Abstract
The Asian citrus psyllid, Diaphorina citri, is the most serious threat to citrus crops worldwide, and its management relies exclusively on frequent applications of chemical insecticides. Entomopathogenic fungi may play an important role for regulating this citrus pest. Two virulent fungal strains, Isaria fumosorosea ESALQ-1296 and Beauveria bassiana ESALQ-PL63, were selected among 17 fungal isolates under laboratory conditions and caused 77.8 and 78.4% adult mortality, respectively, while in semifield trials the adult mortality reached up to 83.5% with B. bassiana and 80.6% with I. fumosorosea. The bioefficacy of these two fungi on D. citri adults was assessed through monthly applications during 1 year in a commercial citrus grove located in Itapetininga, São Paulo, Brazil. The application of I. fumosorosea ESALQ-1296 and B. bassiana ESALQ-PL63 was as effective as the chemical insecticides in most trials. The fungal-induced mortality of D. citri adults ranged from 96.1% in December 2011 to 57.8% in October 2012. This mortality level tended to build up with the increase in the maximum relative humidity; the percentage of sporulated cadavers was positively correlated with both higher relative humidity and rainfall but negatively associated with maximum temperatures. These results support the use of I. fumosorosea or B. bassiana as an eco-friendly biopesticide for the integrated management of D. citri.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
The potential of Isaria fumosorosea ESALQ-1296 and Beauveria bassiana ESALQ-PL63 was demonstrated against Diaphorina citri adults under laboratory, semifield and field conditions.
-
The efficacy of these two fungi on D. citri adults in a commercial citrus grove was as effective as the chemical insecticides in most trials.
-
The fungal efficacy was positively correlated with higher relative humidity.
-
These mycopesticides are an alternative to reduce insect populations in citrus orchards and bordering vegetation where D. citri control has been neglected.
Introduction
The Asian citrus psyllid, Diaphorina citri Kuwayama (1907) (Hemiptera: Liviidae), is the most serious threat to citrus crops worldwide as it vectors the phloem-limited bacteria Candidatus Liberibacter asiaticus and Candidatus Liberibacter americanus, the causal agents of citrus Greening disease or Huanglongbing (HLB) (Halbert et al. 2000). HLB is probably the most destructive and deadly citrus disease, resulting in severe yield losses, poor fruit quality and a shortened plant lifespan (Bové 2006; Kuchment 2013). Diaphorina citri has been found widespread throughout Brazil since 1940 (Costa Lima 1942) and emerged as a primary insect pest of Brazilian citrus groves in 2004, after HLB was first reported (Coleta-Filho et al. 2004; Teixeira et al. 2005). Considering that Brazil is the world leader in orange production, representing 63% of the total amount of orange juice exported worldwide (Belasque et al. 2010), Brazil is undoubtedly of key importance in the design of long-term management programs to prevent the spread of HLB by effectively controlling its insect vector, among other phytosanitary measures.
According to surveys conducted by the Foundation of Citrus Growers of São Paulo State (Fundecitrus), HLB has spread quickly throughout citrus-producing areas in the State of São Paulo. Disease incidence levels have risen from 3.4 to 64.1% in commercial citrus groves from 2004 to 2012 (www.fundecitrus.com.br), and no short- or midterm sustainable solutions for this critical issue have been proposed (Belasque et al. 2010).
To date, chemical control has been the basis of D. citri management programs, but this strategy strongly relies upon frequent applications of a few chemical insecticides throughout the crop season (Yamamoto et al. 2009; Tiwari et al. 2011). However, the exclusive use of chemical insecticides is expected to disrupt natural enemy populations and increase not only the selection pressure for resistant D. citri populations but also the risk of residues on fruits (Ortelli et al. 2005; Tiwari et al. 2011; Monzo et al. 2014). To counteract this stand-alone approach, a focus on reduced insecticide reliance; on integrated approaches involving a consortium of different control tactics, with emphasis on biological control; and on selective insecticides is critical for the sustainable management of both the vector and HLB.
Among the natural enemies of D. citri (Yang et al. 2006; Michaud 2010), entomopathogenic fungi stand out as promising candidates because they can be easily mass-produced in vitro and are capable of actively infecting all life stages, through the host cuticle. These biocontrol agents are commercially available as formulated mycopesticides and represent an effective control option that not only aids in mitigation of insecticide resistance in citrus psyllid populations but also reduces the effects on some natural enemies compared with chemical insecticides (Cardoso et al. 2007). The following numerous entomopathogenic fungi have been reported as potential regulators of D. citri: Isaria fumosorosea (Wize), Hirsutella citriformis Speare, Lecanicillium lecanii Zimm., Beauveria bassiana (Bals.-Criv.), Metarhizium anisopliae, Cladosporium sp. nr. oxysporum and Capnodium citri (Subandiyah et al. 2000; Meyer et al. 2007; Stauderman et al. 2012). In China, Acrostalagmus aphidum Oudem. and Isaria javanica (Friederichs & Bally) have also been implicated as naturally occurring fungal pathogens of D. citri (Yang et al. 2006).
Few studies have determined the virulence of entomopathogenic fungi against D. citri nymphs under laboratory conditions (Padulla and Alves 2009; Ferreira Pinto et al. 2012); information regarding the effect of these fungi on the adult stage is even rarer (Lezama-Gutiérrez et al. 2012, 2014; Orduño-Cruz et al. 2015). With the aim of developing the first fungal-based bioinsecticide against D. citri in Brazil, virulent strains were selected among a wide range of entomopathogenic fungal candidates, and the efficacy of two selected strains was compared using an economically feasible single concentration test under laboratory and semifield conditions. Furthermore, the volume rates and concentrations of two previously selected fungal strains were tested under field conditions throughout 1 year, during which mortality and mycosis were also correlated with macroclimatic variables.
Materials and methods
Insect colony
Diaphorina citri originally obtained from Piracicaba, SP, Brazil, was reared on Murraya paniculata (Rutaceae) seedlings inside steel cages (60 cm length × 60 cm width × 50 cm depth) under greenhouse conditions at the University of São Paulo (USP), campus of the College of Agriculture “Luiz de Queiroz” (ESALQ), Piracicaba, SP. Murraya paniculata was used as the preferred host for the feeding and oviposition of D. citri (Nava et al. 2007).
Fungal origin and preparation
The entomopathogenic fungi were obtained from the entomopathogen collection of the Laboratory of Pathology and Microbial Control of Insects at the “Luiz de Queiroz” College of Agriculture (ESALQ), University of São Paulo (USP), located in Piracicaba, SP, Brazil (Table 1). For the laboratory studies, the Isaria and Lecanicillium species were grown on 9-cm petri dishes containing Sabouraud dextrose agar medium supplemented with yeast extract (SDYA) (Goettel and Inglis 1997), whereas the other fungal species were cultivated on potato dextrose agar (PDA, Difco®). The fungi were cultivated in different media to meet their differential nutritional requirements for profuse sporulation. All the cultures were maintained in an environmentally controlled growth chamber set to 26 ± 2 °C under a 12 h photophase for 10 days.
Production of entomopathogenic fungi on rice
Aerial conidia of I. fumosorosea ESALQ-1296 and B. bassiana ESALQ-PL63 were produced on precooked parboiled rice using the plastic bag method (Faria and Magalhães 2001) to provide different inoculum batches for semifield and field experimentation. After 7 days of incubation in polypropylene bags at 26 °C under a 12 h photophase, a mixture of fully sporulated fungus + rice matrix was placed on plastic trays (51 cm length × 33 cm width × 8 cm depth) for an additional 2 days incubated at 18 °C for desiccation using a dehumidifier. Dried conidia (<13% w/w final moisture) were mechanically harvested from the fungus + rice mixture using an electrically vibrating sieve containing a set of three 20-cm round sieves of 32 mesh (pore size 500 µm) (BERTEL Indústria Metalúrgica Ltda., São Paulo, Brazil). Afterward, different batches of dried conidial powder were vacuum sealed and stored at −20 °C until their use in experiments.
The quality control of these conidia was assessed by enumerating the conidial concentration in a hemacytometer (Neubauer chamber, K5-0111 model; KASVI, Curitiba, PR, Brazil) and determining conidial germination on 4 ml of PDA amended with 0.001% (v/v) of the fungicide Derosal® 500 SC (Carbendazim; Bayer CropScience, SP, Brazil) poured on plastic Rodac® plates. This fungicide in low concentrations acts as a fungistatic agent, allowing germ tube formation but preventing the further development of hyphae and consequently the overgrowth of the fungus on nutrient agar media (Oliveira et al. 2015). Conidial viability based on counts of 200 random conidia per plate was measured after 18 h of incubation at 26 ± 2 °C and expressed as percent germination, with a conidium scored as germinated when the length of an elongating germ tube measured twice the size of its diameter. Conidial viability was checked prior to each experiment and reached >90% germination in all cases, unless specifically stated otherwise.
Screening of fungal strains against D. citri adults
To select the most virulent fungal strains, D. citri adults were exposed to a single conidial concentration of 17 entomopathogenic fungi under laboratory conditions (Table 1). The fungi were cultivated in PDA and SDAY media, as previously mentioned, and incubated at 26 ± 2 °C under a 12 h photophase for 10 days. The conidia were harvested from the medium’s surface by scraping with a metallic spatula, and the conidial suspensions of all the fungal strains were prepared with 0.075% (v/v) KBRAdj surfactant (Koppert Sistemas Biológicos LTDA, Piracicaba, SP, Brazil—“Pending registration”) to obtain a desired standard concentration of 1 × 107 conidia/ml. The controls consisted of distilled water and the 0.075% KBRAdj surfactant solution. The experimental cage consisted of a plastic cup (14 cm height × 7 cm diameter and 500 ml volume) with lateral square openings (11 cm height × 5 cm length) covered with sheer net-like fabric (voile) to allow ventilation. A single 10-cm lime seedling (Citrus limonia Osbeck, var. Cravo) (canopy with 7–10 leaves) grown in black plastic tubes (20 cm height × 1.5 cm diameter and 50 ml volume) containing potting mix substrate (pine bark and peat) was transferred to the plastic cup. Twenty unsexed adults of D. citri (sex ratio 1:1) of 10–15 days old (i.e., 10–15 days postemergence) from the insect colony (item 2.1) were transferred to individual plastic cups containing a single lime seedling. Each seedling was topically sprayed with 400 µl conidial suspension of each fungal strain or with water + surfactant in the controls using a handheld airbrush sprayer (SW-168 model, Pneumatic SAGYMA Tools®). This volume provided a uniform coverage of seedlings without runoff. After spraying, these ventilated cages were kept in a growth chamber at 25 ± 2 °C with 75–90% RH under a 12 h photophase. The dead insects were recorded daily over a 10-day incubation period, and the cadavers were transferred to 24-cell well culture plates containing moistened cotton with sterile distilled water (humid chamber) and kept at 25 ± 2 °C for 3 days to check for fungus sporulation. The identification of fungal species was performed by checking their morphological features according to the classification of Humber (2012). The assay followed a randomized experimental design with three replicates per treatment, and the whole experiment was independently conducted twice on different dates.
Laboratory bioefficacy of B. bassiana and I. fumosorosea
Two strains were selected from the study above (B. bassiana ESALQ-PL63 and I. fumosorosea ESALQ-1296) and tested under a lower and economically feasible conidial concentration of 5 × 106 conidia/ml. Adults of D. citri (n = 20, sex ratio 1:1) were sprayed with 400 µl, as described in item 2.4. The insects from two control groups were treated with the 0.075% surfactant solution and distilled water, separately. Insect mortality was determined after 10 days of incubation at 25 ± 2 °C with 75–90% RH under a 12 h photophase, and the cadavers were placed in humid chambers (26 °C and ~100% RH) for 3 days to allow sporulation. The assay followed a randomized experimental design with three replicates per treatment, and the whole experiment was independently conducted four times on different dates.
Semifield bioefficacy of B. bassiana and I. fumosorosea
The bioefficacy of B. bassiana ESALQ-PL63 and I. fumosorosea ESALQ-1296 under semifield conditions was assessed against adult D. citri reared on 2-m high-citrus plants (Citrus sinensis, var. Pera). The plants were protected from rainfall by transparent polyethylene plastic film placed 4 m above them. These plants had been transplanted from 10-kg pots directly into the soil and cultivated in the ground for 1.5 years prior to experimentation. The planting arrangement was linear with a spacing of 2 m × 2 m. One branch per plant was infested with 20 D. citri adults (sex ratio 1:1) of 10–15 days postemergence confined in a bag made of sheer net-like fabric (voile) (20 height × 15 cm length) with mesh size of 0.68 mm height × 0.76 mm length. Aerial conidia of I. fumosorosea ESALQ-1296 and B. bassiana ESALQ-PL63 were produced on precooked parboiled rice (as described in item 2.3). Fungal suspensions for both strains were prepared with dried conidia harvest from fungus-colonized rice and adjusted to 5 × 106 conidia/ml to deliver a rate of 14 ml per plant during late afternoon using a handheld sprayer (Mixyou® 500 ml volume). The experimental unit consisted of 20 adults per branch confined by a voile bag. The control insect groups was sprayed with 0.075% surfactant (KBRAdj) or water only. After 10 days, the branches involved by the voile bag were cut and transported to laboratory to quantify the number of dead and healthy adults. The cadavers were placed in humid chambers (26 °C and ~100% RH) for 3 days to check fungal sporulation as described in item 2.4. The daily temperature and relative humidity were recorded by a datalogger (IDEL Manaus HT-4000) within the plant canopy. The experiment followed a randomized design with five replicates per treatment with a total of 20 plants per experiment, and the whole trial was independently conducted three times on different dates.
Bioefficacy of B. bassiana and I. fumosorosea in a commercial citrus grove
The bioefficacy of the fungal entomopathogens against D. citri adults was later assessed in a commercial citrus grove (var. Valência) through a series of trials conducted throughout 12 months. The experimental field was located in Itapetininga, São Paulo, Brazil, and consisted of a 16.4-ha citrus plot with a density of 470 7-year-old trees per hectare planted 3.1 m apart within rows and 6.8 m apart between the rows. In total, the fungal applications were performed on 12 dates from December 2011 to January 2013 (once per month) using a motorized air blast sprayer (Jacto ARBUS 4000) with a capacity of 4000 l.
Aerial conidia of I. fumosorosea ESALQ-1296 and B. bassiana ESALQ-PL63 were produced on precooked parboiled rice (item 2.3). Four fungal treatments were tested at two volume rates and two concentrations but keeping a constant concentration per hectare (5 × 1012 conidia/ha). The fungal treatments were represented by I. fumosorosea ESALQ-1296 at 2000 l/ha of a suspension containing 2.5 × 106 conidia/ml, I. fumosorosea ESALQ-1296 at 1000 l/ha of a 5 × 106 conidia/ml suspension, B. bassiana ESALQ-PL63 at 2000 l/ha of a 2.5 × 106 conidia/ml suspension, and B. bassiana ESALQ-PL63 at 1000 l/ha of a 5 × 106 conidia/ml suspension. In addition, a chemical insecticide was included as a standard and was represented by the commercial product Provado® SC (Imidacloprid, 200 g a.i./l; Bayer CropScience, São Paulo, SP, Brazil) at a label rate of 0.40 l of commercial product (c.p.) in 1000 l/ha through May to August of 2012. In the subsequent months, we used Dimexion® EC (Dimethoate, 400 g a.i./l; Cheminova, São Paulo, SP, Brazil) instead of Provado® at a label rate of 2.0 l c.p. diluted in 1000 l/ha. Fungus- and insecticide-free controls consisted of trees sprayed with only water at a volume rate of 1000 l/ha.
Due to the devastating effect of HLB, there is zero tolerance for D. citri in commercial citrus crops, and farmers only allow studies using confined insects. The experimental unit consisted of a citrus branch covered with a bag made of sheer net-like fabric (voile) (20 height × 15 cm length) with mesh size of 0.68 mm height × 0.76 mm length containing 20 unsexed D. citri adults (sex ratio 1:1) of 10–15 days postemergence according the methodology of Ribeiro et al. (2015) and Sanches et al. (2009). Young citrus branches (V3–V4 stages) (Medina et al. 2005) were selected according the vegetative phenology, and voile bags were placed in the middle stratum of each tree (2 m height) at the outer side of the canopy in northern direction.
The field plots were located inside the citrus orchard with extremely low infestation of D. citri observed visually in the area during 14 months. No pesticide was applied 7 days before or 7 days after any fungal/insecticide application. The applications of pesticides and fungicides to other pests and diseases in the area were performed according to monitoring and the pesticide application schedule of the farmer.
The six treatments were distributed in a completely randomized block design with nine replicates (i.e., three plants × three blocks). The experiment consisted of 12 trials (1 per month) conducted over a 14-month period in the same area, but the different trials used different randomly chosen trees to assess the performance of the fungal treatments over time under varying ambient environmental conditions. After 10 days of applications, the branches confined in the voile bags were cut off and transported to laboratory for quantification of the number of dead and healthy adults. The dead adults were counted 10 days after application, and the cadavers were incubated in humid chambers to assess the presence of sporulation (used as an indicator of mycosis). For each experimental trial, a datalogger (IDEL Manaus HT-4000) was placed next to the trunk of each citrus tree to record the temperature and relative humidity levels every 30 min. Representative daily maximum and minimum values were recorded for each variable and trial by averaging the ten higher and ten lower values, respectively, that were recorded each day. Then, to calculate the reported monthly maximum and minimum temperature and RH, and the data obtained daily for each variable in each trial along 10 days after application were averaged (n = 100). The cumulative precipitation (rainfall) was also recorded for the 10 days after each spraying from an on-site meteorological weather station.
Statistical analysis
The binomial response variables were represented by the occurrence of death (% mortality) and mycosed insects (% sporulation). The laboratory and semifield mortality data sets were analyzed using logistic regression in a generalized linear mixed model (GLMM) for a binomial distribution using the logit link function; the fixed effects were the fungal treatments, while the random effects were the trials (i.e., repeated experiments on different dates) (Warton and Hui 2011). For the field mortality data, the same statistical approach was used, but the random effects were blocks within trials, and the interaction term was treatment by trial. When the interaction term treatment by trial or only the treatment effect was significant in the model, multiple comparisons were performed to separate the treatment means by Tukey HSD test, with P values adjusted using the false discovery rate (fdr) method and scored as significant at P < 0.05.
Additionally, for the laboratory screening studies involving single-concentration time-response assays and those fungal strains causing more than 50% mortality of D. citri adults, the median lethal time (LT50, time required to kill 50% of the insect population) and respective 95% confidence interval (CI) were estimated by fitting survival data (proportions of insects surviving over time) to a generalized log-logistic model with binomial distribution using the package ‘drc’ (Ritz and Streibig 2008). Since overdispersion (i.e., variance heterogeneity) was detected in this data set, we implemented extrabinomial variation in the model enabling more accurate estimates of model parameters and standard errors. The two-parameter log-logistic model was chosen as it yielded the best goodness-of-fit based on the Bayesian information criterion (BIC) among several models tested, and it can be written as the following:
where b is slope, x is time (in days), and e is the inflexion point of the curve. Median lethal times (LT50) were estimated for all fungal isolates and then considered statistically different according to pairwise Student's t test at P < 0.05.
To investigate the effect of weather (continuous variables) and fungal treatments (categorical variable) as a predictor of a response variable (e.g., mortality), we performed model building with multivariate logistic regression in a generalized linear model (GLM) assuming a binomial distribution for errors with a logit link function. Thus, our purpose here was to develop a model to relate the insect binary response (dead vs. alive) to the predictors represented by the weather variables, such as RHmax, RHmin, T max, T min and precipitation (P p) and by the effect of fungal treatments. When additional extrabinomial variation [i.e., overdispersion (Φ)] was found in the data sets (e.g., residual deviance greater than the degrees of freedom), we employed the penalized maximum likelihood estimation method to appropriately compute the model variance and covariance to obtain accurate coefficients and respective P values. All the statistical analyses were performed with the statistical software R version 2.15.2 (R Development Core Team 2012), and the results were considered significant at P < 0.05.
Results
Screening of entomopathogenic fungi on D. citri adults
The susceptibility of adult D. citri varied significantly among the 17 fungal isolates tested, showing different mortality levels and median lethal times (LT50) (χ 2 = 242.6, df = 17, P < 0.001). Among these 17 fungal strains, 8 caused mortality levels above 50% during the 10-day period of incubation after spraying: B. bassiana ESALQ-PL63 (78.4%), I. fumosorosea ESALQ-1296 (77.8%), B. bassiana ESALQ-1432 (73.4%), Purpureocillium lilacinum ESALQ-1205 (62.5%), M. anisopliae ESALQ-1037 (59.1%), Colletotrichum nymphaeae ESALQ-1393 (57.2%), Lecanicillium sp. ESALQ-949 (55.5%) and M. anisopliae ESALQ-E9 (54.5%) (Fig. 1). The adult mortality in the control (water) and 0.075% surfactant solution alone was 7.6 and 32.4%, respectively. The proportion of cadavers presenting sporulation (mycosis) was also higher (χ 2 = 255.9, df = 16, P < 0.001) in I. fumosorosea ESALQ-1296 (65.5%) than in all the other fungi except B. bassiana ESALQ-PL63 (58.3%). The other fungal strains induced mycosis in D. citri on citrus, with sporulation levels that ranged from 4.7 to 45.9% (Fig. 1). According to log-logistic regression analysis, the lowest LT50 value was 5.9 days, which corresponded to I. fumosorosea ESALQ1296, but it did not differ from LT50 values spanning from 6.2 to 8.1 days achieved with two B. bassiana strains, P. lilacinum ESALQ-1405, Lecanicillium sp. ESALQ-949, C. nymphaeae ESALQ-1393 and M. anisopliae ESALQ1037. Although the other fungal pathogens produced LT50 values greater than 9 days (Table 1), there was a significant effect of all fungal pathogens reducing survival rates of D. citri adults when compared to the control groups (χ 2 = 142.8, df = 16, P = 0.001). I. fumosorosea ESALQ-1296 was among the pathogens of D. citri that killed the fastest, inflicting high mortality and sporulation levels on this host. For this reason, this fungal strain was selected for use in further experiments. The B. bassiana ESALQ-PL63 was selected since it has consistently caused high levels of mortality and sporulation in D. citri adults and is the commercial strain of the formulated mycopesticide Boveril® (Koppert Sistemas Biológicos LTDA, Piracicaba, São Paulo, Brazil).
Laboratory screening of entomopathogenic fungal isolates against Diaphorina citri adults sprayed at a standard concentration of 1 × 107 conidia/ml and mortality/sporulation assessed after 10 days of incubation at 26 °C. Bars (mean ± standard error) indicated by the same letters, in lower case for mortality and capital letters for sporulation, are not significantly different by the Tukey HSD test (P < 0.05). Bb represents B. bassiana, If is I. fumosorosea, Pl is P. lilacinus, Ma is M. anisopliae, Cn is Colletotrichum nymphaeae, Lsp is Lecanicillium sp., Lm is L. muscarium, Ia is I. amoenorosea, Llo is L. longisporum, Lle is L. lecanii, Ht is H. thompsonii, and S is Simplicillium sp. Control = application of water only
Bioefficacy of B. bassiana and I. fumosorosea under laboratory and semifield conditions
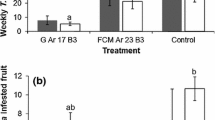
Under laboratory conditions, both B. bassiana ESALQ-PL63 and I. fumosorosea ESALQ-1296 were effective in killing adult D. citri by direct contact, resulting in higher mortality levels (60 and 63%, respectively) than that of the uninoculated controls (χ 2 = 685.8, df = 3, P < 0.001). The proportion of mycosed insects did not vary between the fungal strains tested (χ 2 = 1.31, df = 1, P = 0.253), with 37.9–41.1% of the total number of D. citri cadavers showing sporulation (Fig. 2a).
Laboratory (a) and semifield (b) experiments with Isaria fumosorosea ESALQ-1296 (If) and Beauveria bassiana ESALQ-PL63 (Bb) applied at 5 × 106 conidia/ml against adults of Diaphorina citri. Adjuvant means the application of 0.075% KBRAdj. Control means the application of water. Bars (mean ± SE, n = 24) topped by the same letters, lower case letters for mortality and upper case letters for sporulation, are not significantly different by the Tukey HSD test (P < 0.05)
Semifield trials revealed that the mortality of adult D. citri was significantly affected by directly spraying the fungal treatments (χ 2 = 915.1, df = 3, P < 0.001), which showed superior insecticidal activity compared with the control groups, as indicated the achievement of high mortality levels, up to 83.5% with B. bassiana ESALQ-PL63 and 80.6% with I. fumosorosea ESALQ-1296. The two fungi caused sporulation on similar percentages of adult cadavers (χ 2 = 1.53, df = 1, P = 0.216): 58.3% for I. fumosorosea ESALQ-1296 and 54.4% for B. bassiana ESALQ-PL63 (Fig. 2b).
Bioefficacy of B. bassiana and I. fumosorosea in commercial citrus crops
The fungal and chemical treatments for the 12 open field trials conducted on a commercial citrus farm caused high adult mortality during each month of evaluation (χ 2 = 143.6, df = 32, P < 0.001) (Fig. 3). At the same field concentration of 5 × 1012 conidia/ha, the treatments with B. bassiana ESALQ-PL63 caused mortality levels ranging from 95.6% (May 2012) to 68.9% (September 2012) when applied at 2.5 × 106 conidia/ml using a volume rate of 2000 l/ha, but 87.8% (May 2012) to 61.7% (September 2012) when applied at double the concentration (5 × 106 conidia/ml) and half the volume rate (1000 l/ha). The I. fumosorosea ESALQ-1296 fungus induced D. citri mortality ranging from 96.1% (December 2011) to 69.4% (September 2012) when applied at 2.5 × 106 conidia/ml using a volume rate of 2000 l/ha compared with 94% (June 2012) to 57.8% (October 2012) when applied at 5 × 106 conidia/ml using 1000 l/ha. The applications of chemical insecticides at 1000 l/ha yielded adult mortality between 93.9% (May 2012) and 73.3% (September 2012) throughout the entire experimental period. The mortality levels recorded in the controls (water) were lower in all cases compared with those in the insecticidal or fungal treatments, with a range from 32.2% (June 2012) to 17.2% (November 2012) (Fig. 3).
Performance of direct spray applications using two volume rates and two concentrations (1000 l/ha at 5 × 106 viable conidia/ml or 2000 l/ha at 2.5 × 106 conidia/ml) of Isaria fumosorosea ESALQ-1296 (If) and Beauveria bassiana ESALQ-PL63 (Bb) on the percent mortality and sporulation of Diaphorina citri adults. The chemical insecticide and the control (water) were sprayed at 1000 l/ha. Within months, differences between bars (mean ± SE) topped by the same letters or by ns are not significant by the Tukey HSD test at P < 0.05. Weather variables represented by relative humidity (mean ± 95% CI), temperature (mean ± 95% CI) and rainfall (average) recorded for each experiment conducted in a commercial citrus grove in Itapetininga, SP, Brazil. The asterisk indicates a missing set of observations
The mortality induced by B. bassiana ESALQ-PL63 in adult D. citri was significantly lower in comparison with that in the chemical treatment during the trials performed in December 2011 (1000 l/ha), January 2012 (1000 and 2000 l/ha) and March 2012 (1000 l/ha) (Fig. 3). Similarly, the fungus I. fumosorosea exhibited lower efficacy against D. citri compared with that of the chemical insecticide for the trials conducted in January 2012 (1000 l/ha), April 2012 (1000 and 2000 l/ha), August 2012 (2000 l/ha) and October 2012 (1000 l/ha). In the remaining monthly trials, however, comparable mortality levels were obtained for the fungal and insecticidal treatments (Fig. 3).
During the experimental period, the proportion of sporulated D. citri assessed under laboratory conditions varied significantly among the fungal treatments in five of the 12 months evaluated (i.e., in December 2011, August 2012, September 2012, October 2012 and November 2012), and a significant interaction was found between fungal treatment and month (χ 2 = 116.45, df = 32, P < 0.001) (Fig. 3). The percentage of D. citri showing sporulation induced by B. bassiana ranged from 57.8% (March 2012) to 20% (September 2012) for 2.5 × 106 conidia/ml sprayed using 2000 l/ha and from 60.0% (January and March 2012) to 12.2% (September 2012) for 5 × 106 conidia/ml using 1000 l/ha. The percentage of D. citri showing sporulation induced by I. fumosorosea ranged from 69.5% (Dec/2011) to 7.8% (September 2012) for 2.5 × 106 conidia/ml using 2000 l/ha and 68.9% (Mar/2012) to 14.4% (September 2012) for 5 × 106 conidia/ml using 1000 l/ha. Overall, a significantly lower proportion of sporulated insects was found during the drier months (May 2012 to September 2012).
After pooling data across the 12 field trials (months), the D. citri mortality varied significantly among the treatments (χ 2 = 130.7, df = 5, P < 0.001). The efficacy of the fungal species applied at 2000 l/ha did not differ significantly from that of the chemical insecticide (1000 l/ha) (Fig. 4). Interestingly, the higher (2000 l/ha) compared with the lower volume rate (1000 l/ha) resulted in a greater overall D. citri mortality exclusively for B. bassiana, indicating that the efficacy based on the volume rate was dependent on the fungal species tested (Fig. 4). Conversely, the efficacy of I. fumosorosea against D. citri was unaffected by the volume rate tested, resulting in mortality levels between 79.6 and 82.5%. The proportion of sporulated insects was not significantly affected by the fungal treatments (χ 2 = 0.33, df = 3, P = 0.9537), with an overall sporulation rate ranging from 40.1 to 41.6% (Fig. 4).
Overall effect of direct spray applications using two volume rates (1000 or 2000 l/ha) of Isaria fumosorosea ESALQ-1296 (If) and Beauveria bassiana ESALQ-PL63 (Bb) at 5 × 106 conidia/ml on the percentage mortality and sporulation in Diaphorina citri adults. The chemical insecticide and the control (water) were sprayed at 1000 l/ha. Within variables, differences between bars (mean ± SE, n = 108) topped by the same letters or by ‘ns’ are not significant according to Tukey HSD test at P < 0.05
The adult mortality tended to increase with an increase in the maximum relative humidity (RHmax) according to the coefficients obtained from the multiple logistic regression models (Table 2). In general, the percentage of sporulated cadavers was positively associated with high RH (max and min) and rainfall and was negatively correlated with maximum temperatures.
A multiple logistic regression was performed to predict the occurrence of mycosed D. citri based on the weather variables, including RHmax, precipitation and T max. These weather variables accurately predicted sporulation on adults (i.e., mycosed adults found in the field + sporulation from dead adults incubated in the laboratory) by B. bassiana (P < 0.001). High RHmax (χ 2 = 194.7, P < 0.001), rainfall (χ 2 = 102.9, P < 0.001) and low T max (χ 2 = 10.43, P = 0.015) contributed greatly toward explaining the sporulation levels on adults. Similarly, RHmax (χ 2 = 275.20, P < 0.001) and precipitation (χ 2 = 114.12, P < 0.001) but not T max (χ 2 = 6.9, P = 0.072) were the most meaningful weather variables correlated with percent sporulation on adults by I. fumosorosea (Fig. 5). Rainfall was the factor most strongly correlated with sporulation (coefficients of 0.0428 and 0.0434 for I. fumosorosea and B. bassiana, respectively); thus, the model predicted that a one-unit increase in precipitation would increase the sporulation level by approximately 4.4%. Therefore, the results clearly showed that an increase in rainfall was associated with a higher RHmax and that these conditions were associated with an increased frequency of adult cadavers showing sporulation (Fig. 5). When including the effect of both fungi in the model, there was no significant difference in sporulation rates between I. fumosorosea and B. bassiana (χ 2 = 1.28, P = 0.416).
Multiple logistic regression analysis explaining the relationship between mortality due to fungus expressed as sporulation (mycosis) of D. citri adults (i.e., total insects that showed sporulation in the field or after incubation in the laboratory) and weather variables [e.g., maximum relative humidity (RHmax), precipitation (P p) and maximum temperature (T max)], after direct spray application with Beauveria bassiana ESALQ-PL63 (a) or Isaria fumosorosea ESALQ-1296 (b)
Discussion
The first step in the development of a fungal biopesticide against D. citri resides in the selection of virulent candidate strains. Several parameters were investigated such as production of conidia on rice, resistance to UV radiation and sporulation on other hosts (data not presented); here, we demonstrated the virulence, defined in terms of the killing speed, of 17 fungal strains belonging to different species, some of which have not previously been reported to be pathogenic to citrus psyllids. For example, the fungal entomopathogens C. nymphaeae, H. thompsonii, Simplicillium sp., I. amoenorosea and P. lilacinum (formerly Paecilomyces lilacinus) were here first described as pathogenic to adult psyllids and exhibited variable median lethal time (LT50) values (Table 1).
The efficacy of certain fungal species against nymphs and adults of citrus psyllids has been reported (Padulla and Alves 2009; Hoy et al. 2010; Avery et al. 2011; Casique-Valdes et al. 2011; Hunter et al. 2011; Ferreira Pinto et al. 2012; Lezama-Gutiérrez et al. 2012; Stauderman et al. 2012). However, most of these studies were performed under laboratory conditions testing few fungal isolates or reported only scattered results from field trials. In the present study, we demonstrate the successful use of I. fumosorosea ESALQ-1296 and B. bassiana ESALQ-PL63 in inducing high mortality levels of D. citri adults under laboratory, semifield and field conditions. The studies conducted under semifield conditions were designed to reduce the concentration of conidia, considering the high volume of water required for pesticide applications in citrus. The concentrations used in the laboratory and in other studies, ≥107 conidia/ml, would not be economically feasible for use in citrus orchards. Remarkably, the mortalities observed in the field were relatively higher than those observed in the laboratory and semifield trials.
A few isolates of I. fumosorosea and I. javanica (Ifr AsCP, Apopka-97, ARSEF 3581, FE 9901 and Ifr 4) have shown encouraging results for controlling citrus psyllids (Hoy et al. 2010; Avery et al. 2011; Hunter et al. 2011; Lezama-Gutiérrez et al. 2012; Stauderman et al. 2012). Our results demonstrate that I. fumosorosea ESALQ-1296 is virulent to adult D. citri, inflicting mortality rates up to 77.8%, with 65.5% of those insects showing mycosis under laboratory conditions. The LT50 of I. fumosorosea ESALQ-1296 (1 × 107 conidia/ml) against D. citri adults was estimated to be 5.9 days, which fell into the LT50 range (4.5–6.7 days) reported by early studies at the same inoculum rate (Stauderman et al. 2012). Although a direct lethal effect caused by infection is the most evident trait sought in fungal entomopathogens against insect pests, researchers have suggested that D. citri adults infected with I. fumosorosea decrease their feeding activity before their death, which could lead to a reduction in the transmission of bacteria responsible for the HLB disease in citrus crops (Avery et al. 2011). We suspect that fungal disease may negatively affect adult psyllid fitness by reducing female fecundity and inducing behavioral changes, although this hypothesis needs clarification.
The fungus B. bassiana has also been reported as a potential biocontrol agent against D. citri nymphs and adults, with relatively satisfactory results achieved in the laboratory with strains IBCB 66, Cb 108 and ESALQ-PL63 (Padulla and Alves 2009, Ferreira Pinto et al. 2012; Lezama-Gutiérrez et al. 2012). A previous study showed that third instar nymphs of D. citri were highly susceptible when topically exposed to B. bassiana ESALQ-PL63, with an LT50 of 4.71 days at 2.3 × 107 conidia/ml (Padulla and Alves 2009). In the present study, the direct application of ESALQ-PL63 (1 × 107 conidia/ml) caused an adult mortality of 78.4%, with LT50 of 6.3 days, and nymphs been have shown to be even more susceptible than adults (unpublished data). ESALQ-PL63 is the strain of the commercial mycopesticide registered in Brazil under the name Boveril®, and in our study, we provide so far unreported evidence of its bioefficacy against D. citri, although the commercial formulation differs from the technical preparation used here.
Greenhouse studies have documented reductions of 50% in D. citri populations, with only 20% presenting mycosis, upon the application of the Apopka-97 strain of I. javanica (commercially available as PFR-97®, Certis, WI, USA) (Stauderman et al. 2012). In the present study, we demonstrated that applications of either I. fumosorosea ESALQ-1296 or B. bassiana ESALQ-PL63 can cause high adult mortality (80.6 and 83.5%, respectively) as well as high sporulation levels (58.3 and 54.4%, respectively) in citrus plants, suggesting more promising results than those reported previously. A study conducted in a Citrus latifolia grove (4 years old) located in Colima, Mexico, with four fungal applications of M. anisopliae (Ma 65 and Ma 14 strains), B. bassiana (Cb 108) and I. fumosorosea (Ifr 4) during a period of 64 days at intervals of 15 days resulted in lower nymph and adult counts in comparison with the control, reaching mortality levels of 35–60% in nymphs and 22–50% in adults (Lezama-Gutiérrez et al. 2012).
The population dynamics of D. citri in citrus orchards in São Paulo, Brazil, are extremely dependent on chemical pest control interventions and environmental factors (Belotti et al. 2013). In most citrus orchards, the highest density of D. citri occurs in the end of the spring time (August–September) and in the beginning of the summer (January–February) (Yamamoto et al., 2001). The D. citri management programs in commercial citrus groves in São Paulo are carried out with chemical insecticides that are frequently applied (1 or 2 per month) year-round. In an attempt to prevent D. citri migration into farmers’ groves, citrus farms with intensive D. citri management have used proactive control measurements to keep the insect population down even in neighboring areas where D. citri control has been neglected. Because there is zero tolerance for D. citri, farmers only allow studies using confined insects. To check the effect of the use of voil bags on insect mortality and sporulation, a preliminary study was performed on citrus trees to check the microclimatic conditions inside and outside of the voile bags using two dataloggers during 28 h (Supplementary Figure 1). The results demonstrated that the microclimatic conditions were similar between inside and outside the voile bag, especially when RH was high, but a slightly difference was observed only at midday (when RH <40% and temperatures were higher than 35 °C). During this period, temperatures were 5–7 °C lower and RH 11–15% higher inside than outside the voile bag (Supplementary Figure 2). This is probably due to the higher direct exposition of the equipment (datalogger) to sunlight outside the voile. The mesh size of the voile tissue was large enough to allow air circulation, and a small increase in RH inside the voile bag had an insignificant effect on the fungal infection and sporulation in D. citri adults.
Our open field study, which used a total of 12 fungal applications of I. fumosorosea ESALQ-1296 and B. bassiana ESALQ-PL63 sprayed once a month at two concentrations (2.5 and 5 × 106 viable conidia/ml) and two volume rates (1000 l/ha and 2000 l/ha) in a commercial citrus grove, demonstrated that both fungi were as effective as the standard chemical insecticides (Imidacloprid or Dimethoate) toward D. citri adults in most of the trials carried out. Overall, no difference occurred between the 1000 l/ha and 2000 l/ha applied volumes of I. fumosorosea ESALQ-1296, which achieved mean mortality rates of 79.6 and 82.5%, respectively. However, the application of B. bassiana ESALQ-PL63 at 1000 l/ha was slightly influenced by the volume rates tested, with mortalities reaching 76.4 and 82.3% for 1000 l/ha and 2000 l/ha, respectively. These results suggest that bioefficacy against D. citri in the field can be influenced by the volume rate of application and the fungal species.
There is a wealth of literature discussing the influence of environmental conditions, including rainfall, relative humidity, temperature and solar radiation, on the development of disease induced by fungal entomopathogens (Tanada and Kaya 1993; Inglis et al. 1997; Jaronski 2010). Sporulation on the fungal-killed D. citri appeared to be strongly affected by environmental factors, mainly RH and temperature, as expected, but interestingly, consistent mortality levels (>57%) were found even during relatively drier conditions of the year. During the winter season (June to September), when comparatively dry (<50% UR) and cold (12–18 °C) conditions were recorded, sporulation in D. citri was considerably reduced in comparison with summertime (December to March), which featured a wetter atmosphere (>95% RH) and warmer temperatures (25–30 °C). The efficacy of the fungal treatments even during drier months could be partly attributed to the characteristics of the surfactant used. Although reductions in mortality were observed during drier compared with wetter months, such reductions were also observed in the chemical pesticide treatment and may be associated with the evaporation of water droplets before reaching the plant and insects. A higher proportion of sporulated D. citri detected on citrus plants was positively correlated with greater rainfall and higher RHmax (see Fig. 5). This tendency for increased numbers of insects showing fungal sporulation could be somewhat advantageous through the creation of new infection foci for susceptible citrus pests and thus contributing to the overall performance of microbial control in citrus crops. Conceschi et al. (2016) demonstrated that biological control of citrus pests can be enhanced by transmission and dissemination of conidia of B. bassiana ESALQ-PL63 and I. fumosorosea ESALQ-1296 between D. citri and Toxoptera citricida that share the same habitat and are gregarious (Tsai and Liu 2000; Toledo et al. 2006). This behavior may favor the transfer of conidia between individuals, consequently increasing the efficacy of entomopathogenic fungi in controlling D. citri in citrus orchards.
The potential of I. fumosorosea ESALQ-1296 revealed in these studies supports the development of a new biopesticide under registration in Brazil with the commercial name PUMA (Koppert Sistemas Biológicos LTDA). The use of this mycopesticide in integrated pest management (IPM) programs in commercial citrus orchards represents an eco-friendly strategy to minimize chemical insecticide applications and mitigate the development of insecticide resistance by D. citri.
The efficacy of I. fumosorosea and B. bassiana toward D. citri adults was comparable to chemical insecticides tested here, the only control measure employed so far. Therefore, these mycopesticides have potential for good management in citrus orchards and bordering vegetation. Nevertheless, additional field studies are needed on the impact of I. fumosorosea and B. bassiana on D. citri in different regions and cropping systems.
Author contributions
JJSA, CPD, MRC and IDJ conceived and designed the experiments. JJSA, CPD and MRC conducted the experiments. GMM analyzed the data. All authors wrote and approved the manuscript.
References
Avery PB, Wekesa VW, Hunter WB, Hall DG, Mckenzie CL, Osborne LS, Powell CA, Rogers ME (2011) Effects of the Isaria fumosorosea (Hypocreales: Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci Technol 21(9):1065–1078
Belasque JJ, Bassanezi RB, Yamamoto PT, Ayres AJ, Tachibana A, Violante AR, Tank AJ Jr, Di Giorgi F, Tersi FEA, Menezes GM, Dragone J, Jank RH Jr, Bové JM (2010) Lessons from huanglongbing management in São Paulo state, Brazil. J Plant Pathol 92:285–302
Belotti VH, Rugno GR, Felippe MR, Carmo-uehara A, Garbim LF, Godoy WAC, Yamamoto PT (2013) Population dynamics of Diaphorina citri Kuwayama (Hemiptera: Liviidae) in orchards of ‘Valencia’ orange, ‘Ponkan’ mandarin and ‘Murcott’ tangor trees. Fla Entomol 96(1):173–179
Bové JM (2006) Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J Plant Pathol 88:7–37
Cardoso ER, Freitas S, Nunes HT, Pessoa LGA (2007) Seletividade de Lecanicillium lecanii e Metarhizium anisopliae para larvas de primeiro instar de Ceraeochrysa cincta (Neuroptera: Chrysopidae) em laboratório. Acta Sci Agron 29(4):563–568
Casique-Valdes R, Reyes-Martinez AY, Sanchez-Peña SR, Bidochka MJ, Lopez-Arroyo JL (2011) Pathogenicity of Hirsutella citriformis (Ascomycota: Cordycipitaceae) to Diaphorina citri (Hemiptera: Psyllidae) and Bactericera cockerelli (Hemiptera: Triozidae). Fla Entomol 94:703–705
Coleta-Filho HD, Targon MLPN, Takita MA, De Negri JD, Pompeu JRJ, Machado MA (2004) First report of the causal agent of Huanglongbing (‘Candidatus Liberibacter asiaticus’) in Brazil. Plant Dis 88:1382
Conceschi MR, D’Alessandro CP, Moral RA, Demétrio CGB, Delalibera IJ (2016) Transmission potential of the entomopathogenic fungi Isaria fumosorosea and Beauveria bassiana from sporulated cadavers of Diaphorina citri and Toxoptera citricida to uninfected D. citri adults. Biocontrol 61:567–577
Costa Lima AM (1942) Homópteros. Insetos do Brasil. Escola Nacional de Agronomia, Rio de Janeiro, vol 3, pp 1–327
Faria MR, Magalhães BP (2001) O uso de fungos entomopatogênicos no Brasil. Biotecnol Ciênc Desenvolv 4:18–21
Ferreira Pinto AP, Batista Filho A, Almeida JEM, Wenzel IM (2012) Patogenicidade de Beauveria bassiana ao psilídeo Diaphorina citri e compatibilidade do fungo com produtos fitossanitários. Pesq Agropec Bras 47:1673–1680
Goettel MS, Inglis DG (1997) Fungi: hyphomicetes. In: Lacey LA, Kaya HK (eds) Field manual of techniques in invertebrate pathology, 2nd edn. Springer, Dordrecht, pp 249–264
Halbert SE, Sun X, Dixon WN (2000) Asian citrus psyllid and citrus greening disease. Citrus Ind 91:22–24
Hoy MA, Sing R, Rogers ME (2010) Evaluations of a novel isolate of Isaria fumosorosea for control the Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Psyllidae). Fla Entomol 93:24–32
Humber RA (2012) Identification of entomopathogenic fungi. In: Lacey LA (ed) Manual of techniques in invertebrate pathology. Academic Press, London, pp 151–187
Hunter WB, Avery PB, Pick D, Powell CA (2011) Broad spectrum potential of Isaria fumosorosea against insect pests of citrus. Fla Entomol 94(4):1051–1054
Inglis GD, Johnson DL, Goettel MS (1997) Effects of temperature and sunlight on mycosis (Beauveria bassiana) of grasshoppers under field conditions. Environ Entomol 26:400–409
Jaronski ST (2010) Ecological factors in the inundative use of fungal entomopathogens. Biocontrol 55:159–185
Kuchment A (2013) The end of orange juice. Scientific American, New York, pp 52–59
Lezama-Gutiérrez R, Molina-Ochoa J, Chávez-Flores O, Ángel-Sahagún CA, Skoda SR, Reyes-Martínez G, Barba-Reynoso M, Rebolledo-Domínguez O, Ruíz-Aguilar GML, Foster JE (2012) Use of the entomopathogenic fungi Metarhizium anisopliae, Cordyceps bassiana and Isaria fumosorosea to control Diaphorina citri (Hemiptera: Psyllidae) in Persian lime under field conditions. Int J Trop Insect Sci 32(1):39–44
Lezama-Gutiérrez R, Ramírez-Mancilla A, Castrejón-Agapito H, Peralta-Manzo JJ, Rebolledo-Domínguez O (2014) Uso de Metarhizium anisopliae y Cordyceps bassiana (Ascomycetes) para el control de Diaphorina citri (Hemiptera: Psyllidae) en Limón Mexicano. Entomol Mex 1:219–224
Medina CL, Rena AB, Siqueira DL, Machado EC (2005) Fisiologia dos citros. In: Matos Jr D, De Negri JD, Pio RM, Pompeu Jr J (eds) Citros. Campinas, São Paulo. pp 146–195
Meyer JM, Hoy MA, Boucias DG (2007) Morphological and molecular characterization of a Hirsutella species infecting the Asian citrus psyllid, Diaphorina citri Kumayama (Hemipstera: Psyllidae), in Florida. J Invertebr Pathol 95:101–109
Michaud JP (2010) Natural mortality of the Asian citrus psyllid (Homoptera: Psyllidae) in central Florida. Biol Control 29:260–269
Monzo C, Qureshi JA, Stansly PA (2014) Insecticide sprays, natural enemy assemblages and predation on Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Bull Entomol Res 104:576–585
Nava DE, Torres MLG, Rodrigues MDL, Bento JMS, Parra JRP (2007) Biology of Diaphorina citri (Hem., Psyllidae) on different hosts and at different temperatures. J Appl Entomol 131:709–715
Oliveira DGP, Pauli G, Mascarin GM, Delalibera I (2015) A protocol for determination of conidial viability of the fungal entomopathogens Beauveria bassiana and Metarhizium anisopliae from commercial products. J Microbiol Methods 119:44–52
Orduño-Cruz N, Guzmán-Franco AW, Rodríguez-Leyva E, Alatorre-Rosas R, González-Hernández H, Mora-Aguilera G (2015) In vivo selection of entomopathogenic fungal isolates for control of Diaphorina citri (Hemiptera: Liviidae). Biol Control 90:1–5
Ortelli D, Edder P, Corvi C (2005) Pesticide residues survey in citrus fruits. Food Addit Contam 22:423–428
Padulla LFL, Alves SB (2009) Suscetibilidade de ninfas de Diaphorina citri a fungos entomopatogênicos. Arq Inst Biol 76:297–302
Ribeiro LP, Santos MS, Padoan GL, Vendramim JD (2015) Toxicity of an acetogenin-based bioinsecticide against Diaphorina citri (Hemiptera: Liviidae) and its parasitoid (Hymenoptera: Eulophidae). Fla Entomol 98:835–842
Ritz C, Streibig JC (2008) Nonlinear regression with R. useR! series. Springer, New York, p 148
Sanches AL, Felippe MR, Uehara-Carmo A, Rugno GR, Yamamoto PT (2009) Eficiência de inseticidas sistêmicos, aplicados em mudas cítricas, em préplantio, no controle de Diaphorina citri (Kuwayama) (Hemiptera: Psyllidae). BioAssay 4:1–7
Stauderman K, Avery P, Aristizábal L, Arthur S (2012) Evaluation of Isaria fumosorosea (Hypocreales: Cordycipitaceae) for control of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci Technol 22:747–761
Subandiyah S, Nikoh N, Sato H, Wagiman F, Tsuyumyu S, Fukatsu T (2000) Isolation and characterization of two entomopathogenic fungi attacking Diaphorina citri (Homoptera: Psylloidea) in Indonesia. Mycoscience 41:509–513
Tanada Y, Kaya HK (1993) Insect pathology. Academic Press, London
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Teixeira DC, Saillard C, Eveillard S, Danned JL, Costa PI, Ayres AJ, Bové J (2005) ‘Candidatus Liberibacter americanus’, associated with citrus Huanglongbing (Greening disease) in São Paulo State, Brazil. Int J Syst Evol Microbiol 55:1857–1862
Tiwari S, Mann RS, Rogers ME, Stelinski LL (2011) Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag Sci 67:1258–1268
Toledo FR, Barbosa JC, Yamamoto PT (2006) Distribuição espacial de Toxoptera citricida Kirkaldy (Hemiptera: Aphididae) na cultura de citros. Rev Bras Frutic 28(2):194–198
Tsai JH, Liu YH (2000) Biology of Diaphorina citri (Homoptera: Psyllidae) on four host plants. J Econ Entomol 93(6):1721–1725
Warton DI, Hui FKC (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10
Yamamoto PT, Paiva PEB, Gravena S (2001) Flutuação Populacional de Diaphorina citri Kuwayama (Hemiptera: Psyllidae) em Pomares de Citros na Região Norte do Estado de São Paulo. Neotrop Entomol 30(1):165–170
Yamamoto PT, Felippe MR, Sanches AL, Coelho JHC, Garim LF, Ximenes NL (2009) Eficácia de inseticidas para o manejo de Diaphorina citri Kuwayama (Hemiptera: Psyllidae) em citros. BioAssay 4:1–9
Yang Y, Huang M, Andrew G, Beattie C, Xia Y, Ouyang G, Xiong J (2006) Distribution, biology, ecology and control of the psyllid Diaphorina citri Kuwayama, a major pest of citrus: a status report for China. Int J Pest Manage 52:343–352
Acknowledgements
We thank the Foundation of Citrus Growers of São Paulo State (Fundecitrus), Koppert Sistemas Biológicos LTDA and the São Paulo State Research Foundation (FAPESP Project No. 2011/51556-3) for financial support. The first author was a recipient of a scholarship from the Coordination of Improvement of Higher Education Personnel (CAPES PEC/PG—Brazil 2010–2014).
Funding
This study was funded by the Foundation of Citrus Growers of São Paulo State (Fundecitrus), Koppert Sistemas Biológicos LTDA and the São Paulo State Research Foundation (FAPESP Project No. 2011/51556-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by E. Quesada-Moraga.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Saldarriaga Ausique, J.J., D’Alessandro, C.P., Conceschi, M.R. et al. Efficacy of entomopathogenic fungi against adult Diaphorina citri from laboratory to field applications. J Pest Sci 90, 947–960 (2017). https://doi.org/10.1007/s10340-017-0846-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-017-0846-z