Abstract
This study seeks to investigate whether users activate cognitive representations of their partner’s action when they are involved in tactile collaborative tasks. The social Simon effect is a spatial stimulus–response interference induced by the mere presence of a partner in a go/nogo task. It has been extensively studied in the visual and auditory sensory modalities, but never before in the tactile modality. We compared the performances of 28 participants in three tasks: (1) a standard Simon task where participants responded to two different tactile stimuli applied to their fingertips with either their left or right foot, (2) an individual go/nogo task where participants responded to only one stimulus and (3) a social go/nogo task where they again responded to only one stimulus, but were partnered with another person who responded to the complementary stimulus. The interference effect due to spatial incongruence between the side where participants received the stimulus and the foot used to answer increased significantly in the standard Simon task compared to the social go/nogo task. Such a difference was not observed between the social and individual go/nogo tasks. Performances were nevertheless enhanced in the social go/nogo task, but irrespectively of the stimulus–response congruency. This study is the first to report a negative result for the social Simon effect in the tactile modality. Results suggest that cognitive representation of the co-actor is weaker in this modality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Action performed jointly between two or more human partners has spurred much debate in the field of cognitive sciences. Gallotti and Frith (2013) advocate for the existence of a specific mode of functioning, which they called the “we-mode” (p. 160), that appears when individuals are involved in collective actions. One of the core mechanisms that give rise to the “we-mode” is our spontaneous tendency to be influenced by actions performed by co-actors. Sebanz et al. (2003) reported an experiment that illustrated how one’s motor planning ability was affected by a co-actor’s actions. It has become a prominent paradigm, referred to as the social or joint Simon effect, which has been extensively used to study joint actions between two co-actors (Dittrich et al. 2012; Iani et al. 2011; Klempova and Liepelt 2016; Kuhbandner et al. 2010; Liepelt et al. 2011, 2013; Stenzel et al. 2012, 2014; Tsai and Brass 2007; Vlainic et al. 2010; Welsh 2009; Welsh et al. 2013). It derives from the standard Simon task (Simon 1969) that induces a spatial stimulus–response interference effect whereby participants respond faster to stimuli that are presented on the same side as the limb they use to answer, even though the location of the stimuli is task-irrelevant. For instance, either a blue or green circle appears to the left or right of the participant who is to press a key on her/his left for the green circle and a key on her/his right for the blue circle (Hommel et al. 2009). Participants’ response times will decrease when the location of the stimulus is congruent with the location of the response key. This effect disappears if participants are instructed to perform a simple go/nogo task where they respond to only one of the two stimuli with a single key. The interference effect however reappears de novo when the participant is partnered with another individual who responds to the alternative stimulus. The partner’s action in this social go/nogo condition thus influences the participant’s motor planning. The goal of the study reported here was to test whether this effect can still be observed when the stimuli are delivered on the tactile sensory modality. This issue is expected to be highly relevant for the design of collaborative tactile interfaces.
The social Simon effect has been shown to depend on the degree of perceived interdependence between the co-actors (Colzato et al. 2012; Iani et al. 2011; Ruys and Aarts 2010). It is enhanced when the co-actor is seen as friendly and cooperative compared to intimidating and competitive (Hommel et al. 2009; Iani et al. 2011). The social Simon effect has been classically explained by our spontaneous tendency to represent actions performed by others within our own sensory-motor system (Sebanz et al. 2003; Sebanz et al. 2006), although alternative accounts emphasize the importance of the spatial arrangement of the two co-acting partners with respect to the stimuli (Dittrich et al. 2012, 2013; Guagnano et al. 2010) and the attention-grabbing events caused by the co-actor’s actions (Dolk et al. 2013; Klempova and Liepelt 2016). Despite the different theoretical frameworks that are used to account for the effect, the social Simon effect has been robustly reproduced across various settings and has been used in numerous imaging studies to examine neural networks associated with joint action (Costantini et al. 2013; de la Asuncion et al. 2015; Dolk et al. 2012; Sebanz et al. 2006, 2007; Tsai et al. 2008). Yet, to our knowledge, until now, experiments on the social Simon effect have always used either visual or auditory stimuli, but have never been conducted in the tactile modality.
The small number of studies that implemented the standard Simon task in the tactile modality has been consistent in reporting the expected interference effect. Hasbroucq and Guiard (1992) applied mechanical taps on the index fingers and thumbs of the two hands and found shorter response times when the stimulation was congruent with the hand with which participants had to answer. In the study by Medina et al. (2014), participants received vibrotactile stimuli on their middle fingers and had to respond using foot pedals. They responded faster on trials where the finger receiving the stimulus and the foot releasing the pedal were somatotopically congruent. Salzer et al. (2014) exerted vibrotactile stimulations on the left and right part of the back of the torso. Once again, they observed an interference effect when participants had to answer with the hand opposite to the side where they perceived the tactile stimulus.
In the present experiment, we applied vibrotactile stimulations on the index fingertips of participants, who had to respond by pressing foot pedals. Following a classical experimental design for studying the social Simon effect, we compared three tasks: (1) a standard Simon task where participants received two different types of tactile vibration and had to respond with their two feet; (2) an individual go/nogo task where participants still received the two types of tactile vibration, but responded to only one of them; (3) a social go/nogo task where participants responded in the same way as in the individual go/nogo task, while another person sitting next to them responded to the complementary stimulus. We hypothesized that the congruency between the side where the vibrotactile stimulation was applied and the foot with which participants had to respond would have an effect on response times in the standard Simon task and in the social go/nogo task, but not in the individual go/nogo task. Additionally, participants were administered the Autism-Spectrum Quotient (AQ) questionnaire (Baron-Cohen et al. 2001), which assesses autism spectrum traits in the general population. This questionnaire provided a metric that we intended to correlate with the amplitude of the hypothesized interference effect in the social Simon go/nogo task. A previous study (Sebanz et al. 2005) reported that the social Simon effect was unaltered in individuals with autism spectrum disorder (ASD). However, given the profound impairments in the ability to spontaneously represent others’ intentions in action that are associated with ASD (Senju et al. 2009) and the theory linking the social Simon effect to a spontaneous representation of others’ action (Sebanz et al. 2003, 2006), we tentatively hypothesized a negative correlation between the social Simon effect and autism spectrum traits given the social nature of the experimental manipulation.
Methods
Participants
Twenty-eight adults (14 males and 14 females) participated in the experiment. Their age range was 21–39 years with a mean of 27.4 years (SD = 5.1). A power analysis was performed prior to the experiment to estimate the required minimum sample size based on data reported by former studies (Hommel et al. 2009; Liepelt et al. 2011). The computation was carried out with the G*Power application (Faul et al. 2007) setting the significance threshold to 0.05 and the power to 0.9. It yielded a minimum sample size of 16. Participants were free of any known psychiatric or neurologic symptoms, non-corrected visual or auditory deficits and recent use of any substance that could impede concentration. This research complied with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board at Université Paris-Descartes. Informed consent was obtained from each participant.
Material
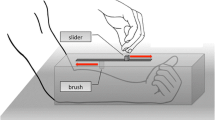
The tactile stimulations were produced by two linear resonant actuators (LRA) from Precision MicroDrive™ that produced vibrations. The actuators were monitored with the National Instrument Emission/Acquisition cards (NI 9265 and NI 9205). The input signals were amplified and powered by home-made electronic cards. The entire experimental systems were controlled with a home-made program coded in the Python language. The LRAs were fixed on a table and positioned on an axis that was parallel to the edge of the table. Participants would sit on a comfortable chair in front of the table, in between the two LRAs, and would place their right and left index fingertips on the LRA that was on the same side as their hand. The vibrotactile stimuli were provided by a 205-Hz vibration of either (a) 1.5-μm displacement amplitude or (b) 3.7-μm displacement amplitude. The vibration was continuous and lasted 250 ms. The 1.5-μm amplitude vibration was referred to as the “low” stimulus signal, and the 3.7-μm amplitude vibration was the “high” stimulus signal. Participants responded to the tactile stimuli by pushing pedals that were located under the table. One pedal was on the left side of the participant, and the other one was on the right. Each pedal was associated with a given vibration amplitude (either high or low) that was indicated on the pedal. The vibration noise was totally eliminated by having participants wear a noise-cancelling headphones playing pink noise. Hence, the sense of touch was the only sensory modality that participants could rely on to discriminate between the two vibration amplitudes.
Procedure
Participants sat next to the experimenter who was on their left. A computer screen was placed on the table in between the participant and the experimenter. Instructions were provided verbally and by writing. The written version was accessible throughout the experiment. Participants were instructed to respond as fast as they could to the tactile stimuli. They had to place their feet symmetrically with respect to their body. Their feet were separated by a distance equivalent to the size of their hips or distance from shoulder to shoulder, according to what was the most natural posture for them. The pedals were placed besides each one of their foot, either under it or next to it, in the most comfortable and easy to reach positions for every individual participant. Participants were then introduced the low and high stimuli on each LRA. For half of the participants, the “high” pedal, which was to be pressed when perceiving a high stimulus, was on their right side and the “low” pedal on their left side. The reverse configuration was used for the other half of participants.
As in Salzer et al. (2014), each trial began with a fixation cross that was displayed at the center of the screen for 250 ms. When the fixation cross disappeared, one of the LRA delivered a vibrotactile stimulus for 250 ms. Once the vibrotactile stimulus began, participants had 1500 ms to respond by pressing one of the two pedals. After the participants’ response, a “right” or “wrong” feedback message was displayed during 300 ms. No feedback was provided if participants had not responded fast enough. The feedback message was followed by a black screen that lasted until the next trial began. The intertrial interval duration varied randomly between 1000 and 1500 ms. Figure 1 summarizes the event flow during a trial.
The experiment was composed of 5 blocks that were separated by short breaks. The first block was used to train participants in perceiving the two different vibrotactile stimulations. It contained 60 trials that were not included in the analyses. The four following blocks comprised 120 trials each and were used to collect experimental data, that is, reaction time and accuracy (number of errors). The reaction time was measured from the stimulus onset until the participant’s response. During each block, the LRAs produced an equal number of low- and high-amplitude stimuli that were equally distributed on the left and on the right. The left/right positions and low/high amplitudes of the stimuli were randomly allocated. As explained above, participants were to respond to the low or high stimuli with either their left foot or right foot depending on where the low and high pedals had been placed. When the stimulus appeared on the same side as the pedal to be pressed, the trial was said to be congruent. It was incongruent when the stimulus appeared on the opposite side.
The four experimental blocks presented three different tasks: One block was dedicated to the standard Simon task, one block to the individual go/nogo task and two blocks for the social go/nogo task (Fig. 2). To neutralize the effect of the tasks’ order, their sequential order was counterbalanced across participants using the Latin square method. As there were three tasks, there were six possible counterbalancing sequences and similar numbers of participants were allotted to each possible sequence (4 to 6 participants per sequence).
Upper view of the experimental setups for the three tasks. In each task, the participant received two tactile stimuli from two LRA. Top: standard Simon task: the participant responded to two stimuli with two pedals; middle: individual go/nogo task: the participant responded to only one stimulus with one pedal; bottom: social go/nogo task: the experimenter and the participant each responded to a different stimuli with one pedal. The position of the response pedal in the individual and social go/nogo tasks was counterbalanced across participants. LRA linear resonant actuator
In the standard Simon task, participants had to respond to the two amplitudes of vibration stimuli (high and low) by pressing the matching pedal. In the individual and social go/nogo tasks, participants would either respond exclusively to the low-amplitude stimuli for the first 60 trials of each block and to the high-amplitude stimuli for the next 60 trials, or they would respond first to the high-amplitude stimuli and then to the low-amplitude stimuli. The order in which participants were to respond to vibration amplitudes was counterbalanced across participants. The position of the pedal which they had to press was also counterbalanced across participants.
The only difference between the individual go/nogo condition and the social go/nogo condition was that the experimenter took part in the task during the social go/nogo condition. In the latter condition, the experimenter responded to the amplitude of the vibration stimuli that the participant was asked not to respond to. For instance, if the participant had to respond to the low-amplitude stimuli, the experimenter responded to the high-amplitude stimuli and vice versa. The instructions explicitly specified that the participant and the experimenter were to cooperate in performing the task. The experimenter placed her fingertips on a second set of LRAs that reproduced the vibrotactile stimuli sent to the participant. The experimenter used the same foot as the participant to press the response pedal. The experimenter was the same for all participants. As she was a female, the participant’s gender was taken into account in the statistical analysis. The experimenter was required to respond evenly with every participants and not adjust to the participants’ performances.
At the end of the experiment, participants were asked to fill in the Autism-Spectrum Quotient (AQ) questionnaire (Baron-Cohen et al. 2001). The AQ is a psychometric instrument used to screen autistic-like traits in the general population. The AQ focuses on questions related to social and communicative skills, imagination and flexibility.
Results
Response times and error were analyzed using repeated-measures analyses of variance (ANOVA) with participants’ gender as an adjustment factor to account for possible effects due to the fact that the experimenter was always a female. Three ANOVAs were conducted for each measure. Every ANOVA had two within factors: the experimental task and the congruency of the trial. Congruent trials were those where the stimulus appeared on the same side as the response pedal. Incongruent trials were those where the stimulus appeared on the opposite side.
The experimental design included three tasks: the standard Simon task, the individual go/nogo task and the social go/nogo task. To test whether there was a social Simon effect, we performed one ANOVA that compared the social go/nogo task with the individual go/nogo task and another ANOVA comparing the social go/nogo task with the standard Simon task. The social Simon effect entailed that congruency would affect response times and errors in the standard Simon task and the social go/nogo task, while it would not in the individual go/nogo task. We therefore hypothesized that the ANOVA comparing the social go/nogo task with the individual go/nogo task would yield an interaction between the task and congruency factors, but that no such interaction would be observed in the ANOVA comparing the social go/nogo task with the standard Simon task. Post hoc t test was performed using the Tukey procedure. The analyses were carried out with Statistica software (www.statsoft.com).
Although the sequential order of the tasks had been counterbalanced across participants, we tested for a possible order effect by adding an additional adjustment factor representing the order in which tasks had been administered. Yet, the tasks’ order did not yield any significant differences except for the percentage of errors in the ANOVA comparing the social go/nogo task with the standard Simon task and, even in this case, this additional adjustment factor did not change the pattern of results. Hence, to facilitate readability, we did not include this additional factor in the analyses presented below.
Number of errors
We computed the percentage of erroneous trials in each task. A trial was considered erroneous when the participant pressed the wrong pedal. There were generally few errors, and thus, the distribution of the percentage of errors was skewed toward zero. To normalize the distribution, we used a Box-Cox transformation (Sakia, 1992) before applying the ANOVA. The ANOVA comparing the individual go/nogo task with the social go/nogo task did not yield any significant main effect, nor interaction between task and congruency, F(1,26) = 0.52, p = 0.48, η 2 p = 0.02. The ANOVA comparing the standard Simon task with the social go/nogo task revealed a significant main effect of task, F(1,26) = 16.08, p ≤ 0.001, η 2 p = 0.38. There were more errors in the standard Simon task (median = 9.17%, interquartile range = 4.58%) than in the social go/nogo task (median = 4.17%, interquartile range = 3.75%). There was no main effect of congruency, nor interaction between congruency and task. The number of errors in each block is plotted in Fig. 3.
Response time
The ANOVA comparing the individual go/nogo task with the social go/nogo task yielded main effects for the task factor, F(1,26) = 14.61 p ≤ 0.001 η2 = 0.36, and the congruency factor, F(1,26) = 6.15 p = 0.02 η2 = 0.19. Response times were longer in the individual go/nogo task (mean = 716 ms, SE = 23 ms) than in the social go/nogo task (mean = 652 ms, SE = 11 ms). Responses were shorted in the congruent trials (mean = 673 ms, SE = 16 ms) compared to the incongruent trials (mean = 694 ms, SE = 17 ms). The interaction between task and congruency was not significant, F(1,26) = 0.258 p = 0.62 η2 ≤ 0.01. The ANOVA comparing the standard Simon task with the social go/nogo task showed main effects for task, F(1,26) = 19.71 p < 0.001 η2 = 0.43, and congruency, F(1,26) = 14.62 p ≤ 0.001 η2 = 0.36. There was also an interaction between task and congruency, F(1,26) = 15.92 p ≤ 0.001 η2 = 0.38. The increase in response times due to congruency was larger in the standard Simon task (congruent: mean = 717 ms, SE = 24 ms; incongruent: mean = 768 ms, SE = 24 ms) than in the social go/nogo task (congruent: mean = 643 ms, SE = 13 ms; incongruent: mean = 660 ms, SE = 11 ms). Post hoc t test showed that the difference between congruent and incongruent trials was significant in each task (all p ≤ 0.05). Response time data are shown in Fig. 4.
Correlations with Autism-Spectrum Quotient (AQ) scores
As explained earlier, we additionally sought to test whether the amplitude of the social Simon effect would correlate with AQ scores. The AQ scores of the participants ranged from 7 to 35 (the maximum possible score is 50) with a mean of 18.2 (SD = 6.9). The amplitude of the social Simon effect was computed as the difference between response times in the incongruent and congruent trials. Pearson’s correlation coefficients were not significant: r = − 0.03 p = 0.89.
Correlations with the experimenter’s response times
Given the unexpected shorter response times in the social go/nogo task, we conducted further analyses to qualify the effect of the partnership. We correlated the response times of the experimenter with those of the participants during this task. Pearson’s correlation was significant, r = 0.87 p < 0.001. We also verified whether there were significant differences in response times between the experimenter and the participants during the social go/nogo task with a Student’s t test. The difference was not significant, t(27) = 1.74 p = 0.09.
Discussion
The results of the present study confirmed the existence of a Simon effect for tactile stimulations, but they did not support our hypothesis of a reappearance of this effect when the action was distributed between two partners. Participants responded faster to congruent trials than to incongruent trials in the standard Simon task where they had to react to the two different vibrotactile amplitudes. When comparing the individual and social versions of the go/nogo task where participants responded to only one vibrotactile amplitude, we did not find the expected difference in the effect of congruency. By contrast, the effect of congruency was superior in the standard Simon condition compared to the social go/nogo condition. In other words, the influence of congruency was reduced in the social go/nogo task to a degree that was not dissimilar to the individual go/nogo task. Response times thus showed patterns that were opposite to what the social Simon effect predicted. Given that our study was dimensioned according to previous studies carried out with visual stimuli, this outcome tentatively suggests that the social Simon effect may depend on the sensory modality of the stimuli. If so, a reduced social Simon effect in the tactile modality is not well accounted for by the current theories explaining this effect. It may be that representing another person’s action within one’s own sensory-motor system (Sebanz et al. 2003) or response coding scheme (Dittrich et al. 2017; Dolk et al. 2013) does not spontaneously occur when stimulations are in the tactile sensory modality. The sense of touch is contingent on one’s local skin contact with an object. It is therefore more personal and does not yield a sensory environment that can be straightforwardly shared. In the social go/nogo task of our experiment, the two partners received the same vibrotactile stimulations, but they originated from different (although identical) sources. The lack of shared sensory space may have hindered the natural tendency of participants to activate sensory-motor representations of their partner’s actions. This interpretation is consistent with neural imaging evidence that emphasizes the important role of shared attention mechanisms in the social Simon effect (Costantini et al. 2013).
The Autism-Spectrum Quotient (AQ) did not correlate with the response time difference between the incongruent and congruent trials in the social go/nogo condition. This result is not surprising given the absence of a social Simon effect.
Despite the absence of the expected social Simon interference, the social go/nogo condition did have an effect: Performances increased independently of the congruency of the stimuli, as shown by the reduced response times in the social go/nogo task compared to the standard Simon and individual go/nogo tasks. This decrease in response times did not come at the expense of accuracy. The percentage of erroneous trials was actually lower in the social go/nogo task compared to the standard Simon task.
One could argue that participants could have used a strategy whereby they relied on the experimenter’s responses, that is, responding only when the experimenter did not respond and inhibiting their response when the experimenter responded. Such a strategy entails that participants would have been waiting for the experimenter’ response, or lack of response, before they would initiate a response. If this was the case, then their response times would be superior to the upper range of the experimenter’s response times. However, response times of the participants were not significantly different from those of the experimenter. Additionally, waiting for the experimenter’s response should have increased the cognitive load of the social go/nogo task compared to the individual go/nogo task, which seems at odds with the fact that processing time was actually reduced in the social go/nogo task.
Altogether, the data showed that performing the task with a partner boosted performances. A similar result was reported in the study on the social Simon effect by Liepelt et al. (2011). The performance boost observed in our experiment cannot be merely attributed to the attendance of another person alongside the participant. Indeed, the experimenter sat next to the participants in every experimental conditions. The only variation introduced by the social go/nogo condition was that the experimenter took part in the task. The enhancing effect on performances in this condition may be explained by the classical effect of social facilitation induced by engaging in the same activity as a partner (Zajonc 1965). Additionally, the response times of the participants correlated with those of the experimenter. The participants and the experimenter thus appeared to have adjusted their processing time when performing the task as partners. This observation tends to support the view of social facilitation induced by the partnership.
In the present experiment, as participants were partnered with the experimenter in the social go/nogo task, they may have considered her as a reference that they should try to match. This could explain why their performances were boosted in the social go/nogo condition. Further research would be warranted to verify whether or not the observed enhancement of performances in the social go/nogo task would have also occurred if the partner had been another randomly selected participant. Despite this limitation, the present study contributes to the current knowledge on joint action by indicating that the social Simon effect may be hindered when the stimulations are in the tactile modality. This outcome suggests that coordination between co-actors might be challenging in this modality as their natural tendency to activate sensory-motor representations of their partner’s actions could be less spontaneous than in other modalities. We tentatively attributed this failure to a lack of shared sensory space. This hypothesis could be tested by future experiments in which the tactile stimulations would be provided to the two partners via the same vibrotactile devices.
References
Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E (2001) The autism-spectrum quotient (AQ): evidence from asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. J Autism Dev Disord 31(1):5–17. https://doi.org/10.1023/A:1005653411471
Colzato LS, de Bruijn ERA, Hommel B (2012) Up to “Me” or up to “Us”? The impact of self-construal priming on cognitive self-other integration. Front Psychol 3:341. https://doi.org/10.3389/fpsyg.2012.00341
Costantini M, Vacri AD, Chiarelli AM, Ferri F, Romani GL, Merla A (2013) Studying social cognition using near-infrared spectroscopy: the case of social Simon effect. J Biomed Op 18(2):025005. https://doi.org/10.1117/1.JBO.18.2.025005
de la Asuncion J, Docx L, Morrens M, Sabbe B, de Bruijn ERA (2015) Neurophysiological evidence for diminished monitoring of own, but intact monitoring of other’s errors in schizophrenia. Psychiatry Res 230(2):220–226. https://doi.org/10.1016/j.psychres.2015.08.043
Dittrich K, Rothe A, Klauer KC (2012) Increased spatial salience in the social Simon task: a response-coding account of spatial compatibility effects. Atten Percept Psychophys 74(5):911–929. https://doi.org/10.3758/s13414-012-0304-1
Dittrich K, Dolk T, Rothe-Wulf A, Klauer KC, Prinz W (2013) Keys and seats: spatial response coding underlying the joint spatial compatibility effect. Atten Percept Psychophys 75(8):1725–1736. https://doi.org/10.3758/s13414-013-0524-z
Dittrich K, Bossert M-L, Rothe-Wulf A, Klauer KC (2017) The joint flanker effect and the joint Simon effect: on the comparability of processes underlying joint compatibility effects. Q J Exp Psychol 70(9):1808–1823. https://doi.org/10.1080/17470218.2016.1207690
Dolk T, Liepelt R, Villringer A, Prinz W, Ragert P (2012) Morphometric gray matter differences of the medial frontal cortex influence the social Simon effect. NeuroImage 61(4):1249–1254. https://doi.org/10.1016/j.neuroimage.2012.03.061
Dolk T, Hommel B, Prinz W, Liepelt R (2013) The (not so) social Simon effect: a referential coding account. J Exp Psychol Hum Percept Perform 39(5):1248
Faul F, Erdfelder E, Lang A-G, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191. https://doi.org/10.3758/BF03193146
Gallotti M, Frith CD (2013) Social cognition in the we-mode. Trends Cognitive Sci 17(4):160–165. https://doi.org/10.1016/j.tics.2013.02.002
Guagnano D, Rusconi E, Umiltà CA (2010) Sharing a task or sharing space? On the effect of the confederate in action coding in a detection task. Cognition 114(3):348–355. https://doi.org/10.1016/j.cognition.2009.10.008
Hasbroucq T, Guiard Y (1992) The effects of intensity and irrelevant location of a tactile stimulation in a choice reaction time task. Neuropsychologia 30(1):91–94. https://doi.org/10.1016/0028-3932(92)90017-G
Hommel B, Colzato LS, van den Wildenberg WPM (2009) How social are task representations? Psychol Sci 20(7):794–798. https://doi.org/10.1111/j.1467-9280.2009.02367.x
Iani C, Anelli F, Nicoletti R, Arcuri L, Rubichi S (2011) The role of group membership on the modulation of joint action. Exp Brain Res 211(3–4):439. https://doi.org/10.1007/s00221-011-2651-x
Klempova B, Liepelt R (2016) Do you really represent my task? Sequential adaptation effects to unexpected events support referential coding for the joint Simon effect. Psychol Res 80(4):449–463. https://doi.org/10.1007/s00426-015-0664-y
Kuhbandner C, Pekrun R, Maier MA (2010) The role of positive and negative affect in the “mirroring” of other persons’ actions. Cogn Emot 24(7):1182–1190. https://doi.org/10.1080/02699930903119196
Liepelt R, Wenke D, Fischer R, Prinz W (2011) Trial-to-trial sequential dependencies in a social and non-social Simon task. Psychol Res 75(5):366–375. https://doi.org/10.1007/s00426-010-0314-3
Liepelt R, Wenke D, Fischer R (2013) Effects of feature integration in a hands-crossed version of the social Simon paradigm. Psychol Res 77(2):240–248. https://doi.org/10.1007/s00426-012-0425-0
Medina J, McCloskey M, Branch H, Rapp B (2014) Somatotopic representation of location: evidence from the Simon effect. J Exp Psychol Hum Percept Perform 40(6):2131–2142. https://doi.org/10.1037/a0037975
Ruys KI, Aarts H (2010) When competition merges people’s behavior: interdependency activates shared action representations. J Exp Soc Psychol 46(6):1130–1133. https://doi.org/10.1016/j.jesp.2010.05.016
Salzer Y, Aisenberg D, Oron-Gilad T, Henik A (2014) In touch with the Simon effect *the FIRST two authors contributed equally. Exp Psychol 61(3):165–179. https://doi.org/10.1027/1618-3169/a000236
Sebanz N, Knoblich G, Prinz W (2003) Representing others’ actions: just like one’s own? Cognition 88(3):B11–B21. https://doi.org/10.1016/S0010-0277(03)00043-X
Sebanz N, Knoblich G, Stumpf L, Prinz W (2005) Far from action-blind: representation of others’ actions in individuals with Autism. Cognitive Neuropsychol 22(3–4):433–454. https://doi.org/10.1080/02643290442000121
Sebanz N, Knoblich G, Prinz W, Wascher E (2006) Twin peaks: an ERP study of action planning and control in coacting individuals. J Cogn Neurosci 18(5):859–870. https://doi.org/10.1162/jocn.2006.18.5.859
Sebanz N, Rebbechi D, Knoblich G, Prinz W, Frith CD (2007) Is it really my turn? An event-related fMRI study of task sharing. Soc Neurosci 2(2):81–95. https://doi.org/10.1080/17470910701237989
Senju A, Southgate V, White S, Frith U (2009) Mindblind eyes: an absence of spontaneous theory of mind in Asperger syndrome. Science 325(5942):883–885. https://doi.org/10.1126/science.1176170
Simon JR (1969) Reactions toward the source of stimulation. J Exp Psychol 81(1):174
Stenzel A, Chinellato E, Tirado A, del Pobil ÁP, Lappe M, Liepelt R (2012) When humanoid robots become human-like interaction partners: corepresentation of robotic actions. J Exp Psychol Hum Percept Perform 38(5):1073–1077. https://doi.org/10.1037/a0029493
Stenzel A, Dolk T, Colzato LS, Sellaro R, Hommel B, Liepelt R (2014) The joint Simon effect depends on perceived agency, but not intentionality, of the alternative action. Front Hum Neurosci 8:595. https://doi.org/10.3389/fnhum.2014.00595
Tsai C-C, Brass M (2007) Does the human motor system simulate pinocchio’s actions?: coacting with a human hand versus a wooden hand in a dyadic interaction. Psychol Sci 18(12):1058–1062. https://doi.org/10.1111/j.1467-9280.2007.02025.x
Tsai C-C, Kuo W-J, Hung DL, Tzeng OJL (2008) Action co-representation is tuned to other humans. J Cogn Neurosci 20(11):2015–2024. https://doi.org/10.1162/jocn.2008.20144
Vlainic E, Liepelt R, Colzato LS, Prinz W, Hommel B (2010) The virtual co-actor: the social simon effect does not rely on online feedback from the other. Front Psychol 1:208. https://doi.org/10.3389/fpsyg.2010.00208
Welsh TN (2009) When 1 + 1=1: the unification of independent actors revealed through joint Simon effects in crossed and uncrossed effector conditions. Hum Mov Sci 28(6):726–737. https://doi.org/10.1016/j.humov.2009.07.006
Welsh TN, Kiernan D, Neyedli HF, Ray M, Pratt J, Potruff A, Weeks DJ (2013) Joint simon effects in extrapersonal space. J Mot Behav 45(1):1–5. https://doi.org/10.1080/00222895.2012.746635
Zajonc RB (1965) Social facilitation. Science 149(3681):269–274
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Handling editor: Katsumi Watanabe (Waseda University Tokyo); Reviewers: Marcello Costantini (University of Chieti) and a second reviewer who prefers to remain anonymous.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pérusseau-Lambert, A., Anastassova, M., Boukallel, M. et al. The social Simon effect in the tactile sensory modality: a negative finding. Cogn Process 20, 299–307 (2019). https://doi.org/10.1007/s10339-019-00911-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10339-019-00911-4