Abstract
Many behaviors are associated with host selection by arthropod pests. The treatment of a host, such as with a pesticide, may impact behaviors involved in this selection whose understanding yields opportunities for pest management. AC–DC electropenetrography (EPG) allows real-time monitoring of insect behaviors, but its use has emphasized feeding activities of hemipteroid insects. Recent improvement in electropenetrography (AC–DC) has made it amenable for use with non-hemipteroid species, such as the invasive spotted wing drosophila (Drosophila suzukii). Therefore, AC–DC EPG was used for the first quantitative study of a non-hemipteroid insect to monitor behaviors of spotted wing drosophila on strawberry fruits treated with either the fungicide fenhexamid or the insecticide spinetoram, in addition to a non-treated control. EPG was used to characterize three behavioral phases of the insect: non-probing (i.e., resting, grooming, and walking), feeding, and egg-laying. The first two phases were affected by sublethal pesticide exposure, but egg-laying was not. Both pesticides decreased the number of non-probing events, but increased their overall durations, while the opposite took place with feeding, especially in spinetoram-treated strawberry. Regarding feeding activity, both pesticides compromised insect dabbing and ingestion with particularly strong impairment by spinetoram, which also compromised how long the females survived (i.e., longevity). EPG revealed valuable insights regarding the behavioral assessment of pesticide-treated hosts by an insect pest. Specifically, the feeding of female of spotted wing drosophila was significantly impaired on strawberries treated with spinetoram compromising female longevity. Though deserving further attention, the fungicide fenhexamid exhibited a relatively mild effect on feeding, but did not affect adult longevity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
AC–DC electropenetrography was used for the first quantitative study of a non-hemipteroid insect to monitor behaviors of spotted wing drosophila on pesticide-treated strawberry fruits
-
The fungicide fenhexamid and the insecticide spinetoram decreased the number of non-probing events, but increased their overall durations, while the opposite took place with feeding, especially with spinetoram
-
Both pesticides compromised insect dabbing and ingestion, particularly spinetoram, which also compromised female longevity
-
Fenhexamid exhibited mild effect on feeding not affecting adult longevity.
Introduction
Insects usually exhibit close association with their host, as is the case with herbivores and their host plants and exemplified by the range of adaptations developed for this interaction (Jaenike 1990; Bernays 1991; Gatehouse 2002). Therefore, it is no wonder that host substrate largely mediates arthropod–plant interactions, allowing underlying links between physiology and behavior of both organisms. Consequently, the host substrate is an important management concern due to its association with the arthropod’s behavioral repertoire (Gatehouse 2002; Simpson et al. 2015). Changes in the host substrate are likely to elicit relevant responses in arthropods either favoring or preventing colonization, depending on the change taking place.
Host plants and plant parts colonized as substrates for herbivorous arthropods are subjected to anthropogenic and environmental influences that potentially interfere with this interaction. Regarding crop cultivation practices, insecticides are arguably the main determinant of plant colonization by insect pest species. However, the potential impact of insecticide use extends beyond the intended lethality (Guedes et al. 2016, 2017). Other pesticides, like fungicides, can also interfere with plant–arthropod interaction via substrate contamination (Guedes et al. 2016), although much less is known on this subject.
Agricultural pesticides may directly interact with the crop plant, the arthropod pest species, or the signal(s) mediating this interaction (Holopainen and Blande 2013; Sih 2013; Jurgens and Bischoff 2016). Indirect interference by pesticides may also take place as a consequence of direct effect on another (directly) affected species, thereby enhancing the complexity of the cascade of possible effects and reaching higher hierarchical levels, including community (Guedes et al. 2016, 2017). Regardless, the plant–insect interaction may be disturbed, either enhancing or compromising the insect response, particularly its behavioral response when sublethal pesticide exposure is considered (Desneux et al. 2007; Walker 2000; Sih 2013; Blande et al. 2014; Guedes et al. 2016). Emphasis on sublethal exposure is justifiable because the initial (lethal) pesticide residue can degrade over time to sublethal levels, which last for much longer, and/or may generate derivative compounds of lower biological activity, and/or will reach nontarget species likely less susceptible than the targeted one(s) (Guedes et al. 2016, 2017).
Understanding the dynamics of plant–arthropod interactions aids pest management and environmental safety when pesticidal compounds are present in the system, as usually is the case in agroecosytems. Nonetheless, the study of these dynamics is challenging, particularly for small organisms. This challenge can be overcome with the use of high-resolution electronic recording of insect activity on the host substrate (Backus and Bennett 1992; Cole et al. 1993; Walker 2000; Itskov et al. 2014). Historically, the prevailing technique for this purpose, electropenetrography or electrical penetration graph (both abbreviated EPG), has been used primarily for monitoring feeding activity by phytophagous hemipteroid insects because EPG instruments were initially designed for these insects (Cole et al. 1993; Calatayud et al. 2001; Xue et al. 2009; Rangasamy et al. 2015; Cervantes et al. 2017a, b). Such electronic monitoring (now termed electropenetrography) was originally conceived in the early 1960s (McLean and Kinsey 1964) and has undergone 50+ years of development culminating with the current third generation of electropenetrographs (McLean and Kinsey 1964; Tjallingii 1978; Backus and Bennett 1992; Backus et al. 2019). The latest generation, the AC–DC electropenetrograph, was designed for greater flexibility of settings, potentially allowing recording for a much broader range of species and conditions than previous instruments, and thus encouraging its use for other types of close–substrate interactions (e.g., walking, grooming, egg-laying), and for non-hemipteroid species such as Drosophila fruit flies.
The spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), is an invasive pest species of soft-skinned fruits that has proven amenable to EPG recording due to its close association with fruit substrates and its mouth part analogy with those of hemipteroid insects (Labandeira 1997; Blanke et al. 2015; Guedes et al. 2019). This pest species has demanded increasing attention since its introduction into Europe and the USA by 2008, and subsequent spread throughout the Americas and beyond (Hauser 2011; Cini et al. 2012; Deprá et al. 2014; Asplen et al. 2015). The adults of this species are not very active in their routine or trivial movements, with limited walking activity and flight endurance except when undergoing long-range dispersal (Tait et al. 2018; Wong et al. 2018). However, both adults and larvae are attracted to undamaged ripening fruits, particularly berries (Bellamy et al. 2013; Rota-Stabelli et al. 2013; Lee et al. 2011, 2016). The consequence is a close association of the insect with its substrate for extended periods of time, and this host association plays a determinant role in the life history of this pest species (Lihoreau et al. 2016; Plantamp et al. 2017). Thus, substrate alterations and particularly insecticide treatment will likely affect the dynamics of the fly–berry association. This subject has been explored in studies of feeding activity among hemipteroid insects since the 1990s (Losel and goodman 1993; Harrewijn 1997; Daniels et al. 2009; Boina et al. 2011; Garzo et al. 2015; Civolani et al. 2014; Tariq et al. 2017), but not yet expanded to other species.
Pesticidal compounds other than insecticides may also affect plant–arthropod interactions in general, and spotted wing drosophila–berries in particular. Although this possibility is commonly overlooked, agroecosystems are quite complex chemical landscapes where the use of fungicides and insecticides commonly co-occurs (Sih 2013; Smith et al. 2014; Jurgens and Bischoff 2016; Guedes et al. 2018). Furthermore, when the post-harvest scenario is added to the pre-harvest concern with pest species, the use of fungicides also becomes more important, increasing the likelihood that insect pest species like the spotted wing drosophila could be exposed to both types of chemicals (Guedes et al. 2018). Curiously though, nothing is really known about the interaction between the fungicide-treated fruit surface and the spotted wing drosophila, which is also true for insecticide-treated fruits. Our study directly addresses this shortcoming. Detailed study of the interaction between spotted wing drosophila and pesticide-treated host fruits will aid in understanding potential pesticide impact on this species, thus aiding regulatory acceptance and future use of this resource.
Our goal was to use AC–DC electropenetrography to electronically monitor the interaction between adults of this pest species and strawberry fruits treated with either the fungicide, fenhexamid, or the insecticide, spinetoram. The latter is an important management tool against spotted wing drosophila (Bruck et al. 2011; Van Timmeren and Isaacs 2013; Smirle et al. 2017). Our objectives were to: 1) determine survival time on treated fruit to optimize the duration of the EPG studies, 2) correlate different waveforms with observed behaviors, and 3) compare behaviors on differently treated fruit surfaces. We expected to recognize the earlier waveforms described in EPG recordings with this species and their quantification (Guedes et al. 2019). We hypothesized that spinetoram-treated strawberry would impact the behavior of D. suzukii because it is neurotoxic and interferes with insect activity (Salgado and Sparks 2010; Casida and Durkin 2013), whereas fenhexamid-treated strawberry would not impact behavior because of its specific mode of action blocking the biosynthesis of ergosterol in fungi cell membranes (Leroux 1996; Yang et al. 2011).
Materials and methods
Insects and insecticides
Adults of spotted wing drosophila were from a laboratory colony reared in nylon enclosures (Bug Dorm-2®, BioQuip, Rancho Dominguez, CA, USA), under controlled conditions of 25 ± 2 °C temperature, 70 ± 10% relative humidity, and 16-h photophase. Insects were reared using a cornmeal–sucrose–agar–yeast diet provided in Petri dishes for both egg-laying and larvae development, as detailed elsewhere (Walse et al. 2012; Guedes et al. 2018).
The carboxamide fungicide, fenhexamid, and the spynosin insecticide, spinetoram, were used at maximum label rates in California (USA) with spray volume of 1000 L/ha, as follows: fenhexamid at 850 g a.i./ha (Elevate® 50 WDG; 50.5%, Arysta Lifescience, San Francisco, CA, USA) and spinetoram at 105.1 g a.i./ha (Delegate® 25 WG; Dow AgroSciences, Indianapolis, IN, USA). Both agrochemicals are currently used in strawberry production, fenhexamid for controlling the gray mold fungal pathogen (Botrytis cinerea Pers.), and spinetoram for spotted wing drosophila control. These pesticides were provided via artificial diet in time–mortality bioassays, as described below, and by fruit immersion in insecticide solution for 30 s in the EPG experiments, as also detailed below.
Time-survival bioassays
Time-survival bioassays were conducted using adult insects to determine the (sublethal) exposure for subsequent EPG analyses, and also to determine adult longevity on pesticide-treated surface (i.e., how long the adults survived under such exposure). The bioassays for each pesticide were conducted at respective maximum label rates, in addition to a non-treated control, dosed with only water. Four independent replicates were used for each pesticide and non-treated control, each encompassing a disposable Petri dish (9 cm diameter) containing 15 ml of artificial diet treated with the respective pesticide at the label rate considering the spray volume indicated above (or only water, if the control), added after the diet cooled but before it was poured. Twenty-five adult flies were chilled and subsequently transferred to each Petri dish, which was maintained under rearing conditions. Survival was recorded at 15-min intervals for 2 h after release, at 30-min intervals for four more hours, and twice a day afterward. Mortality was recognized when the insects were unable to walk a body length.
EPG bioassays
Insect wiring
Gravid adult females (4 to 7 days old; Hamby et al. 2016) were randomly collected from the rearing cages, starved for 1 h, and chilled for wiring. The wiring and whole EPG bioassays were performed as described by Guedes et al. (2019). Briefly, a gold wire (2.5 cm long and 25.4 µm diameter [sold as 0.001 inch; Sigmund Cohn, Mt. Vernon, NY, USA) was glued to the insect pronotum using silver glue (recipe in Cervantes and Backus 2018) under stereomicroscope (MZ125, Leica, Heerbrugg, Switzerland). The other end of the wire was attached to an EPG stub (copper wire soldered onto a brass escutcheon pin) for insertion into the BNC plug of the EPG head stage amplifier.
Electropenetrography
A four-channel, third-generation AC–DC electropenetrograph from EPG Technologies (Gainesville, FL, USA; andygator3@gmail.com) (similar to the one-channel correlation instrument previous published; Backus and Bennett 2009) was used for the recordings, which were performed within a Faraday cage to minimize electrical noise. Each wired fly was connected to an individual head stage amplifier and placed on an individual strawberry fruit (i.e., one fly per fruit), treated or not with pesticide (one block) via 30-s immersion in pesticide solution. Insects of each treatment were simultaneously recorded side by side in a randomized complete design. Between 26 and 30 flies were used for each treatment. Each strawberry had a 1-cm-long copper “plant” electrode inserted and connected to the electropenetrograph. Insect activity on the strawberry fruit surface closed the circuit and allowed recording the voltage variation as waveforms characteristic of each behavior.
Recordings used 109 Ω input impedance/resistance (Ri) and 20 mV alternating current (AC) for 4 h, usually starting by mid-morning (10:00 am), as previously determined (Guedes et al. 2019). Variation in analog electrical voltage was amplified, rectified, and digitally sampled at a rate of 100 Hz per channel using a DI-720 analog–digital board (Dataq Instruments, Akron, OH, USA). The digitized signal was recorded on a desktop computer through the WinDaq Pro+ software (Dataq). Pre- and post-rectification signals were simultaneously recorded and checked to ensure that waveform foldover by the rectifier was properly avoided through the offset function of the instrument; only the post-rectification signal was measured for the recordings. EPG controller gain was 3000×, and WindDaq gain was 8×.
Individual adult females interacting with strawberry fruit during the EPG recordings were also observed with a digital DFC7000T camera coupled with a MZ125 stereomicroscope connected to a desktop computer equipped with the LAS X image recording software (all from Leica). The objective was to correlate visual observations of behavioral activities with corresponding EPG waveforms, although behaviors were not video-recorded due to time synchronization problems.
Behavior (and waveform) quantification
The waveforms were recognized per Guedes et al. (2019) and thus named following the hierarchical conventions phase then family within each phase, as earlier proposed (Backus 2000; Backus et al. 2007). The waveforms were quantified based on continuous stereotypical patterns recognized as waveform events, whose numbers and durations were measured using the WinDaq Waveform Browser software (Dataq). Three main, non-sequential response variables were calculated following Backus et al. (2007), which were: (1) (mean) number of waveform events per insect (NWEI); (2) (mean) waveform duration per insect (WDI), which is the sum of all events of a given waveform averaged per insect; and (3) (mean) duration of waveform events per insect (WDEI).
Statistical analyses
Time-survival results were subjected to survival analyses using Kaplan–Meier estimators assessing the adult insects until they died, including those of the control (PROC LIFETEST, SAS; SAS Institute, Cary, NC, USA). The procedure allowed determination of how long the insects survived (adult longevity) and estimation of the respective median survival/lethal times (LT50s) for each individual replicate (i.e., their longevity) and treatment, which were subjected to analysis of variance and Fisher’s least significant difference (LSD) test (PROC GLM, SAS).
The results from the EPG bioassays were used for calculation of the descriptive statistic response variables described above, using the Ebert 2.0 SAS program (for the first three, compilation steps) and Backus 2.0 SAS program (for analysis); both are available at http://www.crec.ifas.ufl.edu/extension/epg/epg_workshop.shtml (Backus et al. 2007; Ebert et al. 2015). Thus, analyses of variance with restricted maximum likelihood estimation (REML-ANOVA) were performed using the procedure GLIMMIX from SAS and Fisher’s LSD test to recognize treatment differences, when suitable. These analyses were performed at the phase level and also at the family level when necessary. Data transformation was used to improve homoscedasticity of the following variables: WDI and WDEI (log), and NWEI (square root) for phases, and the same variables and transformations for the waveform families within the feeding phase.
Results
Time-survival
Survival time (or longevity) of the adult insects provided with pesticide-treated diet and non-treated diet (control) differed significantly (log-rank χ2 = 64.05, df = 2, P < 0.001). Consequently, the estimated median lethal times (LT50s; or median adult longevity) were also significantly different among the treated and non-treated diets (F2,9 = 371.66, P < 0.001). While the spotted wing drosophila adults exposed to non-treated or fenhexamid-treated diet exhibited similar median survival around 4 days (3.83 ± 0.17 days and 3.58 ± 0.08 days, respectively), insects exposed to spinetoram-treated diet exhibited median survival near the 4-h range (0.13 ± 0.01 days; Fig. 1). Therefore, a recording duration of 4 h was used in the EPG bioassays; no female mortality was observed during the EPG recording period.
Electropenetrography
The EPG recording exhibited a high success rate (100%) following attainment of proper insect preparation and wiring skills. The recording output obtained is detailed below.
Waveform confirmation
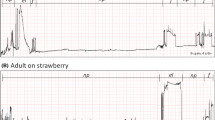
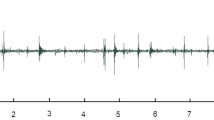
The strawberry-associated behaviors of adult female spotted wing drosophila were observed using stereomicroscopy and correlated with waveform structure recorded via simultaneous EPG, allowing the recognition of three waveform phases and eight families (as described in Table 1 and representatively depicted in Fig. 2). The three observed phases were: (1) non-probing phase, (2) feeding phase, and (3) egg-laying phase. An additional phase (i.e., other) was also used to refer to a waveform representing a brief interruption between feeding and/or egg-laying events reaching baseline, or near so, and lasting no more than 1.5 s, which was coded as N.
Overview of EPG waveforms from female spotted wing drosophila (Drosophila suzukii) on strawberry with 20 mV AC applied signal using 109 Ω (Ri). Monitor gain was set at 3000×, and Windaq gains are indicated in each recording, as are the x-axis compressions. a Waveform phases: non-probing (np), feeding (f), and egg-laying (el). b Waveforms from the non-probing phase: resting (Z), grooming (G), and walking (W); while those from the feeding phase are dabbing (D), and ingesting (I); waveforms from the egg-laying phase are probing (P), and egg-laying per se (L). Interruption of feeding and/or egg-laying is coded as N. Each division lasted 4 s in A and 0.6 s in B
The non-probing phase included three sets of behaviors with respective waveforms (coded as in parenthesis): resting (Z), grooming (G), and walking (W). The feeding and egg-laying phases consisted of two behaviors and set of corresponding waveforms each—dabbing (D) and ingestion (I) for the feeding phase, and abdominal probing (P) and egg-laying per se (L) for the egg-laying phase (Table 1, Fig. 2). These same waveform phases and families were earlier characterized electrically, defined behaviorally, and named (Guedes et al. 2019). Detailed descriptions of these waveform phases and families are briefly summarized below.
The waveforms of the non-probing phase were characterized by very low amplitude, or often, as a flat line representing resting (Z; the baseline recording), or as low-amplitude rounded waveforms, which were also irregular, representing grooming (G). Grooming waveforms were not as tall as walking waveforms (W), which were also represented by acute peaks and valleys of high amplitude reaching up to those of the feeding phase. The latter two waveforms were irregular, particularly grooming, because the leg movements necessary to remove foreign material from the body surface may take place on different body parts. Dabbing (D) and ingestion (I) within the feeding phase were characterized by rectangular-shaped waveforms with a flat plateau reaching about half the height (i.e., amplitude) of the egg-laying waveforms; their difference was the duration because dabbing seldom lasted more than a second (< 1.5 s), unlike ingestion, which is a sustained activity. Finally, the waveforms from the egg-laying phase were of similar amplitude, lasting several seconds for egg-laying and relatively shorter for abdominal probing. Egg-laying represented not only the ovipositor insertion into the fruit, but also a partial or complete insertion of an egg (Fig. 2).
Waveform quantification
Pesticide-treated and non-treated strawberries led to only marginal differences in numbers and durations of non-probing events (NWEI and WDI, respectively; P < 0.10), with the pesticides reducing the former and increasing the latter variables (Fig. 3). In contrast, significant differences were observed for number of feeding events (NWEI; P < 0.001). Both pesticides reduced the number of events (Fig. 4a), and spinetoram also reduced the overall duration of feeding (WDI; Fig. 4b) and the duration of each individual feeding event per insect (WDEI; Fig. 4c). In contrast, behaviors on fenhexamid resembled those on the non-treated strawberry. No differences were observed during the egg-laying phase (F2,42 = 2.19, P > 0.61), which were relatively rare events; probing occurred 6.72 ± 2.24 times on average for the 4-h recording of each insect, and egg-laying occurred even less frequently (2.85 ± 1.13 times 4-h recording).
The feeding patterns of dabbing and ingestion differed greatly among flies on pesticide-treated and non-treated strawberries (P < 0.001). Both pesticides similarly reduced the number (NWEI) of dabbing events compared with flies on non-treated strawberry (F2,83 > 4.89, P = 0.001; Fig. 5a), and also the overall length of time (WDI) spent on dabbing (F2,83 > 6.33, P < 0.001; Fig. 5b), but not the duration of each dabbing event (0.52 ± 0.7 s; F2,83 > 1.85, P = 0.16). Regarding ingestion, the number of events reduced by pesticide treatment with spinetoram was lowest (F2,83 > 13.02, P < 0.001; Fig. 5c), while the time spent ingesting was significantly smaller only for spinetoram-treated strawberries, not fenhexamid-treated ones (F2,83 > 4.51.89, P = 0.001; Fig. 5d). The treatment of a strawberry with either pesticide did not alter the average duration of ingestion events (25.92 ± 6.21 s; F2,83 > 0.96, P = 0.39).
Bar plots of frequency (a, c) and duration (mean ± SE) (b, d) of dabbing (waveform D; plots a, b) and ingestion of ingestion (waveform I; plots c, d) associated with the feeding phase of females of spotted wing drosophila (Drosophila suzukii) on pesticide-treated and non-treated strawberry. Different letters indicate significant difference by Fisher’s LSD test (P < 0.05)
Discussion
The behavior of adult female spotted wing drosophila on pesticide-treated strawberries was analyzed with EPG. The goal was to recognize and quantify behavioral features with EPG in the context of understanding the impact of pesticide treatments on this invasive pest. We expected to differentiate the behaviors of adult females on strawberries treated with the insecticide spinetoram, while we expected no difference between non-treated strawberries and those treated with the fungicide fenhexamid. As defined in more detail in previous work (Guedes et al. 2018), high-resolution AC–DC EPG of fly activity was successful. Our main hypothesis regarding spinetoram was confirmed. Curiously though, and contrasting with our hypothesis, fenhexamid also interfered with insect feeding, although only mildly.
Electropenetrography (EPG) is an affordable technique (complete equipment costs less than US$8000) whose feasibility of use in spotted wing drosophila affords some important strategic advantages compared with conventional techniques, aiding rather than replacing the need for behavioral and fitness studies (Backus and Bennett 1992; Walker 2000; Backus et al. 2019). In fact, EPG is valuable in at least three major ways. First, EPG enhances the study output while allowing simultaneous observation of a larger set of individuals and with higher resolution, which is not really feasible for small insects such as SWD when solely relying on direct observation. Second, EPG provides important information on the biomechanics of the feeding/egg-laying processes. This is because the resistance and biopotentials generated by the insect provide very detailed information about types and amounts of fluid flow, mouthpart or ovipositor movements, and other details recorded in higher resolution than via video. Third, because of the second property above, EPG allows recording of salivation (not usually detectable visually) as well as ingestion of food, sensing the substrate prior to oviposition, egg-laying, and other minute behaviors. These minute behaviors are not apparent with direct observation (e.g., depth of egg insertion into the substrate and strength required for that). Therefore, EPG use for non-hemipteroid insects extends to this group study possibilities previously used only for hemipteroid species.
Seven characteristic waveforms were associated with three activity phases of spotted wing drosophila—non-probing, feeding, and egg-laying. An eighth waveform was associated with the interruption of feeding and/or egg-laying. These waveforms are broadly consistent with those of other species (e.g., Cervantes et al. 2017a, b), and were also recently recognized and described in detail for spotted wind drosophila associated with artificial diet and strawberry fruits (Guedes et al. 2019). All of the observed waveforms were detected using low applied voltage (20 mV AC) at high Ri (109 Ω). At that Ri level, waveform fluctuations were probably generated mostly by biopotentials (or electromotive force [emf] component) (Walker 2000; Backus et al. 2018). Further study of spotted wing drosophila waveforms, especially a waveform library, would be warranted to determine whether additional minute behaviors can be resolved via EPG.
Waveform differences during the non-probing phase were milder than expected, but consistently showed an increase in overall duration, primarily due to reduction in number of same-duration events on pesticide-treated strawberries. However, because flies exhibited extensive grooming behavior with an irregular and varied spectrum of low-amplitude waveforms associated with cleaning of different body parts (i.e., head, abdomen, other legs, and wings), future recognition of waveform subfamilies within grooming may increase resolution and likelihood of detection of eventual effects on this subset of behaviors (Guedes et al. 2019). A similar rationale is also valid for walking, which has been recorded in few insect species, e.g., mirid bugs and the Asian citrus psyllid (Backus et al. 2007; Youn et al. 2011). Regardless, both behaviors are potentially important for pesticide residue pickup from the substrate surface and may lead to divergent trends in fly response when contrasted with resting. Such type (subfamily) waveform categorization of non-probing therefore deserves future attention, particularly when contact-acting compounds are involved, as in our study. What little has been explored so far is presently circumscribed to whiteflies (He et al. 2013; Civolani et al. 2014).
The egg-laying phase was characterized by abdominal probing and egg-laying per se, which are also behaviors that further promote contact and transfer of pesticides on treated surfaces, in addition to contact and ingestion. Certainly, there was the potential that egg-laying could be affected by these compounds. Nonetheless, we were unable to detect a significant effect of pesticides in such behavior in the present study with female spotted wing drosophila. This does not necessarily mean that such effects do not exist. They were actually reported in whiteflies (He et al. 2013), although the waveform dynamics of egg-laying on pesticide-treated host surfaces were not recorded. Drosophila egg-laying waveforms exhibited the same structure on treated and pesticide-treated fruits; however, variations in the number and duration of such waveforms were minimal in our study, probably due to the relatively small number of egg-laying events registered in the 4-h recordings. Longer recording times and/or better targeting of the peak window of egg-laying should allow the recording of significantly higher numbers of egg-laying events and eventual detection of pesticide-mediated differences in the egg-laying. The findings by Lin et al. (2014) provide support for the latter approach.
The main differences detected in this study occurred during the feeding phase, whose number and overall duration declined on pesticide-treated strawberries. Coincidentally, feeding is the phase that has received the bulk of attention in EPG studies since the development of this technique in the 1960s, again essentially targeting hemipteroid insects and particularly vectors of plant pathogens (Cole et al. 1993; Harrewijn et al. 1996; Calatayud et al. 2001; Kindt et al. 2003; Xue et al. 2009; Rangasamy et al. 2015; Cervantes et al. 2017a). Pesticide interference with feeding by these insects has also been an increasing target of attention, but focusing mainly on systemic insecticides, including neonicotinoids and sulfoximines (Nisbet et al. 1993; Daniels et al. 2009; Garzo et al. 2015), the pyridine azomethine pymetrozine (Harrewijn 1997; Boina et al. 2011), and the pyridinecarboxamide flonicamid (Morita et al. 2007; Tariq et al. 2017). The two former ones are competitive modulators of nicotinic acetylcholine receptors (nAChR) in neural synapses, while the latter two are modulators of chordonotal organs. Thus, all of them exhibit neural activity as (neurotoxic) compounds leading to feeding inhibition and at least potentially affecting plant virus transmission. Even azadirachtin, a terpenoid extracted from seeds of the neem tree (Azadirachta indica A. Juss), exhibits some systemic activity and aphid antifeedant effect demonstrated in EPG recordings (Nisbet et al. 1993).
Contact insecticides applied to the surface of host (fruit) substrate are more relevant to non-hemipteroid species, including the spotted wing drosophila, than to hemipteroids. Yet, contact insecticides have received very limited attention with EPG to date. However, behavioral interference was also detected with EPG when organosphophates, carbamates, pyrethroids, and diamides were studied in whiteflies (He et al. 2013; Civolani et al. 2014). Also, spotted wing drosophila possesses spongiform mouthparts. Despite the morphological analogy between spongiform and sucking mouthparts, the feeding dynamic is distinct, with Bractocera fruit flies showing a fluid-centered mechanism of feeding with regurgitation and re-ingestion taking place (Vijaysegaran et al. 1997). The flies release watery fluid containing salivary enzymes and/or symbiotic bacteria from their crop onto the substrate for feeding (Vijaysegaran et al. 1997; Coronado-gonzalez et al. 2008), then re-ingest the fluid and released nutrients from the surface using pressure from the crop cibarial pump (Stoffolano and Haselton 2013). Thus, in addition to tarsal contact, pesticide will be potentially picked up from the plant surface by feeding, via dabbing and mainly ingestion per se. As a result, surface pesticides will reach spotted wing adults and may affect their ingestion.
Spinetoram is a spinosyn that exhibits allosteric modulation of nicotinid acetylcholine receptors (nACHR) of insect neural synapses (Sparks et al. 2001; Salgado and Sparks 2010; Casida and Durkin 2013), whose use is important for managing the spotted wing drosophila (Bruck et al. 2011; Van Timmeren and Isaacs 2013; Smirle et al. 2017). This insecticide also seems to antagonize response to γ-amino butyric ac. (GABA) (Sparks et al. 2001; Salgado and Sparks 2010), an inhibitory neurotransmitter, contributing to its neurotoxic activity. Therefore, feeding impairment was expected and detected when strawberry was treated with this compound, leading to reduced dabbing and ingestion by spotted wing adults. These effects likely contributed to the reduced longevity of adult insects exposed to spinetoram-treated diet, which reflects the acute effect of this compound, and may also impair other life history traits following sublethal exposure. The fungicide fenhexamid presents an interesting contrast.
We were not expecting significant behavioral differences from spotted wing drosophila on fenhexamid-treated strawberry, versus non-treated control berries, due to its basic activity—inhibiting cell-wall formation in fungus (Leroux 1996; Yang et al. 2011). Intriguingly, this fungicide also compromised dabbing and even ingestion by female flies, although mildly when compared with spinetoram. Such fenhexamid-impaired feeding did not compromise adult longevity. However, other life history traits could be affected, including fertility, which we did not assess in our study. Furthermore, the co-occurrence of both insecticide and fungicide residues in the same strawberry fruit surface during pre- and post-harvest is likely common and may spark unexpected responses in EPG studies, perhaps even synergism; thus, pesticide mixtures deserve attention in the future. Insecticide efficacy may very well be enhanced with this simultaneous exposure, but these compounds seem to impair fly–host interaction potentially interfering with sampling and behavior-based management tactics (Guedes et al. 2018).
In conclusion, for the first time for a dipteran insect, electropenetrography was successfully used to quantify the behaviors of spotted wing drosophila on strawberry fruits treated with pesticides. Significant feeding impairment was detected when strawberries were treated with spinetoram, which compromised adult longevity. Surprisingly, treatment of strawberries with the fungicide fenhexamid yielded a mild effect on feeding, although it did not affect longevity. Regardless, subtle responses do occur when pesticide-treated fruits are available for spotted wing drosophila and may interfere with fly survival, reproduction, and sampling. Furthermore, the present study demonstrates that AC–DC electropenetrography is useful as a tool for behavioral quantification that is more detailed than that provided by visual/video observation. Therefore, EPG will be relevant to understand dynamics of many insect–host interactions and sublethal effect of pesticides.
Author’s contribution
RNCG, FAC, EAB, and SSW conceived and designed the study. RNCG, FAC, and EAB established the experimental protocols, and EAB and SSW provided reagents and analytical tools. RNCG performed the experiments and analyzed the data. RNCG, FAC, and EAB structured the manuscript, and RNCG wrote the manuscript. All of the authors read, corrected, and approved the manuscript.
References
Asplen MK, Anfora G, Biondi A et al (2015) Invasion biology of spotted wing Drosophila (Drosohila suzikii): a global perspective and future priorities. J Pest Sci 88:469–494. https://doi.org/10.1007/s10340-015-0681-z
Backus EA (2000) Our own jabberwocky: clarifying the terminology of certain piercing-sucking behaviors of homopterans. In: Walker GP, Backus EA (eds) Principles and applications of electronic monitoring and other techniques in the study of homopteran feeding behavior. Entomological Soceity of America, Annapolis, pp 1–13
Backus EA, Bennett WH (1992) New AC electronic insect feeding monitor for fine-structure analysis of waveforms. Ann Entomol Soc Am 85:437–444. https://doi.org/10.1093/aesa/85.4.437
Backus EA, Bennett WH (2009) The AC–DC correlation monitor: new EPG design with flexible input resistors to detect both R and emf components for any piercing-sucking hemipteran. J Insect Physiol 55:869–884. https://doi.org/10.1016/j.jinsphys.2009.05.007
Backus, EA, Cervantes FA, Godfrey L, Akbarc W, Clark TL, Rojas MG (2018) Certain applied electrical signals during EPG cause negative effects on stylet probing behaviors by adult Lygus lineolaris (Hemiptera: Miridae). J Insect Physiol 105:64–75
Backus EA, Cline AR, Ellerseick MR, Serrano MS (2007) Lygus hesperus (Hemiptera: Miridae) feeding on cotton: new methods and parameters for analysis of nonsequential electrical penetration graph. Ann Entomol Soc Am 100:296–310
Backus EA, Vervantes FA, Guedes RNC, Li AY, Wayadande AC (2019) AC–DC electropenetrography for in-depth studies of feeding and oviposition behaviors. Ann Entomol Soc Am. https://doi.org/10.1093/1esa/saz009
Bellamy DE, Sisterson MS, Walse SS (2013) Quantifying host potentials: indexing postharvest fresh fruits for spotted wing drosophila, Drosophila suzukii. PLoS ONE 8:e61227. https://doi.org/10.1371/journal.pone.0061227
Bernays EA (1991) Evolution of insect morphology in relation to plants. Philos Trans R Soc B Biol Sci 333:257–264. https://doi.org/10.1098/rstb.1991.0075
Blande JD, Holopainen JK, Niinemets Ü (2014) Plant volatiles in polluted atmospheres: stress responses and signal degradation. Plant Cell Environ 37:1892–1904. https://doi.org/10.1111/pce.12352
Blanke A, Ruhr PT, Mokso R et al (2015) Structural mouthpart interaction evolved already in the earliest lineages of insects. Proc R Soc B 282:20151033. https://doi.org/10.1098/rspb.2015.1033
Boina DR, Youn Y, Folimonova S, Stelinski LL (2011) Effects of pymetrozine, an antifeedant of Hemiptera, on Asian citrus psyllid, Diaphorina citri, feeding behavior, survival and transmission of Candidatus Liberibacter asiaticus. Pest Manag Sci 67:146–155. https://doi.org/10.1002/ps.2042
Bruck DJ, Bolda M, Tanigoshi L et al (2011) Laboratory and field comparisons of insecticides to reduce infestation of Drosophila suzukii in berry crops. Pest Manag Sci 67:1375–1385. https://doi.org/10.1002/ps.2242
Calatayud PA, Seligmann CD, Polanía MA, Bellotti AC (2001) Influence of parasitism by encyrtid parasitoids on the feeding behaviour of the cassava mealybug Phenacoccus herreni. Entomol Exp Appl 98:271–278. https://doi.org/10.1023/A:1018947527397
Casida JE, Durkin KA (2013) Neuroactive insecticides: targets, selectivity, resistance, and secondary effects. Annu Rev Entomol 58:99–117. https://doi.org/10.1146/annurev-ento-120811-153645
Cervantes FA, Backus EA (2018) EPG waveform library for Graphocephala atropunctata (Hemiptera: Cicadellidae): Effect of adhesive, input resistor, and voltage levels on waveform appearance and probing behaviors. J Insect Physiol 109:21–40
Cervantes FA, Backus EA, Godfrey L et al (2017a) Ecology and behavior correlation of electropenetrography waveforms from Lygus lineolaris (Hemiptera: Miridae) feeding on cotton squares with chemical evidence of inducible tannins. J Econ Entomol 110:2068–2075. https://doi.org/10.1093/jee/tox198
Cervantes FA, Backus EA, Godfrey L et al (2017b) Behavior characterization of an EPG waveform library for adult Lygus lineolaris and Lygus hesperus (Hemiptera: Miridae) feeding on cotton squares. Ann Entomol Soc Am 109:684–697. https://doi.org/10.1093/aesa/saw039
Cini A, Ioriatti C, Anfora G (2012) A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull Insectol 65:149–160
Civolani S, Cassanelli S, Chicca M et al (2014) An EPG study of the probing behavior of adult Bemisia tabaci Biotype Q (Hemiptera: Aleyrodidae) following exposure to cyantraniliprole. J Econ Entomol 107:910–919. https://doi.org/10.1603/EC13511
Cole RA, Riggall W, Morgan A (1993) Electronically monitored feeding behaviour of the lettuce root aphid (Pemphigus bursarius) on resistant and susceptible lettuce varieties. Entomol Exp Appl 68:179–185
Coronado-gonzalez PA, Vijaysegaran S, Robinson AS (2008) Functional morphology of the mouthparts of the adult Mediterranean fruit fly, Ceratitis capitata. J Insect Sci 8:1–11
Daniels M, Bale JS, Newbury HJ et al (2009) A sublethal dose of thiamethoxam causes a reduction in xylem feeding by the bird cherry-oat aphid (Rhopalosiphum padi), which is associated with dehydration and reduced performance. J Insect Conserv 55:758–765. https://doi.org/10.1016/j.jinsphys.2009.03.002
Deprá M, Poppe JL, Schmitz HJ et al (2004) The first records of the invasive pest Drosophila suzukii in the South American continent. J Pest Sci 87:379–383. https://doi.org/10.1007/s10340-014-0591-5
Desneux N, Decourtye A, Delpuech J-M (2007) The sublethal effects of pesticides on benefitial arthropods. Annu Rev Entomol 52:81–206. https://doi.org/10.1146/annurev.ento.52.110405.091440
Ebert TA, Backus EA, Cid M et al (2015) A new SAS program for behavioral analysis of electrical penetration graph data. Comput Electron Agric 116:80–87. https://doi.org/10.1016/j.compag.2015.06.011
Garzo E, Moreno A, Hernando S et al (2015) Electrical penetration graph technique as a tool to monitor the early stages of aphid resistance to insecticides. Pest Manag Sci 72:707–718. https://doi.org/10.1002/ps.4041
Gatehouse JA (2002) Plant resistance towards insect herbivores: a dynamic interaction. New Phytol 156:145–169. https://doi.org/10.1046/j.1469-8137.2002.00519.x
Guedes RNC, Smagghe G, Stark JD, Desneux N (2016) Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu Rev Entomol 61:43–62. https://doi.org/10.1146/annurev-ento-010715-023646
Guedes RNC, Walse SS, Throne JE (2017) Sublethal exposure, insecticide resistance, and community stress. Curr Opin Insect Sci 21:47–53. https://doi.org/10.1016/j.cois.2017.04.010
Guedes RNC, Corbett S, Rodriguez M, Walse JJGSS (2018) Pesticide-mediated disruption of spotted wing Drosophila flight response to raspberries. J Appl Entomol 142:457–464. https://doi.org/10.1111/jen.12500
Guedes RNC, Cervantes FA, Backus EA, Walse SS (2019) Substrate-mediated feeding and egg-laying by spotted wing drosophila: waveform recognition and quantification via electropenetrography. J Pest Sci 92:495–507. https://doi.org/10.1007/s10340-018-1065-y
Hamby KA, Bellamy DE, Chiu JC, Lee JC, Walton VM, Wiman NG, York RM, Biondi A (2016) Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii. J Pest Sci 89:605–619. https://doi.org/10.1007/s10340-016-0756-5
Harrewijn P (1997) Pymetrozine, a fast-acting and selective inhibitor of aphid feeding. In-situ studies with electronic monitoring of feeding behaviour. Pestic Sci 49:130–140
Harrewijn P, Piron PGM, Mollema C (1996) Electrically recorded probing behaviour of thrips species on optimal and suboptimal hosts. Entomol Exp Appl 80:43–45
Hauser M (2011) A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag Sci 67:1352–1357. https://doi.org/10.1002/ps.2265
He Y, Zhao J, Zheng Y et al (2013) Assessment of potential sublethal effects of various insecticides on key biological traits of the tobacco whitefly, Bemisia tabaci. Int J Biol Sci 9:246–255. https://doi.org/10.7150/ijbs.5762
Holopainen JK, Blande JD (2013) Where do herbivore-induced plant volatiles go? Front Plant Sci 4:1–13. https://doi.org/10.3389/fpls.2013.00185
Itskov PM, Moreira J-M, Vinnik E et al (2014) Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat Commun 5:4560. https://doi.org/10.1038/ncomms5560
Jaenike J (1990) Host specialization in phytophagous insects. Annu Rev Ecol Syst 21:243–273
Jurgens A, Bischoff M (2016) Changing odour landscapes: the effect of anthropogenic volatile pollutants on plant—pollinator olfactory communication. Funct Ecol. https://doi.org/10.1111/1365-2435.12774
Kindt F, Joosten NN, Peters D, Tjallingii WF (2003) Characterisation of the feeding behaviour of western flower thrips in terms of electrical penetration graph (EPG) waveforms. J Insect Physiol 49:183–191. https://doi.org/10.1016/S0022-1910(02)00255-X
Labandeira CC (1997) Insect mouthparts: ascertaining the paleobiology of insect feeding strategies. Annu Rev Entomol 28:153–193
Lee JC, Bruck DJ, Dreves AJ et al (2011) Spotted wing drosophila, Drosophila suzukii, across perspectives. Pest Manag Sci 67:1349–1351. https://doi.org/10.1002/ps.2271
Lee JC, Dalton DT, Swoboda-Bhattarai KA, Bruck DJ, Burrack HJ, Strik BC, Woltz JM, Walton VM (2016) Characterization and manipulation of fruit susceptibility to Drosophila suzukii. J Pest Sci 89:771–780. https://doi.org/10.1007/s10340-015-0718-3
Leroux P (1996) Recent developments in the mode of action of fungicides. Pestic Sci 47:191–197
Lihoreau M, Poissonnier L, Isabel G, Dussutour A (2016) Drosophila females trade off good nutrition with high-quality oviposition sites when choosing foods. J Exp Biol 219:2514–2524. https://doi.org/10.1242/jeb.142257
Lin Q-C, Zhai Y-F, Zhou C-G, Li L-L, Zhuang Q-Y, Zhang X-Y, Zalom FG, Yu Y (2014) Behavioral rhythms of Drosophila suzukii and Drosophila melanogaster. Fla Entomol 97:1424–1433. https://doi.org/10.1653/024.097.0417
Losel PM, Goodman LJ (1993) Effects on the feeding behaviour of Nilaparvata lugens (Stal) of sublethal concentrations of the foliarly applied nitromethylene heterocycle 2-nitromethylene-1,3-thiazinan-3-yl-carbamaldehyde. Physiol Entomol 18:67–74
Morita M, Ueda T, Yoneda T, Koyanagi T (2007) Flonicamid, a novel insecticide with a rapid inhibitory effect on aphid feeding. Pest Manag Sci 973:969–973. https://doi.org/10.1002/ps.1423
Nisbet AJ, Woodford JAT, Strang RHC, Connolly JD (1993) Systemic antifeedant effects of azadirachtin on the peach-potato aphid Myzus persicae. Entomol Exp Appl 68:87–98
McLean DL, Kinsey MG (1964) A technique for electronically recording aphid feeding and salivation. Nature 202:1358–1359
Plantamp C, Estragnat V, Fellous S et al (2017) Where and what to feed? Differential effects on fecundity and longevity in the invasive Drosophila suzukii. Basic Appl Ecol 19:56–66. https://doi.org/10.1016/j.baae.2016.10.005
Rangasamy M, Mcauslane HJ, Backus EA, Cherry RH (2015) Differential probing behavior of Blissus insularis (Hemiptera: Blissidae) on resistant and ausceptible St. Augustinegrasses. J Econ Entomol 108:780–788. https://doi.org/10.1093/jee/tou061
Rota-Stabelli O, Blaxter M, Anfora G (2013) Drosophila suzukii. Curr Biol 23:R8–R9. https://doi.org/10.1016/j.cub.2012.11.021
Salgado VL, Sparks TC (2010) The spinosyns: chemistry, biochemistry, mode of action, and resistance. In: Gilbert LI, Latrou K, Gill SS (eds) Comprehensive molecular insect science. Elsevier, Oxford, pp 137–173
Sih A (2013) Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim Behav 85:1077–1088. https://doi.org/10.1016/j.anbehav.2013.02.017
Simpson SJ, Clissold FJ, Lihoreau M et al (2015) Recent advances in the integrative nutrition of arthropods. Annu Rev Entomol 60:293–311. https://doi.org/10.1146/annurev-ento-010814-020917
Smirle MJ, Zurowski CL, Ayyanath M et al (2017) Laboratory studies of insecticide efficacy and resistance in Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) populations from British Columbia, Canada. Pest Manag Sci 73:130–137. https://doi.org/10.1002/ps.4310
Smith TB, Kinnison MT, Strauss SY et al (2014) Prescriptive evolution to conserve and manage biodiversity. Annu Rev Ecol Evol Syst 45:1–22. https://doi.org/10.1146/annurev-ecolsys-120213-091747
Sparks TC, Crouse GD, Durst G (2001) Natural products as insecticides: the biology, biochemistry and quantitative structure-activity relationships of spinosyns and spinosoids. Pest Manag Sci 57:896–905. https://doi.org/10.1002/ps.358
Stoffolano JG Jr, Haselton AT (2013) The adult dipteran crop: a unique and overlooked organ. Annu Rev Entomol 58:205–228. https://doi.org/10.1146/annurev-ento-120811-153653
Tait G, Grassi A, Pfab F, Crava CM, Dalton DT, Magarey R et al (2018) Large-scale spatial dynmaics of Drosophila suzukii in Trentino, Italy. J Pest Sci 91:1213–1224. https://doi.org/10.1007/s10340-018-0985-x
Tariq K, Noor M, Backus EA et al (2017) The toxicity of flonicamid to cotton leafhopper, Amrasca biguttula (Ishida), is by disruption of ingestion: an electropenetrography study. Pest Manag Sci 73:1661–1669. https://doi.org/10.1002/ps.4508
Van Timmeren S, Isaacs R (2013) Control of spotted wing drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection programs. Crop Prot 54:126–133. https://doi.org/10.1016/j.cropro.2013.08.003
Tjallingii FT (1978) Electronic recording of penetration behaviour by aphids. Ent Exp Appl 24:721–730
Vijaysegaran S, Walter GH, Drew RAI (1997) Mouthpart structure, feeding mechanisms, and natural food sources of adult Bactrocera (Diptera: Tephritidae). Ann Entomol Soc Am 90:184–201
Walker GP (2000) A Beginner’s guide to electronic monitoring. In: Walker GP, Backus EA (eds) Principles and applications of electronic monitoring and other techniques in the study of homopteran feeding behavior. Entomological Soceity of America, Annapolis, pp 14–40
Walse SS, Krugner R, Tebbets JS (2012) Postharvest treatment of strawberries with methyl bromide to control spotted wing drosophila, Drosophila suzukii. J Asia Pac Entomol 15:451–456. https://doi.org/10.1016/j.aspen.2012.05.003
Wong JS, Cave AC, Lightle DM, Mahaffee WF, Naranjo SE, Wiman NG et al (2018) Drosophila suzukii flight performance reduced by starvation but not affected by humidity. J Pest Sci 91:1269–1278. https://doi.org/10.1007/s10340-018-1013-x
Xue K, Wang X-Y, Huang C-H et al (2009) Stylet penetration behaviors of the cotton aphid Aphis gossypii on transgenic Bt cotton. Insect Sci 16:137–146. https://doi.org/10.1111/j.1744-7917.2009.00265.x
Yang C, Hamel C, Vujanovic V, Gan Y (2011) Fungicide : modes of action and possible impact on nontarget microorganisms. ISRN Ecol ID 130289. https://doi.org/10.5402/2011/130289
Youn Y, Backus EA, Serikawa RH, Lukasz L (2011) Correlation of an electrical penetration graph waveform with walking by Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Fla Entomol 94:1084–1087
Acknowledgements
Financial support was provided by the CAPES Foundation (Brazilian Ministry of Education; Finance Code 001) and USDA-ARS, which was greatly appreciated. The research was also supported in part by an appointment to the Agricultural Research Service (ARS) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Agriculture (USDA). ORISE is managed by Oak Ridge Associated Universities (ORAU) under DOE contract number DE-SC0014664. All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of CAPES, USDA, ARS, DOE, or ORAU/ORISE. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were considered in the present study.
Informed consent
The authors of this manuscript accept that the paper is submitted for publication in the Journal of Pest Science, and report that this paper has not been published or accepted for publication in another journal, nor is under consider at another journal.
Additional information
Communicated by A. Biondi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guedes, R.N.C., Cervantes, F.A., Backus, E.A. et al. Electropenetrography of spotted wing drosophila (Drosophila suzukii) on pesticide-treated strawberry. J Pest Sci 93, 91–102 (2020). https://doi.org/10.1007/s10340-019-01124-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-019-01124-6