,9). Further, PCA plot suggested strong correlation between biological replicates within each treatment (Supplementary Doc 10). The clustering of various RNA-Seq replicates for both the nematodes along with differentially expressed transcripts is represented in Fig. 4. A high correlation coefficient value of > 0.9 was obtained among the biological replicates of FF-treated (for 8 h) and Hup A-treated (8 h at 100 ppm) samples as well as the controls for both the nematodes.
Heat maps showing clustering of differentially expressed transcripts in C. elegans and M. incognita. The heat maps from C. elegans representing fungal filtrate treatment and control (a), Huperzine A treatment and control (b) and from M. incognita representing fungal filtrate treatment and control (c), Huperzine A treatment and control (d) were generated by using Log2 fold change values derived from transcriptome data using DESeq R package. The heat maps were produced by hierarchical clustering using R package ggplot2. Vertical axes represent the fold change as indicated, and each row refers to the same transcript. [FF: treatment with fungal filtrate, Hup A: treatment with Huperzine A, Control: treatment with water]
Annotation and differential gene expression profiling of FF- and Hup A-treated C. elegans and M. incognita
In the FF-treated C. elegans samples, 3,643 transcripts were differentially expressed as compared to control, out of which 3,211 were down-regulated and 429 were up-regulated (Fig. 5a–c). The top three up-regulated transcripts were C23G10.4a.2 (24.98 folds, hypoxia domain), F01F1.8b.2 (24.17 folds, glucose-dehydrogenase) and T24B8.3b.2 (23.82 folds, glucosyltransferase). The top three down-regulated transcripts were T2TE9.1D.26 (− 30 folds, mitochondrial carrier), F46H5.3A.1 (− 28.91 folds, catalytic binding) and T04F3.1b (− 27.70, amino-transferase), respectively. Likewise, in Hup A-treated C. elegans samples, 4,901 transcripts were differentially expressed as compared to control, out of which 3,903 were down-regulated and 995 were up-regulated (Fig. 5a–c). The top three up-regulated transcripts—C55A6.2b (26.01 folds), C23g10.4a.2 (25.05 folds) and F01F1.8B.2 (24.81 folds), were annotated as tubulin–tyrosine ligase, cylosome and chaperons, respectively, and the top three down-regulated transcripts were M18.1B.2 (− 28.03 folds), Y105E8B.1E.2 (− 27.18 folds) and F4E25i.1 (− 27.03 folds), annotated as cuticle collagen, tropomyosin and unknown protein, respectively.
Numbers of differentially expressed transcripts in C. elegans and M. incognita. Total number of differentially expressed (a), down-regulated (b) and up-regulated (c) transcripts in C. elegans; and differentially expressed (d), down-regulated (e) and up-regulated (f) transcripts in M. incognita are represented by Venn diagrams. The numerical (percentage value) represents the numbers of genes specific and common between different treatments and controls. [FF: treatment with fungal filtrate, Hup A: treatment with Huperzine A, control: treatment with water]
Gene ontology (GO) analyses of differentially expressed transcripts in C. elegans and M. incognita. The bar graphs represent biological function (in blue), cellular component (in red) and molecular function (in green) categories in C. elegans treated with fungal filtrate (a) and Huperzine A (b), and M. incognita treated with fungal filtrate (c) and Huperzine A (d). [FF: treatment with fungal filtrate, Hup A: treatment with Huperzine A, control: treatment with water]
In the FF-treated M. incognita samples, 1,635 transcripts were differentially expressed, out of which 587 were down-regulated and 1,045 were up-regulated (Fig. 5d–f). The top three up-regulated transcripts—Minc30s019g37445 (9.97 folds), Minc3s00072g03555 (9.87 folds) and Minc3s01805g26440 (9.17 folds), were annotated as unknown or uncharacterized proteins. On the other hand, the three topmost down-regulated transcripts—Minc3s00001208594530 (− 9.40 folds), Minc3s00001g07972 (− 8.311 folds) and Minc3s00001g08596 (− 8.04 folds)—were annotated as hypothetical proteins. In the Hup A-treated samples, 1,079 transcripts were differentially expressed, out of which 597 were down-regulated and 482 were up-regulated (Fig. 5d–f). The top three up-regulated transcripts were Minc3s00300330g10349 (22.3 folds), Minc3s00522g13690 (22.1 folds) and Minc3s02477g3021 (12.2 folds), whereas Minc3s01840g26666 (− 7.48 folds), Minc3s00076g03724 (− 7.26 folds) and Minc3s00698g16246 (− 7.17 folds) were the top three down-regulated transcripts. All these up- and down-regulated proteins were unannotated.
Our analyses revealed that 180 up-regulated and 1,575 down-regulated transcripts were common between FF- and Hup A-treated C. elegans nematodes (Fig. 5b, c). Similarly, in case of M. incognita, 184 up-regulated and 93 down-regulated transcripts were common between FF- and Hup A-treated worms (Fig. 5e, f). In C. elegans, the highest up-regulated transcript was C23G10.4a.2 (25.05 folds) annotated as proteasome/cyclosome protein, whereas the highest down-regulated transcript was T27E9.1d.26 (− 30.87 folds) annotated as mitochondrial carrier protein. Interestingly, in M. incognita, both the highest up-regulated and down-regulated transcripts, i.e., Minc3s00237g08286 (7.461 folds) and Minc3s10547g44158 (− 9.41 folds), were annotated as hypothetical proteins.
Gene ontology (GO) and KEGG pathway analyses of the differentially expressed transcripts
The DEGs were functionally characterized into GO categories of molecular functions, biological processes and cellular components. Considering the biological processes, the FF-treated C. elegans samples showed GO:009792 (embryo development egg hatching), GO:00003 (reproduction) and GO:0002119 (nematode larval development) as the most enriched categories with 763, 705 and 539 transcripts, followed by GO:0040011 (locomotion) and GO:0007165 (signal transduction) with 569 and 296 transcripts. In case of Hup A-treated C. elegans samples, GO:009792, GO:0040011 and GO:00003 (embryo development egg hatching, locomotion and nematode larval development) were most enriched with 951, 705 and 704 transcripts, followed by GO:008152 (metabolic process) and GO:008340 (determination of adult life span) with 502 and 426 transcripts, respectively. However, in the FF-treated M. incognita samples, GO:0002119 (nematode larval development) and GO:000003 (reproduction) were most enriched with 78 transcripts, followed by GO:0001852 (metabolic process) with 57 transcripts. Among the molecular GO categories (Fig. 6), 43% (153 transcripts) transcripts belonged to multicellular organism processes and 43% (105 transcripts) belonged to binding category of the molecular functional group. Interestingly, there were very few transcripts (39%, 100 transcripts) under cellular components for both the nematodes.
The differentially expressed transcripts common between HUF- and FF-treated nematodes were further subjected to pathway analyses represented by KEGG Automatic Annotation Server (KAAS). In C. elegans, 334 transcripts (out of 1,765) could be assigned to various pathways and the most enriched KEGG pathways were metabolic pathway (cel01100, 118 transcripts), purine metabolism (cel00230, 33 transcripts) and neuroactive ligand (cel04080, 23 transcripts). Interestingly, in M. incognita, a total of 164 transcripts (out of 297) were annotated, and the most important KEGG pathways were lysosomes (ko04142, 28 transcripts), oxidative phosphorylation (ko00190, 17 transcripts), spliceosomes (ko03040, 15 transcripts) and MAPK signaling pathway (ko04010, 10 transcripts). The topmost mapped pathways are shown in Table 4.
Additionally, our transcriptome data showed significant activation of neurosignaling and other pathways in nematodes treated with FF and Hup A. Out of total 1,765 genes, 89 C. elegans genes were involved in various signaling pathways, including 15 involved in calcium signaling pathways and 15 involved in neuroactive ligand receptor showed down-regulated expression. Again, 12 genes associated with phosphatidylinositol signaling exhibited both up- and down-regulation, and 12 genes involved in mitogen-activated protein kinase (MAPK) pathways showed down-regulation in the treated samples. Notably, 8 Erb pathway genes and 3 mTOR pathway genes showed up-regulation. The other genes involved in Wnt, FOXO and Jak-STAT signaling pathways were down-regulated.
Further, 30 M. incognita genes (out of 297) were involved in neural response, out of which 1 involved in acetylcholinesterase (AChE) and 6 associated with neuropeptide receptors (NPR) showed down-regulated expression. Several other genes were associated with notch, MAPK, Wnt, FoxO and mTOR signaling as well as longevity regulating pathways in the FF and Hup A treatments. Additionally, genes involved to calcium signaling and neuroactive ligand interaction also showed up-regulation.
Interestingly, no gene was found to be directly related to autophagy in C. elegans, but the 8 genes related to lysosomes and 7 genes related to phagosomes showed down-regulation. Similarly, in M. incognita, 17 autophagy pathway related genes were down-regulated. Some of these genes are directly involved in autophagy, whereas others were involved via phagosomes and lysosomes. Notably, 4 autophagy-related genes were found to be cysteine protease-related proteins, 1 gene involved in death associated protein kinase and 2 genes related to MAPK signaling.
Validation of RNA-seq expression patterns of differentially expressed genes in C. elegance and M. incognita by qRT PCR
Twenty-two transcripts (11 each from down- and up-regulated categories of DEGs) from both C. elegance and M. incognita were validated for expression patterns. The expression of most of the tested transcripts was found to be in conformity with the expression patterns in the RNA-Seq data (Supplementary Doc 11). For 10 of the 44 DEGs, qRT PCR showed baseline expression instead of a significant up- or down-regulation (Supplementary Doc 11). It was observed that in general the RNA-Seq fold change expression of a particular transcript was much higher or lower (especially for C. elegans) as compared to level detected by qRT PCR (Supplementary Doc 11).
Discussion
Globally, RKNs are considered to be the most notorious PPNs responsible for massive yield losses. Although a handful of cultural, mechanical and physical management tactics are deployed for RKN control depending upon the crop and its economic importance, there lies a continuous demand for novel and ecofriendly management options. In this regard, biological control has been widely used for RKN management with promising success and fungi are known to be one of the propitious candidates in this run. The top list of commercial biocontrol fungal agents used against the RKNs includes Trichoderma spp., Arthrobotrys spp., Pochonia spp. and Paecilomyces (and Purpureocillium) spp. (Abd-Elgawad and Askary 2018). But, native strains of any new biocontrol agents are preferred from ecological perspective for field applications due to high adaptability in the given ecosystem, and we can also avoid some of the challenges posed by introduction of exotic species from different ecological regime. In view of this, the present study was undertaken to explore and identify the diversity of potential and novel rhizosphere dwelling Paecilomyces spp. from several geographical and agroecological regimes of India aiming to manage M. incognita. In-depth morphological and molecular characterization identified the present species as P. tenuis, designated as isolate Pt_RK.
To the best of our knowledge, this is the first report of P. tenuis infecting any of the RKN species. Previously, P. lilacinus (Moreno-Gavíra et al. 2020), P. variotii (Perveen and Shahzad 2013), P. formosus (Baazeem et al. 2021) and P. fumosoroseus (Tiganomilani et al. 1995; Perveen and Shahzad 2013) were shown to infect the PPN juveniles and eggs. Additionally, Chan et al. (2010) found that CaMV35S promoter-mediated transfer of chitinase gene PjCHI-1, isolated from P. javanicus, in heat-tolerant tomato could inhibit the M. incognita egg production and embryonic development. However, any record of P. tenuis infecting a PPN species is completely unknown. Besides, we have confirmed the presence of Hup A in our P. tenuis isolate by chromatographic analyses. Of late, Hup A has gained huge attention as an AChE inhibitor and N-methyl d-aspartate (NMDA) receptor agonist having insecticidal and antifeedant properties (Tang et al. 1999; Ainge et al. 2002; Coleman et al. 2008). Further, it is largely used in medical sectors to treat neurodegenerative diseases (Zhang et al. 1991; Zangara 2003). Hup A from our P. tenuis Pt_RK isolate was found to have the same retention time as standard. Quantification by UFLC analysis showed that our isolate contained 433.56 mg L−1 Hup A, which is much higher as compared to 21 µg L−1 in P. tenuis YS-13 (Su and Yang 2015), 8.32 µg L−1 in Acremonium sp., 327.8 µg L−1 in Shiraia sp., 37.63 µg L−1 in Trichoderma sp. (Le et al. 2019) and 319.8 ± 0.17 mg L−1 in T. harzianum NSW-V (Wen-Xia et al. 2020) isolates. To date, the report by Wen-Xia et al. (2020) stood highest for any fungal isolate biosynthesizing Hup A in liquid culture. But, in the present study our isolate showed the highest Hup A biosynthesis (433.56 mg mL−1) as on now. The AChE inhibition assay of the Hup A from P. tenuis Pt_RK (IC50 value: 2.85 ± 0.12 mg mL−1) was similar to that of standard Hup A and thus confirmed its ability to inhibit the enzyme.
In the present investigation, while evaluating the efficacy of P. tenuis Pt_RK against M. incognita, we initially examined the effect of fungal filtrate (FF) and compared it with pure Hup A, a known AChE inhibitor, on worm mortality, behavior and hatching inhibition potential under in vitro conditions. The effect of FF on egg hatching inhibition may be attributed by several individual reasons or a combination of them. Firstly, P. tenuis Pt_RK contains Hup A, an AChE inhibitor (Tang et al. 1999). AChE gene is expressed in Meloidogyne eggs (Piotte et al. 1999), and disruption of the ace gene function by creating mutants in model nematode C. elegans induces hatching inhibition including developmental arrest (Johnson et al. 1988). Thus, reduced hatching of M. incognita eggs by our isolate is an indicative of inhibition of AChE activity by Hup A present in P. tenuis Pt_RK. Secondly, P. tenuis secretes lipases (Sornakili et al. 2020), an important enzyme of perivitelline fluid of nematode eggs (Mkandawire et al. 2022). Soaking in P. tenuis FF might also disrupt the enzymatic titer of the lipases resulting in reduced hatching of the juveniles. Thirdly, acetic acid is a known nematode egg hatching inhibitor of the Meloidogyne spp. (Djian et al. 1991; Ntalli et al. 2016), which was found to be present in our fungal filtrate by GC-MS analysis. The reduced egg hatching by P. tenuis Pt_RK isolate may involve the nematicidal activity of acetic acid. The treatment of M. incognita with P. tenuis Pt_RK FF and Hup A also showed behavioral anomalies in terms of uncoordinated movement of the J2s while finding host root. The treated worms represented random circular tracks reflecting confused locomotion, as compared to normal sinusoidal movement of the worms. Piotte et al. (1999) showed AChE activity in amphids of the J2s where the nervous system plays pivotal part in transmitting sensory perception thus helping to locate host and movement during migration in and outside the root. Treatment with our P. tenuis isolate could have disrupted the AChE activity due to the presence of Hup A thereby resulting in aberrant migration of the J2s. Further, this might also aid in reduced penetration of the treated J2s inside root that ultimately affected the establishment of the nematode species and its pathogenic ability. Corroborating this, similar results were also observed earlier where disruption of neuropeptidergic genes associated with FMRF-amide-like peptide and neuropeptide-like protein in M. incognita also affected host finding and pathogenic ability of the worms (Maule et al. 2002; Papolu et al. 2013; Dash et al. 2017). Moreover, the impaired migration and reduced penetration may also be attributed due to acetic acid and other bioactive compounds present in the fungal isolate that act as a selective nematicide (Djian et al. 1991; Ntalli et al. 2016). Lastly, P. tenuis Pt_RK could also directly parasitize the infective J2s under laboratory conditions. This could be advantageous in reducing initial soil nematode population density thus protecting the crop at early stage which is very crucial for ensuring yields.
Application of P. tenuis Pt_RK FF resulted in promotion of plant growth with an increase in root and shoot dry weight. In this regard, Khan et al. (2012) reported that inoculation of P. formosus in cucumber significantly enhanced the shoot length and allied growth characteristics under salinity stress due to elevation of endogenous gibberellin content. Similar observations were recorded by Bilal et al. (2017) who showed application of P. formosus enhanced the growth characteristics of soybean affecting endogenous phytohormones. It has also been observed that the consortium of endophytic P. formosus LHL10 and Sphingomonas sp. LK11 exerts potent growth and tolerance responses against abiotic stresses (combined Al and Zn stress) (Bilal et al. 2018). Moreno-Gavíra et al. (2020) reported high germination percentage, seedling vigor, root and shoot length by application of P. variotii on tomato and pepper due to biostimulation effect after colonization. The enhanced plant growth with application of our isolate also indicates that P. tenuis has a precise plant growth-promoting ability in occurrence of biotic stresses. However, the precise mechanism of this growth enhancing was not studied here.
We have studied the transcriptome profile of M. incognita and C. elegans after being treated with the P. tenuis Pt_RK fungal filtrate and Hup A to conceptualize the underlying mechanism of toxicity. Here, we used the model nematode C. elegans to obtain better information since comprehensive annotation of genes and pathways is available for this nematode, and followed up with transcriptome of M. incognita for validation of the mechanisms. To protect the integrity of total RNA extracted from the soaked worms, concentration and exposure time was combined in such a way that no worm dies; instead, they exhibit toxicity symptoms (Hada et al. 2021). Following FF treatment, the transcripts encoding hypoxia domain protein, glucose-dehydrogenase and glucosyltransferase were highly up-regulated in C. elegans along with the heat-shock proteins. The Hup A treatment, on the other hand, resulted in up-regulation of cyclosome, chaperons and tubulin ligase to a large extent. In M. incognita, the top-listed up-regulated transcripts were unannotated. Hup A is an AChE inhibitor, and inhibition of this neurotransmitter activity in vivo or by exogenous drug delivery induces hypoxic condition in animals (Furukawa et al. 2014). At cellular level, the hypoxia domain-containing proteins are activated under oxygen stress to maintain the proteome in a functional state and are also involved in developmental and morphological activities (Fawcett et al. 2015). The chaperons and heat-shock proteins are simultaneously activated by hypoxia in C. elegans to maintain the proteostasis (Powell-Coffman 2010). These results clearly indicate that similar mechanisms may be involved in our case where the Hup A inhibited the AChE activity in the worm resulting in perturbation of the downstream-acting transcripts. Further, the anaphase-promoting complex or cyclosome is also up-regulated in our study. In C. elegans, cyclosome regulates the eukaryotic cell cycle (Frazier et al. 2004), and knockdown of transcriptional intermediary factor 1γ (a cyclosome-interacting protein) resulted in failure of cell to undergo metaphase-to-anaphase transition (Sedgwick et al. 2014). Glucose dehydrogenase and glucosyltransferase activity is associated with cell growth and proliferation through production of NADPH by pentose phosphate pathway and glucosylation, respectively (Tian et al. 1998; Mehboob and Lang 2021). Perturbation of these transcripts validates the egg hatching disruption potential of Hup A by affecting the underlying pathways, which was manifested by reduced hatching of the nematode eggs following FF and Hup A treatment in our case.
The transcripts coding for mitochondrial carrier protein, amino-transferases and catalytic binding domain-containing proteins, cuticle collagen and tropomyosin were all down-regulated in C. elegans following FF and Hup A treatment. The family of mitochondrial carrier proteins, arginine kinase F46H5.3 and amino-transferases have role in embryonic energy metabolism having the ATP and nucleotide-binding ligands (Tsang and Lemire 2003). Disruption of the corresponding transcripts suggests that the FF (together with action of Hup A) attenuated the energy metabolism of the developing embryo that resulted in reduced hatching of the worms. Further, down-regulation of the collagen may also aid in this process by targeting the collagen synthesis pathways in the developing nematodes (Thein et al. 2003). The mis-functioning of cellular energy metabolism pathways and cuticular collagen synthesis might result in worm mortality, as observed post-treatment with FF and Hup A. In this regard, the tropomyosin-associated transcripts were also down-regulated, which indicates the possible inhibition of muscular contractility aiding in uncoordinated movement of the worms (Barnes et al. 2018). The mortality and uncoordinated movement may also be ensued by AChE inhibition by Hup A present in the FF. Inhibition of AChE leads to cholinergic stress affecting muscarinic and nicotinic ACh receptors (Rand 2007), and innumerable examples are present about organocarbamate and organophosphate molecules (both are AChE inhibitors) causing paralysis and death of nematodes. The organocarbamate molecules also affect migration of the nematodes toward host by targeting the neurosignaling (Hough and Thomason 1975). Hence, the mortality and behavioral anomalies manifested post-FF and -Hup A treatment may be a combinatorial effect of interference of neurosignaling, muscular activity and energy supply in the worms.
Our study revealed various active pathways in M. incognita and C. elegans upon treatment with FF and Hup A. Out of them, a considerable (almost 50%) were active in both the worms and notable among them include proteasomal pathways, metabolic pathways, cytochrome P450s, ErB signaling, mTOR signaling, Wnt signaling, Jak-STAT signaling, FoxO signaling, MAPK signaling, phosphatidylinositol signaling, Hedgehog signaling, phagosomes and lysosomes. In C. elegans, proteasomal complex takes part in detoxification and immune responses (Keith et al. 2016; Sladowska et al. 2021). Upon activation of proteasomal activity, mitochondrial chaperones and proteases get stimulated enhancing the metabolic pathways (Lin et al. 2016a, b). Here, the increased proteasomal transcripts possibly redirect the metabolic pathways aiming detoxification of the xenobiotic toxins in the worm. The cytochrome P450s are found in almost all living organisms including the nematodes and are chiefly engaged in detoxification of exogenous drugs and pesticide molecules (Laing et al. 2015). Other pathways, such as ErbB signaling (Yarden and Sliwkowski 2001), mTOR signaling (Aramburu et al. 2014), Wnt signaling (Silva-García et al. 2014), Jak-STAT signaling (Tanguy et al. 2017), FoxO signaling (Hesp et al. 2015), MAPK signaling (Kim et al. 2004), phosphatidylinositol signaling (Morris et al. 1996) and Hedgehog signaling (Hsia et al. 2015; Lin et al. 2016a, b) primarily act in stress responses and also participate in embryonic development and dauer induction of C. elegans. Phagosomal and lysosomal activity targets the xenobiotics and toxins to degrade by vesicular trafficking (De Voer et al. 2008). All these active pathways suggest a general response to a xenobiotic in both these nematodes upon exposure to Hup A and FF.
In conclusion, the present study finds a P. tenuis isolate from India capable of imparting mortality, egg hatching inhibition, J2 parasitization and behavioral anomalies in M. incognita, thereby reducing its multiplication and pathogenic potential. Additionally, the fungus was also found to enhance the plant growth and produce Hup A. Previously, the endophytic fungi were given much attention for large-scale commercial production of Hup A (Sang et al. 2020). But the present study unfolds new avenues to elaborately examine the soil fungal diversity for commercial production of Hup A. Besides, biocontrol approaches offer a safer substitute for pest management that can help to overcome major problems of resistance development and non-target effects generally associated with synthetic molecules. Though various Paecilomyces spp. are widely used for PPN biocontrol across the globe, P. lilacinus, P. variotti and P. javanicus are still known to cause fatal pneumonia, oculomycosis, fungemia, endocarditis and peritonitis in the immunosuppressed humans (Pastor and Guarro 2006; Steiner et al. 2013; Turner and Conrad 2015). Despite the ill-reputation of few Paecilomyces spp. for being human pathogenic, any clinical report of P. tenuis infecting human is not known till date (Moreira et al. 2018). Hence, a biocontrol agent like P. tenuis Pt_RK with known and efficient nematophagous and nematicidal action along with possibility of plant growth promotion provides an advantage with regards to its prospective commercial and field adoption.
Data availability
The raw reads were deposited in the NCBI Sequence Read Archive (SRA) database under the BioProject no. PRJNA800591; study accession no. SRP356762; Biosample accession nos. SAMN25258043 and SAMN25258042; and SRA accession nos. SRR17756040–SRR17756055.
References
Abd-Elgawad MM, Askary TH (2018) Fungal and bacterial nematicides in integrated nematode management strategies. Egypt J Biol Pest Control 28:1–24. https://doi.org/10.1186/s41938-018-0080-x
Adhikari M, Kim S, Kim HS, Lee HB, Lee YS (2016) Sixteen new records of ascomycetes from crop field soil in Korea. Korea J Mycol 44:271–288. https://doi.org/10.4489/KJM.2016.44.4.271
Ainge GD, Lorimer SD, Gerard PJ, Ruf LD (2002) Insecticidal activity of huperzine A from the New Zealand clubmoss, Lycopodium varium. J Agric Food Chem 50:491–494. https://doi.org/10.1021/jf0106087
Aramburu J, Ortells MC, Tejedor S, Buxadé M, López-Rodríguez C (2014) Transcriptional regulation of the stress response by mTOR. Sci Signal 7:re2–re2. https://doi.org/10.1126/scisignal.2005326
Atanasova L, Le Crom S, Gruber S, Coulpier F, Seidl-Seiboth V, Kubicek CP, Druzhinina IS (2013) Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genom 14:1–15. https://doi.org/10.1186/1471-2164-14-121
Baazeem A, Alorabi M, Manikandan P, Alotaibi SS, Almanea A, Abdel-Hadi A, Vijayaraghavan P, Raj SRF, Kim YO, Kim HJ (2021) Paecilomyces formosus MD12, a biocontrol agent to treat Meloidogyne incognita on brinjal in green house. J Fungi 7:632. https://doi.org/10.1111/j.1744-7348.1989.tb06792.x
Bailey F, Gray NF (1989) The comparison of isolation techniques for nematophagous fungi from soil. Ann Appl Biol 114:125–132. https://doi.org/10.1111/j.1744-7348.1989.tb06792.x
Barnes DE, Watabe E, Ono K, Kwak E, Kuroyanagi H, Ono S (2018) Tropomyosin isoforms differentially affect muscle contractility in the head and body regions of the nematode Caenorhabditis elegans. J Mol Cell Biol 29:1075–1088. https://doi.org/10.1091/mbc.E17-03-0152
Baron NC, de Souza Pollo A, Rigobelo EC (2020) Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ 8:e9005. https://doi.org/10.7717/peerj.9005
Bilal S, Khan AL, Shahzad R, Asaf S, Kang SM, Lee IJ (2017) Endophytic Paecilomyces formosus LHL10 augments Glycine max L. adaptation to Ni-contamination through affecting endogenous phytohormones and oxidative stress. Front Plant Sci 8:870. https://doi.org/10.3389/fpls.2017.00870
Bilal S, Shahzad R, Khan AL, Kang S-M, Imran QM, Al-Harrasi A, Yun B-W, Lee I-J (2018) Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Front Plant Sci 9:1273. https://doi.org/10.3389/fpls.2018.01273
Byrd DW Jr, Kirkpatrick T, Barker K (1983) An improved technique for clearing and staining plant tissues for detection of nematodes. J Nematol 15:142–143
Chan YL, Cai D, Taylor PW, Chan MT, Yeh KW (2010) Adverse effect of the chitinolytic enzyme PjCHI-1 in transgenic tomato on egg mass production and embryonic development of Meloidogyne incognita. Plant Pathol 59:922–930. https://doi.org/10.1111/j.1365-3059.2010.02314.x
Chen S, Zhou Y, Chen Y, Gu J (2018) Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Coleman BR, Ratcliffe RH, Oguntayo SA, Shi X, Doctor BP, Gordon RK, Nambiar MP (2008) [+]-Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats. Chem Biol Interact 175:387–395. https://doi.org/10.1016/j.cbi.2008.05.023
Conesa A, Götz S (2008) Blast2GO: a comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics 2008:619832. https://doi.org/10.1155/2008/619832
Contina JB, Dandurand LM, Knudsen GR (2017) Use of GFP-tagged Trichoderma harzianum as a tool to study the biological control of the potato cyst nematode Globodera pallida. Appl Soil Ecol 115:31–37. https://doi.org/10.1016/j.apsoil.2017.03.010
Dahlin P, Eder R, Consoli E, Krauss J, Kiewnick S (2019) Integrated control of Meloidogyne incognita in tomatoes using fluopyram and Purpureocillium lilacinum strain 251. Crop Prot 124:104874. https://doi.org/10.1016/j.cropro.2019.104874
Dash M, Dutta TK, Phani V, Papolu PK, Shivakumara TN, Rao U (2017) RNAi-mediated disruption of neuropeptide genes, nlp-3 and nlp-12, cause multiple behavioral defects in Meloidogyne incognita. Biochem Biophys Res Commun 490:933–940. https://doi.org/10.1016/j.bbrc.2017.06.143
Davies JT, Ireson JE, Allen GR (2005) The impact of gorse thrips, ryegrass competition, and simulated grazing on gorse seedling performance in a controlled environment. Biol Control 32:280–286. https://doi.org/10.1016/j.biocontrol.2004.10.007
De Voer G, Peters D, Taschner PE (2008) Caenorhabditis elegans as a model for lysosomal storage disorders. Biochem Biophys Acta Mol Basis Dis 1782:433–446. https://doi.org/10.1016/j.bbadis.2008.04.003
Den Belder E, Boekestein A, Van Esch JWJ, Thiel F (1993) Low temperature scanning electron microscopy in fungus-nematode interaction. Scanning 15:37–42. https://doi.org/10.1002/sca.4950150106
Djian C, Pijarowski L, Ponchet M, Arpin N, Favre-Bonvin J (1991) Acetic acid: a selective nematicidal metabolite from culture filtrates of paecilomyces lilacinus (Thom) samson and Trichoderma longibrachiatum Rifai. Nematologica 37:101–112. https://doi.org/10.1163/187529291X00105
Dong LH, Fan SW, Ling QZ, Huang BB, Wei ZJ (2014) Identification of huperzine A-producing endophytic fungi isolated from Huperzia serrata. World J Microbiol Biotechnol 30:1011–1017. https://doi.org/10.1007/s11274-013-1519-6
Douda O, Manasova M, Zouhar M, Hnatek J, Stejskal V (2021) Field validation of the effect of soil fumigation of ethanedinitrile (EDN) on the mortality of Meloidogyne hapla and carrot yield parameters. Agronomy 11:208. https://doi.org/10.3390/agronomy11020208
Du B, Xu Y, Dong H, Li Y, Wang J (2020) Phanerochaete chrysosporium strain B-22, a nematophagous fungus parasitizing Meloidogyne incognita. PLoS ONE 15:e0216688. https://doi.org/10.1371/journal.pone.0216688
Ehwaeti ME, Phillips MS, Trudgill DL (1998) Dynamics of damage to tomato by Meloidogyne incognita. Fundam Appl Nematol 21:627–635
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Faske TR, Hurd K (2015) Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to fluopyram. J Nematol 47:316–321
Fawcett EM, Hoyt JM, Johnson JK, Miller DL (2015) Hypoxia disrupts proteostasis in C. elegans. Aging Cell 14:92–101. https://doi.org/10.1111/acel.12301
Flatt T, Heyland A (2011) Mechanisms of life history evolution: the genetics and physiology of life history traits and trade-offs. OUP Oxford. https://doi.org/10.1093/acprof:oso/9780199568765.001.0001
Forghani F, Hajihassani A (2020) Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Front Plant Sci 11:1125. https://doi.org/10.3389/fpls.2020.01125
Frazier T, Shakes D, Hota U, Boyd L (2004) Caenorhabditis elegans UBC-2 functions with the anaphase-promoting complex but also has other activities. J Cell Sci 117:5427–5435. https://doi.org/10.1242/jcs.01417
Furukawa S, Yang L, Sameshima H (2014) Galantamine, an acetylcholinesterase inhibitor, reduces brain damage induced by hypoxia-ischemia in newborn rats. Int J Dev Neurosci 37:52–57. https://doi.org/10.1016/j.ijdevneu.2014.06.011
Glass NL, Donaldson GC (1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol 61:1323–1330. https://doi.org/10.1128/aem.61.4.1323-1330.1995
Hada A, Dutta TK, Singh N, Singh B, Rai V, Singh NK, Rao U (2020) A genome-wide association study in Indian wild rice accessions for resistance to the root-knot nematode Meloidogyne graminicola. PLoS ONE 15:e0239085. https://doi.org/10.1371/journal.pone.0239085
Hada A, Singh D, Satyanarayana KKVV, Chatterjee M, Phani V, Rao U (2021) Effect of fluensulfone on different functional genes of root-knot nematode Meloidogyne incognita. J Nematol 53:e2021. https://doi.org/10.21307/jofnem-2021-073
Han YF, Zhang YW, Liang JD, Liang ZQ (2007) A novel Paecilomyces species isolated from soil in China. Mycotaxon 102:51–56. https://doi.org/10.5248/114.25
Hesp K, Smant G, Kammenga JE (2015) Caenorhabditis elegans DAF-16/FOXO transcription factor and its mammalian homologs associate with age-related disease. Exp Gerontol 72:1–7. https://doi.org/10.1016/j.exger.2015.09.006
Hough A, Thomason IJ (1975) Effects of aldicarb on the behavior of Heterodera schachtii and Meloidogyne javanica. J Nematol 7:221–229. https://doi.org/10.1007/s11515-015-1343-5
Hsia EY, Gui Y, Zheng X (2015) Regulation of Hedgehog signaling by ubiquitination. Front Biol 10:203–220. https://doi.org/10.1007/s11515-015-1343-5
Johnson CD, Rand JB, Herman RK, Stern BD, Russell RL (1988) The acetylcholinesterase genes of C. elegans: identification of a third gene (ace-3) and mosaic mapping of a synthetic lethal phenotype. Neuron 1:165–173. https://doi.org/10.1016/0896-6273(88)90201-2
Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WM (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14:946–961. https://doi.org/10.1111/mpp.12057
Kearn J, Ludlow E, Dillon J, O’Connor V, Holden-Dye L (2014) Fluensulfone is a nematicide with a mode of action distinct from anticholinesterases and macrocyclic lactones. Pestic Biochem Physiol 109:44–57. https://doi.org/10.1016/j.pestbp.2014.01.004
Keith SA, Maddux SK, Zhong Y, Chinchankar MN, Ferguson AA, Ghazi A, Fisher AL (2016) Graded proteasome dysfunction in Caenorhabditis elegans activates an adaptive response involving the conserved SKN-1 and ELT-2 transcription factors and the autophagy-lysosome pathway. PLoS Genet 12:e1005823. https://doi.org/10.1371/journal.pgen.1005823
Khan AL, Hamayun M, Kang SM, Kim YH, Jung HY, Lee J-H, Lee IJ (2012) Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: an example of Paecilomyces formosus LHL10. BMC Microbiol 12:3. https://doi.org/10.1186/1471-2180-12-3
Kim ES, Kim MS, Moon A (2004) TGF-β-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol 25:1375–1382. https://doi.org/10.3892/ijo.25.5.1375
Kim D, Paggi JM, Park C, Bennett C, Salzberg SL (2019) Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37:907–915. https://doi.org/10.1038/s41587-019-0201-4
Lahm GP, Desaeger J, Smith BK, Pahutski TF, Rivera MA, Meloro T, Kucharczyk R, Lett RM, Daly A, Smith BT (2017) The discovery of fluazaindolizine: a new product for the control of plant parasitic nematodes. Bioorg Med Chem Lett 27:1572–1575. https://doi.org/10.1016/j.bmcl.2017.02.029
Lai Y, Liu K, Zhang X, Zhang X, Li K, Wang N, Shu C, Wu Y, Wang C, Bushley KE, Xiang M, Liu X (2014) Comparative genomics and transcriptomics analyses reveal divergent lifestyle features of nematode endoparasitic fungus Hirsutella minnesotensis. Genome Biol Evol 6:3077–3093. https://doi.org/10.1093/gbe/evu241
Laing R, Bartley DJ, Morrison AA, Rezansoff A, Martinelli A, Laing ST, Gilleard JS (2015) The cytochrome P450 family in the parasitic nematode Haemonchus contortus. Int J Parasitol 45:243–251. https://doi.org/10.1016/j.ijpara.2014.12.001
Le TTM, Hoang ATH, Le TTB, Vo TTB, Van Quyen DV, Chu HH (2019) Isolation of endophytic fungi and screening of Huperzine A-producing fungus from Huperzia serrata in Vietnam. Sci Rep 9:16152. https://doi.org/10.1038/s41598-019-52481-2
Le TTM, Hoang ATH, Nguyen NP, Le TTB, Trinh HTT, Vo TTB, Van Quyen DV (2020) A novel huperzine A-producing endophytic fungus Fusarium sp. Rsp5.2 isolated from Huperzia serrate. Biotechnol Lett 42:987–995. https://doi.org/10.1007/s10529-020-02836-x
Li J, Zou C, Xu J, Ji X, Niu X, Yang J, Huang X, Zhang K-Q (2015) Molecular mechanisms of nematode-nematophagous microbe interactions: basis for biological control of plant-parasitic nematodes. Ann Rev Phytopath 53:67–95. https://doi.org/10.1146/annurev-phyto-080614-120336
Li XQ, Xu K, Liu XM, Zhang PA (2020) Systematic review on secondary metabolites of Paecilomyces species: chemical diversity and biological activity. Planta Med 86:805–821. https://doi.org/10.1055/a-1196-1906
Lin EH, Kao YR, Lin CA, Kuo TY, Yang SP, Hsu C-F, Chou T-Y, Ho C-C, Wu C-W (2016a) Hedgehog pathway maintains cell survival under stress conditions, and drives drug resistance in lung adenocarcinoma. Oncotarget 7:24179–24193. https://doi.org/10.18632/oncotarget.8253
Lin XW, Tang L, Yang J, Xu WH (2016b) HIF-1 regulates insect lifespan extension by inhibiting c-Myc-TFAM signaling and mitochondrial biogenesis. Biochim Biophys Acta 1863:2594–2603. https://doi.org/10.1016/j.bbamcr.2016.07.007
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. https://doi.org/10.1186/s13059-014-0550-8
Maule AG, Mousley A, Marks NJ, Day TA, Thompson DP, Geary TG, Halton DW (2002) Neuropeptide signaling systems-potential drug targets for parasite and pest control. Curr Top Med Chem 2:733–758. https://doi.org/10.2174/1568026023393697
Mehboob M, Lang M (2021) Structure, function, and pathology of protein O-glucosyltransferases. Cell Death Dis 12:71. https://doi.org/10.1038/s41419-020-03314-y
Mishra J, Bhardwaj A, Pal M, Rajput R, Misra K (2018) High performance thin layer chromatography hyphenated with electrospray mass spectrometry for evaluation of nucleobases in two traditional Chinese medicinal mushrooms: a metabolomic approach. J Liq Chromatogr Relat 41:910–918. https://doi.org/10.1080/10826076.2018.1539672
Mitkowski NA, Abawi GS (2003) Root-knot nematodes. Plant Health Instr. https://doi.org/10.1094/PHI-I-2003-0917-01
Mkandawire TT, Grencis RK, Berriman M, Duque-Correa MA (2022) Hatching of parasitic nematode eggs: a crucial step determining infection. Trends Parasitol 38:174–187. https://doi.org/10.1016/j.pt.2021.08.008
Moreira DC, Oliveira MME, Borba CM (2018) Human Pathogenic Paecilomyces from Food. Microorg 6:64. https://doi.org/10.3390/microorganisms6030064
Moreno-Gavíra A, Diánez F, Sánchez-Montesinos B, Santos M (2020) Paecilomyces variotii as a plant-growth promoter in horticulture. Agronomy 10:597. https://doi.org/10.3390/agronomy10040597
Moriya Y, Itoh M, Okuda S, Yoshizawa AC, Kanehisa M (2007) KAAS: an automatic genome annotation and pathway reconstruction server. Nucleic Acids Res 35:W182–W185. https://doi.org/10.1093/nar/gkm321
Morris JZ, Tissenbaum HA, Ruvkun G (1996) A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature 382:536–539. https://doi.org/10.1038/382536a0
Morton O, Hirsch P, Kerry B (2004) Infection of plant-parasitic nematodes by nematophagous fungi-a review of the application of molecular biology to understand infection processes and to improve biological control. J Nematol 6:161–170. https://doi.org/10.1163/1568541041218004
Ntalli N, Ratajczak M, Oplos C, Menkissoglu-Spiroudi U, Adamski Z (2016) Acetic acid, 2-undecanone, and (e)-2-decenal ultrastructural malformations on Meloidogyne incognita. J Nematol 48:248–260. https://doi.org/10.21307/jofnem-2017-033
Pandit R, Patel R, Patel N, Bhatt V, Joshi C, Singh PK, Kunjadia A (2017) RNA-Seq reveals the molecular mechanism of trapping and killing of root-knot nematodes by nematode-trapping fungi. World J Microbiol Biotechnol 33:65. https://doi.org/10.1007/s11274-017-2232-7
Papolu PK, Gantasala NP, Kamaraju D, Banakar P, Sreevathsa R, Rao U (2013) Utility of host delivered RNAi of two FMRF amide like peptides, flp-14 and flp-18, for the management of root knot nematode. Meloidogyne Incognita Plos One 8:e80603. https://doi.org/10.1371/journal.pone.0080603
Pastor FJ, Guarro J (2006) Clinical manifestations, treatment and outcome of Paecilomyces lilacinus infections. Clin Microbiol Infect 12:948–960. https://doi.org/10.1111/j.1469-0691.2006.01481.x
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL (2016) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol 33:290–295. https://doi.org/10.1038/nbt.3122
Perveen Z, Shahzad SA (2013) comparative study of the efficacy of Paecilomyces species against root-knot nematode Meloidogyne incognita. Pak J Nematol 31:125–131
Phani V, Shivakumara TN, Davies KG, Rao U (2017) Meloidogyne incognita fatty acid-and retinol-binding protein (Mi-FAR-1) affects nematode infection of plant roots and the attachment of Pasteuria penetrans endospores. Front Microbiol 8:2122. https://doi.org/10.3389/fmicb.2017.02122
Phani V, Somvanshi VS, Shukla RN, Davies KG, Rao U (2018) A transcriptomic snapshot of early molecular communication between Pasteuria penetrans and Meloidogyne incognita. BMC Genom 19:850. https://doi.org/10.1186/s12864-018-5230-8
Phani V, Khan MR, Dutta TK (2021) Plant-parasitic nematodes as a potential threat to protected agriculture: current status and management options. Crop Prot 144:105573. https://doi.org/10.1016/j.cropro.2021.105573
Piotte C, Arthaud L, Abad P, Rosso MN (1999) Molecular cloning of an acetylcholinesterase gene from the plant parasitic nematodes, Meloidogyne incognita and Meloidogyne javanica. Mol Biochem Parasitol 99:247–256. https://doi.org/10.1016/s0166-6851(99)00033-x
Powell-Coffman JA (2010) Hypoxia signaling and resistance in C. elegans. Trends Endocrinol Metab 21:435–440. https://doi.org/10.1016/j.tem.2010.02.006
Rand JB (2007) Acetylcholine. WormBook. https://doi.org/10.1895/wormbook.1.131.1
Sang X, Yang M, Su J (2020) Research on endophytic fungi for producing huperzine A on a large-scale. Crit Rev Microbiol 6:654–664. https://doi.org/10.1080/1040841X.2020.1819771
Sedgwick B, Riches K, Bageghni SA, O’Regan DJ, Porter KE, Turner NA (2014) Investigating inherent functional differences between human cardiac fibroblasts cultured from nondiabetic and Type 2 diabetic donors. Cardiovasc Pathol 23:204–210. https://doi.org/10.1016/j.carpath.2014.03.004
Shu S, Zhao X, Wang W, Zhang G, Cosoveanu A, Ahn Y, Wang M (2014) Identification of a novel endophytic fungus from Huperzia serrata which produces huperzine A. World J Microbiol Biotechnol 30:3101–3109. https://doi.org/10.1007/s11274-014-1737-6
Siddiqui IA, Shaukat SS (2004) Systemic resistance in tomato induced by biocontrol bacteria against the root-knot nematode, Meloidogyne javanica is independent of salicylic acid production. J Phytopathol 152:48–54. https://doi.org/10.1046/j.1439-0434.2003.00800.x
Silva SD, Carneiro RM, Faria M, Souza DA, Monnerat RG, Lopes RB (2017) Evaluation of Pochonia chlamydosporia and Purpureocillium lilacinum for suppression of Meloidogyne enterolobii on tomato and banana. J Nematol 49:77–85. https://doi.org/10.21307/jofnem-2017-047
Silva-García O, Valdez-Alarcón JJ, Baizabal-Aguirre VM (2014) The Wnt/β-catenin signaling pathway controls the inflammatory response in infections caused by pathogenic bacteria. Med Inflamm 2014:310183. https://doi.org/10.1155/2014/310183
Sladowska M, Turek M, Kim MJ, Drabikowski K, Mussulini BHM (2021) Proteasome activity contributes to pro-survival response upon mild mitochondrial stress in Caenorhabditis elegans. PLoS Biol 19:e3001302. https://doi.org/10.1371/journal.pbio.3001302
Slomczynska U, South MS, Bunkers G, Edgecomb D, Wyse-Pester D, Selness S, Ding Y, Christiansen J, Ediger K, Miller W (2014) Tioxazafen: a new broadspectrum seed treatment nematicide. In: Maienfisch P, Stevenson TM (eds) ACS symposium series, discovery and synthesis of crop protection products, vol 1204. Monsanto Corporation, Chesterfield, pp 129–147. https://doi.org/10.1021/bk-2015-1204.ch010
Sornakili A, Thankappan S, Sridharan AP, Nithya P, Uthandi S (2020) Antagonistic fungal endophytes and their metabolite-mediated interactions against phytopathogens in rice. Physiol Mol Plant Pathol 112:101525. https://doi.org/10.1016/j.pmpp.2020.101525
Steiner B, Aquino VR, Paz AA, Silla LM, Zavascki A, Goldani LZ (2013) Paecilomyces variotii as an emergent pathogenic agent of pneumonia. Case Rep Infect Dis 2013:273848. https://doi.org/10.1155/2013/273848
Su J, Yang M (2015) Huperzine A production by Paecilomyces tenuis YS-13, an endophytic fungus isolated from Huperzia serrata. Nat Prod Res 29:1035–1041. https://doi.org/10.1080/14786419.2014.980245
Tang XC, He XC, Bai DL (1999) Huperzine A: a novel acetylcholinesterase inhibitor. Drugs Fut 24:647–664. https://doi.org/10.1358/dof.1999.024.06.545143
Tanguy M, Véron L, Stempor P, Ahringer J, Sarkies P, Miska EA (2017) An alternative STAT signaling pathway acts in viral immunity in Caenorhabditis elegans. Mbio 8:e00924-e1017. https://doi.org/10.1128/mBio.00924-17
Thein MC, McCormack G, Winter AD, Johnstone IL, Shoemaker CB, Page AP (2003) Caenorhabditis elegans exoskeleton collagen COL-19: an adult-specific marker for collagen modification and assembly, and the analysis of organismal morphology. Dev Dynam 226:523–539. https://doi.org/10.1002/dvdy.10259
Tian WN, Braunstein LD, Pang J, Stuhlmeier KM, Xi QC, Tian X, Stanton RC (1998) Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J Biol Chem 273:10609–10617. https://doi.org/10.1074/jbc.273.17.10609
Tiganomilani MS, Carneiro RG, Defaria MR, Frazão HS, McCoy CW (1995) Isozyme characterization and pathogenicity of Paecilomyces fumosoroseus and P. lilacinus to Diabrotica speciosa (Coleoptera: Chrysomelidae) and Meloidogyne javanica (Nematoda: Tylenchidae). Biol Control 5:378–382. https://doi.org/10.1006/bcon.1995.1044
Tsang WY, Lemire BD (2003) The role of mitochondria in the life of the nematode, Caenorhabditis elegans. Biochim Biophys Acta 1638:91–105. https://doi.org/10.1016/S0925-4439(03)00079-6
Turner LD, Conrad D (2015) Retrospective case-series of Paecilomyces lilacinus ocular mycoses in Queensland. Aust BMC Res Notes 8:627. https://doi.org/10.1186/s13104-015-1591-0
Wang R, Tang XC (2005) Neuroprotective effects of huperzine A. Neurosignals 14:71–82. https://doi.org/10.1159/000085387
Wang C, Lower S, Williamson VM (2009) Application of Pluronic gel to the study of root-knot nematode behavior. Nematology 11:453–464. https://doi.org/10.1163/156854109X447024
Wang Y, Zeng QG, Zhang ZB, Yan RM, Wang LY, Zhu D (2015) Isolation and characterization of endophytic huperzine A-producing fungi from Huperzia serrata. J Ind Microbiol Biotechnol 38:1267–1278. https://doi.org/10.1007/s10295-010-0905-4
Wen-Xia H, Zhong-Wen H, Min J, Han Z, Wei-Ze L, Li-Bin Y, Fei L, Lu H, Ning Z, Xiao-Feng L (2020) Five novel and highly efficient endophytic fungi isolated from Huperzia serrata expressing huperzine A for the treatment of Alzheimer’s disease. Appl Microbiol Biotechnol 104:9159–9177. https://doi.org/10.1007/s00253-020-10894-4
Whitehead AG, Hemming JRA (1965) comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann Appl Biol 55:25–38. https://doi.org/10.1111/j.1744-7348.1965.tb07864.x
Wu Q, Gu Y (2006) Quantification of huperzine A in Huperzia serrata by HPLC-UV and identification of the major constituents in its alkaloid extracts by HPLC-DAD-MS-MS. J Pharm Biomed 40:993–998. https://doi.org/10.1016/j.jpba.2005.07.047
Yang WN, Han H, Hu XD, Feng GF, Qian YH (2013) The effects of perindopril on cognitive impairment induced by d-galactose and aluminum trichloride via inhibition of acetylcholinesterase activity and oxidative stress. Pharmacol Biochem Behav 114:31–36. https://doi.org/10.1016/j.pbb.2013.10.027
Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2:127–137. https://doi.org/10.1038/35052073
Zangara A (2003) The psychopharmacology of huperzine A: an alkaloid with cognitive enhancing and neuroprotective properties of interest in the treatment of Alzheimer’s disease. Pharmacol Biochem Behav 75:675–686. https://doi.org/10.1016/S0091-3057(03)00111-4
Zelík P, Lukešová A, Voloshko LN, Štys D, Kopecký J (2009) Screening for acetylcholinesterase inhibitory activity in cyanobacteria of the genus Nostoc. Enzyme Inhib Med Chem 24:531–536. https://doi.org/10.1080/14756360802234836
Zhang RW, Tang XC, Han YY, Sang GW, Zhang YD (1991) Drug evaluation of huperzine A in the treatment of senile memory disorders. Acta Pharmacol Sin 12:250–252
Zhang ZB, Zeng QG, Yan RM, Wang Y, Zou ZR, Zhu D (2011) Endophytic fungus Cladosporium cladosporioides LF70 from Huperzia serrata produces Huperzine A. World J Microbiol Biotechnol 27:479–486. https://doi.org/10.1007/s11274-010-0476-6
Zhang FF, Wang MZ, Zheng YX, Liu HY, Zhang XQ, Wu SS (2015) Isolation and characterization of endophytic Huperzine A-producing fungi from Phlegmariurus phlegmaria. Microbiology 84:701–709. https://doi.org/10.1134/S0026261715050185
Zhu D, Wang J, Zeng Q, Zhang Z, Yan R (2010) A novel endophytic Huperzine A–producing fungus, Shiraia sp. Slf14, isolated from Huperzia serrata. J Appl Microbiol 109:1469–1478. https://doi.org/10.1111/j.1365-2672.2010.04777.x
Acknowledgements
The authors sincerely acknowledge Dr. Jayarama D. Bhat, D. Sc., Formerly Professor of Botany, Goa University, Goa, India, for his constructive comments and suggestions during the manuscript preparation and final drafting. We also acknowledge Mr. Purshotam and Mr. Vivek for helping in culture maintenance.
Funding
This work was supported by the Genomics Assisted Crop Improvement and Management project under World Bank and Indian Council of Agricultural Research sponsored National Agricultural Higher Education Project-Centre for Advanced Agricultural Science and Technology (NAHEP-CAAST) project.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by RK, NJ, AH, JY, MC, CGB, JM, VSR and GC; analyses were performed by UR, VP, RK, RB, JG, AK and VSS; the first draft of the manuscript was written by RK, VP, NJ, VSS and UR; final drafting and critical revision of the manuscript were done by UR, VP and VSS. All authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Aurelio Ciancio.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kassam, R., Jaiswal, N., Hada, A. et al. Evaluation of Paecilomyces tenuis producing Huperzine A for the management of root-knot nematode Meloidogyne incognita (Nematoda: Meloidogynidae). J Pest Sci 96, 723–743 (2023). https://doi.org/10.1007/s10340-022-01521-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-022-01521-4