Abstract
Insects exhibit complex symbiotic interactions with microorganisms, which provide an opportunity for developing novel pest management strategies. Closely related to Drosophila melanogaster, which is commonly used as a model to explore insect–microbe interactions, Drosophila suzukii is an important invasive insect pest of fruit crops in the Americas and Europe. We provide an overview of Drosophila–microbe interactions and review current research with D. suzukii. Recent studies revealed yeast and bacterial species associated with D. suzukii flies, fly guts and infested fruit. The ecological importance of these insect–microbe interactions is under investigation. Microbes have a strong impact on insect physiology and D. suzukii responds both positively and aversively to microbial volatiles. We highlight potential pest management strategies that take advantage of D. suzukii–microbe ecology, including improved monitoring as well as management using behavioural manipulation, phagostimulants and biotechnology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Symbiotic microorganisms play important roles in insects’ life cycles, and yeasts and bacteria impact Drosophila food quality, development time and reproductive output.
-
Despite an affinity for ripening or ripe fresh fruit, D. suzukii shows associations with bacteria and yeasts, similar to other drosophilids.
-
We review recent work describing D. suzukii–microbe ecology and areas where these relationships can potentially be used to improve D. suzukii pest management.
Introduction
Microbes are found in insect habitats, on their food substrates, on and inside the insect body, and within insect cells (Phaff et al. 1956; Begon 1982; Chandler et al. 2011; Douglas 2007). Notably, most of these microbial associations are not pathogenic, but rather beneficial or apparently benign (Douglas 2007). The ecological status of insect-associated microorganisms vary from taxa that maintain substantial free-living populations outside their associates to taxa that are insect species specific and generally do not develop outside the insect host (Douglas 2015). Insects disperse free-living microorganisms between external environmental habitats (Douglas 2015) and in special cases even “farm” mutualistic microbes on plant substrates such as leaves or fruit (Mueller et al. 2005; Stamps et al. 2012). Insect-associated microbes comprise a significant portion of the known microbial flora, as has been impressively demonstrated for yeasts by the hundreds of species isolated from beetles (Suh et al. 2005).
Insect–microbe associations have historically been studied using culture-dependent approaches; however, advances in high-throughput sequencing have provided new tools to supplement these approaches. Targeted rRNA gene sequencing of 16S for bacteria, 18S for eukaryotes, and the internal transcribed spacer region for fungi has been used to profile microbial communities (Bokulich and Mills 2013; Segata et al. 2013). Genome-wide sequencing approaches, including metagenomics, metatranscriptomics, metametabolomics and metaproteomics are becoming increasingly popular as technology advances and the cost of high-throughput sequencing decreases (Knief 2014). These approaches detect both culturable and unculturable microbes from within the community, and can provide information not only at a phylogenetic or taxonomic level, but also can provide information on gene function. Indeed, the whole meta-genome shotgun sequencing technique has been validated and bioinformatic procedures have been developed to answer taxonomic and metabolic profiling questions (Segata et al. 2013; Escobar-Zepeda et al. 2015). As more studies are performed and technology improves, these techniques will continue to improve our understanding of microbial community systems biology, including insect–microbe interactions.
Microbial products have long since been used as lures in entomology and pest management (Landolt and Hammond 2001, and references therein). Torula yeast (Candida utilis), as a prominent example, has been applied as a food lure to trap adult Tephritid fruit flies (Lopez-D et al. 1971; Daane and Johnson 2010; Leblanc et al. 2010). It has long been noted that torula yeast and other food lures targeting tephritids also capture non-target organisms, many of which are saprophagous flies, such as: calliphorids, ceratopogonids, chloropids, drosophilids, lonchaeids, muscids and sarcophagids (Thomas 2003; Leblanc et al. 2010). The richness of attracted taxa illustrates the potential of yeast as a powerful attractant on the one hand and challenges application aiming at high target specificity on the other. Favourably, insects show clear species-specific responses to different blends of fermentation compounds (Landolt and Alfaro 2001, and references therein). Understanding of mechanisms underlying specific ecological insect microbe interactions will help us to develop targeted microbe-based pest control techniques. Given that many insects are associated with specific microbes, and these interactions can be critically important for insects’ fitness, in particular close interactions like symbioses can be a resource for developing novel, species-specific pest management tactics (Douglas 2007). As a proof of concept, the recognition of insect–microbe interactions has lead to a control method exploiting the association between the codling moth Cydia pomonella with yeasts, and an insect-pathogenic virus (Witzgall et al. 2012; Knight and Witzgall 2013).
Drosophila species typically develop in decaying plant material like overripe fruit as well as in mushrooms and other fungi (Starmer 1981; Begon 1982; Markow and O’Grady 2005). Drosophila–yeast associations are among the best studied of insect–microbe interactions, and are a model system for interactions between free-living microbes and insects. Yeast communities have been collected from Drosophila for many years (Phaff et al. 1956; Chandler et al. 2012). Unlike most other drosophilids the spotted wing drosophila, Drosophila suzukii, develops in ripening and ripe fruit rather than overripe and rotting fruit (Walsh et al. 2011) but still is closely associated with yeasts (Hamby et al. 2012). Adult females oviposit directly into fruit with their sclerotized and serrated ovipositor, and cause economic damage to susceptible small and stone fruit in North America, Asia and Europe (Cini et al. 2012; Walsh et al. 2011; Asplen et al. 2015). The worldwide economic impacts due to D. suzukii are significant, and new management strategies are needed (Asplen et al. 2015).
Here we provide a brief summary of Drosophila–microbe ecology including the impacts of microbes on Drosophila physiology and behaviour as well as the role Drosophila plays in yeast dispersal and ecology. We then review the current literature on D. suzukii–microbe interactions, and, finally, we emphasize how insect interactions with free-living microorganisms can be exploited for pest management.
Drosophila-microbe ecology, physiology and behaviour
Many dipterans are saprophagous, feeding upon microbe-rich host substrates. Drosophila species often use fermenting plant material where larvae and adult flies feed on the nutritious microbial flora (Starmer 1981; Begon 1982). Microbes live closely associated with drosophilids, and use larval as well as adult flies as their hosts (Ganter 2006; Douglas 2007; Chandler et al. 2011).
Drosophila yeast and bacterial associations
Drosophila species with diverse habits and hosts are characterized by distinct assemblages of associated yeast microbes on which they feed (Phaff et al. 1956, Begon 1982; Chandler et al. 2012; Stamps et al. 2012; Lam and Howell 2015). Yeast proteins and lipids improve the food quality of plant substrates and support fly survival and development (Bos et al. 1976; Begon 1982; Yamada et al. 2015). Moreover, D. melanogaster females require a complex diet or yeast for ovarian maturation (Bownes et al. 1988). Saccharomyces cerevisiae is sufficient for D. melanogaster development and more flies develop on fermenting grapes that contain yeast, relative to grapes without yeast (Becher et al. 2012).
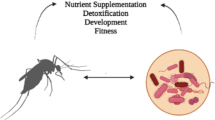
Fermenting substrates like overripe fruits are not only used as food resource but also as mating site, explaining attraction of virgin females and males to fermentation odours (Fig. 1). Indeed, yeasts are integral elements of Drosophila ecology that influence fly physiology and behaviour. Larvae of various Drosophila species prefer yeasts that result in high survivorship to adulthood (Lindsay 1958, Anagnostou et al. 2010). Despite potential adaptations for a novel ripening fruit niche, when yeast is removed from standard laboratory Drosophila diet, fewer D. suzukii survive to adulthood relative to the standard diet at low population density (Hardin et al. 2015). Adult D. suzukii flies readily feed on yeast and fruit blossoms in the laboratory, and microbes together with floral nectar or pollen likely are important food resources especially during early spring when fruit may be sparse (Tochen et al. 2016; Mori et al. accepted). Similar to other drosophilids, D. suzukii hosts a specific yeast flora, with Hanseniaspora uvarum most frequently cultured from field-collected adults and larvae, followed by Issatchenkia terricola (formerly Pichia) and P. kluyveri (Hamby et al. 2012). D. suzukii has shown a preference for H. uvarum in a laboratory multiple choice assay when given the choice between six species (Scheidler et al. 2015). Therefore, it is likely that D. suzukii has similar relationships with yeasts as other drosophilids.
Illustration of interactions between Drosophila and microbes and their utility for pest management. a Infestation of ripening fruit by Drosophila suzukii. Larvae develop inside the fruit, pupate and emerge as adult flies. b Drosophila females of various species require food for ovarian maturation and are attracted to food odours like yeast volatiles. c Such volatiles lead female as well as male drosophilids to traps used for monitoring or attract-and-kill strategies, or d guide the flies to natural resources. e Overripe fruit is a food resource and habitat for many Drosophila species, and D. suzukii also visits fermenting fruit. Drosophilds vector yeasts and bacteria to f damaged and g fresh fruit. h Sexually mature females can infest fresh fruit. i As male and female D. suzukii are attracted to fermentation odours, yeast can be applied as attractant and phagostimulant in combination with killing agents
Although fly genotype, diet, and host bacterial community impact the gut bacterial community associated with Drosophila, the bacterial microbiome of different populations and species are dominated by few bacterial groups such as Enterobacteriales, Rhodospirillales and Lactobacillales (Chandler et al. 2011; Broderick and Lemaitre 2012; Chandler et al. 2012; Wong et al. 2011). Interestingly, bacterial communities of D. suzukii were reported to be dominated by Tatumella, a genus of enterobacteria previously not considered a common associate of drosophilids (Chandler et al. 2014). Moreover, Acetobacteraceae were abundantly associated with D. suzukii (Chandler et al. 2014) with different species differing in their odour profiles and attractiveness to the flies (Mazzetto et al. 2016). Similar to yeasts, bacteria ingested with food or inhabiting the gut influence Drosophila larval growth and development (Shin et al. 2011; Ridley et al. 2012; Newell and Douglas 2014), and may even be capable of influencing Drosophila mating behaviour (Sharon et al. 2010). Additionally, Wolbachia bacteria, maternally transmitted intracellular endosymbionts that are widespread in Drosophila species, can have a diversity of effects on Drosophila physiology such as reproductive manipulation or increased longevity (Zug and Hammerstein 2015). In D. suzukii, Wolbachia infections have been suggested to be mutualistic but our understanding of mechanisms underlying such interactions and effects on fecundity need further investigation (Hamm et al. 2014; Mazzetto et al. 2015).
Odour-mediated responses to microbial volatiles
Considering the value of yeasts and bacteria as food resources and potential indicators of habitat quality, it is not surprising that microbial odours induce strong attraction in Drosophila larvae and flies (Fishilevich et al. 2005; Becher et al. 2010, 2012; Venu et al. 2014; Dweck et al. 2015; Scheidler et al. 2015). Like most drosophilids, D. suzukii is strongly attracted to yeast (Iglesias et al. 2014; Scheidler et al. 2015) and specific fermentation compounds (Landolt et al. 2011; Cha et al. 2012; Kleiber et al. 2014). Such behaviours are the output of chemosensory processes and various microbial volatiles have been shown to induce responses in D. suzukii antennae (Cha et al. 2012; Abraham et al. 2015; Keesey et al. 2015; Revadi et al. 2015; Scheidler et al. 2015).
Evidently, Drosophila chemosensory receptors are sensitive to microbial metabolites. For example, fermentation products like esters, alcohols, acids or carbon dioxide are ligands of Drosophila olfactory (Stensmyr et al. 2003), ionotropic (Ai et al. 2013) and gustatory receptors (Kwon et al. 2007; Wisotsky et al. 2011; Charlu et al. 2013). Microbial metabolites both positively and aversively affect feeding, mating and oviposition behaviours. Glycerol provides a sensory cue for fermentation processes, which influences adult D. melanogaster feeding responses towards yeast (Wisotsky et al. 2011). Additionally, fermentation products enhance Drosophila responses to the male pheromone during courtship (Bartelt et al. 1985; Lebreton et al. 2012, 2015), increase female sexual receptivity (Gorter et al. 2016) and mediate oviposition behaviour (Joseph et al. 2009; Becher et al. 2012). In contrast, microbial odours also induce aversive behaviours as demonstrated for geosmin, which negatively affects attraction, feeding and oviposition in D. melanogaster (Becher et al. 2010; Stensmyr et al. 2012). In D. suzukii, geosmin as well as 1-octen-3-ol induce aversion as described further below (Wallingford et al. 2015).
Profit for microbes
Microbes also benefit from their association with drosophilids, suggesting that for some interacting species the relations are mutualistic. As in other insects, microbes generally profit from drosophilds as hosts (Janson et al. 2008) exploiting in particular the insect gut as a habitat (Engel and Moran 2013). In addition, microbes benefit from transportation and dispersal to new substrates (Gilbert 1980; Buser et al. 2014), particularly yeasts which generally are only poorly dispersed by wind. On the host substrate, Drosophila larval feeding positively affects yeast density, and decreases the development of mould fungi (Wertheim et al. 2002; Stamps et al. 2012; Caballero Oritz et al. 2013). Yeast spores were found to survive the passage through the D. melanogaster gut facilitating dispersal and outcrossing between different yeast strains (Pulvirenti et al. 2002, Reuter et al. 2007).
Volatile signals emitted by microbes possibly coevolve together with insect sensory systems (Scheidler et al. 2015). There is increasing support that attraction of insect vectors is a prime function of yeast volatiles. Indeed the aroma gene ATF1, which encodes an acetate ester synthase promotes dispersal of yeast while deletion of ATF1 shows no other negative effect than decreased vectoring by D. melanogaster when tested in the lab (Christiaens et al. 2014).
Implications of Drosophila-microbe ecology for pest management
Current D. suzukii management relies on repeated applications of broad-spectrum insecticides (Beers et al. 2011; Bruck et al. 2011; Van Timmeren and Isaacs 2013). Alternative pest management strategies are required to develop successful Integrated Pest Management (IPM) programs. Symbioses with microorganisms represent an untapped resource for pest management (Douglas 2007, 2015). While obligate symbionts are particularly promising, facultative symbionts that have significant impacts on the pest’s life history can also be exploited (Douglas 2007). We describe pest management tactics where Drosophila–microbe ecology may be leveraged for improved D. suzukii management.
Monitoring pest populations
Monitoring insect populations to evaluate their phenology, population dynamics and the risk of crop damage is an important component of IPM. For many insects, monitoring is achieved using traps baited with attractive semiochemicals. Sweet baits and fermentation products have traditionally been used as attractants for a broad range of insects (Ditman and Cory 1933; Landolt and Hammond 2001). Indeed, fermentation-based attractants have been developed for D. suzukii (Cha et al. 2012; Landolt et al. 2012; Burrack et al. 2015). Actively fermenting baker’s yeast (S. cerevisiae) baits are often among the highest capturing attractants when compared to other fermentation products such as apple cider vinegar and wine (Hamby et al. 2014; Iglesias et al. 2014). However, despite efforts to improve both trap designs and attractants (Lee et al. 2012; Landolt et al. 2012; Lee et al. 2013), D. suzukii monitoring remains difficult for growers to implement (Burrack et al. 2015). Current monitoring systems suffer from inconsistent efficacy, and they exhibit variability in trap captures depending on crop type, crop phenology and D. suzukii phenology. It is difficult to relate trap captures to infestation. Therefore, it is difficult to use trap captures to schedule time-sensitive IPM actions. Perhaps the largest constraint to widespread adoption of D. suzukii monitoring by stakeholders is the lack of selectivity. Because non-target drosophilids are also captured, users of the current monitoring system must identify trap captures under magnification which is time consuming and cumbersome. Additionally, separating D. suzukii from other drosophilids can be challenging for nonexperts (Burrack et al. 2015). Therefore, developing trapping systems with increased selectivity is an important research priority.
One avenue for attractant development is microbial volatile emissions. Microbial volatiles are used by many insect species to locate resources, and play an important role in insect behavioural ecology (Davis et al. 2013). In a survey of insects that are attracted to fungal headspace odours from Aureobasidium pullulans in spearmint fields, 65 % of trapped insects were dipterans (Davis and Landolt 2013). Moreover, drosophilids and other dipterans were among the insects significantly attracted to traps baited with live yeasts in an apple orchard (Andreadis et al. 2015). Recent work suggests that Drosophila species can distinguish between the volatile emissions produced by different yeast species and strains, and that closely related Drosophila (D. melanogaster compared to D. suzukii) differentially respond to specific microbial volatile constituents (Arguello et al. 2013; Scheidler et al. 2015). Therefore, microbial volatile emissions may be useful for improving attractant selectivity. A successful monitoring system for D. suzukii would selectively attract D. suzukii adults prior to commercial infestation, effectively track population activity throughout the season and accurately relate to larval densities in the fruit.
Behavioural manipulation for D. suzukii management
Behavioural manipulation using insect semiochemicals is an important component of sustainable pest management. Repellents, attract-and-kill and mass trapping have been used against various insect pests to reduce economic damage (El-Sayed et al. 2006, 2009). Recent work has shown that repellent chemicals can be used to reduce D. suzukii oviposition in host fruit. In the laboratory, a significant reduction in D. suzukii oviposition in blueberries was achieved when the blueberries were painted with butyl anthranilate, a DEET-substitute compound that is approved for human consumption (Pham and Ray 2015). Wallingford et al. (2015) evaluated two compounds that are commonly associated with bacterial and fungal volatile emissions, 1-octen-3-ol and geosmin. Both compounds repelled adult female D. suzukii in laboratory gated-trap choice tests. Field trials were conducted in red raspberries using odorant dispensers affixed to plants and the repellent 1-octen-3-ol. Fruit near the dispensers were sampled for infestation and significantly lower infestation was observed in the fruit near the repellent dispensers versus the solvent controls. Microbial volatiles could be used to develop repellent compounds for D. suzukii pest management. However, the compounds must be safe for human consumption and cannot affect fruit quality and taste.
Attract-and-kill and mass trapping are similar approaches that are separated by the method of killing the insect after attraction, typically a toxicant for attract-and-kill compared with a physical killing system such as an adhesive or drowning solution for mass trapping (El-Sayed et al. 2006, 2009). Both strategies are effective for low density insect populations, and would be most useful during the early season before the D. suzukii population builds. A very competitive lure that outcompetes the natural odour source or host signal is required for either of these techniques to work (Fig. 1c). These strategies diverge in their formulation and release strategy with attract-and-kill systems requiring sufficient contact with the toxicant that the insect is killed, and these formulations are commonly patented (e.g. GF-120 and SPLAT). Mass trapping systems require experiments on effective trap design and trap density. Indeed, preliminary mass trapping experiments using an apple cider vinegar–yeast–flour bait for management of D. suzukii reported that blueberry infestation was significantly higher where traps were deployed, with increased D. suzukii activity near the attractant traps (Hampton et al. 2014). Both mass trapping and attract-and-kill systems have been used successfully to manage various Tephritid fruit fly pests (El-Sayed et al. 2006, 2009); therefore, they may have potential as management strategies for D. suzukii with an improved attractant and effective deployment technology. Indeed, mass trapping has been integrated with other D. suzukii pest management techniques in Italian and Swiss fruit systems (De Ros et al. 2015; Baroffio 2015). Microbial volatiles are underutilized in behavioural manipulation strategies, and are likely a good resource for such strategies since these volatiles are often chemically distinct from the pervading background of plant volatiles (Witzgall et al. 2012).
Sterile insect technique
Recent work in Tephritid fruit fly sterile insect technique (SIT) has demonstrated the importance of understanding insect–microbe interactions for successfully developing novel pest management strategies. For example, Bactrocera oleae sterile insect technique programmes were practically abandoned in the 1980’s due to a lack of understanding of the fly’s basic biology, including its microbe interactions (Estes et al. 2012). Bactrocera oleae possesses gut evaginations that house their bacterial endosymbiont ‘Candidatus Erwinia dacicola’, which is important for adult and larval nutrition and helps larvae to overcome host defence (Ben-Yosef et al. 2010, 2015). Additionally, transiently acquired free-living bacteria are common in wild B. oleae and likely benefit B. oleae health. Laboratory flies reared on artificial media exhibit declines in endosymbiont populations and significant changes in their transient bacterial microbiome (fewer and different taxa) compared to wild flies. Because one of the biggest challenges for B. oleae SIT is mass rearing high-quality flies (necessary for competitiveness in the wild), facilitating these bacterial interactions during rearing (e.g. removing antibiotics from artificial media which has been successful) has been highlighted as important for the success of future SIT programmes (Estes et al. 2012). In Ceratitis capitata the irradiation process to produce sterile males affects the gut bacterial community. These males are less competent in attracting and mating with wild females, and providing diets containing bacteria significantly improved the sterile male performance in copulatory tests (Ben Ami et al. 2010). Transgenic genetic technologies are being developed in D. suzukii using the piggyBac transposon vector (Schetelig and Handler 2013) and CRISPER/Cas9-mediated gene editing (Li and Scott 2016). In the future, these technologies could be used to develop a SIT or a gene drive system for suppression of D. suzukii populations (Schetelig and Handler 2013; Li and Scott 2016), and mass rearing high-quality D. suzukii would be important.
Phagostimulants and RNA interference
Phagostimulants can be used to improve the efficacy of insecticides and microbial control agents that require ingestion (Bell and Kanavel 1977; Williams et al. 2004; Knight et al. 2015). In some cases, phagostimulants can even significantly reduce the amount of active ingredient necessary for successful management. These lower rates increase safety for workers and beneficials (Williams et al. 2004). Sucrose has been evaluated as a phagostimulant for D. suzukii, and both contact active and primarily ingestion active insecticides exhibited increased efficacy when used with sucrose (Cowles et al. 2015). Additionally, combinations of sucrose with yeast (S. cerevisiae) and yeast-like fungus (A. pullulans) have been evaluated as phagostimulants to improve the efficacy of cyantraniliprole and spinosad (Knight et al. 2015). Interestingly, A. pullulans is commercially available as Blossom Protect (Westbridge Ag Products, Vista, CA) and registered for control of fireblight Erwinia amylovora. In some cases, addition of a yeast-feeding stimulant with sugar improved activity (evaluated by adult mortality, egg and larval infestation) relative to sugar alone; however, the effect varied by insecticide, yeast species and the yeast formulation (Knight et al. 2015). Yeast species that are attractive to D. suzukii for feeding could be used as phagostimulants to improve insecticide efficacy (Fig. 1i). Recent findings suggest that this strategy could facilitate attract-and-kill control of D. suzukii (Mori et al. accepted).
RNA interference (RNAi) co-opts a cellular mechanism that likely evolved to protect eukaryotes from RNA viruses, introducing exogenous double-stranded RNA (dsRNA) to activate the cellular machinery to degrade or suppress the translation of gene transcripts complementary to the dsRNA (Ding 2010; Burand and Hunter 2013; Gu and Knipple 2013). Recent advancements in RNAi have developed higher throughput and cheaper methods for RNAi target selection, target screening and the synthesis of dsRNA, increasing the feasibility of using RNAi as a tool for pest management (Gu and Knipple 2013; Zotti and Smagghe 2015; Murphy et al. 2016a). Specifically, dsRNA delivery systems involving in vivo expression of dsRNA using vector constructs that contain a target gene sequence (a gene vital to the insect and localized in the insect gut) that use bacteria, host plants or viruses (host plant or insect) to host the vector and express the dsRNA have been successful (Burand and Hunter 2013; Gu and Knipple 2013). However, delivery of intact dsRNA to the target site remains a challenge (Zotti and Smagghe 2015; Taning et al. 2016). Oral application has proven promising to increase gene silencing and D. suzukii mortality (Taning et al. 2016). One novel approach to dsRNA delivery takes advantage of D. suzukii–yeast interactions (Murphy et al. 2016b). By introducing the vector construct to yeast, this system couples an attractive food source with a readily transformed and cultured in vivo dsRNA expression system (Murphy et al. 2016b). This “yeast biopesticide” was shown to significantly decrease larval survivorship, reduce adult locomotor activity and reduce reproductive output in a species-specific manner (no significant effects on the closely related D. melanogaster). Therefore, D. suzukii–yeast ecology may be leveraged to develop new pest management biotechnologies such as RNAi.
Author contribution
K. A. H. and P. G. B. equally contributed to the writing of the manuscript.
References
Abraham J, Zhang A, Angeli S, Abubeker S, Michel C, Feng Y, Rodriguez-Saona C (2015) Behavioral and antennal responses of Drosophila suzukii (Diptera: Drosophilidae) to volatiles from fruit extracts. Environ Entomol 44:356–367
Ai M, Blais S, Park J-Y, Min S, Neubert TA, Suh GSB (2013) Ionotropic glutamate receptors IR64a and IR8a form a functional odorant receptor complex in vivo in Drosophila. J Neurosci 33:10741–10749
Anagnostou C, Dorsch M, Rohlfs M (2010) Influence of dietary yeasts on Drosophila melanogaster life-history traits. Entomol Exp Appl 136:1–11
Andreadis SS, Witzgall P, Becher PG (2015) Survey of arthropod assemblages responding to live yeasts in an organic apple orchard. Front Ecol Evol 3:121
Arguello JR, Sellanes C, Lou YR, Raguso RA (2013) Can yeast (S. cerevisiae) metabolic volatiles provide polymorphic signaling? PLoS One 8:e70219
Asplen MK, Anfora G, Biondi A, Choi D-S, Chu D et al (2015) Invasion biology of spotted wing Drosophila (Drosophila suzukii): a global perspective and future priorities. J Pest Sci 88:469–494
Baroffio C (2015) Four years of experience with SWD in Switzerland. Presentation at the Nordic Seminar, Copenhagen
Bartelt RJ, Schaner AM, Jackson LL (1985) Cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J Chem Ecol 11:1747–1756
Becher PG, Bengtsson M, Hansson BS, Witzgall P (2010) Flying the Fly: long-range flight behavior of Drosophila melanogaster to attractive odors. J Chem Ecol 36:599–607
Becher PG, Flick G, Rozpędowska E, Schmidt A, Hagman A, Lebreton S, Larsson MC, Hansson BS, Piškur J, Witzgall P, Bengtsson M (2012) Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Funct Ecol 26:822–828
Beers EH, Van Steenwuk RA, Shearer PW, Coates WW, Grant JA (2011) Developing Drosophila suzukii management programs for sweet cherry in the western United States. Pest Manag Sci 67:1386–1395
Begon M (1982) Yeasts and Drosophila. In: Ashburner M, Carson HL, Thompson J (eds) The genetics and biology of Drosophila, vol 3a. Academic Press, London, pp 345–384
Bell MR, Kanavel RF (1977) Field tests of a nuclear polyhedrosis virus in a bait formulation for control of pink bollworm and Heliothis spp. in cotton in Arizona. J Econ Entomol 70:625–629
Ben Ami E, Yuval B, Jurkevitch E (2010) Maniupation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. ISME J 4:28–37
Ben-Yosef M, Aharon Y, Jurkevitch E, Yuval B (2010) Give us the tools and we will do the job: symbiotic bacteira affect olive fly fitness in a diet-dependent fashion. Proc R Soc Lond B 277:1545–1552
Ben-Yosef M, Pasternak Z, Jurkevitch E, Yuval B (2015) Symbiotic bacteria enable olive fly larvae to overcome host defences. R Soc Open Sci 2:150170
Bokulich NA, Mills DA (2013) Improved selection of internal transcribed spacer-specific primers enables quantitative, ultra-high-throughput profiling of fungal communities. Appl Environ Microbiol 79:2519–2526
Bos M, Burnet B, Farrow R, Woods RA (1976) Development of Drosophila on sterol mutants of the yeast Saccharomyces cerevisiae. Genet Res 28:163–176
Bownes M, Scott A, Shirras A (1988) Dietary components modulate yolk protein gene transcription in Drosophila melanogaster. Development 103:119–128
Broderick NA, Lemaitre B (2012) Gut-associated microbes of Drosophila melanogaster. Gut Microbes 3:307–321
Bruck DJ, Bolda M, Tanigoshi L, Klick J, Kleiber J, DeFrancesco J, Gerdeman B, Spitler H (2011) Laboratory and field comparisons of insecticides to reduce infestation of Drosophila suzukii in berry crops. Pest Manag Sci 67:1375–1385
Burand JP, Hunter WB (2013) RNAi: future in insect management. J Invertebr Pathol 112:S68–S74
Burrack HJ, Asplen M, Bahder L, Collins J, Drummond FA, Guédot C, Isaacs R, Johnson D, Blanton A, Lee JC, Loeb G, Rodriguez-Saona C, Van Timmeren S, Walsh D, McPhie DR (2015) Multi-state comparison of attractants for monitoring Drosophila suzukii (Diptera: Drosophilidae) in blueberries and caneberries. Environ Entomol. doi:10.1093/ee/nvv022
Buser CC, Newcomb RD, Gaskett AC, Goddard MR (2014) Niche construction initiates the evolution of mutualistic interactions. Ecol Lett 17:1257–1264
Caballero Oritz S, Trienens M, Rohlfs M (2013) Induced fungal resistance to insect grazing: reciprocal fitness consequences and fungal gene expression in the Drosophila-Aspergillus model system. Plos One 8:e74951
Cha DH, Adams T, Rogg H, Landolt PJ (2012) Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing drosophila, Drosophila suzukii. J Chem Ecol 38:1419–1431
Chandler JA, Lang JM, Bhatnagar S, Eisen JA, Kopp A (2011) Bacterial communities of diverse Drosophila species: ecological context of a host-microbe model system. PLoS Genet 7:e1002272
Chandler JA, Eisen JA, Kopp A (2012) Yeast communities of diverse Drosophila species: comparison of two symbiont groups in the same hosts. Appl Environ Microbiol 78:7327–7336
Chandler JA, James PM, Jospin G, Lang JM (2014) The bacterial communities of Drosophila suzukii collected from undamaged cherries. PeerJ 2:e474
Charlu S, Wisotsky Z, Medina A, Dahanukar A (2013) Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nat Commun 4:2042
Christiaens JF, Franco LM, Cools TL, De Meester L, Michiels J, Wenseleers T, Hassan BA, Yaksi E, Verstrepen KJ (2014) The fungal aroma gene ATF1 promotes dispersal of yeast cells through insect vectors. Cell Rep 9:425–432
Cini A, Ioriatti C, Anfora G (2012) A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull Insectol 65:149–160
Cowles RS, Rodriguez-Saona C, Holdcraft R, Loeb GM, Elsensohn JE, Hesler SP (2015) Sucrose improves insecticidal activity against Drosophila suzukii (Diptera: Drosophilidae). J Econ Entomol 108:640–653
Daane KM, Johnson MW (2010) Olive fruit fly: managing an ancient pest in modern times. Annu Rev Entomol 55:151–169
Davis TS, Landolt PJ (2013) A survey of insect assemblages responding to volatiles from a ubiquitous fungus in an agricultural landscape. J Chem Ecol 39:860–868
Davis TS, Crippen TL, Hofstetter RW, Tomberlin JK (2013) Microbial volatile emissions as insect semiochemicals. J Chem Ecol 39:840–859
De Ros G, Conci S, Pantezzi T, Savini G (2015) The economic impact of invasive pest Drosophila suzukii on berry production in the Province of Trento, Italy. J Berry Res 5(2):89–96
Ding SW (2010) RNA-based antiviral immunity. Nat Rev Immunol 10:632–644
Ditman LP, Cory EN (1933) The response of corn earworm moths to various sugar solutions. J Econ Entomol 26:109–115
Douglas AE (2007) Symbiotic microorganisms: untapped resources for insect pest control. Trends Biotechnol 25:338–342
Douglas AE (2015) Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol 60:17–34
Dweck HKM, Ebrahim SAM, Farhan A, Hansson BS, Stensmyr MC (2015) Olfactory proxy detection of dietary antioxidants in Drosophila. Curr Biol 25:1–12
El-Sayed AM, Suckling DM, Wearing CH, Byers JA (2006) Potential of mass trapping for long-term pest management and eradication of invasive species. J Econ Entomol 99:1550–1564
El-Sayed AM, Suckling DM, Byers JA, Jang EB, Wearing CH (2009) Potential of “lure and kill” in long-term pest management and eradication of invasive species. J Econ Entomol 102:815–835
Engel P, Moran NA (2013) The gut microbiota of insects—diversity in structure and function. FEMS Microbial Rev 37:699–735
Escobar-Zepeda A, Vera-Ponce de León A, Sanchez-Flores A (2015) The road to metagenomics: from microbiology to DNA sequencing technologies and bioinformatics. Front Genet 6:348
Estes AM, Nestel D, Belcari A, Jessup A, Rempoulakis P, Economopoulos AP (2012) A basis for the renewal of sterile insect technique for the olive fly, Bactrocera oleae (Rossi). J Appl Entomol 136:1–16
Fishilevich E, Domingos AI, Asahina K, Naef F, Vosshall LB, Louis M (2005) Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr Biol 15:2086–2096
Ganter PF (2006) Yeast and invertebrate associations. In: Rosa CA, Péter G (eds) The yeast handbook: biodiversity and ecophysiology of yeast. Springer, Berlin, pp 303–370
Gilbert DG (1980) Dispersal of yeasts and bacteria by Drosophila in a temperate forest. Oecologia 46:135–137
Gorter JA, Jagadeesh S, Gahr C, Boonekamp JJ, Levine JD, Billeter JC (2016) The nutritional and hedonic value of food modulate sexual receptivity in Drosophila melanogaster females. Sci Rep 6:19441
Gu L, Knipple DC (2013) Recent advances in RNA interference research in insects: implications for future insect pest management strategies. Crop Prot 45:36–40
Hamby KA, Hernández A, Boundy-Mills K, Zaloma FG (2012) Associations of Yeasts with Spotted-Wing Drosophila (Drosophila suzukii; Diptera: Drosophilidae) in Cherries and Raspberries. Appl Environ Microbiol 78:4869
Hamby KA, Bolda MP, Sheehan ME, Zalom FG (2014) Seasonal monitoring for Drosophila suzukii (Diptera: Drosophilidae) in California commercial raspberries. Environ Entomol 43:1008–1018
Hamm CA, Begun DJ, Vo A, Smith CCR, Perot S, Shaver AO, Jaenike J, Turelli M (2014) Wolbachia do not live by reproductive manipulation alone: infection polymorphism in Drosophila suzukii and D. subpulchrella. Mol Ecol 23:4871–4885
Hampton E, Koski C, Barsoian O, Faubert H, Cowles RS, Alm SR (2014) Use of early ripening cultivars to avoid infestation and mass trapping to manage Drosophila suzukii (Diptera: Drosophilidae) in Vaccinium corymbosum (Ericales: Ericaceae). J Econ Entomol 107(5):1849–1857
Hardin JA, Kraus DA, Burrack HJ (2015) Diet quality mitigates intraspecific larval competition in Drosophila suzukii. Entomol Exp Appl 56:59–65
Iglesias LE, Nyoike TW, Liburd OE (2014) Effect of trap design, bait type, and age on captures of Drosophila suzukii (Diptera: Drosophilidae) in berry crops. J Econ Entomol 107:1508–1518
Janson EM, Stireman JO III, Singer MS, Abbot P (2008) Phytophagous insect-microbe mutualisms and adaptive evolutionary diversification. Evolution 62:997–1012
Joseph RM, Devineni AV, King IFG, Heberlein U (2009) Ovipostion preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc Natl Acad Sci USA 106:11352–11357
Keesey IW, Knaden M, Hansson BS (2015) Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J Chem Ecol 41:121–128
Kleiber JR, Unelius R, Lee JC, Suckling DM, Qian MC, Bruck DJ (2014) Attractiveness of fermentation and related products to spotted wing Drosophila (Diptera: Drosophilidae). Environ Entomol 43:439–447
Knief C (2014) Analysis of plant microbe interactions in the era of next generation sequencing technologies. Front Plant Sci 5:216
Knight A, Witzgall P (2013) Combining mutualistic yeast and pathogenic virus—a novel method for codling moth control. J Chem Ecol 39:1019–1026
Knight AL, Basoalto E, Witzgall P (2015) Improving the performance of the granulosis virus of codling moth (Lepidoptera: Tortricidae) by adding the yeast Saccharomyces cerevisiae with sugar. Environ Entomol 44:252–259
Kwon JY, Dahanukar A, Weiss LA, Carlson JR (2007) The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA 104:3574–3578
Lam SSTH, Howell KS (2015) Drosophila-associated yeast species in vineyard ecosystems. FEMS Microbiol Lett 362(20):1–7
Landolt PI, Alfaro JF (2001) Trapping Lacanobia subjuncta, Xestia c-nigrum, and Mamestra configurata (Lepidoptera: Noctuidae) with acetic acid and 3-methyl-1-butanol in controlled release dispensers. Environ Entomol 30:656–662
Landolt PI, Hammond PC (2001) Species’ composition of moths captured in traps baited with acetic acid and 3-methyl-1-butanol, in Yakima County, Washington. J Lepidopterists Soc 55:53–58
Landolt PJ, Adams T, Rogg H (2011) Trapping spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. J Appl Entomol 136:148–154
Landolt PJ, Adams T, Rogg H (2012) Trapping spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. J Appl Entomol 136:148–154
Leblanc L, Vargas RI, Rubinoff D (2010) A comparison of nontarget captures in biolure and liquid protein food lures in Hawaii. Proc Hawaii Entomol Soc 42:15–22
Lebreton S, Becher PG, Hansson BS, Witzgall P (2012) Attraction of Drosophila melanogaster males to food-related and fly odours. J Insect Physiol 58:125–129
Lebreton S, Trona F, Borrero-Echeverry P, Bilz F, Grabe V, Becher PG, Carlsson MA, Nässel DR, Hansson BS, Sachse S, Witzgall P (2015) Feeding regulates sex pheromone attraction and courtship in Drosophila females. Sci Rep 5:13132
Lee JC, Burrack HJ, Barrantes LD, Beers EH, Dreves AJ, Hamby KA, Haviland DR, Isaacs R, Richardson TA, Shearer PW, Stanley CA, Walsh DB, Walton VM, Zalom FG, Bruck DJ (2012) Evaluation of monitoring traps for Drosophila suzukii (Diptera: Drosophilidae) in North America. J Econ Entomol 105:1350–1357
Lee JC, Shearer PW, Barrantes L, Beers E, Burrack H, Dalton DT, Dreves AJ, Gut LJ, Hamby KA, Haviland DR, Isaacs R, Nielsen AL, Richardson T, Rodriguez-Saona C, Stanly CA, Walsh DB, Walton VM, Yee WL, Zalom FG, Bruck DJ (2013) Trap designs for monitoring Drosophila suzukii (Diptera: Drosophilidae). Environ Entomol 42:1348–1355
Li F, Scott MJ (2016) CRISPER/Cas9-mediated mutagenesis of the white and Sexlethal loci in the invasive pest, Drosophila suzukii. Biochem Biophys Res Commun 469:911–916
Lindsay SL (1958) Preferences of Drosophila larvae. Am Nat 92:279–285
Lopez-D F, Steiner LF, Holbrook FR (1971) A new yeast hydrolysate-borax bait for trapping the Caribbean fruit fly. J Econ Entomol 64:1541–1543
Markow TA, O’Grady PM (2005) Evolutionary genetics of reproductive behavior in Drosophila: connecting the dots. Annu Rev Genet 39:263–291
Mazzetto F, Gonella E, Alma A (2015) Wolbachia infection affects female fecundity in Drosophila suzukii. Bull Insectol 68:153–157
Mazzetto F, Gonella E, Crotti E, Vacchini V, Syrpas M, Pontini M, Mangelinckx S, Daffonchio D, Alma A (2016) Olfactory attraction of Drosophila suzukii by symbiotic acetic acid bacteria. J Pest Sci. doi:10.1007/s10340-016-0754-7
Mori BA, Whitener AB, Leinweber Y, Revadi S, Beers EH, Witzgall P, Becher PG (accepted) Enhanced yeast feeding following mating facilitates control of the invasive fruit pest Drosophila suzukii. J Appl Ecol
Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR (2005) The evolution of agriculture in insects. Annu Rev Ecol Evol Syst 36:563–569
Murphy KA, West JD, Kwok RS, Chiu JC (2016a) Accelerating research on Spotted Wing Drosophila management using genomic technologies. J Pest Sci. doi:10.1007/s10340-016-0741-z
Murphy KA, Tabuloc CA, Cervantes KR, Chiu JC (2016b) Ingestion of genetically modified yeast symbiont reduces fitness of an insect pest via RNA interference. Sci Rep 6:22587
Newell PD, Douglas AE (2014) Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster. Appl Environ Microbiol 80:788–796
Phaff HJ, Miller MW, Recca JA, Shifrine M, Mrak EM (1956) Studies on the ecology of Drosophila in the Yosemite Region of California. II Yeasts found in the alimentary canal of Drosophila. Ecology 37(3):533–538
Pham CK, Ray A (2015) Conservation of olfactory avoidance in Drosophila species and identification of repellents for Drosophila suzukii. Sci Rep 5:11527
Pulvirenti A, Zambonelli C, Todaro A, Giudici P (2002) Interspecific hybridisation by digestive tract of invertebrates as a source of environmental biodiversity within the Saccharomyces cerevisiae. Ann Microbiol 52:245–255
Reuter M, Bell G, Greig D (2007) Increased outbreeding in yeast in response to dispersal by an insect vector. Curr Biol 17:R81–R83
Revadi S, Vitagliano S, Stacconi MVR, Ramasamy S, Mansurian S, Carlin S, Vrhovsek U, Becher PG, Mazzoni V, Rota-Stabelli O, Angeli S, Dekker T, Anfora G (2015) Olfactory responses of Drosophila suzukii females to host plant volatiles. Physiol Entomol 40:54–64
Ridley EV, Wong AC-N, Westmiller S, Douglas AE (2012) Impact of the resident microbiota on the nutritional phenotype of Drosophila melanogaster. PLoS One 7:e36765
Scheidler NH, Liu C, Hamby KA, Zalom FG, Syed Z (2015) Volatile codes: correlation of olfactory signals and reception in Drosophila-yeast chemical communication. Sci Rep 5:14059
Schetelig MF, Handler AM (2013) Germline transformation of the spotted wing drosophild, Drosophila suzukii, with a piggyBac transposon vector. Genetica 141:189–193
Segata N, Boernigen D, Tickle TL, Morgan XC, Garrett WS, Huttenhower C (2013) Computational meta’omics for microbial community studies. Mol Syst Biol 9:666
Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E (2010) Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci USA 107:20051–20056
Shin SC, Kim S-H, You H, Kim B, Kim AC, Lee K-A, Yoon J-H, Ryu J-H, Lee W-J (2011) Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334:670–674
Stamps JA, Yang LH, Morales VM, Boundy-Mills KL (2012) Drosophila regulate yeast density and increase yeast community similarity in a natural substrate. PLoS One 7:e42238
Starmer WT (1981) A comparison of Drosophila habitats according to the physiological attributes of the associated yeast communities. Evolution 35:38–52
Stensmyr MC, Giordano E, Balloi A, Angioy A-M, Hansson BS (2003) Novel natural ligands for Drosophila olfactory receptor neurons. J Exp Biol 206:715–724
Stensmyr MC, Dweck HKM, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, Wicher D, Sachse S, Knaden M, Becher PG, Seki Y, Hansson BS (2012) A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151:1345–1357
Suh SO, McHugh JV, Pollock DD, Blackwell M (2005) The beetle gut: a hyperdiverse source of novel yeasts. Mycol Res 109:261–265
Taning CNT, Christiaens O, Berkvens N, Casteels H, Maes M, Smagghe G (2016) Oral RNAi to control Drosophila suzukii: laboratory testing against larval and adult stages. J Pest Sci. doi:10.1007/s10340-016-0736-9
Thomas DB (2003) Nontarget insects captured in fruit fly (Diptera: Tephritidae) surveillance traps. J Econ Entomol 96:1732–1737
Tochen S, Walton VM, Lee JC (2016) Impact of floral feeding on adult Drosophila suzukii survival and nutrient status. J Pest Sci (in press)
Van Timmeren S, Isaacs R (2013) Control of spotted wing drosophila, Drosophila suzukii by specific insecticides and by conventional and organic crop protection programs. Crop Prot 54:125–133
Venu I, Durisko Z, Xu J, Dukas R (2014) Social attraction mediated by fruit flie’s microbiome. J Exp Biol 217:1346–1352
Wallingford AK, Hesler SP, Cha DH, Loeb GM (2015) Behavioral responses of spotted-wing drosophila, Drosophila suzukii Matsumura, to aversive odors and a potential oviposition deterrent in the field. Pest Manag Sci. doi:10.1002/ps.4040
Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee J, Bruck DJ, Walton VM, O’Neal SD, Zalom FG (2011) Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J Integr Pest Manag 2:G1–G7
Wertheim B, Marchais J, Vet LM, Dicke M (2002) Allee effect in larval resource exploitation in Drosophila: an interaction among density of adults, larvae, and micro-organisms. Ecol Entomol 27:608–617
Williams T, Cisneros J, Penagos DI, Valle J, Tamez-Guerra P (2004) Ultralow rates of spinosad in phagostimulant granules provide control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in Maize. J Econ Entomol 97:422–428
Wisotsky Z, Medina A, Freeman E, Dahanukar A (2011) Evolutionary differences in food preference rely on Gr64e, a receptor for glycerol. Nat Neurosci 12:1534–1542
Witzgall P, Proffit M, Rozpedowska E, Becher PG, Andreadis S, Coracini M, Lindblom TUT, Ream LJ, Hagman A, Bengtsson M, Kurtzman CP, Piškur J, Knight A (2012) “This is not an apple”—yeast mutualism in codling moth. J Chem Ecol 38:949–957
Wong CNA, Ng P, Dougal AE (2011) Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol 13:1889–1900
Yamada R, Deshpanade SA, Bruce KD, Mak EM, Ja WW (2015) Microbes promote amino acid harvest to reduce undernutrition in Drosophila. Cell Rep 10:865–872
Zotti MJ, Smagghe G (2015) RNAi technology for insect management and protection of beneficial insects from diseases: lessons, challenges and risk assessments. Neotrop Entomol. doi:10.1007/s13744-015-0291-8
Zug R, Hammerstein P (2015) Bad guys turned nice? A critical assessment of Wolbachia mutualism in arthropod hosts. Biol Rev 90:89–111
Acknowledgments
KAH was funded by the North American Bramble Growers Research Foundation to perform research on D. suzukii microbe interactions in 2015. PGB was supported by the Swedish Research Council Formas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This review complies with ethical principles.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by N. Desneux.
Special Issue: Spotted Wing Drosophila.
Rights and permissions
About this article
Cite this article
Hamby, K.A., Becher, P.G. Current knowledge of interactions between Drosophila suzukii and microbes, and their potential utility for pest management. J Pest Sci 89, 621–630 (2016). https://doi.org/10.1007/s10340-016-0768-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-016-0768-1