Abstract
Dicyphus bolivari Lindberg and Dicyphus errans (Wolff) (Hemiptera: Miridae) are naturally widespread in many crops with low-pesticide pressure, where they prey upon several arthropods, including the tomato pinworm Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). However, their efficacy as biological control agents (BCAs) of this pest needs further investigations. Therefore, in this study the predatory efficacy of D. bolivari and of D. errans on T. absoluta was evaluated on tomato in laboratory and greenhouse trials. Their functional response to different numbers of T. absoluta eggs (up to 350) offered to single females or 5th-instar nymphs for 24 h was assessed in laboratory. Females and nymphs of both predators showed a high voracity and a type II functional response, with an estimated maximum predation rate per day of 189 and 194 eggs for D. bolivari females and nymphs, respectively, and 197 and 179 eggs for D. errans females and nymphs, respectively. The predators showed similar predation rates of T. absoluta eggs on plants in cage trials. However, our greenhouse trial showed that the commonly used Macrolophus pygmaeus (Rambur) (Hemiptera: Miridae), which has a lower individual predation capacity than D. bolivari and D. errans, was more effective in controlling T. absoluta than D. errans and D. bolivari because of its stronger numerical response to densities of T. absoluta and supplemental food than the other two predator species. This shows that long-term greenhouse trials, which include functional and numerical responses to pest densities, are essential to evaluate the efficacy of an omnivorous predator.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
The potential of Dicyphus bolivari and Dicyphus errans as biological control agents of Tuta absoluta was evaluated
-
In laboratory, females and 5th-instar nymphs of both mirids showed a type II functional response to eggs of T. absoluta
-
In greenhouse, both predators were able to reduce the population of T. absoluta, but less effectively than Macrolophus pygmaeus, which showed the strongest numerical response
-
Functional response studies should be combined with population dynamics and multiple-prey studies to evaluate omnivorous predator efficacy
Introduction
Tomato crop [Solanum lycopersicum L. (Solanaceae)] is affected by several pests (e.g., aphids, leaf miners, spider mites, thrips, whiteflies). Recently, in the Mediterranean area, the exotic tomato pinworm Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) has become a serious threat for this crop, and chemical control is often ineffective because of its cryptic habits and high pesticide resistance (Desneux et al. 2010; Tropea Garzia et al. 2012; Campos et al. 2017; Biondi et al. 2018; Roditakis et al. 2018). To reduce both pest infestations and pesticide use, sustainable control can be achieved using effective biological control agents (BCAs).
Hemipteran predators, such as anthocorids, geocorids, mirids, nabids, and pentatomids, have been identified as potential BCAs against T. absoluta (Ferracini et al. 2019). Omnivorous mirids were used soon after the pests’ arrival, through augmentative and inoculative releases in fields and plant nurseries, sometimes supported by conservation strategies (e.g., using banker plants) (Biondi et al. 2018). Several species of mirid predators belonging to the tribe Dicyphini (Heteroptera: Miridae) have been reported in many crops with low-pesticide pressure preying upon various pests (Ingegno et al. 2008, Leman et al. 2019). However, only a few dicyphine species [i.e., Macrolophus pygmaeus (Rambur) and Nesidiocoris tenuis (Reuter)] are commercially produced and released, while many other species belonging to the genus Dicyphus have been observed in several crops (Ingegno et al. 2017a; Sanchez and Cassis 2018). Among them, the Mediterranean Dicyphus bolivari Lindberg [previously named Dicyphus maroccanus Wagner (Sanchez and Cassis 2018)] and the Palaearctic Dicyphus errans (Wolff) live omnivorously on various host plants and pests (Ingegno et al. 2008; Voigt et al. 2007; Abbas et al. 2014). Recently, both predators have been reported to prey upon T. absoluta in open field and greenhouse crops (Zappalà et al. 2013; Abbas et al. 2014). Moreover, D. errans showed potential as a BCA of this exotic pest under laboratory conditions (Ingegno et al. 2013, 2017a, b).
A fundamental aspect in multitrophic interactions and population dynamics in a predator–prey system is the capability of a BCA to find, kill and consume prey (Nachman 2006). Predator’s feeding rate related to changes in prey density, the so-called functional response, is one of the key components to assess its effectiveness in controlling pest populations and the stability of prey–predator dynamics (Abrams 1982; Fernández-Arhex and Corley 2003). The numerical response (increase in densities) of the predator to prey densities is another key component for evaluating its potential as BCA, and biological control eventually represents the combined results of functional and numerical responses to pests (Coll and Ridgway 1995; Van Den Meiracker and Sabelis 1999).
The main aim of this study was to further evaluate, under laboratory and greenhouse conditions, the potential of D. bolivari and D. errans as BCAs of T. absoluta by comparing their predatory traits through assessment of: (i) functional responses of females and nymphs to increasing egg densities in arena trials; (ii) daily predation of eggs on infested tomato plants in cage trials; (iii) population dynamics of T. absoluta and the predators in a greenhouse trial with a time period long enough to observe the numerical response of the released predators. The commonly used predator M. pygmaeus was included as a reference in this greenhouse trial.
Materials and methods
Insect and plant rearing
Colonies of D. bolivari were started from individuals collected on tomato in the Valencia region, Spain, in 2015. They were reared on pods of the flat bean Phaseolus vulgaris L. (Fabaceae) in transparent plastic cylinder cages (H 27 cm, ∅ 25 cm; JET 107 PM, Jokey plastic GmbH, Sohland, Germany) with the drilled lid covered with a fine net mesh. Colonies of D. errans were started from individuals collected on the European black nightshade Solanum nigrum L. (Solanaceae) in Piedmont region, NW Italy, in 2015. They were reared on this plant species, as well as on tomato and tobacco Nicotiana tabacum L. (Solanaceae), in insect cubic cages (47.5 cm edge; BugDorm, MegaView, Taiwan). Both dicyphine species were supplied with eggs of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) mixed with dehydrated and decapsuled cysts of Artemia sp. (Anostraca: Artemiidae) (Entofood, Koppert B.V. Berkel en Rodenrijs, The Netherlands). Colonies of M. pygmaeus were established starting from individuals purchased from Koppert Biological Systems (Berkel en Rodenrijs, The Netherlands) and reared on flat bean pods inside transparent plastic cylinder boxes (H 27 cm, ∅ 25 cm, JET 107 PM, Jokey Plastik GmbH, Sohland, Germany). Ventilation was possible through a hole in the lid covered with insect gauze (mesh size 80 µm). All cultures were kept in climatic chambers at 25 ± 1 °C, 70 ± 5% RH, 16:8 L:D.
Colonies of T. absoluta were established from individuals provided by Bioplanet laboratories (Bioplanet s.c.a., Cesena, Italy). A continuous rearing was maintained on tomato plants in net cages (W 150 × L 150 × H 110 cm) consisting of a stainless steel frame structure supporting an insect-proof net (mesh 0.23 × 0.23 mm). The rearing was carried out in an experimental heated greenhouse at 27 ± 3 °C and 55 ± 23% RH.
For plant growing, seeds from seed companies were used, except for seeds of S. nigrum, which were collected from wild plants grown in Piedmont region. Seeds were sown in plastic pots (∅ 14 cm), watered daily and fertilized. Plants were grown in an experimental heated greenhouse at 27 ± 3 °C and 55 ± 23% RH.
Functional response trials
In laboratory, 1-week-old females of D. bolivari and D. errans were used to assess their functional response to T. absoluta egg densities. After starving them for 16 h, to standardize individual behaviour, single females were exposed to definite prey densities for 24 h. Specifically, eight treatments consisting of different amounts of T. absoluta eggs offered as prey on tomato leaflets (i.e., 5, 10, 25, 50, 90, 150, 250 and 350 eggs) were set up to fit the functional response curve. The experiments were carried out in artificial arenas with excised leaflets, which were shown to be representative for functional response studies on a plant level for the related M. pygmaeus (Maselou et al. 2014). Tuta absoluta eggs were offered on three tomato leaflets, with stalks placed into 2-ml plastic tubes containing water and sealed with Parafilm®, inside a Petri dish (∅ 20 cm). After 24 h, the female was removed, and the leaflets were inspected under a stereomicroscope to count predated eggs. Five replicates were performed for each treatment. Experiments were carried out in climatic chambers at 24 ± 1 °C, 65 ± 5% RH and 16:8 L:D.
The functional response of juvenile predators on T. absoluta eggs was assessed by using 5th-instar nymphs of D. bolivari and D. errans with the same experimental conditions and procedure above reported for females. Ten treatments consisting of different amounts of T. absoluta eggs offered as prey on tomato leaflets (i.e., 5, 10, 15, 20, 40, 50, 100, 150, 200, 300 eggs) were set up. Five replicates were performed for each treatment.
Predation trials on plants
In laboratory, the predatory efficacy of D. bolivari and D. errans on tomato plants infested with T. absoluta was investigated through cage trials. Three treatments were compared: (1) tomato infested with T. absoluta; (2) tomato infested with T. absoluta plus one female of D. bolivari, (3) tomato infested with T. absoluta plus one female of D. errans. A tomato plant (H 40 cm, with 6–7 leaves) was infested with a constant amount of 20 T. absoluta eggs (< 24 h), gently transferred with a brush, every 24 h per 3 days (for a total of 60 eggs) and isolated in a net insect tent cage (W 60 × L 60 × H 60 cm, BugDorm, MegaView, Taiwan). After 24 h from first egg insertion, in the treatment 2 and in the treatment 3, one female of D. bolivari or of D. errans was introduced and removed after 72 h, respectively, while the treatment 1 was kept as control without predators. The number of T. absoluta eggs was checked at 24, 48 and 72 h from the predator introduction, and the number of T. absoluta larvae was counted at the last egg checking (96 h from the first egg introduction). Ten replicates were performed for each treatment. The experiment was carried out in climatic chambers at 24 ± 1 °C, 65 ± 5% RH and 16:8 L:D.
Population dynamics greenhouse trials
A greenhouse trial was set up to evaluate the effects of pre-established populations of D. bolivari and D. errans on T. absoluta establishment and population increase. The commonly used M. pygmaeus was included in this trial as a reference treatment. The experiment was conducted in a greenhouse compartment of 98 m2 at Wageningen University & Research in Bleiswijk, The Netherlands. Three-week-old tomato plants (with each four developed leaves), cv Brioso (Rijk Zwaan, The Netherlands), were grown individually in rock wool blocks, and each block was placed on a 50 cm rock wool slab. The young plants were vaccinated with a mild isolate of the Pepino mosaic virus (PepMV) (PMV®-01, DCM, Belgium), to offer protection against more aggressive isolates, which is a common practice for Dutch tomato growers. Nutrients for the plants were provided through drip irrigation. Each tomato plant was subsequently enclosed in a mesh cage (60 × 60 × 180 cm) made of fine gauze (mesh size 500 µm, Vermandel, The Netherlands) representing an experimental unit. The experimental units were distributed within the greenhouse using a randomized block design with the following treatments: (A) no predators (control), (B) D. bolivari, (C) D. errans and (D) M. pygmaeus. There were five replicates of each treatment, thus 20 experimental units were used in total.
The predators were introduced 2, 3 and 4 weeks after placing the plants in the cages in densities of, respectively, 3, 3 and 6 couples of 1-week-old adults (12 females and 12 males in total). The establishment and population growth of the predators were supported by adding weekly 0.5 gr of sterilized E. kuehniella eggs per cage (Koppert B.V. Berkel en Rodenrijs, The Netherlands). The eggs were spread all over the plant with a fine brush and applied for 7 weeks, starting in the week of the first predator introduction. Tuta absoluta was introduced as adults, starting in the same week of the last predator introduction. The introduction was spread over 4 weeks to ensure that eggs and first larval stages (vulnerable for predation) were present over a longer time period. Young mated couples of T. absoluta were weekly introduced in densities of 1, 2, 1 and 3 couples per cage (7 females and 7 males in total). Densities of predatory mirids and T. absoluta were monitored every 2 weeks, during a 6-week period, by counting the total number of each species per cage (nymphs and adults of predatory mirids and larvae, pupae and adults of T. absoluta).
Temperature and relative humidity in the greenhouse compartment were recorded every 5 min using a climate recorder (Hoogendoorn Growth Management, Vlaardingen, The Netherlands) throughout the experiment. The average temperature and relative humidity during the experiment (from the time predators were introduced) were 21.9 °C (range 14.9–39.1 °C) and 64% (range 32–89%), respectively.
Statistical analyses
Functional response type and parameters of attack rate and handling time showed by D. bolivari and D. errans females and 5th-instar nymphs were estimated using the general approach proposed by Okuyama (2012a). This approach includes the application of a model selection index (i.e., Akaike information criterion, AIC) directly to candidate models. We used the maximum likelihood approach to obtain parameter estimates of the Holling’s type II (Eq. 1), Rogers random predator equation (Eq. 2) and Holling’s type III (Eq. 3) (Bolker 2008; Okuyama 2012b). The maximum likelihood method is less used than the typical estimation method of least squares, but it has been recently been accounted for better performances in estimating functional response parameters than the estimation method of least squares (Okuyama 2012b). The three functional response models were then used to fit the data regarding the consumption of T. absoluta eggs, using the R package bbmle for maximum likelihood estimation (mle); since Na is on both left and right side of the equation, the Eq. 2 has been modified using LambertW function (Bolker 2008; Haddaway et al. 2012). Rogers random predator equation describes a type II functional response but accounts for prey depletion, that is, the case of our experiments, since mirid predators completely consume preyed eggs. The best model for each mirid species and life stage was selected using the AIC test (lower AIC means a better model fit), from which the coefficients a (attack rate) and Th (handling time) were obtained and compared using confidence intervals (95%). Mean values of Th were used to calculate the maximum attack rate as T/Th (Hassell 2000), representing the maximal number of T. absoluta eggs that could be attacked during the considered time interval (in our case T = 1 day). Statistical analyses were performed using the statistical software R (R Core Team 2018).
Equation 1 Holling’s type II functional response model
Equation 2 Rogers random predator equation
Equation 3 Holling’s type III functional response model
Data on cage trials were analysed by performing one-way ANOVA since data satisfied normality and homogeneity criteria (Shapiro–Wilk and Levene tests). When significantly different, means were separated by Tukey’s test (P < 0.05). A Student t test was performed for comparing the two tested species. Statistical analyses were performed using the statistical software SPSS, 25th edition (IBM Corp., NY, USA).
Population dynamics of predators and T. absoluta in the greenhouse trial were analysed with repeated measures ANOVA. Predator densities were based on the cumulative numbers of nymphs and adults; T. absoluta densities were based on the cumulative numbers of larvae, pupae and adults. Data were prior to the analyses log(+ 1) transformed to fit a normal distribution. Differences among treatments were tested with Fisher’s least significant difference (LSD) test (P < 0.05). These analyses were performed using the software package Genstat, 18th edition.
Results
Functional response trials
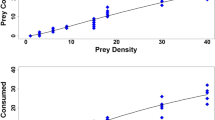
The two mirids D. bolivari and D. errans presented very similar functional response curves showing a type II functional response, which is inverse density dependent, both for the adult and nymphal stage (Fig. 1). The model fits were compared and are reported in Table 1. Rogers random predator function presented lower values for females and 5th-instar nymphs of both predator species, which indicates that accounting for prey depletion results in models of better fit. Parameters estimated by Rogers random predator equation were all significantly different from zero, showing a marked increase in consumed eggs with an increasing number of offered eggs. Comparing the confidence intervals of functional response parameters, D. bolivari and D. errans did not differ significantly in attack rate of T. absoluta eggs (Table 2), although D. bolivari showed a trend towards a greater attack rate compared to D. errans, for both females and 5th-nymphs. The two mirids also did not differ in handling time of T. absoluta eggs (Table 2), regardless of the considered life stage. These results led to similar maximum attack rates: 188.52 and 194.18 eggs day−1 for D. bolivari females and 5th-instar nymphs, respectively, and 197.24 and 178.58 eggs day−1 for D. errans females and 5th-instar nymphs, respectively. In our experiment, when exposed to the highest density of 350 T. absoluta eggs, predator females consumed on average more than 130 eggs per day.
Predation trials on plants
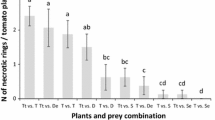
No differences in the number of daily preyed eggs of T. absoluta were found between the two predators D. bolivari and D. errans (Student t test; df = 18; after 24 h: F = 0.109, P = 0.745; after 48 h: F = 1.580, P = 0.225; after 72 h: F = 0.027, P = 0.871). Except for the first 24 h, both predators were able to prey around 12–14 eggs per day with a continuous daily offer of 20 eggs (Table 3). The total number of T. absoluta eggs consumed over 3 days of exposure by both predators was also similar, namely 29.0 ± 2.1 for D. bolivari and 33.5 ± 2.6 for D. errans (Student t test; df = 18; F = 0.732; P = 0.404). The total number of viable T. absoluta eggs after 3 days of exposure to both predators was significantly different from that one of the control without predators (ANOVA; df = 2; F = 36.634; P < 0.0001) (Table 3). Also the number of emerged larvae at the end of the trial (i.e., 96 h after the first egg insertion) was significantly different between treatments with predators and without predators (ANOVA; df = 2; F = 5.601; P < 0.01) (Table 3).
Population dynamics greenhouse trials
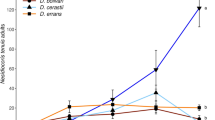
Densities of T. absoluta were significantly different among treatments through time (F3,12 = 19.12, P < 0.001). The predators D. bolivari and D. errans both equally reduced the population increase in T. absoluta compared to the treatment without predators, but M. pygmaeus clearly better suppressed T. absoluta (Fig. 2A). Densities of predators were also significantly different among species (F2,8 = 10.41, P = 0.006), with the highest densities observed for M. pygmaeus and comparable lower densities for D. bolivari and D. errans (Fig. 2B).
Population growth in greenhouse trials of (A) Tuta absoluta in treatments with no predators (untreated) and three species of mirid predators, and (B) the three species of the mirid predators. Data shown are the mean (± SE) densities of (A) larvae, pupae and adults of T. absoluta and (B) nymphs and adults of mirid predators per experimental unit. Predators were released in week 1, 2 and 3 and T. absoluta in week 3, 4, 5 and 6. Different letters indicate significant differences among treatments through time (Fisher’s LSD test, P < 0.05)
Discussion
The functional responses of D. bolivari and D. errans to T. absoluta eggs, which were compared here for the first time, show that these mirids can consume large quantities of T. absoluta eggs. The high-estimated maximum attack rate (i.e., 180–200 eggs per day) for both predators and the short handling time highlight the voracity of these predator species. The predatory response of females and 5th-instar nymphs of D. bolivari and D. errans to increasing densities of T. absoluta eggs was described by a type II functional response, consistent with the results reported in most studies involving dicyphine species (Alvarado et al. 1997; Foglar et al. 1990; Montserrat et al. 2000; Fantinou et al. 2008; Maselou et al. 2014, 2015). The type II functional response is often characteristic of invertebrate predators that provide efficient pest control, even though it is often associated with unstable predator–prey population dynamics (Juliano 2001; Briggs and Hoopes 2004; Nachman 2006). However, these fluctuating dynamics are less crucial for omnivores that stabilize their densities by feeding on plants and/or on provided alternative food sources (Messelink et al. 2014).
Several studies have also reported that dicyphine predators show a type III functional response (Enkegaard et al. 2001; Hamdan 2006). A recent study on three Neotropical dicyphine species showed a type II functional response for one species and type III for the other two species to egg densities of T. absoluta (van Lenteren et al. 2016). The different outcomes achieved by these studies may be due to difficulties in discriminating readily between type II and III functional response curves or to biological differences in both prey and predator species investigated. At relatively low prey densities, it may be difficult to distinguish between different types of functional responses (Fantinou et al. 2008). In the present study, we provided a large range of densities of eggs to females (from 5 to 350 eggs) and to 5th-instar nymphs (from 5 to 300 eggs) of both predator species. This enabled us to clearly define the response type as type II, with the average proportion of consumed eggs steadily declining as the number of offered eggs increased.
Previous studies showed a preference by the mirid predators D. errans, M. pygmaeus and N. tenuis for T. absoluta eggs rather than larvae (Ingegno et al. 2013; Jaworski et al. 2013; Urbaneja et al. 2009), probably because of the concealing behaviour of the larvae. Van Lenteren et al. (2016) reported Macrolophus basicornis (Stål) as the best candidate for control of the tomato pinworm in Brazil, compared to other two tested species. At the highest provided egg density (256), this species consumed 100 eggs per day, whereas the other two predator species Campyloneuropsis infumatus (Carvalho) and Engytatus varians (Distant) consumed 51 and 91 eggs per day, respectively. In our study, D. bolivari and D. errans were able to prey around 115–125 eggs at similar prey density (250 offered eggs), respectively, showing even better performances compared to their Neotropical relatives. These two predator species show also higher maximum predation rates than that one reported for the commonly used predator M. pygmaeus (ca. 90 eggs day−1, Michaelides et al. 2018).
Mirid predator preference towards eggs was especially evident for nymphal stages of M. pygmaeus and N. tenuis, probably due to their small size (Jaworski et al. 2013; Urbaneja et al. 2009). In our study, 5th-instar nymphs of D. bolivari and D. errans were able to predate more than 100 eggs per day when exposed to the highest density of T. absoluta eggs (i.e., 300). Comparing the maximum attack rates, females and nymphs showed to prey almost the same amount of T. absoluta eggs, even though females are larger than 5th-instar nymphs and have different feeding requirements, also related to the biomass needed for egg development (Fellowes et al. 2007). Nevertheless, 5th-instar nymphs of both predator species consistently preyed T. absoluta eggs at each offered density, thus highlighting the strong predatory capabilities of the late nymphal instar of these mirids on the pest.
Dicyphus bolivari and D. errans showed their predation capability on T. absoluta also in cage and in greenhouse trials, being able to reduce the population of T. absoluta. On plants in cage, after a period of adaptation of 24 h, both predators preyed on average 12–14 eggs of the tomato pinworm over the 20 fresh eggs daily offered, as estimated by the trend of the functional response curves (~ 15) (Fig. 1). Tuta absoluta usually has an oviposition peak in the first 3 days after adult emergence, during which each female can lay up to 12–14 eggs per day (Lee et al. 2014), although higher peaks were also recorded (Pereyra and Sánchez 2006). Therefore, at the beginning of infestation, a 1:1 predator/prey ratio could lead to a successful control of the pest. However, since the viable eggs were not daily removed, the number of available eggs per day per female raised the quote of 20 with a consequent higher hypothetic expected number of consumed eggs. This discrepancy between real and predicted values of preyed eggs can be attributed to the different experimental scales. The presence of an entire plant could have influenced several aspects, such as phytophagy and searching time, compared to arena scale with excised leaflets, in contrast with what observed in Maselou et al. (2014).
The high predation rates of T. absoluta eggs we observed for D. bolivari and D. errans suggest that these predators can potentially be more effective in controlling T. absoluta than the commonly used M. pygmaeus. However, the greenhouse trial showed the opposite effect: M. pygmaeus was clearly more effective in controlling the tomato pinworm than D. bolivari and D. errans. At the same time, we observed a much faster population increase for this predator compared to D. bolivari and D. errans, which is a likely explanation for the difference in suppression of T. absoluta. This shows that lower predation capacity per individual can soon be compensated by a stronger population increase, which increases the predation capacity of the total predator population. The predation capacity per individual probably had a stronger influence at the start of the experiment when equal numbers of predators were released, but in a longer time period the numerical response of the predators was eventually a more defining factor. Yet, it needs to be considered that these results were achieved in the summer period with high temperature peaks. The numerical response of the predators may have been different under other climatic conditions, for example at lower temperatures, which would have changed the outcome of the experiment. Also, a longer time period to build-up predator densities prior to the introduction of T. absoluta could have resulted in a more effective control of T. absoluta by D. bolivari and D. errans than achieved in the trial of this study.
The results of the greenhouse study show that functional response studies for omnivorous predators are relevant to assess their behaviour at different prey densities and their potential predation capacity, but they are not completely defining their predation efficacy to evaluate them as BCAs. Since they are omnivores, their functional response to a specific pest will also be strongly affected by the presence of other prey. For example, increases in the numbers of one prey often decrease the predator’s functional response to other prey, either due to satiation or switching (Murdoch 1969). Additionally, increases in the density of one prey increase predator numbers (numerical response), which can reduce the numbers of a second prey species (Holt 1977). For these reasons, the functional response to a single pest can be considered as a starting point to evaluate omnivorous predators but it should go together with studies on population dynamics, which allow assessing numerical response during the experimental time period, and multiple-prey studies. Moreover, the nutritional quality of their host plant will also affect predation rates by changing their extent of plant feeding and flexible feeding behaviour (Castañé et al. 2011; Biondi et al. 2016). Plant quality may also indirectly affect the functional response to pests through the accumulation of secondary metabolites in prey (Koller et al. 2007).
In conclusion, our study shows the potential of D. bolivari and D. errans as BCAs of T. absoluta, but further investigations are still needed to evaluate their ability of establishment, reproduction rate, developmental time and survival in different environmental and agronomic conditions and behaviour in multiple-prey communities that are common in tomato crops. Moreover, also their degree of phytophagy and potential crop damage should be studied as a part of their evaluation for use in biological control.
Author contributions statement
BLI and GM conceived, designed the trials and wrote the manuscript. NB analysed the functional response data. BLI, AI, LD, JBW and AL conducted the experiments. LT supervised the trials and contributed to manuscript writing. All authors read and approved the manuscript.
References
Abbas S, Pérez-Hedo M, Colazza S, Urbaneja A (2014) The predatory mirid Dicyphus maroccanus as a new potential biological control agent in tomato crops. BioControl 59:565–574. https://doi.org/10.1007/s10526-014-9587-6
Abrams PA (1982) Functional responses of optimal foragers. Am Nat 120:382–390. https://doi.org/10.1086/283996
Alvarado P, Baltà O, Alomar O (1997) Efficiency of four Heteroptera as predators of Aphis gossypii and Macrosiphum euphorbiae (Hom.: Aphididae). Entomophaga 42:215–226. https://doi.org/10.1007/BF02769899
Biondi A, Zappalà L, Di Mauro A, Tropea Garzia G, Russo A, Desneux N, Siscaro G (2016) Can alternative host plant and prey affect phytophagy and biological control by the zoophytophagous mirid Nesidiocoris tenuis? BioControl 61(1):79–90. https://doi.org/10.1007/s10526-015-9700-5
Biondi A, Guedes RNC, Wan F-H, Desneux N (2018) Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: past, present, and future. Annu Rev Entomol 63:239–258. https://doi.org/10.1146/annurev-ento-031616-034933
Bolker BM (2008) Ecological models and data in R. Princeton University Press, Princeton, pp 351–356 (ISBN 0691125228)
Briggs CJ, Hoopes MF (2004) Stabilizing effects in spatial parasitoid–host and predator–prey models: a review. Theor Popul Biol 65:299–315. https://doi.org/10.1016/j.tpb.2003.11.001
Campos MR, Biondi A, Adiga A, Guedes RNC, Desneux N (2017) From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J Pest Sci 90:787–796. https://doi.org/10.1007/s10340-017-0867-7
Castañé C, Arnó J, Gabarra R, Alomar Ò (2011) Plant damage to vegetable crops by zoophytophagous mirid predators. Biol Control 59:22–29. https://doi.org/10.1016/j.biocontrol.2011.03.007
Coll M, Ridgway RL (1995) Functional and numerical responses of Orius insidiosus (Heteroptera: Anthocoridae) to its prey in different vegetable crops. Ann Entomol Soc Am 88:732–738. https://doi.org/10.1093/aesa/88.6.732
Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S, Narvaez-Vasquez CA, Gonzalez-Cabrera J, Catalán Ruescas D, Tabone E, Frandon J, Pizzol J, Poncet C, Cabello T, Urbaneja A (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, geographic expansion and prospects for biological control. J Pest Sci 83:197–215. https://doi.org/10.1007/s10340-010-0321-6
Enkegaard A, Brødsgaard HF, Hansen DL (2001) Macrolophus caliginosus: functional response to whiteflies and preference and switching capacity between whiteflies and spider mites. Entomol Exp Appl 101:81–88. https://doi.org/10.1046/j.1570-7458.2001.00893.x
Fantinou AA, Perdikis DC, Maselou DA, Lambropoulos PD (2008) Prey killing without consumption: Does Macrolophus pygmaeus show adaptive foraging behaviour? Biol Control 47:187–193. https://doi.org/10.1016/j.biocontrol.2008.08.004
Fellowes MDE, Van Alphen JJM, Jervis MA (2007) Foraging Behaviour. In: Jervis MA (ed) Insects as natural enemies. A practical perspective. Springer Netherlands, Amsterdam, pp 1–72 (ISBN 978-1-4020-2625-6)
Fernández-Arhex V, Corley JC (2003) The functional response of parasitoids and its implications for biological control. Biocontrol Sci Technol 13:403–413. https://doi.org/10.1080/0958315031000104523
Ferracini C, Bueno VHP, Dindo ML, Ingegno BL, Luna MG, Salas Gervassio NG, Sánchez NE, Siscaro G, van Lenteren JC, Zappalà L, Tavella L (2019) Natural enemies of Tuta absoluta in the Mediterranean basin. Biocont Sci Technol Eur S Am. https://doi.org/10.1080/09583157.2019.1572711
Foglar H, Malausa JC, Wajnberg E (1990) The functional response and preference of Macrolophus caliginosus [Heteroptera: Miridae] for two of its prey: Myzus persicae and Tetranychus urticae. Entomophaga 35:465–474. https://doi.org/10.1007/BF02375272
Haddaway NR, Wilcox RH, Heptonstall REA, Griffiths HM, Mortimer RJG, Christmas M, Dunn AM (2012) Predatory functional response and prey choice identify predation differences between native/invasive and parasitised/unparasitised crayfish. PLoS ONE 7:e32229. https://doi.org/10.1371/journal.pone.0032229
Hamdan A-JS (2006) Functional and numerical responses of the predatory bug Macrolophus caliginosus Wagner fed on different densities of eggs of the greenhouse whitefly, Trialeurodes vaporariorum (Westwood). J Biol Res 6:147–154
Hassell MP (2000) Host–parasitoid population dynamics. J Anim Ecol 69:543–566. https://doi.org/10.1046/j.1365-2656.2000.00445.x
Holt RD (1977) Predation, apparent competition, and the structure of prey communities. Theor Popul Biol 12:197–229. https://doi.org/10.1016/0040-5809(77)90042-9
Ingegno BL, Goula M, Navone P, Tavella L (2008) Distribution and host plants of the genus Dicyphus in the Alpine valleys of NW Italy. Bull Insectol 61:139–140 (ISSN 1721-8861)
Ingegno BL, Ferracini C, Gallinotti D, Alma A, Tavella L (2013) Evaluation of the effectiveness of Dicyphus errans (Wolff) as predator of Tuta absoluta (Meyrick). Biol Control 67:246–252. https://doi.org/10.1016/j.biocontrol.2013.08.002
Ingegno BL, Candian V, Psomadelis I, Bodino N, Tavella L (2017a) The potential of host plants for biological control of Tuta absoluta by the predator Dicyphus errans. Bull Entomol Res 107:340–348. https://doi.org/10.1017/S0007485316001036
Ingegno BL, Bodino N, Leman A et al (2017b) Predatory efficacy of Dicyphus errans on different prey. Acta Hortic 1164:425–430. https://doi.org/10.17660/ActaHortic.2017.1164.55
Jaworski CC, Bompard A, Genies L, Amiens-Desneux E, Desneux N (2013) Preference and prey switching in a generalist predator attacking local and invasive alien pests. PLoS ONE 8:e82231. https://doi.org/10.1371/journal.pone.0082231
Juliano SA (2001) Nonlinear curve fitting: predation and functional response curves, p. 159–182. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments, 2nd edn. Chapman and Hall, New York, p 373
Koller M, Knapp M, Schausberger P (2007) Direct and indirect adverse effects of tomato on the predatory mite Neoseiulus californicus feeding on the spider mite Tetranychus evansi. Entomol Exp Appl 125:297–305. https://doi.org/10.1111/j.1570-7458.2007.00625.x
Lee MS, Albajes R, Eizaguirre M (2014) Mating behaviour of female Tuta absoluta (Lepidoptera: Gelechiidae): polyandry increases reproductive output. J Pest Sci 87:429–439. https://doi.org/10.1007/s10340-014-0576-4
Leman A, Ingegno BL, Tavella L, Janssen A, Messelink GJ (2019) The omnivorous predator Macrolophus pygmaeus, a good candidate for the control of both greenhouse whitefly and poinsettia thrips on gerbera plants. Insect Sci. https://doi.org/10.1111/1744-7917.1265
Maselou DA, Perdikis DC, Sabelis MW, Fantinou AA (2014) Use of plant resources by an omnivorous predator and the consequences for effective predation. Biol Control 79:92–100. https://doi.org/10.1016/j.biocontrol.2014.09.002
Maselou DA, Perdikis DC, Fantinou A (2015) Effect of hunger level on prey consumption and functional response of the predator Macrolophus pygmaeus. Bull Insectol 68:211–218 (ISSN 1721-8861)
Messelink GJ, Bennison J, Alomar O, Ingegno BL, Tavella L, Shipp L, Palevsky E, Wäckers FL (2014) Approaches to conserving natural enemy populations in greenhouse crops: current methods and future prospects. BioControl 59:377–393. https://doi.org/10.1007/s10526-014-9579-6
Michaelides G, Sfenthourakis S, Pitsillou Seraphides N (2018) Functional response and multiple predator effects of two generalist predators preying on Tuta absoluta eggs. Pest Manag Sci 74:332–339. https://doi.org/10.1002/ps.4703
Montserrat M, Albajes R, Castañé C (2000) Functional response of four Heteropteran predators preying on greenhouse whitefly (Homoptera: Aleyrodidae) and western flower thrips (Thysanoptera: Thripidae). Environ Entomol 29:1075–1082. https://doi.org/10.1603/0046-225X-29.5.1075
Murdoch WW (1969) Switching in general predators: experiments on predator specificity and stability of prey populations. Ecol Monogr 39:335–354. https://doi.org/10.2307/1942352
Nachman G (2006) A functional response model of a predator population foraging in a patchy habitat: functional response in a patchy environment. J Anim Ecol 75:948–958. https://doi.org/10.1111/j.1365-2656.2006.01114.x
Okuyama T (2012a) On selection of functional response models: Holling’s models and more. BioControl 58:293–298. https://doi.org/10.1007/s10526-012-9492-9
Okuyama T (2012b) A likelihood approach for functional response models. Biol Control 60:103–107
Pereyra PC, Sánchez NE (2006) Effect of two solanaceous plants on developmental and population parameters of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop Entomol 35:671–676. https://doi.org/10.1590/s1519-566x2006000500016
R Development Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (ISBN: 3-900051-07-0)
Roditakis E, Vasakis E, García-Vidal L, del Rosario Martínez-Aguirre M, Rison JL, Haxaire-Lutun MO, Nauen R, Tsagkarakou A, Bielza P (2018) A four-year survey on insecticide resistance and likelihood of chemical control failure for tomato leaf miner Tuta absoluta in the European/Asian region. J Pest Sci 91:421–435. https://doi.org/10.1007/s10340-017-0900-x
Sanchez JA, Cassis G (2018) Towards solving the taxonomic impasse of the biocontrol plant bug subgenus Dicyphus (Dicyphus) (Insecta: Heteroptera: Miridae) using molecular, morphometric and morphological partitions. Zool J Linn Soc. https://doi.org/10.1093/zoolinnean/zly005 (epub ahead of print)
Tropea Garzia G, Siscaro G, Biondi A, Zappalà L (2012) Tuta absoluta, a South American pest of tomato now in the EPPO region: biology, distribution and damage. EPPO Bull 42:205–210. https://doi.org/10.1111/epp.2556
Urbaneja A, Monton H, Mollá O (2009) Suitability of the tomato borer Tuta absoluta as prey for Macrolophus pygmaeus and Nesidiocoris tenuis. J Appl Entomol 133:292–296. https://doi.org/10.1111/j.1439-0418.2008.01319.x
Van Den Meiracker RAF, Sabelis MW (1999) Do functional responses of predatory arthropods reach a plateau? A case study of Orius insidiosus with western flower thrips as prey. Entomol Exp Appl 90:323–329. https://doi.org/10.1046/j.1570-7458.1999.00452.x
Van Lenteren JH, Hemerik L, Lins JC Jr, Bueno VHP (2016) Functional responses of three neotropical mirid predators to eggs of Tuta absoluta on tomato. Insects 7:34. https://doi.org/10.3390/insects7030034
Voigt D, Gorb E, Gorb S (2007) Plant surface–bug interactions: Dicyphus errans stalking along trichomes. Arthropod-Plant Interact 1:221–243. https://doi.org/10.1007/s11829-007-9021-4
Zappalà L, Biondi A, Alma A, Al-Jboory IJ, Arnò J, Bayram A, Chailleux A, El-Arnaouty A, Gerling D, Guenaoui Y, Shaltiel-Harpaz L, Siscaro G, Stavrinides M, Tavella L, Vercher Aznar R, Urbaneja A, Desneux N (2013) Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J Pest Sci 86(4):635–647. https://doi.org/10.1007/s10340-013-0531-9
Acknowledgements
The functional response and predation cage trials were partly supported by the Italian national project “Insects and globalization: sustainable control of exotic species in agro-forestry ecosystems (GEISCA)” of the Italian Ministry of University and Research. The greenhouse trial of Wageningen University & Research was funded by the Dutch top sector Horticulture and Starting Materials. The research was partly carried out during a Short Term Scientific Mission of COST Action FA1105 “Towards a sustainable and productive EU organic greenhouse horticulture”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by A. Biondi & N. Desneux.
Rights and permissions
About this article
Cite this article
Ingegno, B.L., Messelink, G.J., Bodino, N. et al. Functional response of the mirid predators Dicyphus bolivari and Dicyphus errans and their efficacy as biological control agents of Tuta absoluta on tomato. J Pest Sci 92, 1457–1466 (2019). https://doi.org/10.1007/s10340-019-01079-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-019-01079-8