Abstract
Insect pests often exhibit predictable seasonal population dynamics in response to temperature and other environmental drivers. Understanding these dynamics is critical to developing effective integrated pest management strategies. Here we studied the seasonal phenology and feeding activity of two wireworm species that are major pests of wheat crops in the Pacific Northwestern United States, Limonius californicus and L. infuscatus. We conducted monthly sampling of the damaging larval stages of both species in commercial spring wheat fields in Washington and Idaho throughout 2013 and 2014. These data were used to model the seasonal phenology and feeding activity of each species in relation to soil temperature. We found larvae of both species were most abundant relatively early in the season, with total wireworms captures in soil cores declining as the season progressed. Larvae of both species were collected predominantly in the top 70 cm of the soil profile, suggesting that they primarily feed on plant roots and seeds up to this depth. While patterns of seasonal abundance of both species were similar, feeding activity varied significantly between the two species. Our results indicate that as spring moves into summer L. californicus feeds more aggressively, whereas the activity of L. infuscatus decreases as the crop season progresses. These differences might help explain why L. californicus is generally a more economically damaging pest that also threatens winter crops, while damage from L. infuscatus is generally limited to the spring. Accordingly, management strategies for each species should be tailored to their specific seasonal dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
We have relatively little knowledge on the seasonal population dynamics of wireworm species in the Pacific Northwestern United States.
-
We found that two prevalent wireworm species in the genus Limonius had significantly different seasonal activity patterns in Pacific Northwest wheat cropping systems.
-
Models developed here can aid growers understand wireworm life cycles and evaluate variation in damage caused by different species of wireworms throughout a season.
Introduction
Understanding pest population dynamics is the foundation of integrated pest management (IPM). Temperature mediates insect development, and the seasonal activity of pest life stages can often be predicted by degree days (Cammell and Knight 1992; Pedigo 1999; van Asch and Visser 2007; Jones et al. 2009). In turn, phenology models can optimize the timing of controls in IPM (Welch et al. 1978; Pruess 1983; Logan et al. 2006; Nietschke et al. 2007). For example, phenology models predict when eggs of the codling moth, Cydia pomonella, will hatch; incorporation of these models into decision-support tools facilitates timely insecticide applications for this pest (Jones et al. 2013). More broadly, characterizing pest population dynamics can help explain why all herbivore species are not equally damaging pests or equally susceptible to control strategies.
Phenology models could help understand the dynamics of soil-dwelling pests (Allsopp and Butler 1987; Villani and Wright 1990; Johnson et al. 2007), which are difficult to sample due to their cryptic habitats (Villani and Gould 1986; Hunter 2001; Chahartaghi et al. 2005). Management of many subterranean insect pests often relies on prophylactic seed-applied insecticides (Albajes et al. 2003; Koch et al. 2005; Cox et al. 2007; Wilde et al. 2004). However, phenology models based on soil temperature have been effective for predicting the behavior and dynamics of soil-dwelling pests (Freckman and Caswell 1985; Blossey and Hunt-Joshi 2003; Johnson et al. 2007; Goldson and Gerard 2008). Such models allow for greater efficiency in sampling and monitoring and allow crop producers to better understand which species have the greatest pest potential by evaluating overlap between key pest and crop growth stages.
One major soil-dwelling pest group of major economic importance for many field crops worldwide is wireworms, the larvae of click beetles (Marske and Ivie 2003; Vernon et al. 2008, 2009; Barsics et al. 2013; Traugott et al. 2015). In many cereal cropping systems, most conventional growers use prophylactic neonicotinoid seed treatments for wireworm control (Alvarez 2004; Horton 2006). While neonicotinoids may provide economic benefits for wheat growers (Esser et al. 2015), there is room for improvement in their use (Furlan 2004). IPM of wireworms could be improved by understanding the activity of wireworm species across seasons. This would allow producers to assess variation in economic damage between species and effectively deploy control strategies (Cherry 2007; Landl et al. 2010; Willis et al. 2010; Furlan 2014). For example, if certain wireworm species feed primarily in the spring, growers might avoid using prophylactic insecticides in winter-planted crops. Similarly, growers might be able to adjust their insecticide rates to target particularly aggressive wireworm species in spring-planted crops based on knowledge of their phenology and feeding activity (Esser et al. 2015).
Here we investigated the two most prevalent wireworm species in the Pacific Northwestern US, Limonius infuscatus and L. californicus (Esser et al. 2015). Specifically, we assessed the role of temperature in mediating the phenology and feeding activity of these two species in commercial spring wheat crops. Wheat fields were monitored because wireworms have become the primary insect pest of cereal crops in the Pacific Northwestern United States, with up to 70 % yield losses in individual farms (Morales-Rodriguez et al. 2014; Higginbotham et al. 2014). Development of temperature-based models for wireworms and other soil-dwelling insects in wheat crops would allow us to better understand the basic biology of these pest species, providing a foundation for the development of more effective IPM programs.
Materials and methods
Study sites and wireworms
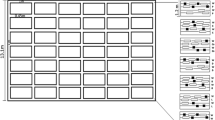
We monitored wireworm populations in 21 commercial spring wheat (Triticum aestivum) fields in eastern Washington and northern Idaho during 2013 (11 fields) and 2014 (10 fields) (Fig. 1). From samples, these fields contained high abundances of only L. californicus, only L. infuscatus, or both species (Fig. 1). These 21 farms used similar production practices typical of the region (Fig. 1), and all were managed by growers. Standard agronomic practices include 3-year rotations of either (1) winter wheat–spring wheat–spring wheat or (2) winter wheat–spring wheat–summer fallow or (3) winter wheat–spring wheat–legume crops (Schillinger et al. 2006). Each sampled farm grew spring wheat (variety ‘Louise’) in the year wireworms were sampled and had grown winter wheat the prior season. The seeding rate varied by location and production methods, with a range of seeding rates from 67 to 112 kg/ha. Seven of the farms used no-till production methods, while the other 14 used conventional tillage. Conventional tillage practices included fall moldboard plow, followed by a secondary tillage and seedbed preparation, and double-disk drill seeding with 18-cm row spacing. No-till practices involved direct double-disk seed placement with row spacing of 30.5 cm. Each farm used seed-applied neonicotinoid insecticides (i.e., thiamethoxam or imidacloprid) at planting with rates of 7–12 g active ingredient per 100 kg seed, a standard recommended chemical control method for wireworms in cereal crops (Esser et al. 2015; Higginbotham et al. 2014). All of the farms were situated in 300 to 680 mm annual precipitation zones (AgWeatherNet 2015) and did not use irrigation. Average soil moisture throughout the growing season was 15.7 % (SE = 0.51). Each farm had a silt loam soil and all but one had alkaline soil (mean pH 7.60; SE = 0.16).
Map of fields in Washington and Idaho states that were sampled over the 2013 and 2014 growing seasons for wireworms. The shape of the symbol denotes the wireworm species that were collected at the site and the color denotes the survey year. The majority of sites had only one wireworm species present, although some sites in each year had both species present
Data collection
Wireworms were sampled in each field using modified solar bait traps and soil cores (Esser et al. 2015). Bait traps are indicative of feeding activity, while soil cores are indicative of population size. Initial wireworm densities using both methods were measured in each field in late April or early May prior to seeding, and then monthly through August. At each sampling date soil moisture content and pH were measured on-site using sensors (General Digital Soil Moisture Meter with a 20-cm probe; Luster Leaf® Rapitest Digital Soil pH meter).
At each farm site, a total of 10 bait traps were deployed each month to evaluate wireworm feeding activity. Each bait trap consisted of 120 cm3 of a corn and wheat seed blend (ratio 50:50) placed in a nylon stocking. These baits were submerged in water for 24 h prior to deployment to start seed germination, as wireworms are attracted to CO2 produced by germinating seeds (Doane et al. 1975). Baits were placed in approximately the same locations of fields each month of sampling, but at least 10 m from any previous sample to avoid localized attrition of wireworms. The first bait was placed 50 m from a field edge to limit edge effects, and subsequent baits were located 50 m apart in a zig-zag pattern toward the center of the farm. Each bait was carefully placed in a 20-cm deep hole, which was dug between the wheat rows and covered with soil to minimize disruption of the surrounding wheat plants. Traps were retrieved after 8 days, and wireworms were recovered by hand. We identified all larvae to species using taxonomic keys (Lanchester 1946). We also recorded the number of large larvae (≥9 mm) and the number of small larvae (<9 mm); these classes represent differences in larval age.
In addition to bait traps, we also sampled each field for wireworms using soil cores. This method estimated population size and the vertical distribution of wireworms in the soil profile over the course of the season. Soil core scouting occurred on the same days when bait traps were deployed. At each farm site, we collected soil from 20 locations (two soil cores were taken 3–5 m from each bait trap) at each of three depths: top (0–35 cm), middle (36–70 cm), and bottom (71–105 cm). To extract soil samples, we used a one-piece soil auger that was 10 cm in diameter and 135 cm in length. Excavated soil was subdivided in the field using soil-sorting sieves (16.5 cm in diameter; three mesh sizes: 4, 2, and 0.25 mm) and placed into plastic containers after which it was examined for wireworms by hand. Collected wireworms were stored in 95 % ethanol, transported to the laboratory, and identified to species, and their size was recorded.
Data analyses
We first analyzed whether the two species differed in their abundance at varying depths in the soil profile over the growing season. To analyze the vertical distribution of wireworms in the soil profile over time, we used repeated measures generalized linear models with species, month, soil depth, and all two-way interactions as explanatory variables. Repeated measurements were the counts of wireworms at each soil depth in soil cores at each site every month. Our original model also included the effects of year, tillage, soil pH, soil moisture, and the number of species at each site (one or two). However, these variables were never significant (see Results), and our final model was simplified to only include the variables species, time, depth, and all two-way interactions. Models were fit with a negative binomial distribution based on the wireworm count data. Separate analyses were conducted for each wireworm species. All statistical analyses were conducted in SAS (version 9.2, SAS Institute 2009).
We next analyzed whether the two wireworm species differed in their feeding activity over time using repeated measures generalized linear models with species, time, and their interaction as explanatory variables. Repeated measurements were the counts of wireworms in bait traps at each site each month. Our original model also included the effects of year, tillage, soil pH, soil moisture, the number of wireworm species collected (one or two), and all two-way interactions. However, these variables were never significant (see “Results” section), and our final model was simplified to only include the explanatory variables species, time, and their interaction. Models were fit with a negative binomial distribution based on the observed data. Separate models were run for each of the three response variables (number of total, large, and small larvae).
Finally, we analyzed the seasonal phenology of wireworms in relation to soil temperature by plotting cumulative soil core catch against accumulated degree days for all sites. Cumulative soil core catch was calculated for each field and sampling date as a proportion of the total number collected at each location over the course of the growing season. Degree-day accumulations, based upon field-specific soil temperatures, were calculated from January 1st of each year using the average method (Damos and Savopoulou-Soultani 2012):
where T average is the average daily soil temperature and T base is the lower development threshold. For each site, daily average soil temperature data at 20 cm depth were retrieved from the nearest meteorological station (AgWeatherNet 2015). We used a lower development threshold of 4 °C, the lowest soil temperature at which we have observed Limonius larvae feeding on cereal crops in our region (Milosavljević, personal observation). We then modeled cumulative soil core catch based on accumulated degree days using a four-parameter cumulative Weibull distribution:
where y is the cumulative percentage of wireworms collected in soil cores, x is the cumulative degree days observed in the field, x 0 is cumulative degree days at the midpoint (point at which 50 % of wireworms were collected), and a, b, and c are scale (denotes the statistical dispersion of the distribution), location (determines the shift of a distribution and indicates the minimum value of x), and shape (denotes how symmetrical the curve is) parameters, respectively (Royo-Esnal et al. 2015). Separate models were fit for total, small, and large wireworms of each species.
Results
Wireworm feeding activity
A total of 1312 L. infuscatus and 3651 L. californicus were captured in bait traps. The number collected in bait traps was not significantly affected by year, tillage soil pH, soil moisture, or the number of species collected, or any interactions with these variables (P > 0.15 for all models). However, the number of wireworms collected in traps was affected by the wireworm species and month (Table 1). The number of L. californicus collected was significantly greater than the number of L. infuscatus for total, small, and large wireworms (Table 1 species effect, Fig. 2a–c). The differences in feeding activity between the two species increased as the season progressed (Table 1 interaction effect; Fig. 2). The majority of L. infuscatus in bait traps were collected during May and June (Fig. 2), with few collected in the months of July or August (Fig. 2). In contrast, the number of L. californicus collected in bait traps remained fairly consistent throughout the four month sampling period (Fig. 2).
The abundance of a, d total wireworms, b, e small wireworms (≤9 mm), and c, f large wireworms (>9 mm) collected over the growing season (averaged across 2013 and 2014). Shown are the mean (±SE) for L. infuscatus (Li) and L. californicus (Lc) abundance collected per ten traps (a, b, c) and twenty soil cores (d, e, f) at all locations in different time intervals
Wireworm abundance and seasonal dynamics
Similar to bait trapping, the numbers of wireworms collected in soil cores were not affected by year, tillage soil pH, soil moisture, or the number of species collected, or any interactions with these variables (P > 0.18 for all models). However, the number of wireworms collected in soil cores was affected by the wireworm species, month, and depth (Table 2). We found significantly more L. californicus per soil core sample than L. infuscatus (Table 2 species effect, Fig. 2d–f). However, the trends in abundance over time were similar for both species (Table 2 interaction effect, Fig. 2d–f). The majority of L. infuscatus larvae in soil cores were collected in the first two months of sampling (May and June) (Fig. 2), with 50 and 90 % captured by 220 and 1200 cumulative degree days (Fig. 3). Similarly, the highest abundance of L. californicus in soil cores was recorded in May and June (Fig. 2), with 50 and 90 % of the total population captured by 320 and 1300 degree days (Fig. 3).
Cumulative soil core catch of (a, b, c) L. infuscatus and (d, e, f) L. californicus in relation to cumulative degree days in the soil. Shown are cumulative soil core catch of a total, b small, and c large L. infuscatus larvae, and d total, e small, and f large L. californicus larvae. The symbols represent the raw data, and the curves show the best-fit Weibull model for each species. The dashed lines indicate the number of cumulative degree days where 50 and 90 % of the total population was collected
Distribution of wireworms in the soil profile
Soil depth had a significant effect on the number of wireworms collected for both species (Table 2 depth effect, Figs. 4, 5). The highest abundance of L. infuscatus larvae occurred between 35 and 70 cm, with the lowest densities collected between 71 and 105 cm (Figs. 4, 5). Similarly, significantly more L. californicus larvae were found between 35 and 70 cm than at any other depth (Table 2; Figs. 4, 5); significantly more L. californicus were also found in the top soil layer between 0 and 35 cm compared to the layer from 70 to 105 cm (Table 2; Figs. 4, 5). The ratio of wireworms of both species that were collected at different soil depths was consistent over time (Table 2 interaction terms).
The abundance of wireworms collected at different depths in the soil profile over the growing season (averaged across 2013 and 2014). Shown are the mean abundance (+SE) for a L. infuscatus and b L. californicus. Within each panel, different letters above the bars indicate significant differences (α = 0.05)
Proportions of wireworms collected at different depths in the soil profile over the growing season (averaged across 2013 and 2014). Shown are the proportions of a total, c small, and e large L. infuscatus larvae, and b total, d small, and f large L. californicus larvae collected per twenty soil cores at all locations in different time intervals
Discussion
Multiple studies have shown that the seasonal feeding activity of wireworms varies considerably across species, mediated by the crop type and environmental conditions (Jansson and Seal 1994; Cherry 2007; Kuhar and Alvarez 2008; Vernon and van Herk 2012). For example, in sugarcane fields in Florida, Glyphonyx bimarginatus and Conoderus spp. are most active in the summer, while the feeding activity of Melanotus communis declines from spring to summer (Cherry 2007). However, in Missouri cornfields, Conoderus spp. have been shown to cause the most damage early in the season, with feeding activity significantly declining in the summer due to unfavorable conditions (Kuhar and Alvarez 2008). Our results suggest that the seasonal feeding activity (from bait traps) differs significantly between species in the genus Limonius, despite similarities in the population dynamics (from soil cores) of these species.
We observed that the feeding activity of L. infuscatus occurred primarily early in the season, from April to June. This is also the period this species can cause the most damage to newly planted wheat seeds or young roots (Esser et al. 2015). Previous research has shown that larvae of a related species, L. canus, also have peak feeding activity periods early in the season, after which they move down in the soil profile to avoid dry soil surfaces and unbearably high soil temperatures (Jones and Shirck 1942; Horton 2006). In contrast, the feeding activity of L. californicus remained consistent as the crop season progressed. This might be attributed to the ability of L. californicus to effectively adjust to ambient temperatures. For example, Campbell (1937) showed that L. californicus larvae never cease their feeding activity completely and can change their preference for soil temperatures (lower or higher) based on previous experience. These differences allow L. californicus populations to be more active as the season progresses despite rising soil temperatures.
Differences in feeding activity we observed might also be due to variation in the ability of each species to tolerate seed-applied neonicotinoids, which were applied in all of our study fields. We observed that L. infuscatus had peak activity periods soon after planting, when seed-applied neonicotinoids are expressed at the highest concentrations in seeds and roots (Cox et al. 2007; Elbert et al. 2008; Vernon et al. 2009). This was followed by a sharp decline in feeding activity as the crop season progressed. This suggests that L. infuscatus larvae may be killed or rendered moribund for a prolonged period during feeding on neonicotinoid-treated seed early in the season. In contrast, we observed that L. californicus larvae actively fed throughout the growing season. This suggests wireworms of this species might be less impacted by seed-applied neonicotinoids over the course of a growing season, possibly because this species is less affected by neonicotinoids than L. infuscatus or is able to overcome insecticide-induced morbidity more rapidly than L. infuscatus. Our study is representative of wireworms in highly modified agroecosystems where 100 % of wheat fields are treated. While wireworm behavior in this scenario may differ from untreated fields, our results provide us with a better understanding of the biology of these two species in cropping systems managed conventionally, which is necessary to develop appropriate IPM programs for wireworms.
Differences in the seasonal feeding activity patterns of the two Limonius species might underlie variation in damage caused to wheat crops. On-farm experimental trials in Pacific Northwest wheat crops show that L. californicus is more damaging to crop yields, and less impacted by seed-applied neonicotinoids than L. infuscatus (Esser et al. 2015). These trials also showed that the insecticide rate that produces maximum returns differs depending on the actual species present in the field. Our results here suggest a mechanism that may explain why L. californicus is more damaging for fall-planted crops than L. infuscatus because it actively feeds throughout the season while L. infuscatus generally does not. More research is therefore needed to explore whether growers could move away from complete reliance on prophylactic treatments of seed-applied neonicotinoids in fall-planted crops in areas where only L. infuscatus is present.
Our investigation of the seasonal dynamics of wireworms was based on two commonly used sampling techniques: bait traps and soil cores. Multiple studies have shown that bait traps are the most efficient tool for monitoring wireworms in bare fields prior to seeding (Parker 1994, 1996; Furlan 2014). Yet, our food sources (i.e., crop roots) later in the season can confound larvae and decrease trapping efficiency (Landl et al. 2010). In turn, declining bait trap catch of L. infuscatus over the course of a season could be due to overall lower activity levels but might also be confounded by decreased efficiency of bait traps as the wheat crop established. However, we recorded increased trap captures of L. californicus larvae over time, suggesting that this pest species was actively feeding on baits regardless of the wheat stand during the course of the growing season. Overall, while bait trapping may be confounded by the presence of crop plants, such that our estimates of wireworm potential damage might be conservative, our results clearly show that the differences in feeding activity between the two species increased significantly over the growing season.
The vertical distribution of wireworms might also impact damage associated with particular species (Toba and Turner 1983; Benefer et al. 2010). If wireworms move to lower soil depths as a season progresses, due to dry conditions and high-temperatures in the upper soil or due to insecticide exposure, they might be less impactful as pests. However, this was not observed for either species. While our soil core sampling yielded significantly fewer wireworms than our bait-sampling, it did reveal that the distributions of wireworms were fairly consistent over the course of the season. Thus, conditions were favorable for both species to feed in the upper-layers of the soil profile throughout the season.
In addition to the bait trap data, our soil core sampling reinforced the differences in the biology of the two pests that may underlie their differences in pest status. The majority of the root system of wheat plants develops within the upper 35 cm of soil over the first 60 days after planting, with only a few tap roots reaching soil depths below this level (Weaver 1926). Thus, although wireworms of both species may impact wheat plants in the upper soil profile early in the season, only L. californicus, which continues to actively feed throughout the field season is likely to also have a strong impact on wheat roots below a depth of 35 cm. In contrast, our results show that L. infuscatus decreases in feeding activity over time, before the majority of wheat roots reach deeper soil profiles, potentially diminishing their impact. The observed decrease in the feeding activity of this species later in the season may be due to insecticide-induced mortality and/or long-term morbidity or because L. infuscatus larvae switched their dietary preference from wheat to other organic matter over time.
In addition to collecting information on the total wireworm populations, we also collected data on small/younger (≤9 mm) and large/older (>9 mm) wireworms at each site. These data allow us to differentiate wireworms that are neonates (resulting from egg-laying in the current year) from residents (larvae that hatched in previous season and have continued development) (Vernon et al. 2009). Such delineation of size classes is important when estimating the seasonal dynamics of wireworm species that have multi-year life cycles (e.g., Agriotes spp. and Limonius spp.), since the number of neonates can vary significantly from year to year and location to location. We found that small L. californicus larvae increased in their feeding activity as the growing season progressed, while larger wireworms remained consistently active across the season. In contrast, both small and large L. infuscatus larvae diminished in feeding activity during the months of July and August. These results may be due to the production of new neonates which would be occurring and feeding through the growing season and might also indicate possible differences in mating and oviposition behavior between these two species.
Previous research has shown that in some Elaterid species both sexes are capable of mating only once (e.g., Ctenicera (=Selatosomus) destructor) (Zacharuk 1962), whereas in others, mating can be repeated multiple times in a lifetime (e.g., A. ustulatus) (Furlan 1996). In addition, egg hatching period in A. ustulatus is influenced by environmental conditions and can last from 15 days to approximately 2 months (Furlan 1998). Moreover, L. californicus larvae might need from 3 to 7 weeks to hatch and start feeding on crops depending on the ambient conditions (EMPPO 2005). Similarly, our results show that neonate L. californicus larvae increase in their feeding on crops throughout the growing season. This might be caused by the extended egg hatching or neonate activity periods, or might indicate that the adults of this species are active later in the season compared to L. infuscatus or are capable of mating multiple times in their lifetime. Although our data do not allow us to test these hypotheses, they provide further support that the differences in the feeding activity between these two species might underlie differences in their crop impacts.
Subterranean insect pests such as wireworms are difficult to monitor because of their cryptic life histories, and outbreaks can occur with little warning. This can drive crop producers to make management decisions based on minimal information, limiting the effectiveness of pest management. In the present study, we show that differences in the seasonal feeding activity of two Limonius species may mediate their varying impacts on spring wheat crops, such that each species requires a targeted IPM approach. For example, a properly used one-time control application early in the season might be effective in preventing economic damage by L. infuscatus but not for L. californicus larvae. Accordingly, our previous research showed that pesticide rate that generates highest returns may differ from location to location depending on the wireworm species present in a given field. Moreover, only L. californicus is likely to have major impacts on fall-planted crops, and growers applying prophylactic treatments of seed-applied neonicotinoids for L. infuscatus in fall-planted crops may not receive a suitable return. In turn, the results of our and similar studies provide us with a better understanding of the ecology of wireworm species and highlight the differences between the two wireworm species in their impacts on wheat production. Understanding species-specific phenology and feeding activity of wireworms could have broad impacts for understanding which species are expected to cause the most damage to particular crops, and provide a foundation for developing IPM strategies.
Author contribution statement
IM, AE and DC conceived and designed the research. IM and AE conducted the experiments. IM and DC analyzed the data. IM wrote the manuscript with edits from AE and DC.
References
AgWeatherNet (2015) Washington State University’s AgWeatherNet. Prosser, WA, USA. http://weather.wsu.edu/awn.php. Accessed 23 September 2015
Albajes R, López C, Pons X (2003) Predatory fauna in cornfields and response to imidacloprid seed treatment. J Econ Entomol 96:1805–1813
Allsopp PG, Butler DG (1987) Estimating day-degrees from daily maximum-minimum temperatures: a comparison of techniques for a soil-dwelling insect. Agric For Meteorol 41:165–172
Alvarez JM (2004) Potato insect pests. In: Capinera JL (ed) Encyclopedia of entomology. Kluwer Academic Press, Dordrecht, pp 1803–1816
Barsics F, Haubruge E, Verheggen FJ (2013) Wireworms’ management: an overview of the existing methods, with particular regards to Agriotes spp. (Coleoptera: Elateridae). Insects 4:117–152
Benefer C, Andrew P, Blackshaw R, Ellis J, Knight M (2010) The spatial distribution of phytophagous insect larvae in grassland soils. Appl Soil Ecol 45:269–274
Blossey B, Hunt-Joshi TR (2003) Belowground herbivory by insects: influence on plants and aboveground herbivores. Annu Rev Entomol 48:521–547
Cammell ME, Knight JD (1992) Effects of climatic change on the population dynamics of crop pests. Adv Ecol Res 22:117–162
Campbell RE (1937) Temperature and moisture preferences of wireworms. Ecology 18:479–489
Chahartaghi M, Langel R, Scheu S, Ruess L (2005) Feeding guilds in Collembola based on nitrogen stable isotope ratios. Soil Biol Biochem 37:1718–1725
Cherry R (2007) Seasonal population dynamics of wireworms (Coleoptera: Elateridae) in Florida sugarcane fields. Fla Entomol 90:426–430
Cox WJ, Cherney JH, Shields E (2007) Clothianidin seed treatments inconsistently affect corn forage yield when following soybean. Agron J 99:543–548
Damos P, Savopoulou-Soultani M (2012) Temperature-driven models for insect development and vital thermal requirements. Psyche. doi:10.1155/2012/123405
Doane JF, Lee YW, Klinger J, Westcott ND (1975) The orientation response of Ctenicera destructor and other wireworms (Coleoptera: Elateridae) to germinating grain and carbon dioxide. Can Entomol 107:1233–1252
Elbert A, Haas M, Springer B, Thielert W, Nauen R (2008) Applied aspects of neonicotinoid uses in crop protection. Pest Manag Sci 64:1099–1105
Esser AD, Milosavljević I, Crowder DW (2015) Effects of neonicotinoids and crop rotation for managing wireworms in wheat crops. J Econ Entomol 108:1786–1794
European and Mediterranean Plant Protection Organization (EMPPO) (2005) Limonius californicus. Bull OEPP 35:377–379
Freckman DW, Caswell EP (1985) The ecology of nematodes in agroecosystems. Annu Rev Phytopathol 23:275–296
Furlan L (1996) The biology of Agriotes ustulatus Schaller (Col., Elateridae). I. Adults and oviposition. J Appl Entomol 120:269–274
Furlan L (1998) The biology of Agriotes ustulatus Schaller (Col., Elateridae). II. Larval development, pupation, whole cycle description and practical implications. J Appl Entomol 122:71–78
Furlan L (2004) The biology of Agriotes sordidus Illiger (Col., Elateridae). J Appl Entomol 128:696–706
Furlan L (2014) IPM thresholds for Agriotes wireworm species in maize in Southern Europe. J Pest Sci 87:609–617
Goldson SL, Gerard PJ (2008) Using biocontrol against root-feeding pests, with particular reference to Sitona root weevils. In: Johnson SN, Murray PJ (eds) Root feeders: an ecosystem perspective. CABI, Wallingford, pp 115–133
Higginbotham RW, Froese PS, Carter AH (2014) Tolerance of wheat (Poales: Poaceae) seedlings to wireworm (Coleoptera: Elateridae). J Econ Entomol 107:833–837
Horton DR (2006) Quantitative relationship between potato tuber damage and counts of Pacific coast wireworm (Coleoptera: Elateridae) in baits: seasonal effects. J Entomol Soc Br Columbia 103:37–48
Hunter MD (2001) Out of sight, out of mind: the impacts of root-feeding insects in natural and managed systems. Agric For Entomol 3:3–9
Institute SAS (2009) SAS/STAT®, version 9.2 user’s guide, 2nd edn. SAS Institute, Cary
Jansson RK, Seal DR (1994) Biology and management of wireworms on potato. In: Zehnder GW, Powelson ML, Jansson RK, Raman KV (eds) Advances in potato pest biology and management. American Phytopathological Society Press, St Paul, pp 31–53
Johnson SN, Zhang X, Crawford JW, Gregory PJ, Young IM (2007) Egg hatching and survival time of soil-dwelling insect larvae: a partial differential equation model and experimental validation. Ecol Model 202:493–502
Jones EW, Shirck FH (1942) The seasonal vertical distribution of wireworms in the soil in relation to their control in the Pacific Northwest. J Agric Res 65:125–142
Jones VP, Unruh TR, Horton DR, Mills NJ, Brunner JF, Beers EH, Shearer PW (2009) Tree fruit IPM programs in the western United States: the challenge of enhancing biological control through intensive management. Pest Manag Sci 65:1305–1310
Jones VP, Hilton R, Brunner JF, Bentley WJ, Alston DG, Barrett B, Van Steenwyk RA, Hull LA, Walgenbach JF, Coates WW, Smith TJ (2013) Predicting emergence of codling moth, Cydia pomonella (Lepidoptera: Tortricidae) in North America. Pest Manag Sci 69:1393–1398
Koch RL, Burkness EC, Hutchison WD, Rabaey TL (2005) Efficacy of systemic insecticide seed treatments for protection of early-growth-stage snap beans from bean leaf beetle (Coleoptera: Chrysomelidae) foliar feeding. Crop Prot 24:734–742
Kuhar TP, Alvarez JM (2008) Timing of injury and efficacy of soil-applied insecticides against wireworms on potato in Virginia. Crop Prot 27:792–798
Lanchester HP (1946) Larval determination of six economic species of Limonius (Coleoptera: Elateridae). Ann Entomol Soc Am 39:619–626
Landl M, Furlan L, Glauninger J (2010) Seasonal fluctuations in Agriotes spp. (Coleoptera: Elateridae) at two sites in Austria and the efficiency of bait trap designs for monitoring wireworm populations in the soil. J Plant Dis Protect 117:268–272
Logan JD, Wolesensky W, Joern A (2006) Temperature-dependent phenology and predation in arthropod systems. Ecol Model 196:471–482
Marske KA, Ivie MA (2003) Beetle fauna of the United States and Canada. Coleopt Bull 57:495–503
Morales-Rodriguez A, O’Neill RP, Wanner KW (2014) A survey of wireworm (Coleoptera: Elateridae) species infesting cereal crops in Montana. Pan-Pac Entomol 90:116–125
Nietschke BS, Magarey RD, Borchert DM, Calvin DD, Jones E (2007) A developmental database to support insect phenology models. Crop Prot 26:1444–1448
Parker WE (1994) Evaluation of the use of food baits for detecting wireworms (Agriotes spp., Coleoptera: Elateridae) in fields intended for arable crop production. Crop Prot 13:271–276
Parker WE (1996) The development of baiting techniques to detect wireworms (Agriotes spp., Coleoptera: Elateridae) in the field, and the relationship between bait-trap catches and wireworm damage to potato. Crop Prot 15:521–527
Pedigo LP (1999) Entomology and pest management, 3rd edn. Prentice Hall, New Jersey
Pruess KP (1983) Day-degree methods for pest management. Environ Entomol 12:613–619
Royo-Esnal A, García AL, Torra J, Forcella F, Recasens J (2015) Describing Polygonum aviculare emergence in different tillage systems. Weed Res 55:387–395
Schillinger WF, Papendick RI, Guy SO, Rasmussen PE, van Kessel C (2006) Dryland cropping in the western United States. In: Peterson GA, Unger PW, Payne WA (eds.) Dryland agriculture, 2nd ed. Agronomy monograph no 23. ASA, CSSA, and SSSA, Madison, WI, pp 365–393
Toba HH, Turner JE (1983) Evaluation of baiting techniques for sampling wireworms (Coleoptera: Elateridae) infesting wheat in Washington. J Econ Entomol 76:850–855
Traugott M, Benefer CM, Blackshaw RP, van Herk WG, Vernon RS (2015) Biology, ecology, and control of elaterid beetles in agricultural land. Annu Rev Entomol 60:313–334
van Asch M, Visser ME (2007) Phenology of forest caterpillars and their host trees: the importance of synchrony. Annu Rev Entomol 52:37–55
Vernon RS, van Herk WG (2012) Wireworms as pests of potato. In: Giordanengo P, Vincent C, Alyokhin A (eds) Insect pests of potato: global perspectives on biology and management. Academic Press, Elsevier, pp 103–164
Vernon RS, van Herk WG, Tolman J, Saavedra HO, Clodius M, Gage B (2008) Transitional sublethal and lethal effects of insecticides following dermal exposures to five economic species of wireworms (Coleoptera: Elateridae). J Econ Entomol 101:367–374
Vernon RS, van Herk WG, Clodius M, Harding C (2009) Wireworm management I: stand protection versus wireworm mortality with wheat seed treatments. J Econ Entomol 102:2126–2136
Villani MG, Gould F (1986) Use of radiographs for movement analysis of the corn wireworm, Melanotus communis (Coleoptera: Elateridae). Environ Entomol 15:462–464
Villani MG, Wright RJ (1990) Environmental influences on soil macroarthropod behavior in agricultural systems. Ann Rev Entomol 35:249–269
Weaver JE (1926) Root development of field crops. McGraw-Hill Book Co., New York
Welch SM, Croft BA, Brunner JF, Michels MF (1978) PETE: an extension phenology modeling system for management of multi-species pest complex. Environ Entomol 7:487–494
Wilde G, Roozebooom K, Claassen M, Janssenand K, Witt M (2004) Seed treatment for control of early-season pests of corn and its effect on yield. J Agric Urban Entomol 21:75–85
Willis RB, Abney MR, Kennedy GG (2010) Survey of wireworms (Coleoptera: Elateridae) in North Carolina sweetpotato fields and seasonal abundance of Conoderus vespertinus. J Econ Entomol 103:1268–1276
Zacharuk RY (1962) Distribution, habits, and development of Ctenicera destructor (Brown) in Western Canada, with notes on the related species C. aeripennis (Kby) (Coleoptera: Elateridae). Can J Zool 40:539–552
Acknowledgments
We thank Elias Bloom for helping construct Fig. 1. We also thank the many wheat growers we provided access to their farms for this research project. This project was supported by grants from the Washington Grain Commission to DC and AE.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Milosavljević, I., Esser, A.D. & Crowder, D.W. Seasonal population dynamics of wireworms in wheat crops in the Pacific Northwestern United States. J Pest Sci 90, 77–86 (2017). https://doi.org/10.1007/s10340-016-0750-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-016-0750-y