Abstract
The effects of furoic acid and furfuryl alcohol on the detection of furfural in transformer oil were studied using the traditional liquid–liquid extraction combined with high-performance liquid chromatography (LLE-HPLC) technology, in which, the methanol used as extraction agent and the C18 column and ultraviolet−visible detector were used as the separation and detection units for the determination of the furfural. The results show that the presence of furfuryl alcohol or furoic acid increases the extraction efficiency of furfural from 42.07 to 73.21% and 65.33%, respectively. The extraction efficiency of furfural decreases when the concentrations of furfuryl alcohol increase from 0.2 to 0.6 mg L−1, but the extraction efficiency of furfural increases with the concentrations of furoic acid from 0.2 to 0.6 mg L−1. This indicated that the accuracy of detection and extraction efficiency of furfural in transformer oil by LLE-HPLC technology may be affected by the furfuryl alcohol or furoic acid dissolved in transformer oil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ageing of the oil–paper directly affects the safe and stable operation of the transformer and; hence, research on the evaluation of ageing conditions is beneficial and is significant to ensure the safety of power systems [1]. Both China’s power industrial standards and the US IEC standards regard the degree of polymerization (DP) value of the insulation paper as the best indicator of the ageing condition of transformers [2]. It has been shown that the logarithm of the concentration of furfural during the ageing process is approximately linear with the DP of insulating paper [3]. Therefore, the evaluation of insulation ageing condition is usually achieved by detecting the content of furfural dissolved in transformer oil [4,5,6].
High-performance liquid chromatography (HPLC) as the most acceptable detection technique for furfural has been widely used in electric power industry [2, 7, 8]. In this method, the key to the determination of furfural in transformer oil is the separation and enrichment of target analytes from oil matrix using the liquid–liquid extraction (LLE) technology with the advantages of simple operation and low-solvent consumption. However, the degradation products of oil–paper insulation are very complex and more components have been gradually discovered by researchers, such as alcohol (methanol, ethanol, phenol, isopropyl alcohol, and furfuryl alcohol), aldehyde ketone (acetone, methyl ethyl ketone, and furfural), organic acid (carboxylic acid and furoic acid) and ester (methyl acetate and methyl formate) [9, 10]. It has been confirmed that furfural, furoic acid, and furfuryl alcohol are major degradation products of insulation paper [11]. Therefore, in the extraction process, most of the organic solvents used as extract ants can dissolve the components more or less; this will cause high-background interference and influence the extraction efficiency of the extraction agent for separation and enrichment of target matter in oil. In the past years, many studies have been conducted on the analysis and detection method of furfural in transformer oil [2, 8, 12,13,14,15,16], but the effects of other degradation products on the extraction efficiency and detection results of furfural are seldom reported.
In this work, to better apply HPLC technologies on the analysis of furfural in oil and establish a more convenient evaluation method of oil–paper insulation ageing, the liquid–liquid extraction (LLE) procedure was employed to collect the furfural from transformer oil before HPLC analysis using methanol as extract ant and the effects of furoic acid and furfuryl alcohol on the extraction efficiency and detection results of furfural are researched.
Experiments
Chemicals and Reagents
Methanol of HPLC grade was obtained from Tedia Company, Inc. (Fairfield, OH, USA). Furfural, furoic acid, and furfuryl alcohol (AR grade) were purchased from Aladdin Chemistry (Shanghai, China). Water was purified by a Millipore Direct Q3 system (Millipore Corporation, Bedford, MA, USA). The 25# transformer oil was offered by Electric Power Research Institute of Guangxi Power Grid Co., Ltd (Nanning, China).
Instrumentation
The HPLC spectra were measured on a FL 5090 HPLC (Fuli instruments, China) using a Sapphiresil C18 column (250 mm × 4.6 mm, 5 μm, Fuli instruments, China) within 10 min. The injection volume was 20 μL with LC5090 series autosampler (Fuli instruments, China) and the detections of furfural were performed on a LC5090 series dual-wavelength ultraviolet detector at wavelength of 277 nm. Mobile phase consisted of 50% methanol (solvent A) and 50% water (solvent B) and the flow rate and column temperature were 1.0 mL min−1 and 40 °C, respectively.
Preparation of Standard Solutions and Testing Samples
To prepare stock standard solution of furfural with concentration of 1000.0 mg L−1, 0.10 g (accurately weighed to 0.1 mg) of furfural was added into a 100 mL amber volumetric flask and dissolved with methanol and then, sufficient methanol was added to achieve a solution volume of 100 mL. Finally, the flask was shaken until homogeneous and clear solution was formed. Before use, standard working solutions of variable concentrations (0.25, 0.5, 1.0, 2.0 and 4.0 mg L−1) were prepared by diluting appropriate volumes of the stock solution with methanol.
Furfural (0.10 g), furoic acid (0.10 g), and furfuryl alcohol (0.10 g) was individually added into 100 mL transformer oil to obtained oil samples of 1000.0 mg L−1 and then added an appropriate amount of new transformer oil to obtain the diluted oil samples with concentration of 2.0 mg L−1.
In order to obtain oil samples containing different concentrations of furoic acid or furfuryl alcohol, in which the concentration of furfural was kept at 1.0 mg L−1, 1 mL furfural oil sample of 2.0 mg L−1 was added into a 10 mL vial with cap by transfer liquid gun, then different amount (0, 0.2, 0.4, 0.6, and 0.8 mL) of furoic acid or furfuryl alcohol diluted oil samples with concentration of 2.0 mg L−1 were also added into the vial. Finally, an appropriate amount of new transformer oil was added to keep the total volume of oil samples at 2 mL.
Extraction Procedure
The liquid–liquid extraction method was used to extract the furfural from oil with methanol. The 1.5 mL of methanol was added to the vial containing 2 mL oil samples as prepared and then the vial was capped with a vial cap and mixed thoroughly for 5 min with vortex mixer. The vial was then centrifuged for 3 min at 3600 rpm. Using a 30 mL syringe, about 1.2 mL of the extraction fluid was removed, and transferred to a 2 mL autosampler vial for analysis of HPLC. Table 1 lists the number and specifications of extraction samples taken in this study.
The extraction efficiencies of furfural were calculated using the following formula:
where φ is extraction efficiencies of furfural, Ce (mg L−1) is the content of furfural in extraction agent of methanol detected by HPLC, Co (mg L−1) is the content of furfural in transformer oil, Ve (mL) is the volume of methanol used for the extraction of furfural, and Vo (mL) is the volume of oil samples as prepared.
Results and Discussion
In order to realize the peak of furfural in the resulted chromatograms, the standard methanol solution of furfural with concentration of 4.0 mg L−1 firstly was detected by the HPLC as the reference material. The chromatogram graph as shown in Fig. 1, it can be known that the retention time of furfural is 4.3 min.
The reproducibility of the whole approach has been evaluated using the following procedure, in which the analysis of the same standard sample has been done by three different operators in six replicates. The relative standard deviation (RSD) percentages for furfural with concentration of 0.25 mg L−1 and 4.0 mg L−1 have been calculated to be 4.85 and 3.2%, respectively. This shows the good repetition and accuracy of the HPLC.
In order to obtain the calibration curve of furfural, all previously prepared standard methanol solutions of furfural were detected and according to the retention time of the peaks, the peak of furfural will be able to realize in the chromatograms. Table 2 shows the relationship between peak height and different concentrations of the chromatograms. Figure 2 represents the calibration curve of the reference furfural and a fitting equation with a correlation coefficient r = 0.996 was obtained. Using this graph and fitting equation, the concentration of furfural can be calculated from the corresponding peak height in a chromatogram obtained from analyzing an unknown sample by HPLC.
Then, the pure methanol and the extraction of fresh transformer oil were also analyzed by the HPLC spectra and the chromatogram graph as shown in Fig. 3. It can be seen that the positive and negative peaks at about 3 min are caused by methanol. In the spectrum (b) of blank transformer oil extracts, a small peak with a height of 1765 uAU appears at about 4.3 min, which confirmed the existence of trace furfural in transformer oil. According to the calibration curve of furfural (Fig. 2), the concentration of furfural is 0.0173 mg L−1, which can be ignored for the convenience of calculation.
In order to study the influence of furoic acid and furfuryl alcohol on the extraction efficiency of furfural by conventional LLE-HPLC, the transformer oil containing different concentrations of furoic acid or furfuryl alcohol were analyzed, in which, the concentration of furfural was stabilized at 1 mg L−1.
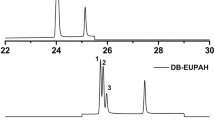
Figure 4 and Table 3 show, respectively, the chromatograms and HPLC results of extraction from oil samples numbered 0–0, 1–1, and 2–2, in which, the concentrations of furfural were kept at 1 mg L−1 and the samples 1–1 and 2–1 contain 0.2 mg L−1 of furfuryl alcohol and furoic acid, respectively. It can be seen that the peak height of furfural increases with the addition of furfuryl alcohol or furoic acid, this indicates that the presence of furfuryl alcohol or furoic acid increases the concentration of furfural in methanol extract and improves the extraction efficiency. Obviously, the analysis results of Table 3 show that the extraction efficiency reaches 73.21% and 65.33%, respectively, in the presence of furfuryl alcohol or furoic acid, while only 42.07% in the absence of furfuryl alcohol or furoic acid. In addition, as can be seen from Fig. 4, the new peaks appearing at about 2.0 and 2.5 min of retention time may be attributed to the peaks of furoic acid and furfuryl alcohol, respectively, which has been reported that the peak of furoic acid appears earlier than peak of furfuryl alcohol, which appears earlier than the peak of furfural [8, 12,13,14].
The effects of furfuryl alcohol concentration on the extraction efficiency of furfural were also carried out in this study, in which the concentration range of furfuryl alcohol is from 0.2 to 0.8 mg L−1. As can be seen from Fig. 5, four chromatographic curves are very similar, in which the retention time of all peaks is almost unchanged. However, as can be seen from Table 4, the peak height of furfural first decreases slightly with the increase in the concentration of furfuryl alcohol and then increases slightly with the increase in the furfuryl alcohol concentration to 0.8 mg L−1. A possible reason is that furfuryl alcohol has good solubility for furfural and the furfural is more easily extracted into methanol when the furfuryl alcohol dissolves into methanol. Therefore, the presence of furfuryl alcohol promotes the extraction ability of methanol to furfural, which can be confirmed from the extraction efficiency calculated in Table 4. Meanwhile, there is a greater interaction between furfuryl alcohol and methanol, which may inhibit the dissolution rate of furfural in methanol and leads to the formation of competition between furfuryl alcohol and furfural. This is why the extraction efficiency of furfural decreases with the increase in concentration of furfuryl alcohol.
Similarly, the concentrations of furfural in oil were kept constant, while the concentration of furoic acid was varied. The chromatographic curves of extraction from oil with different concentration of furoic acid are shown in Fig. 6 and the analysis results and extraction efficiency of furfural by LLE-HPLC method are listed in Table 5. It was shown that the peak height of furfural first increases slightly with increase in the concentration of furoic acid and then decreases slightly with the increase in the furoic acid concentration to 0.8 mg L−1. This might be due to the fact that the interaction between furoic acid and furfural makes furfural easier to be extracted into methanol, but when the amount of furfuryl acid is higher, the furoic acid dissolves into methanol with a greater tendency and inhibit the dissolution rate of furfural. On the other hand, the existence of this interaction also promotes the extraction ability of methanol to furfural, which can be confirmed from the extraction efficiency calculated with a more than 60% value.
Conclusion
The traditional LLE-HPLC technology has been widely used in the separation and analysis of various organic compounds and also is used as a China’s power industrial standard to detect furfural in transformer oil for the evaluation of the ageing of transformers. However, the influence of various organic matters in oil on the extraction and detection of furfur by LLE-HPLC is still unclear, which makes it difficult to correctly evaluate the operation status of transformers. In this work, the effects of furoic acid and furfuryl alcohol on the extraction of furfur dissolved in transformer oil has been study, the results indicated that the presence of furfuryl alcohol or furoic acid increases the extraction efficiency of furfural from 42.07 to 73.21% and 65.33%, respectively, and the extraction efficiency ( or peak height) of furfural first decreases slightly with increase in the concentration of furfuryl alcohol and then increases slightly with the increase in the furfuryl alcohol concentration to 0.8 mg L−1. However, it is in contrast to the trend of the concentration change of furoic acid. Therefore, it can be seen from the results that the accuracy of detection of furfural in transformer oil by LLE-HPLC technology may be affected by the various and complex degradation products of oil–paper insulation.
References
Chen W, Zou J, Wan F, Fan Z, Yang D (2018) AIP Adv 8:035204–35211
Wang Y, Li H, Yang Z, Zhang W, Hua J (2017) J Sep Sci 40:4805–4812
Shroff DH, Stannett AW (1985) IEE Proc Gener Transm Distrib 132:312–319
Scheirs J, Camino G, Avidano M, Tumiatti W (1998) J Appl Polym Sci 69:2541–2547
Hohlein I, Kachler AJ (2005) IEEE Electr Insul M 21:20–24
Okabe S, Ueta G, Tsuboi T (2013) IEEE Trans Dielectr Electr Insul 20:346–355
Emsley AM, Xiao X, Heywood RJ, Ali M (2000) IEE Proc Sci Meas Technol 147:110–114
Lin M, Lin K, Lin A, Gras R, Luong J (2016) J Sep Sci 39:2777–2784
Kohtoh M, Ueta G, Okabe S, Amimoto T (2010) IEEE Trans Dielectr Electr Insul 17:808–818
Okabe S, Kaneko S, Kohtoh M, Amimoto T (2010) IEEE Trans Dielectr Electr Insul 17:302–311
Hill DJT, Le TT, Darveniza M, Saha T (1996) Polym Degrad Stabil 51:211–218
Hong SW, Han HE, Chae KS (1981) J Liq Chromatogr 4:285–292
Jiang J, Shi Q, Chen H (2005) Se Pu 23:110–111
Hai-Yan LI, Wei-Jie Z, Meng HE, Ze-Yu HE (2016) J Instrum Anal 35:311–316
Li J, Xu Y, Zhang M, Wang D (2017) Energy Fuel 31:13769–13774
Shiri F, Hashemi B, Sobhani S (2017) J Anal Chem 72:671–677
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Zhang, Y., Shang, M. et al. The Effects of Furoic Acid and Furfuryl Alcohol on Detection of Furfural in Transformer Oil by High-Performance Liquid Chromatography. Chromatographia 83, 927–932 (2020). https://doi.org/10.1007/s10337-020-03916-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03916-w