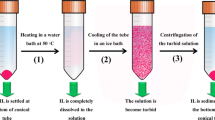
Abstract
The traditional optimization of extraction for sample pre-treatment usually involves only the single-factor experimental design to obtain the best conditions of performance. However, in optimization, only employing the single-factor experimental design becomes less frequent as it ignores the interaction among various factors. To identify the effective or critical factors and interactions among them in a sample pre-treatment, the experimental design should be conducted by the design of experiments (DoE). In this study, lithium bis(trifluoromethanesulfonyl)imide (LiNTf2)-enhanced dual microextraction method, a new type of ionic liquid-based dispersive liquid–liquid microextraction (IL-DLLME) was applied for the simultaneous enrichment and extraction of phthalate (PAE) and metabolites showing weak to moderate polarity in human urine. The conditions were optimized by the single-factor experimental design, full factorial design, and central composite design (CCD) based on DoE methodologies. Under optimal conditions, the proposed method performed high recoveries (95.19–98.3%) and low LODs (0.14–0.46 μg L−1) in the range of 2–500 μg L−1 for six PAE and metabolites in human urine. Extending from this study, this method has excellent potential for the enrichment and extraction of chemical compounds ranging from weak to moderate polarity in environmental and biological matrices as well. Besides, for the optimization of sample pre-treatment, DoE is necessary and vital in analytical chemistry and clinical laboratories to obtain the interactions between various factors instead of using only a single-factor experiment.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In analytical chemistry and clinical laboratories, although more and more advanced types of equipment are used for the analysis, the direct quantification of specific compounds in the complex environmental and biological matrices is still conditionally restricted, wherein the first step usually involves the pre-treatment of samples. For example, solid-phase extraction (SPE) [1], solid-phase microextraction (SPME) [2], liquid-phase microextraction (LPME) [3], and dispersive liquid–liquid microextraction (DLLME) [4] have been applied for the pre-treatment of compounds in different sample matrices. In analytical chemistry and clinical laboratories, it is essential to obtain the optimal conditions of pre-treatment as they ensure accurate quantification, besides saving time and cost. It has gradually become a consensus that the optimization of performance conditions is one of the most critical stages in sample pre-treatment procedure [5].
Effective scientific ways assist in analyzing and optimizing the parameters of various factors to obtain the most useful solution and understand the interactions of various factors on the response (for example, the extraction efficiency). For sample pre-treatment, the traditional optimization usually involves only the single-factor experimental design, which leads to obtaining the best conditions of performance and extraction. A single-factor experiment refers to only one factor to be studied in a particular experiment, or it focuses on the effect of one factor on the response at a time. However, it should be noted that the single-factor experiment does not mean that there is only one factor affecting the experimental results. Owing to this, currently in the method optimization, only using the single-factor experimental design is less frequent as it ignores the interactions among various factors, and there is no correlation between factors by default. In a sample preparation procedure, to identify the effective or critical factors and the interactions among them, the experimental design should be conducted following the design of experiment (DoE) methodology, which is one of the most common ways to design the experiments and optimize the experimental conditions. The methodology for DoE was first proposed by Fisher [6], in which the central idea was to identify key factors using different statistical models (depending on the type of problem) and exclude uncontrollable variables by randomization and replication of the experiments. The applied models include fractional factorial, full factorial, orthogonal array, Plackett–Burman, Box–Behnken, central composite, D-optimal designs, and Doehlert [5]. In general, the DoE operation consists of three steps. First, the factors that may affect the response should be selected based on the general information and previous research and then giving a reasonable and approximate range of values to these factors according to single-factor experimental design. The second step involves finding out the primary factors by full factorial or other appropriate models and excluding uncontrollable variables by randomization and replication of the experiments. Then, central composite design (CCD) or other appropriate design should be selected to set a series of experiments and finally create an appropriate model according to the mathematical relationship between the factors and response. Following this, the main factors are screened out, and finally, an appropriate model is created. The whole process is time-saving, the runs are reduced significantly and more effective, and the most important is that the interactions between various factors could be illustrated by mathematical formulae or intuitive plots. Thus, DoE is a scientific and efficient strategy that should be widely promoted and used in analytical chemistry and clinical laboratories.
In recent years, more attention is being paid to the potential lifetime risks of chronic exposure to phthalate (PAE), which leads to an increase in the development of quantitative methods for PAE and its biological metabolites. For example, SPE, SPME, LPME, and DLLME, coupled with high-performance liquid chromatography (HPLC), mass spectroscopy, and gas chromatography–mass spectroscopy (GC–MS) have been developed [1,2,3,4].
Phthalate consists of a group of chemicals that are widely used as plasticizers in various types of plastic products, such as intravenous tubing and bag, food wrapping bag, wallpaper, and decoration materials [7, 8], and also found in facial cleanser and cosmetics [9]. A correlation between PAE metabolites and diabetes has been shown in recent studies [8, 10, 11]. Despite the development of several methods for the pre-treatment of PAE and metabolites in urine, these methods display few disadvantages such as, tedious processing, time-consuming, requirements of organic solvents, difficulties in the simultaneous quantification of PAE and its metabolites due to the variation in the polarity of the compounds, and the requirement of derivatization steps for GC–MS analysis [12].
Dispersive liquid–liquid microextraction was first proposed by Rezaee et al. [13] to detect organic compounds in aqueous samples. Ionic liquid, known as “green solvent” [14], has been widely used in ionic liquid-based DLLME (IL-DLLME) for the microextraction of various compounds in different matrices [15,16,17]. Till now, at least three types of IL-DLLME have been developed, for example, in a traditional IL-DLLME where organic solvent acts as a dispersing agent [15, 16]; in an in situ IL-DLLME [17] there is simultaneous accomplishment of metathesis reaction and extraction [18]; and “dual microextraction” which was first proposed for the determination of three types of PAE metabolites in urine [12]. Compared to other two types of IL-DLLME, “dual microextraction” gives higher extraction recovery for weak to moderately polar compounds and avoids unnecessary losses of moderately polar compounds in the urine, but does not detect their parent compounds. In this study, “LiNTf2-enhanced, dual microextraction” method has been developed for the determination of PAE metabolites and their parent compounds that include simultaneous detection of six compounds. In this method, 3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide-based ILs ([CnMIM]NTf2) were selected as extractant, 1-butyl-3-methylimidazolium tetrafluoroborate ([C4MIM]BF4) was selected as dispersing agent, and lithium bis(trifluoromethanesulfonyl)imide (LiNTf2) was selected to react with [C4MIM]BF4 to accomplish dual extraction.
The objective of this study is to demonstrate how the sample pre-treatment for PAE and metabolites having different polarity could be optimized according to DoE methodology. In this paper, the optimization starts from the single-factor experiment, then an appropriate screening design was selected and discussed, and lastly, optimization was conducted and analyzed by central composite design [19, 20] (CCD) based on response surface methodology (RSM).
Experimental
Reagents
Bis(2-ethylhexyl) phthalate (DEHP), benzylbutyl phthalate (BBzP) and diisobutyl phthalate (DiBP), monoethylhexyl phthalate (MEHP), monobenzyl phthalate (MBzP), and monoisobutyl phthalate (MiBP) were obtained from AccuStandard Inc. (New Haven, USA), and their purities were > 99.8%. Methanol, acetone, acetonitrile, acetic acid, and HCl were received from Sigma-Aldrich (Shanghai, China). Ultrapure water (18.20 MΩ cm, 25 °C) was obtained from a Milli-Q water system (Millipore, Billerica, MA, USA). Ionic liquids of 1-butyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide ([C4MIM]NTf2), 1-hexyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide ([C6MIM]NTf2), 1-octyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide ([C8MIM]NTf2), 1-butyl-3-methylimidazolium tetrafluoroborate ([C4MIM]BF4), and lithium bis(trifluoromethanesulfonyl)imide (LiNTf2) were purchased from Shanghai Chengjie Chemical Co., Ltd. (Shanghai, China).
HPLC and Other Equipment
An Accela HPLC system (Thermo Fish Scientific™, Waltham, MA, USA) with Accela PDA 80 HZ detector, Accela auto sampler, and 1250 pump were used for chromatographic analysis. A Zorbax Eclipse XDB-C18 column (5 μm, 4.6 mm × 250 mm) (Agilent, Santa Clara, CA, USA) was used as a stationary phase. A gradient mode was set for the mobile phase, which consists of acetonitrile (phase A) and water (phase B), both contain 1% (v/v) acetic acid. The initial condition was 25% A and 75% B for 4 min; phase A increased linearly to 55% A in the first 10 min and then increased linearly to 75% A in another 6 min, then increased linearly to 100% A in 2 min and kept for 10 min, then returned to initial condition in 2 min. The flow rate was 0.8 mL min−1, the column temperature was set at 30 °C, and the detector wavelength was set at 228 nm. An FE28 acidity meter (Mettler Toledo, Switzerland) was used for pH measurements. An LP vortex mixer and ST40R centrifuge (Thermo Scientific™, Waltham, MA, USA) were used for blending the solutions and centrifugation, respectively. A BSA224S precision electronic balance (Sartorius, Goettingen, Germany) was used for measuring the salt precisely. A KQ-500B ultrasonic cleaner (ultrasonic water bath) was obtained from Guangzhou Huruiming Instrument Co., Ltd. (Guangdong, China).
Standard Solutions and Samples
A standard stock solution of each compound was prepared at 100 mg L−1 in acetonitrile and stored at − 20 °C. Working standard solutions were prepared daily at different concentrations using the standard stock solution diluted with acetonitrile. The urine samples were collected in the morning from volunteers without any renal dysfunction from the Department of Clinical Laboratory, Shanghai East Hospital, School of Medicine, Tong Ji University (Shanghai, China). They were collected in 15 mL centrifugal tubes after filtering through a filter of 0.22 μm, and then stored at − 20 °C for further analysis.
Extraction Procedure
The extraction procedure was developed and modified based on our earlier report [12]. The urine samples were thawed at 25 °C for 1 h and centrifuged at 3000 rpm for 10 min before analysis. 1 mL of urine and 4 mL of ultrapure water were added into 10 mL screw cap glass conical tube, followed by acidification to pH 2.4–4.0 using HCl. Shortly after, [CnMIM]NTf2 (n = 4, 6, 8; volume = 20–120 μL), [C4MIM]BF4 (volume = 120–300 μL) and LiNTf2 (0.18–0.46 g) were slowly added into conical tube which immediately formed a cloudy solution. Subsequently, the tube was placed in an ultrasonic water bath at 25 °C for 2–10 min. Then, for the extraction, the tube was placed in an ice bath for 2–10 min, and the cloudy solution was then centrifuged at 3000 rpm for 10 min. Finally, the sedimented phase was collected using microliter syringe and diluted with 200 μL acetonitrile, and filtered using a filter of 0.22 μm before analysis by HPLC–PDA. All measurements were performed in triplicate.
Experimental Design
All experiments were conducted based on DoE methodologies. First, the possible operational factors that affect the extraction efficiency were selected based on the general information and previous research reports, and a reasonable and approximate range of values were given to these factors according to single-factor experimental design. Second, the main factors were found out by full factorial design, which is one of the most important designs to screen several parameters. The effective factors were determined, and their suitable levels were estimated without conducting many experiments [21,22,23]. Then, CCD was selected to set a series of experiments, and finally, an appropriate model was generated according to the mathematical relationship between the factors and response. CCD is one of the most widely used RSM containing three set of design points at five levels (− α, − 1, 0, + 1, + α) for the estimation of curvature: (1) + 1 and − 1 are the cube points representing a two-level full factorial design (Nf = 2f) that include all possible combinations between upper and lower level variables, (2) + α and − α are the axial points (Na = 2f, the α value is between 1 and the square root of the number of factors), and (3) 0 for center points (f is the number of factors) [19, 20]. In this case, the average extraction recovery of six compounds was selected as the “response” for the estimation of extraction efficiency. The recovery was computed using the following Eq. (1):
where ER (%), Csed, Co, Vsed, and Vaq are the average extraction recovery, the concentration of compound found in the sedimented phase, the initial concentration of compound in the aqueous sample, the volume of the sedimented phase, and the volume of the aqueous sample, respectively [24].
Statistical Analysis
Modeling and data processing were conducted using the software package, Design-Expert 8.0.6 (Minneapolis, USA). The data were subjected to analysis of variance (ANOVA), considering significant differences at p ≤ 0.05.
Results and Discussion
Single-Factor Experiment
Based on our previous experiments, the following factors were determined which potentially affect ER (%): type and volume of extractant, sonication time, time of extraction, pH, type of dispersing agent, the volume of the dispersing agent, and the ion-exchange reagent. The effect of one factor on ER (%) was evaluated by experiments conducted under the condition of holding other factors at an appropriate level. This approach is useful for screening factors in the initial steps of the optimization process.
Selection of Extractant
In this study, [CnMIM]NTf2 (n = 4, 6, 8) was selected as the extractant based on their low solubility in water, higher density than water, and good chromatographic behavior. PAE metabolites are relatively more polar than their respective parent compounds [12]. As shown in Fig. 1a, the ER (%) of MiBP and MBzP (< 55%) was significantly lower than that of MEHP and their parent compounds (ca. 80%) using conventional DLLME (the extraction recovery of the moderately polar compound was not enhanced by the addition of LiNTf2). As for the type of IL-based extractant, [C8MIM]NTf2 increased the ER (%) by 30% as compared to [C6MIM]NTf2 and [C4MIM]NTf2. As a result, [C8MIM]NTf2 was selected as the extractant in this study. Figure 1b shows the effect of different volumes of [C8MIM]NTf2 on ER (%), and for further studies, 30 μL was found to be optimum.
Effect of single-factor experiment on extraction recovery. a Effects of different extractant on extraction recoveries. Conditions: 5.0 mL water sample, pH of 3, 8 min sonication time, 30 μL extractant [C8MIM]NTf2, 50 μL [C6MIM]NTf2 and 120 μL [C4MIM]NTf2, 200 μL [C4MIM]BF4 (without addition of LiNTF2), and 8 min extraction time. MEHP, DiBP, BBzP and DEHP are weak polar compounds, they have higher solubility in hydrophobic IL (hydrophobicity: [C8MIM]NTf2 > [C6MIM]NTf2 > [C4MIM]NTf2). b Effects of different volumes of extractant on extraction recoveries. Conditions: 5.0 mL water sample, pH 3, 8 min sonication time, extractant was [C8MIM]NTf2, 200 μL [C4MIM]BF4 (without addition of LiNTF2), 8 min extraction time. The ER (%) of each compound increases slowly by increasing the volume of [C8MIM]NTf2 from 20 to 30 μL, but it is not obvious when increasing the volume of [C8MIM]NTf2 from 35 to 40 μL. c Effects of the different disperser on extraction recoveries. Conditions: 5.0 mL water sample, pH 3, 8 min sonication time, 30μL [C8MIM]NTf2, 500 μL organic disperser solvent, and 200 μL [C4MIM]BF4 (without addition of LiNTF2), 8 min extraction time. The ER (%) of six compounds by organic solvent-based dispersing agents was comparable with that of [C4MIM]BF4, but for moderately polar compounds (MiBP and MBzP), none of them could increase the ER (%) to > 60%. d Effects of the different volume of disperser on extraction recoveries. Conditions: 5.0 mL water sample, pH 3, 8 min sonication time, 30 μL [C8MIM]NTf2, [C4MIM]BF4 was disperser (without addition of LiNTF2), 8 min extraction time. The ER (%) of six compounds increased gradually with an increase in the volume of dispersing agent from 120 to 300 μL. e Effects of ion-exchange reagent LiNTF2 on extraction recoveries. Conditions: 5.0 mL water sample, 30 μL [C8MIM]NTf2, 200 μL [C4MIM]BF4 (with or without addition of LiNTF2), 8 min sonication time and 8 min extraction time. LiNTf2 (+) and LiNTf2 (−) indicate with and without addition of LiNTf2, respectively. The in situ DLLME indicates microextraction performed only by [C4MIM]BF4 and LiNTf2. The ER (%) of all the six compounds is less than 55% by the in situ DLLME, and a higher ER (%) (81–85%) was obtained for four weak polar compounds (MEHP, DiBP, BBzP and DEHP) but not for the rest (MiBP and MBzP, both < 55%) by conventional IL-DLLME (LiNTf2 (−)). However, the ER (%) of all the six compounds is > 90% by the dual microextraction (LiNTf2 (+)) that consists of conventional IL-DLLME and in situ DLLME
Selection of Dispersing Agent
Organic solvents such as methanol, acetonitrile, and acetone are the traditional dispersing agents for the extraction of chemicals. Since it has a high solubility (18.8 mg mL−1) in the aqueous, [C4MIM]BF4 can be a dispersing agent as well [12]. As shown in Fig. 1c, the ER (%) of organic solvents was comparable with that of [C4MIM]BF4, but a higher extraction efficiency (> 90%) can be obtained for moderately polar compounds (MiBP and MBzP) by dual microextraction (Fig. 1e) which was performed by the addition of LiNTf2 after the conventional DLLME. Besides, [C4MIM]BF4 was environmental friendly compared to organic solvents. As a result, [C4MIM]BF4 was selected as the dispersing agent.
Effects of the Volume of Dispersing Agent and Ion-Exchange Reagent
Figure 1d, e show the effects of the volume of dispersing agent and amount of ion-exchange reagent on ER (%), respectively. The ER (%) of six compounds increased gradually with an increase in the volume of dispersing agent from 120 to 300 μL. When LiNTf2 was added, the extraction recoveries of MiBP and MBzP were much higher than those without any addition of LiNTf2 (conventional IL-DLLME) in Fig. 1e (LiNTf2 (+) and (−) indicate with and without addition of LiNTf2, respectively). It has a dual identity that water-miscible [C4MIM]BF4 played not only as dispersing agent but as an extractant in the case of sample pre-treatment. An in situ DLLME was performed between LiNTf2 and [C4MIM]BF4 when LiNTf2 was added (the molar ratio of LiNTf2 to [C4MIM]BF4 is 1:1), and as a result [C4MIM]NTf2 was formed, where the extractant can be separated from the aqueous phase, resulting in a significant increase in the ER (%) of MiBP and MBzP from < 55% to > 90% in the dual microextraction method. However, when compared with the conventional IL-DLLME (without addition of LiNTf2) and dual microextraction method, the ER (%) of each compound obtained by the in situ DLLME method was less than 55%. Generally, ER (%) shows a positive correlation with the ratio of dispersing agent to extractant (Ra) (in this case the volume of extractant was 30 μL). Thus, dual microextraction was accomplished rapidly with [C8MIM]NTf2, [C4MIM]BF4, and LiNTf2, and the sedimented phase was separated in one step instead of two steps in our previous method [12], making it more controllable with time- and cost-saving.
Effects of Sonication Time, Extraction Time, and pH
Supplementary Fig. 2a–c illustrate the effects of sonication time, extraction time, and pH on ER (%) of six compounds. The ER (%) increased gradually from 2 to 10 min of sonication and extraction times, respectively. The effects of pH on ER (%) could be complex, and the possible optimal conditions were in the range of pH 2.7–3.4.
Full Factorial Design
The results from single-factor experiment demonstrated that four main factors (with their acronyms and appropriate level range indicated in parentheses) had the largest effects on the extraction recoveries: ultrasonication time (Ut, 3–8 min), extraction time (Et, 3–8 min), the volume ratio of dispersing agent to extractant (Ra, 4–10, with the same mole of LiNTf2 with [C4MIM]BF4) and pH (P, 2.5–3.6). Then, the two-level full factorial design, including 24 runs were applied for the estimation of the effects of each factor. The design matrix (coded values of variables) and responses (average extraction recovery of six compounds) for the full factorial (24) design contained 16 runs as summarized in Supplementary Table 1, and the experimental sequence was random. The statistical data were analyzed by ANOVA (Supplementary Table 2). The “Model F value” of 844.31 (p value of 0.0012) implied that the model is significant, and the values of “Prob > F” (p values) less than 0.05 indicated that the model terms are significant. In this case, A (Ut), B (Et), C (Ra), and D (P) are significant model terms. The p value of B, C, and D was all less than 0.01, but the p value of A was 0.0220, indicating that B, C, and D were the most important factors compared with A. The normal plot, half-normal plot, and Pareto chart have been displayed in Fig. 2. For further study of the interactions between various factors and to determine if the model contains squared factors, CCD was recommended.
Central Composite Design (CCD)
Interactions among the four factors were analyzed by CCD, and the followed protocol has been shown in the Supplementary material. Independent variables, their acronyms, and levels by CCD are shown in Supplementary Table 3. The design matrix and the responses by CCD have been displayed in Supplementary Table 4. A quadratic function model was applied for regression analysis (Supplementary Table 5), and the factors “AD” and “BD” in Table 1 with lower F value (p value of > 0.05) which indicate these two factors were not significant and could not be removed from this model because of the so-called "Model hierarchy" principle or rule [23]. This model is shown in Eq. (2) in terms of coded values. The model F value of 183.59 (p < 0.0001) implies that the model is extremely significant, and the lack of fit F value of 2.59 (p > 0.2347) signifies that the lack of fit is not significant, and non-significant lack of fit is good. The adjusted coefficient of determination (R2) of 0.9895 was used to evaluate the goodness of fit for this model which elucidates 98.95% of the variance indicating a strong model that fits with the data. In this case, A, B, C, D, AB, AC, BC, CD, A2, B2, C2, and D2 were significant model terms, while AD and BD were not significant model terms. As can be seen in Eq. (2), there were four main effects (A, B, C, and D), six two-factor interaction effects (AB, AC, BC, CD were significant model terms, AD and BD were not significant model terms) and four curvature effects (A2, B2, C2, and D2), where Y is the average extraction recovery of six compounds, b0 is the intercept, and b1 to b14 are the parameter coefficients:
with b0 = 85.08; b1 = 4.22; b2 = 3.55; b3 = 14.95; b4 = − 5.07; b5 = 1.66; b6 = − 2.14; b7 = 0.78; b8 = 1.24; b9 = -0.88; b10 = 1.19; b11 = − 1.44; b12 = − 4.29; b13 = − 6.17; b14 = − 9.09.
To test the prediction ability of this model, four more independent experiments were carried out (1.3 Model validation in Supplementary materials). As shown in Supplementary Table 6, bias between result of model prediction and outcome of experiment was − 1.21 to 4.92%, showing a strong correlation between them.
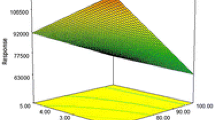
The model graphs of 3D response surfaces and contour lines are shown in Fig. 3, and two factors were investigated in each plot according to the criteria that two of them varied in the range while holding other two at their central levels [25]. The maximum average extraction recovery of each plot and their trends are shown in Table 2. As a result, the optimal conditions have been suggested based on the Design-Expert software as follows: Ra of 10 (300/30, v/v, μL), 0.46 g LiNTf2, 8.00 min of sonication time, 7.43 min of extraction time, and a pH of 2.94.
I Contour lines and three-dimensional (3D) plot for extraction time and sonication time on average extraction recovery under pH 3.05 and Ra of 7; II Contour lines and 3D plot for sonication time and Ra on average extraction recovery under 5.5 min extraction time and pH 3.05; III Contour lines and 3D plot for extraction time and Ra on average extraction recovery under 5.5 min sonication time and pH 3.05. IV Contour lines and 3D plot for pH and Ra on average extraction recovery under 5.5 min sonication time and 5.5 min extraction time
Analytical Performance and Application in the Urine Sample
To evaluate the proposed LiNTf2-enhanced dual microextraction method, a series of experiments were conducted under the optimal experimental conditions for obtaining linearity, the limit of detection (LOD), the limit of quantitation (LOQ), accuracy and precision following the CLSI guidelines (EP15-A3) [26]. This method indicated excellent linearity in the range of 2–500 μg L−1, the coefficients of determination (R2) were in the range of 0.9995–0.9998, and the LODs (S/N = 3) and LOQs (S/N = 10) were in the range of 0.14–0.46 μg L−1 and 0.5–1.45 μg L−1 in the urine, respectively (Table 3).
Repeatability variance (SR) and within-laboratory variance (SWL) were calculated according to the CLSI guidelines (EP15-A3). Two quality control samples in low and high level (20 and 200 μg L−1, respectively) and two urine samples with the fortification of six standard PAE and metabolites at two levels (10 and 60 µg L−1, respectively) were repeatedly detected five times a day for 5 consecutive days, and each sample contained 25 test data. The SR was in a range of 0.69–7.31% for intra-day analysis, and SWL was in a range of 0.69–7.31%. The bias of quality control was in a range of 2.13–6.68% (Table 4). Figure 4 is a typical chromatogram for PAE and metabolites using the proposed method, and all six PAE and metabolites in the unfortified urine samples were detected. The extraction recoveries were in the range of 95.19–98.30% for the six compounds (Table 4). As a result, this optimal method is in high precision and accuracy, which has the potential to determine trace levels of PAE and metabolites in human urine.
Comparison of the Proposed Method with Other Methods for Determination of PAE and Metabolites
The proposed method was compared with other published methods, such as solid-phase extraction coupled with GC–MS (SPE-GC–MS) [1], solid-phase extraction coupled with LC–MS (SPE-LC–MS) [27, 28], solvent-based dispersive liquid–liquid microextraction coupled with GC–MS (SB-DLLME) [29], ionic liquid-based dispersive liquid–liquid microextraction coupled with HPLC (IL-DLLME-HPLC) detection [30], solidification of floating organic drop-based dispersive liquid–liquid microextraction coupled with HPLC (SFO-DLLME) detection [31] and temperature-controlled ionic liquid dispersive liquid–liquid microextraction coupled with HPLC (TC-IL-DLLME) detection [32]. As shown in Table 5, SPE and DLLME are widely used for sample pre-treatment before GC–MS, LC–MS and HPLC detection. The LOD (0.14–0.46 μg L−1) of the proposed method is much lower than that of IL-DLLME (3.3 μg L−1 for DEHP) and SB-DLLME (1 μg L−1 for DEHP), and lower than that of other DLLME methods (ca. 0.6–0.9 μg L−1) except for SPE-GC–MS (0.1 μg L−1) and SPE-LC–MS (0.02–0.2 μg L−1). However, there are several disadvantages in SPE pre-treatment, such as they require a long pre-treatment time and large volume organic solvent, GC–MS and LC–MS are more expensive than HPLC used in this investigation. In comparison, the DLLME pre-treatment takes a much shorter time and is fully compatible with HPLC detection, and only a small volume of organic solvents needs to be used as extractant or dispersing agent. More precisely, the proposed method consists of in situ reaction and conventional DLLME in one step, resulting in a dual microextraction of compounds without using organic solvents. In general, the main advantages of the proposed method are: (1) time and cost-saving, full compatibility with HPLC detection; (2) more environmental friendly and simple to use; (3) high extraction recovery obtained for both weakly and moderately polar compounds simultaneously owing to dual microextraction in one step.
Conclusions
This study developed a LiNTf2-enhanced dual microextraction method for the determination of PAE and metabolites in urine. This proposed method was integrated in situ reaction (LiNTf2 and dispersing agent) with conventional IL-based DLLME to realize dual microextraction in a short time. Consequently, the extraction efficiency was greatly improved for both weakly and moderately polar compounds. The operational conditions were optimized by DoE containing single-factor experimental design, full factorial design, and CCD. Under the optimal experimental conditions, the extraction recovery of all six PAE and metabolites was > 90%, and the LODs and LOQs were in the range of 0.14–0.46 μg L−1 and 0.5–1.45 μg L−1 in in the urine, respectively. The optimal method demonstrated high precision and accuracy according to the experiments of performance validation conducted by the CLSI guidelines (EP15-A3). As a result, LiNTf2-enhanced dual microextraction method has excellent potential for the enrichment and extraction of chemical compounds in environmental and biological matrices. Besides, DoE is a scientific and efficient strategy that should be widely promoted and used in analytical chemistry and clinical laboratories.
References
Brossa L, Marcé RM, Borrull F, Pocurull E (2002) Application of on-line solid-phase extraction-gas chromatography-mass spectrometry to the determination of endocrine disruptors in water samples. J Chromatogr A 963(1):287–294
Psillakis E, Mantzavinos D, Kalogerakis N (2004) Monitoring the sonochemical degradation of phthalate esters in water using solid-phase microextraction. Chemosphere 54(7):849–857
Polo M, Llompart M, Garcia-Jares C, Cela R (2005) Multivariate optimization of a solid-phase microextraction method for the analysis of phthalate esters in environmental waters. J Chromatogr A 1072(1):63–72
He L, Luo X, Xie H, Wang C, Jaing X, Lu K (2009) Ionic liquid-based dispersive liquid–liquid microextraction followed high-performance liquid chromatography for the determination of organophosphorus pesticides in water sample. J Chromatogr A 655(1):52–59
Leila M, Zahra T, Reza KM (2018) Applications and opportunities of experimental design for the dispersive liquid–liquid microextraction method—a review. Talanta 190:335–356
Hinkelman K, Kempthorne O (2005) Design and analysis of experiments: advanced experimental design. Wiley, Hoboken
Hauser R, Calafat AM (2005) Phthalates and human health. Occup Environ Med 62(11):806–818
Svensson K, Hernandez-Ramirez RU, Burguete-Garcia A, Cebrian ME, Calafat AM, Needham LL, Claudio L, Lopez-Carrillo L (2011) Phthalate exposure associated with self-reported diabetes among Mexican women. Environ Res 111(6):792–796
Sathyanarayana S (2008) Phthalates and children's health. Curr Probl Pediatr Adolesc Health Care 38(2):34–49. https://doi.org/10.1016/j.cppeds.2007.11.001
Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH (2007) Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect 115(6):876–882
Lind PM, Zethelius B, Lind L (2012) Circulating levels of phthalate metabolites are associated with prevalent diabetes in the elderly. Diabetes Care 35(7):1519–1524
Wu J, Ye Z, Li X, Wang X, Luo F, Sheng B, Li Y, Lyu J (2016) Optimization of a NH4PF6-enhanced, non-organic solvent, dual microextraction method for determination of phthalate metabolites in urine by high performance liquid chromatography. J Chromatogr B 1014:1–9
Rezaee M, Assadi Y, Hosseini MRM, Aghaee E, Ahmadi F, Berijani F (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116(1–2):1–9
Du FY, Xiao XH, Luo XJ, Li GK (2009) Application of ionic liquids in the microwave-assisted extraction of polyphenolic compounds from medicinal plants. Talanta 78(3):1177–1184
Liu Y, Zhao E, Zhu W, Gao H, Zhou Z (2009) Determination of four heterocyclic insecticides by ionic liquid dispersive liquid–liquid microextraction in water samples. J Chromatogr A 1216(6):885–891
Pena MT, Casais MC, Mejuto MC, Cela R (2009) Development of an ionic liquid based dispersive liquid–liquid microextraction method for the analysis of polycyclic aromatic hydrocarbons in water samples. J Chromatogr A 1216(36):6356–6364
Yao C, Anderson JL (2009) Dispersive liquid–liquid microextraction using an in situ metathesis reaction to form an ionic liquid extraction phase for the preconcentration of aromatic compounds from water. Anal Bioanal Chem 395(5):1491–1502
Sun JN, Chen J, Shi YP (2014) Multiple functional ionic liquids based dispersive liquid–liquid microextraction combined with high performance chromatography for the determination of phenolic compounds in water samples. Talanta 125(11):329–335
Ferreira SLC, Korn MGA, Ferreira HS, da Silva EGP, Araújo RGO, Souza AS, Macedo SM, Lima DDC, de Jesus RM, Amorim FAC (2007) Application of multivariate techniques in optimization of spectroanalytical methods. Appl Spectrosc Rev 42(5):475–491
Box GEP, Wilson KB (1992) On the experimental attainment of optimum conditions. Breakthroughs in statistics. Springer, New York, pp 270–310
Hibbert DB (2012) Experimental design in chromatography: a tutorial review. J Chromatogr B 910(1):2–13
Dejaegher B, Vander Heyden Y (2009) The use of experimental design in separation science. Acta Chromatogr 21(2):161–201
Montgomery DC (2017) Design and analysis of experiments. Wiley, Hoboken
Sereshti H, Heravi YE, Samadi S (2012) Optimized ultrasound-assisted emulsification microextraction for simultaneous trace multielement determination of heavy metals in real water samples by ICP-OES. Talanta 97(16):235–241
Sereshti H, Izadmanesh Y, Samadi S (2011) Optimized ultrasonic assisted extraction-dispersive liquid-liquid microextraction coupled with gas chromatography for determination of essential oil of Oliveria decumbens Vent. J Chromatogr A 1218(29):4593–4598
Neill Carey R (2014) User verification of precision and estimation of bias; Approved Guideline-Third edition: EP15-A3. CLSI. 34(12)
Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, Needham LL, Brock JW (2003) Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography–atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B 789(2):393–404
Heffernan AL, Thompson K, Eaglesham G, Vijayasarathy S, Mueller JF, Sly PD, Gomez MJ (2016) Rapid, automated online SPE-LC-QTRAP-MS/MS method for the simultaneous analysis of 14 phthalate metabolites and 5 bisphenol analogues in human urine. Talanta 151:224–233
Notardonato I, Protano C, Vitali M, Bhattacharya B, Avino P (2019) A method validation for simultaneous determination of phthalates and bisphenol a released from plastic water containers. Appl Sci 9:2945
Sha C, Yi-Sheng Z, Shui-Yuan C, Tian Q, Hao S (2011) Development of an ionic liquid-based dispersive liquid–liquid micro-extraction method for the determination of phthalate esters in water samples. J Sep Sci 34(13):1503–1507
Wu C, Li Y, Chang S, Chen Y, Chen D, Gong J, Xu L (2018) Determination of phthalates in water by dispersive liquid-liquid microextraction based on solidification of floating organic drop followed by high performance liquid chromatography. Chin J Chromatogr 36(5):452–457
Sun JN, Shi YP, Chen J (2013) Simultaneous determination of plasticizer di(2-ethylhexyl)phthalate and its metabolite in human urine by temperature controlled ionic liquid dispersive liquid–liquid microextraction combined with high performance liquid chromatography. Anal Methods 5(6):1427–1434
Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (81671599, 81601407 and 81801616) and the Health and Family Planning Committee of Pudong New Area (PKJ2015-Y18). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Thanks for the linguistic services from Edit Springs (https://www.editsprings.com/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all patients included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ye, Z., Ma, M., Guo, Y. et al. Determination of Phthalate and Metabolites in Human Urine by Lithium Bis(trifluoromethanesulfonyl)imide-Enhanced Dual Microextraction Method Optimized by Central Composite Design. Chromatographia 83, 397–408 (2020). https://doi.org/10.1007/s10337-020-03858-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-020-03858-3