Abstract
A 2 m long open tubular column with a copolymer layer fabricated on the inner surface of silica capillary of 50 μm id has been prepared. A ligand with a terminal halogen was bound to the capillary inner surface via silanol-isocyanate reaction and an initiator moiety was introduced by reacting with the terminal halogen. Then a thick layer of linear copolymer chains was generated on the inner surface of capillary by reversible addition-fragmentation transfer polymerization of styrene and N-phenyl acrylamide. The resultant open tubular column exhibited a remarkably high separation efficiency for the separation of a synthetic mixture of five peptides in liquid capillary chromatography (in the pressure mode). The effect of monomer mixing ratio (styrene versus N-phenyl acrylamide) on the chromatographic separation efficiency has been studied. The column prepared with the optimum mixing ratio produced the number of theoretical plates (N) of 391,200 per column under the optimized elution pressure. The column to column repeatability estimated as relative standard deviation in plate count, retention factor, asymmetry factor, and resolution was found better than 3.0%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Open tubular (OT) capillary columns have been setting their firm position in various areas of separation science such as capillary electrochromatography (CEC), capillary liquid chromatography (CLC), and solid phase extraction. Especially their application in CEC has attracted a lot of attention for their outstanding separation performance [1]. Various formats of OT stationary phase have been used, i.e., in-situ prepared polymers, molecular imprinted polymers (MIP), monoliths, brush ligands, host ligands, block copolymers, nanoparticles, proteins, polysaccharides, aptamers, carbon nanotubes, tentacles, and polyelectrolyte multi-layers [1].
According to the recent OT-CEC reviews [2,3,4,5,6], the traditional methods have been continually used for formation of OT-CEC columns by making use of silica capillaries of 50–100 μm id and some modified raw materials, hardly reporting prominent performance progress of the resultant columns. In recent years (from 2016 to 2018), enormous attention has been devoted to the development of novel stationary phases for CEC, open-tubular columns being the main trend, however, there are no significant breakthroughs in technology and principles in CEC [2]. Applications of OT-CEC in specific areas have also been reviewed, for example, in analysis of peptides and proteins [3], OT stationary phases based on alkylthiol gold nanparticles [4], food analysis [5], and enantio-separation [6].
In the comprehensive overview in 2013 over preparation and application trends of various open tubular capillary columns, CEC was recommended as the major application area of OT capillary columns for its excellent separation efficiency secured by the flat eluent velocity distribution across the capillary diameter [1]. However, CEC/MS is less popular than nano-LC/MS for its more complicated instruments and fussy operation although it is regarded as a powerful analytical method. LC/MS with a miniaturized LC part seems to be the prototype in analysis of modern complicated samples frequently encountered in the real world. In this regard, the desperate goal of separation science is to realize a miniaturized LC column with high separation efficiency still being easy to make and handle. OT-LC columns may be the ultimate answer to such an issue.
As introduced in recent reviews [7,8,9], the general trend of OT-LC columns is to use capillaries as narrow as possible to improve its separation efficiency up to an extreme value (over 10 million plates/m). Under the right conditions, OT-LC columns have the potential to offer superior column efficiency, higher overall peak capacity, and higher column permeability compared to packed capillary and monolithic columns [7]. Although a variety of preparation methods have been discussed for open tubular stationary phases, there is no discussion or suggestion on OT columns based on long linear polymer ligands attached to the capillary inner surface in these reviews.
Fast mass transfer kinetics is critical to good separation efficiency. In the situation of fixed capillary id, fast mass transfer kinetics in OT columns can be secured by realizing flexible and/or thin stationary phase in usual OT silica capillary columns, or by providing enough numbers of mesopores in a well-connected structure in the case of OT columns with thick stationary phase [1].
Recent outstanding results in OTLC have been mostly obtained in the long columns with very narrow id of 10 μm or less. The pioneering work of the Karger group [10,11,12,13,14] in proteomic analysis with porous layer open tubular (PLOT) columns of 10 μm id was followed by the work of the Desmet group [15, 16] with the OT columns of 5 μm id, and the work of the Liu group [17,18,19,20] with the OT columns of 2 μm id.
For example, a value of N (number of theoretical plates) over 10 million/m was obtained with a capillary column modified with a C18 ligand (44 cm effective length, 2 μm id) in the isocratic elution with 80/20 (v/v) 10 mM NH4HCO3/acetonitrile [17]. Using a similar column (80 cm length, 2 μm id), 440 apparent peaks were separated with a peak capacity of 1640 within 172 min for a sample from pepsin/trypsin-digested Escherichia coli cell lysate [18]. Ultrafast gradient separation was also successfully carried out, and a baseline separation was achieved with a 2.7 cm long column for 6 amino acids in less than 0.7 s [19]. Most recently, a 2 μm i.d. × 75 cm long OT column modified with trimethoxy(octadecyl)silane was used for digested E. coli lysates to show abnormally large peak capacities (1900 − 2000 in 3−5 h) routinely [20].
The above approach (long and very narrow OT capillaries) certainly has achieved the best separation efficiency in the history of liquid chromatography. This approach is also very useful for analysis of samples with extremely tiny volume such as a single cell. It may be the only feasible method for analysis of multiple components of a single cell. On the other hand, such excellent separation efficiency could be unnecessarily high for routine analysis of most common samples. Those columns are also rather inconvenient to make and use for high chance of column clogging. Therefore, it would be very helpful in most chromatographic applications if proper OT columns are available showing rather wide id (ca 50 μm) and comparable separation efficiency to the columns of very narrow id.
Recently, a strategy of binding long polymer chains to inner surface of capillary was proposed in our laboratory to accomplish such a goal [21,22,23]. In our previous studies, capillaries of 60–120 mm length (50 μm id) were modified with a monomer mixture by reversible addition-fragmentation transfer (RAFT) polymerization and the resultant columns were used for the separation of derivatized saccharide isomers and tryptic digest of cytochrome C in capillary electrochromatography [21,22,23], and capillary liquid chromatography [23]. OT capillary column has the advantage of low column back pressure, so absolute column efficiency can be improved through increasing the length of column. An open tubular column whose length is at least 2 m would be the right choice in liquid capillary chromatography.
In the present study, we tried to achieve higher column efficiency through preparation of 2 m long OT column adopting the similar preparation protocol to that reported previously [23] with some modification. The successful growth of a thick polymer layer on the inner wall without bulk structure formation (column clogging) is the point of the modification. We adopted to use a heavy-duty syringe pump for delivery of reaction mixture to prevent the risk of column clogging that might be frequently encountered in the course of column preparation through polymerization. We also adopted RAFT polymerization for OT column to ensure anchoring long copolymer chains upon the surface minimizing formation of freed polymer chains. In RAFT polymerization, the surface is first modified with a ligand bearing a halogen terminal, then an initiator moiety is introduced upon it, thus the copolymer chains are grown from the surface. The copolymer layer is composed of styrene and N-phenylacrylamide in this study. The resultant column was used for the separation of five standard peptides to result in an excellent separation efficiency: an average N value of 391,200 per column in capillary liquid chromatography.
Experimental
Chemicals and Materials
All chemicals were of reagent or analytical grade. Sodium hydroxide, hydrochloric acid, glacial acetic acid, 3-chloropropyl isocyanate, dibutyltin dichloride, sodium diethyldithiocarbamate (SDEDTC), anhydrous toluene, tetrahydrofuran (THF), N-phenylacrylamide, ammonium formate and styrene were purchased from Sigma-Aldrich (St. Louis, MO, USA). The peptides (Trp-Gly, Thr-Tyr-Ser, angiotensin I, isotocin, and bradykinin) were also obtained from Sigma-Aldrich (St. Louis, MO, USA). The solvents were used for preparation of reaction mixture and washing of prepared column. Acetonitrile (ACN), methanol, 2-propanol, acetone, and water were purchased from Avantor (Phillipsburg, NJ, USA) and used without further purification. Polyimide coated fused silica capillary with 50 μm id (365 μm od) were purchased from Polymicro Technologies (Phoenix, AZ, USA).
Instrumentation
The polymerization reaction mixture was pumped through capillaries with a heavy duty Chemyx (Stanford, TX, USA) Nexus 6000 syringe pump. Thermally initiated formation of OT columns was carried out in a custom-designed tube furnace made by Daeheung Science Corporation (Incheon, Korea). Capillary liquid chromatography experiments were performed on an Agilent (Waldbronn, Germany) HP3D CE system with a diode array detector and the Chemstation data processing software in the pressure mode. The SEM images of the cross-section of the OT column were captured by a Hitachi (Tokyo, Japan) S-4200 field emission scanning electron microscopy (FE-SEM).
Preparation of OT Columns
Capillary Pretreatment
Fused-silica capillaries were initially pretreated for activation of the surface silanol groups. The pretreatment of fused-silica capillary (50 μm id and 200 cm length) was carried out according to the procedure as reported previously [20] with some modification. Briefly, the capillary was filled with 4 M NaOH solution, plugged with rubber septa at both ends, and left alone at 55 °C for 30 days. The capillary was washed with water followed by 0.1 M HCl, and water again, flushed with acetone for 1 h, and dried with N2 gas at 120 °C for 2 h. Such itching process has been known to result in exposure of an increased number of silanol groups.
Attachment of Initiator Moiety on the Inner Surface of Capillary
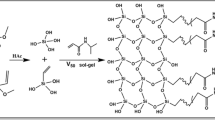
The solution composed of 3-chloropropyl isocyanate (30 μL), anhydrous toluene (3.0 mL), and dibutyltin dichloride (30 mg, catalyst), was pumped through the pretreated capillary at 90 °C for 15 h at a flow rate of 0.20 mL/h. The capillary was flushed with toluene for 10 h followed by acetone for 5 h, and dried with N2 gas for 30 min. The reaction scheme is given in Fig. 1a. The flow of a solution of SDEDTC (100 mg) in anhydrous THF (3.0 mL) was then introduced into the capillary at 60 °C for 15 h at a flow rate of 0.2 mL/h. The capillary was washed with methanol overnight followed by acetone for 5 h, and dried with N2 for 20 min. The reaction scheme is shown in Fig. 1b.
In Situ RAFT Copolymerization
In situ RAFT co-polymerization was performed over the bound initiator on the capillary inner surface to form the co-polymer layer by running a mixture containing 2.0 mL styrene, 180 mg N-phenylacrylamide, 2.0 mL p-xylene, and 2.0 mL 4-methyl-2-pentanone at 100 °C for 48 h at a slow flow rate of 0.125 mL/h. The reaction mixture was sonicated and N2-purged for 30 min and allowed to flow through the capillary by a 0.2 μm Whatman (Maidstone, UK) syringe filter. A series of washing solvents, i.e., toluene, 2-propanol, (50:50, v/v) 2-propanol/water at 50 °C, and acetone at room temperature were allowed to flow one by one through the capillary to remove the residual reaction mixture and freed oligomers. The scheme of formation of polymer chains is illustrated in Fig. 1c.
Before the CLC operation, the OT column was flushed with the mobile phase for 2–3 h to let the stationary phase in dynamic equilibrium. A detection window was created at the position of 8.8 cm from the outlet end by burning the polyimide coating, thus the effective length was 191.2 cm. The OT column was finally installed in the instrument for analysis.
Three SEM images obtained from 3 columns were used to measure the co-polymer layer thickness. The thickness data were collected at many positions to yield the average thickness of 1.5 ± 0.2 μm.
Chromatographic Conditions
The buffer solution (20 mM ammonium formate, ionic strength = 0.02 M) was prepared in distilled water and its pH was adjusted to the desired value by adding formic acid to the stock buffer solution.
The mobile phase was prepared by mixing appropriate amount of ACN with 20 mM ammonium formate (60:40 v/v) followed by degassing in an ultrasonic bath for 30 min. Five standard peptides were dissolved in the buffer to obtain a solution containing 1 mg/mL of each peptide. The sample solution was stored in a refrigerator at 4 °C. The detection wavelength and capillary temperature were set to 214 nm and 25 °C, respectively. Prior to carrying out the chromatographic separation, all solutions were filtered through a 0.22 μm membrane filter and degassed in an ultrasonic bath for 15 min. The stock solution was 20 times diluted by the mobile phase for actual sample injection. KNO3 was used as the void volume marker. The sample was injected for 10 s under 10 mbar (pressure injection). The signal intensity was linear with injection pressure in the pressure range from 2 to 30 mbar.
Results and Discussion
The Optimized Mixing Ratio of Styrene vs N-Phenylacrylamide
The N values obtained with the OT columns prepared with different monomer mixing ratios are comparatively summarized in Table 1. The elution pressure was 18 mbar (see the section of van Deemter plots). The trend of height equivalent to theoretical plates (HETP) with respect to monomer mixing ratio is shown in Fig. 2. As shown in Table 1 and Fig. 2, the co-polymer layer was composed of a major nonpolar component (styrene, 2 mL) accompanied with a minor polar component (N-phenyl acrylamide, 180 mg) in the optimized stationary phase. Styrene is an essential component for radical formation at a high reaction temperature to induce RAFT polymerization. The role of N-phenylacrylamide in the reaction mixture is to provide the required polarity of the stationary phase to yield enough retention of polar analytes. When only styrene was used for modification, the resultant stationary phase was not useful in providing enough retention of peptides. When the amount of N-phenylacrylamide was over 300 mg in 2 mL styrene, the separation efficiency of the OT column was degraded abruptly. Use of such a higher amount N-phenylacrylamide will result in increase of the polarity of the co-polymer chain to cause to serious entangling of the ligands by the stronger ligand-by-ligand interaction, thereby degrading mass transfer kinetics. Peptides have amide groups thus the amide group is selected as the polar group. Including the phenyl group in the polar monomer is intended to improve the compatibility of the polymerization reaction mixture. When acrylamide was used instead of N-phenylacrylamide, the resultant stationary phase showed much inferior separation efficiency.
Inner Surface Architecture of the OT Column
To gain a better understanding of the morphological structure of modified OT column, the FE-SEM images on the inner surface were obtained (Fig. 3). Clearly, the bare silica capillary possessed a neat circular inner surface according to Fig. 3a. A co-polymer layer of about 1.5 μm thickness with considerable fluctuations was well formed over the surface after RAFT polymerization as shown in Fig. 3b–d. If the capillary inner surface is modified with short ligand molecules (such as C18), the layer is not visible in general SEM images. The collapsed polymer chains as observed in FE-SEM images (Fig. 3) will be spread open in the mobile phase causing increased stationary phase surface to show nice retention and resolution of analytes, also causing enhanced mass transfer kinetics to show excellent separation efficiency. This long chain-like character of the ligand contributes to excellent performance of current OT columns.
Chromatographic Performance of 2 m OT Column for Peptides
The data of number of theoretical plates (N) were obtained with the 2 m long OT column over the various elution pressures from 3 to 50 mbar (Table 2). The chromatogram of KNO3 and five peptides obtained with the OT column of the optimized monomer mixing ratio under the optimum elution pressure (18 mbar) is shown in Fig. 4.
The results of column-to-column repeatability based on three OT columns evaluated in terms of retention factor (k), N value, asymmetry factor (As), and peak resolution (Rs) are summarized in Table 3 with the average and relative standard deviation (RSD) values of the measured data. The column to column reproducibility was at least better than 3.0%. Especially, the relative standard deviation (RSD%) values in k, As, and Rs were found better than 2.0%, exhibiting satisfactory repeatability of the OT columns.
As shown in Table 3, the retention factors (k) of the analyzed peptides are in a reasonable range of 1.2–1.7. In addition, the chromatographic resolution (Rs) for any pair of two adjacent peaks was in the range of 6.3–7.7, indicating superb separation performance. All the asymmetry factor values are not greater than 1.10, proving nice symmetrical peak shapes with insignificant tailing for polar peptides, and that was enabled by nice control of the polarity of stationary phase through optimization of the monomer mixing ratio. The average number of theoretical plates obtained with our capillary column over the 5 peptides under the optimized elution pressure was 391,200 per column.
Durability Test of OT Column
Leaching or bleeding of polymer stationary phase from the capillary surface is relatively easy to happen when a pressure is applied in OT-CLC. The optimum elution pressure was applied to drive the mobile phase through the column, and the peptide mixture was injected for separation 10 times a day over a period of 1 month. The temperature was kept at 25 °C throughout. During the period (more than 200 injections), both the retention time and separation performance maintained above 90% of the original values. The OT column of our type requires very low operation pressure (18 mbar). Leaching or bleeding on account of pressure seems negligible for our OT column. Degradation of column performance may be caused by contamination of stationary phase, too. The chance of contamination also seems to be low for our column since it is composed of linear polymer chains providing good flexibility and permeability under the mobile phase flow to prevent sticky adsorption of contaminants in comparison to common crosslinked phase (monolithic porous layer).
van Deemter Plots
Figure 5 shows the van Deemter plots obtained with our OT column. The separation of test solutes was carried out at various pressures from 3 to 50 mbar. The linear velocity of mobile phase was determined by KNO3 as the void volume marker. The optimum linear velocity was 2.82 mm/s under the elution pressure of 18 mbar where the corresponding H value was 4.88 μm (average among different peptides), which is impressive. The sample prepared according the experimental section was introduced by pressure injection for 10 s under 10 mbar.
Comparison with Other OT Columns in the Literature
Resolution and separation efficiency are the two primary factors that should be considered simultaneously in evaluation of chromatographic performance of stationary phases. Resolution is generated and improved by gradual increase of volume of stationary phase. On the other hand, separation efficiency is reduced with increase of stationary phase volume [1]. Silica capillary has a relatively much narrower surface area than porous silica particles or monolithic structures, causing some troubles on modification. If the inner surface is modified with a small (low molecular weight) ligand, the amount of stationary phase should be too low to get enough analyte retention and resolution. Immobilizing polymeric ligands instead to overcome this problem should cause reduced mass transfer kinetics. A PLOT (monolith type) format has been mostly favored among various polymer formats since improved mass transfer kinetics is achieved owing to ample mesopores and macropores therein, and the PLOT layer may be well extended to a higher thickness to enable improvement of solute retention and resolution [1].
On the other hand, the superior strategy of adoption of chemically bonded long polymer chains on the inner surface of silica capillary has not been yet generally acknowledged in the literature. In order to prove superiority of our strategy, the separation efficiency of the columns of current study was compared below to those of other OT columns reported in the literature.
As mentioned already, the column separation efficiency of OT-CEC is always better than that of OT-CLC if the same column is used in both analyses. It has been also clearly proven in our previous study [23]. Nevertheless, it is rare to find OT-CEC results better than the results of current OT-CLC study. Our current column (1.91 m effective column length) prepared with the optimum mixing ratio produced the number of theoretical plates (N) of 391,200 per column (204,800 plates/m) under the optimized elution pressure.
An OT (50 μm id) column was developed for analysis of peptides and proteins by modification with tentacle-type polymer, and the obtained separation efficiency was 144,000–189,000 and 97,000–170,000 plates/m for peptides and proteins in OT-CEC, respectively [24]. A capillary of 20 μm id was etched and modified with a C18 ligand to show separation efficiency of 140,000 plates/m for serotorin in OT-CEC [25]. A gold-nanoparticles-modified silica capillary (75 μm id and 31 cm effective length) was prepared to exhibit only ca 23,000 plates for peptides and proteins [26]. A capillary of 50 μm id was etched and modified with a C18 ligand to show up to 300,000 plates/m for peptides in gradient elution CEC [27]. An OT capillary column was prepared by multilayer-by-multilayer bonding of silica nanoparticles followed by C18 modification, and the resultant column (75 μm id, 20 cm effective length) prepared with three coating cycles showed high separation efficiency up to 269,000 plates/m for toluene in CEC [28]. A uniform porous silica layer (240 nm) OT column was prepared by a single step biphasic reaction, and the resultant column (50 μm id, 40 cm effective length) was applied for CEC separation without further modification to show theoretical plate numbers over 60,000 [29].
The policy of attaching long polymer chains to the inner surface of silica capillary was recently adopted by the Dovichi group in their proteomic research with CEC/MS [30,31,32]. The column was made by filling the capillary (50 μm id, 100 cm length) with a mixture composed of acrylamide, 4,4′-azobis(4-cyanovaleric acid) in an acetate buffer and by incubating at 60 °C [30]. The observed theoretical plate numbers were in the range of 240,000–600,000. Using the capillary column (50 μm id, 100–110 cm length), they were able to identify over 27,000 peptides and 4,400 proteins in a single 120 min run from 220 ng of K562-cell digest [31] and 4405 phosphopeptides from 220 ng of enriched phosphopeptides derived from mouse brain [32]. On the other hand, the observed theoretical plate numbers of peptides in Ref [23] were at least over 1.1 million in CEC. The polymerization reaction mixture was pumped through the capillary (50 μm id) during the reaction as reported previously [23] while the capillary was filled with the reaction mixture and incubated in the study of the Dovichi group [30,31,32].
As for OT-CLC, the capillary columns reported in the literature with id of 15 μm or greater have never shown better separation efficiency than our current capillary columns of 50 μm id. For example, a C8-modified PLOT column (15 μm id, 3 m length) with a 0.5 μm silica porous layer was prepared to show 167,500 plates for propylbenzene [33]. The same group of this work also carried out separation of small analytes by normal phase liquid chromatography using a non-modified porous silica layer open tubular column (15 μm id, 3 m length, 0.5 μm layer thickness) to yield up to 170,000 theoretical plates [34]. A novel chiral PLOT column (75 μm id, 15 cm length) was developed by in-situ polymerization of ethylene dimethacrylate and 3‐chloro‐2‐hydroxypropylmethacrylate and consequent introduction of β-cyclodextrin via the reaction with the reactive terminal chlorine, and the resultant OT column showed theoretical plate numbers up to 26,000 plates/m for enantio-separation [35].
Superiority of Long Chain Ligands in OTLC
Examination of our OT columns for chromatographic performance has resulted in observation of an excellent separation efficiency of 391,200 plates per column corresponding to 204,800 plates/m (4.88 μm HETP). Such excellent performance is believed to be owing to two major factors. First, it seems that accelerated high mass transfer kinetics was enabled by formation of long linear chains on the inner surface through their spreading open in the mobile phase. Second, based on the abnormally outstanding separation efficiency, the following explanation is possible. The long chain ligands will wave and roll, and their movements will interfere with mobile phase flow to induce partial turbulent flow reducing the parabolic flow velocity distribution and accelerating analyte diffusion across the column diameter.
It should be noted that the N value/column is not linear with column length in preparation of OT columns. In our previous work [23], the effective column length (50 μm id) was 1.11 m, and the average N value among 8 peptides was 259,400/column (233,900/m). In current study, the effective column length was 1.91 m, and the average N value among 5 peptides was 391,000/column (204,800/m). The N/m value was decreased to 87.6% for the change of effective column length from 1.11 to 1.91 m. A similar trend was observed when long S-ketoprofen MIP PLOT columns were used for enantio-separation of R, S-ketoprofen in CEC [36]. The measured N/m value for R-ketoprofen was reduced from 1,130,000 to 835,000 and 706,000 for the effective column length of 1.00, 2.00, and 3.00 m, respectively [36].
Such phenomenon means that extension of column length caused some change in the stationary phase structure. We suspect that the rate of crosslinking among the polymer chains may be somewhat increased to degrade mass transfer kinetics as the column length under polymerization is extended. Nevertheless, the separation efficiency of 204,800 plates/m (391,000/column) of this study is still excellent if it is considered that the column id is 50 μm.
Realization of outstanding separation performance and good durability of current study seems to be also obliged to successful formation of rather thick stationary phase. A thick layer of our format enables enough retention of analytes and improved resolution with little degradation of separation efficiency.
In order to make a thicker layer, some changes were made in preparation of OT columns. The pretreatment of silica capillary was carried out in a harsher situation and for a longer time to create more silanol groups on the inner surface. The capillary was filled with 4 M NaOH solution, plugged with rubber septa at both ends, and kept at 55 °C for 30 days. In addition, the polymerization mixture was flown at a lower flow rate for a longer time. In the previous study, the mixture was flown for 8 h [23] while it was flown for 48 h in this study. The layer thickness was ca 0.5 μm in the previous study [23] while it was increased to ca 1.5 μm in this study. The reaction mixture was composed of styrene (1.0 mL), methacrylic acid (50 μL), N-phenylacrylamide (100 mg), anhydrous p-xylene (2.0 mL), and 4-methyl-2-pentanone (1.0 mL) in the previous study while it was composed of 2.0 mL styrene, 180 mg N-phenylacrylamide, 2.0 mL p-xylene, and 2.0 mL 4-methyl-2-pentanone in this study. Methacrylic acid (EOF monomer) was removed in the reaction mixture in this study. This study was devoted to only liquid chromatography, thus methacrylic acid was not necessary. Furthermore, incorporation of methacrylic acid in the reaction mixture may somewhat contribute to band broadening since it is rather too polar.
As for concentration of monomer mixture, it was not found helpful to increase the concentration over that used in this study. More diluted reaction mixture was not tried since the reaction time should be extended over 48 h, which is already long enough.
Pumping the polymerization reaction mixture through the capillary is critical. When the polymerization was carried out by filling the capillary with the mixture followed by plugging both ends with septa and heating, it caused frequent clogging of column or poor chromatographic performance if not clogged. When such process was carried out by filling a diluted reaction mixture to prevent clogging, the layer thickness of the resultant column was too thin to show enough retention and resolution.
Briefly, heavy-duty pumping of polymerization mixture and proper formulation of mixture combined by RAFT polymerization at a slow flow rate for an enough time are the requirements for preparation of useful OT columns of rather convenient id. There should be some formation of crosslinking among polymer chains since RAFT polymerization is also based on a radical mechanism although RAFT polymerization has been known to produce linear polymer chains mostly. Such trouble may be more serious when the polymer chains are grown up. Stable heavy-duty pumping is believed to minimize this trouble. There should be also generation of unwanted freed polymer chains, and they are also well swept out of the column by pumping. The above arguments may be supported by the results of our trial to produce OT-CLC columns by filling the reaction mixture in the silica capillary, sealing the capillary ends, and incubating. The resultant capillary columns showed much inferior separation efficiency with frequent clogging during the run.
Conclusion
In this work, we examined the feasibility of using 2 m long OT columns of rather wide id (50 µm) modified with linear polymer chains. A rather thick co-polymer layer (ca 1.5 µm) was fabricated by carefully controlled RAFT polymerization. It was found that the chromatographic performance was dependent on the mixing ratio of monomers (N-phenyl acrylamide versus polystyrene). With the optimal mixing ratio of 180 mg N-phenyl acrylamide in 2 mL styrene, the resultant OT column with a layer composed of a major nonpolar constituent and a minor polar constituent has shown impressive separation efficiency of 391,200 plates/column. The successful preparation of OT columns of outstanding performance in this study is owing to the synergistic effects of proper formulation of polymerization mixture, stable heavy-duty pumping, and RAFT polymerization at a slow flow rate for an enough time. This study proposes a promising perspective of OT columns of our type (long polymer chain ligand) as useful separation media.
References
Cheong WJ, Ali F, Kim YS, Lee JW (2013) Comprehensive overview of recent preparation and application trends of various open tubular capillary columns in separation science. J Chromatgr A 1308:1–24
Mao ZK, Chen ZL (2019) Advances in capillary electro-chromatography. J Pharm Anal 9:227–237
Mikšík I (2017) Capillary electrochromatography of proteins and peptides (2006–2015). J Sep Sci 40:251–271
Guihen E (2017) Recent highlights in electro-driven separations-selected applications of alkylthiol gold nanoparticles in capillary electrophoresis and capillary electro-chromatography. Electrophoresis 38:2184–2192
D’Orazio G, Asensio-Ramos M, Fanali C, Hernandez-Borges J, Fanali S (2006) Capillary electrochromatography in food analysis. Trends Anal Chem 82:250–267
Christodoulou CPK, Nicolaou AJ, Stavrou IJ (2006) Enantioseparations in open-tubular capillary electrochromatography: Recent advances and applications. J Chromatgr A 1467:145–154
Lam SC, Rodriguez ES, Haddad PR, Paull B (2019) Recent advances in open tubular capillary liquid chromatography. Analyst 144:3464–3482
Li RN, Wang YN, Peng MH, Wang XY, Guo GS (2017) Preparation and application of porous layer open tubular capillary columns with narrow bore in liquid chromatography. Chin J Anal Chem 45:1865–1873
Knob R, Kulsing C, Boysen RI, Macka M, Hearn MTW (2015) Surface-area expansion with monolithic open tubular columns. Trends Anal Chem 67:16–25
Yue GH, Luo QZ, Zhang J, Wu SL, Karger BL (2007) Ultratrace LC/MS proteomic analysis using 10-μm-i.d. porous layer open tubular poly(styrene-divinylbenzene) capillary columns. Anal Chem 79:938–946
Luo QZ, Yue GH, Valaskovic GA, Gu Y, Wu SL, Karger BL (2007) On-line 1D and 2D porous layer open tubular/LC-ESI-MS using 10-μm-i.d. poly(styrene-divinylbenzene) columns for ultrasensitive proteomic analysis. Anal Chem 79:6174–6181
Luo QZ, Gu Y, Wu SL, Rejtar T, Karger BL (2008) Two-dimensional strong cation exchange/porous layer open tubular/mass spectrometry for ultratrace proteomic analysis using a 10 μm id poly(styrenedivinylbenzene) porous layer open tubular column with an on-line triphasic trapping column. Electrophoresis 29:1604–1611
Luo QZ, Rejtar T, Wu SL, Karger BL (2009) Hydrophilic interaction 10 μm I.D. porous layer open tubular columns for ultratrace glycan analysis by liquid chromatography-mass spectrometry. J Chromatgr A 1216:1223–1231
Wang DD, Hincapie M, Rejtar T, Karger BL (2011) Ultrasensitive characterization of site-specific glycosylation of affinity-purified haptoglobin from lung cancer patient plasma using 10 μm i.d. porous layer open tubular liquid chromatography-linear ion trap collision-induced dissociation/electron transfer dissociation mass spectrometry. Anal Chem 83:2029–2037
Hara T, Futagami S, Eeltink S, Malsche WD, Baron GV, Desmet G (2016) Very high efficiency porous silica layer open-tubular capillary columns produced via in-column sol-gel processing. Anal Chem 88:10158–10166
Hara T, Izumi Z, Nakao M, Hata K, Baron GV, Bamba T, Desmet G (2018) Silica-based hybrid porous layers to enhance the retention and efficiency of open tubular capillary columns with a 5 μm inner diameter. J Chromatgr A 1580:63–71
Chen H, Yang Y, Qiao ZZ, Xiang PL, Ren JT, Meng YZ, Zhang KQ, Liu SR (2018) A narrow open tubular column for high efficiency liquid chromatographic separation. Analyst 143:2008–2011
Yang Y, Chen H, Beckner MA, Xiang PL, Lu JJ, Cao CX, Liu SR (2018) Narrow, open tubular column for ultrahigh-efficiency liquid-chromatographic separation under elution pressure of less than 50 bar. Anal Chem 90:10676–10680
Xiang PL, Yang Y, Zhao ZT, Chen ML, Liu SR (2019) Ultrafast gradient separation with narrow open tubular liquid chromatography. Anal Chem 91:10738–10743
Xiang PL, Yang Y, Zhao ZT, Chen AP, Liu SR (2019) Experimentally validating open tubular liquid chromatography for a peak capacity of 2000 in 3 h. Anal Chem 91:10518–10523
Ali F, Cheong WJ (2015) Open tubular capillary electrochromatography with an N-phenylacrylamide-styrene copolymer based stationary phase for the separation of anomers of glucose and structural isomers of maltotriose. J Sep Sci 38:1763–1770
Ali F, Cheong WJ (2016) High efficiency robust open tubular capillary electrochromatography column for the separation of peptides. Bull Korean Chem Soc 37:1374–1377
Ali A, Cheong WJ (2017) Open tubular capillary column of 50 μm internal diameter with a very high separation efficiency for the separation of peptides in CEC and LC. J Sep Sci 50:2654–2661
Zhang XW, Yang J, Liu SF, Xie ZH (2011) Branched polyethyleneimine bonded tentacle-type polymer stationary phase for peptides and proteins separations by open tubular capillary electrochromatography. J Sep Sci 23:3383–3391
Matyska MT, Pesek JJ, Boysen RI, Hearn MTW (2001) Characterization of open tubular capillary electrochromatography columns for the analysis of synthetic peptides using isocratic conditions. Anal Chem 21:5116–5125
Hamer M, Yone A, Rezzano I (2012) Gold nanoparticle-coated capillaries for protein and peptide analysis on open tubular capillary electrochromatography. Electrophoresis 33:334–339
Yang YZ, Boysen RI, Matyska MT, Pesek JJ, Hearn MTW (2007) Open-tubular capillary electrochromatography coupled with electrospray ionization mass spectrometry for peptide analysis. Anal Chem 79:4942–4949
Qu QS, Liu YY, Shi WJ, Yan C, Tang XQ (2015) Tunable thick porous silica coating fabricated by multilayer-by-multilayer bonding of silica nanoparticles for open tubular capillary chromatographic separation. J Chromatogr A 19:25–31
Liu X, Sun SC, Nie RB, Ma JC, Qu QS, Yang L (2018) Highly uniform porous silica layer open-tubular capillary columns produced via in-situ biphasic sol-gel processing for open-tubular capillary electrochromatography. J Chromatogr A 1538:86–93
Zhang ZB, Peuchen EH, Dovichi NJ (2017) Surface-confined aqueous reversible addition-fragmentation chain transfer (SCARAFT) polymerization method for preparation of coated capillary leads to over 10 000 peptides identified from 25 ng HeLa digest by using capillary zone electrophoresis-tandem mass spectrometry. Anal Chem 89:6774–6780
Zhang ZB, Hebert AS, Westphall MS, Qu YY, Coon JJ, Dovichi NJ (2018) Production of over 27 000 peptide and nearly 4400 protein dentifications by single-shot capillary-zone electrophoresis-mass spectrometry via combination of a very-low-electroosmosis coated capillary, a third-generation electrokinetically-pumped sheath-low nanospray interface, an orbitrap fusion Lumos Tribrid mass spectrometer, and an advanced-Peak-determination algorithm. Anal Chem 90:12090–12093
Zhang ZB, Hebert AS, Westphall MS, Coon JJ, Dovichi NJ (2019) Single-shot capillary zone electrophoresis-tandem mass spectrometry produces over 4400 phosphopeptide identifications rom a 220 ng sample. J Proteome Res 18:3166–3173
Forster S, Kolmar H, Altmaier S (2013) Performance evaluation of thick film open tubular silica capillary by reversed phase liquid chromatography. J Chromatogr A 1283:110–115
Forster S, Kolmar H, Altmaier S (2012) Synthesis and characterization of new generation open tubular silica capillaries for liquid chromatography. J Chromatogr A 1265:88–94
Aydoğan C (2018) Chiral separation and determination of amino acid enantiomers in fruit juice by open-tubular nano liquid chromatography. Chirality 30:1144–1149
Zaidi SA, Cheong WJ (2009) Long open tubular molecule imprinted polymer capillary columns with excellent separation efficiencies in chiral and nonchiral separation by capillary electrochromatography. Electrophoresis 30:1603–1607
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) (2016 R1D1A1B03930174 and 2016 M3A9E1918324).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, A., Sun, G., Kim, J.S. et al. Preparation and Evaluation of 2 m Long Open Tubular Capillary Columns of 50 μm Internal Diameter for Separation of Peptides in Liquid Chromatography. Chromatographia 84, 257–266 (2021). https://doi.org/10.1007/s10337-020-04003-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-04003-w