Abstract
Core–shell-type polymers based on a hyperbranched (hb) poly(ethylenimine) core and a shell with a variable maltose content were applied as coating materials for fused silica capillaries. A new, simple, fast, and reproducible way of modifying the capillary walls through the physical adsorption of the core–shell-type polymers using a Cu2+ support was developed. The coating created by this method was found to be very stable compared to the coating created using a solution of the polymer only. Capillaries modified with the core–shell-type polymers were tested by applying them to the electrophoretic separation of catecholamines and proteins. The modified capillaries showed high efficiencies (up to 800,000 theoretical plates per meter for lysozyme) and separation selectivities. The highest efficiency was achieved using capillaries modified with the polymer containing the lowest content of maltose in the shell and the most accessible positively charged core. Various online concentration techniques were also tested as a means to lower detection limits further, making it possible to analyze proteins in biological fluids (saliva) as well as catecholamines in human urine after SPE using activated alumina.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Capillary electrophoresis (CE) has become a powerful separation technique, especially in the field of the analysis of biologically active compounds, due to its high efficiency, use of small sample volumes, ease of automation, and because it can be readily and advantageously coupled to MS detection. However, the analysis of molecules with amino groups (including aminoacids, catecholamines, proteins, and some heterocyclic compounds) is complicated because they can interact with silanol groups in the capillary walls through electrostatic and hydrophobic interactions and intermolecular hydrogen bonds. Well-known and effective methods of overcoming this disadvantage of CE are covalent modification as well as the formation of physically adsorbed coatings on the fused silica capillary walls that block access to silanol groups and thus prevent the sorption of solutes.
These coatings can be developed based on small molecules (silanes, methyl methacrylates with amino and ammonium groups, cationic lipid vesicles) or polymers (HMW polyvinyl alcohols, methylcellulose, polyacrylamide, etc.) [1,2,3,4,5,6,7].
The application of uncharged polymers as coating materials enables electroosmotic flow (EOF) to be suppressed, which in turn increases the analysis time [8]. The development of new coatings for capillary walls for use in CE remains an active area of research; for instance, there were some publications in 2016 [9,10,11] focusing on the possibility of using LMW compounds such as fullerenes, cyclodextrins, and HMW polycationic compounds in coatings.
The stability of covalent coatings is limited by the stability of covalent bonds in acidic and alkaline media. Physically adsorbed coatings are good alternatives to covalent coatings due to their simplicity and because the procedure involved in modifying the capillary walls is relatively rapid. Moreover, such coatings are compatible with MS detection, whereas the surfactants and other additives used in dynamic modification suppress sample ionization and contaminate the ion source in MS detection [12].
The compounds most commonly used to develop physically adsorbed coatings include polycationic polymers that contain a large number of positively charged groups and carbohydrates, as well as polymers such as polyethylenimine, chitosan, polyarginine, Polybrene, and polydiallyldimethylammonium chloride [13,14,15].
Applying a spermine-dextran copolymer as a dynamic coating of the walls of capillaries used for the separation of neurotransmitters led to a considerable increase in analyte separation efficiency and selectivity [16]. Dendritic polymers, which have a micelle-like globular structure and a high terminal group content, are particularly promising materials for developing stable coatings of fused silica capillary walls [17]. They exhibit low viscosity in solution compared to the corresponding linear polymers. Examples of dendritic polymers that have been used in coatings include polyamidoamine, cyclodextrin-derived, and carbosilane dendrimers [18,19,20]. Hyperbranched (hb) polymers possess the same properties as dendritic molecules; however, unlike dendrimers, they are easy to synthesize. Most of the coatings that are described in the literature as using hb polymers were created by simply rapidly rinsing the capillary with solutions of the modifying substances at appropriate concentrations.
The work reported in the present paper focused on the application of hyperbranched “core–shell” polymers consisting of a hyperbranched polyethyleneimine (PEI) core with a mass of 5 kDa and a terminal oligosaccharide shell containing varying contents of maltose (Mal). Three polymers, A–C, were considered with shell maltose contents of 70, 32, and 16%, respectively (Fig. 1). Polymer A predominantly contained tertiary amino groups, while polymers B and C mainly contained secondary and primary groups, respectively. The syntheses of these polymers are described in [21].
Hb polymers based on polyethyleneimine (PEI) surrounded by a maltose shell [20]
These polymers are polyelectrolytes; they have a positively charged polyethylenimine core as well as a high density of terminal groups. The isoelectric point, solubility, and surface properties of PEI-Mal vary depending on the degree of substitution of the terminal amino groups by maltose residues. Polymer structure C possesses an open maltose shell and a high isoelectric point (pI 9.4), whereas structures A and B have denser maltose shells and lower isoelectric points (pI 8.1 and 9.1, respectively). Thus, PEI-Mal is a prospective candidate for the development of non-covalently bonded, physically adsorbed coatings that can be applied to the walls of capillaries used in electrophoresis.
In contrast to our previous research in which some hb polymers were used to develop dynamic and covalent coatings [22], the work discussed here focused on the formation of non-covalently bonded, physically adsorbed coatings in CE. These coatings should possess better stability than dynamic coatings, and should be much easier and faster to develop than covalent coatings.
Materials and Methods
Reagents and Solutions
Copper(II) sulfate, boric acid, ammonium acetate, sodium hydroxide, disodium hydrogen phosphate, monobasic sodium phosphate, dimethylformamide (DMFA), concentrated hydrochloric acid, phosphoric acid, and acetic acid as well as dopamine (DA), epinephrine (E), normetanephrine (NM), norepinephrine (NE), 3,4-dihydroxybenzylamine hydrobromide (DHBA), human serum albumin (pI 4.7, M 66241 g/mol), lysozyme from chicken egg white (pI 11.0, M 14300 g/mol), myoglobin from equine skeletal muscle (pI 7.0, M 17800 g/mol), and transferrin from human blood plasma (pI 5.8, M 79550 g/mol) were purchased from Sigma–Aldrich (analytical grade; St. Louis, MO, USA). 1.0 mg mL−1 stock solutions of catecholamines were prepared with 0.1 M hydrochloric acid, and 4.0 mg mL−1 stock solutions of proteins were prepared with deionized water. The solutions of proteins and cathecolamines were diluted before use to final concentrations of 1 mg mL−1 and 25 μg mL−1, respectively.
Instrumentation
Instrumentation included a capillary electrophoresis system (Capel-105 M, Lumex, St. Petersburg, Russia) equipped with an UV-spectrophotometric detector (wavelength range 190–360 nm), a laboratory pH meter (Seven Easy, METTLER TOLEDO, Greifensee, Switzerland), and an analytical balance (Pioneer PA214C, OHAUS, Parsippany, NJ, USA; e = 1 mg, d = 0.1 mg). Fused-silica capillaries with an external polyimide coating (Polymicro Technologies, Phoenix, AZ, USA; id 50 µm, od 360 µm, effective length 50 cm, total length 60 cm) were also used.
Coating Development
Before the polymer coating was prepared, the capillary was rinsed (for 30 min) with 1.0 M HCl, water, and (for 2 h) with 2 M NaOH to pre-activate the fused-silica surfaces of the capillary walls. Hyperbranched core–shell polymers with different degrees of substitution by maltose were tested as coatings for CE. To this end, the capillary was flushed with a solution of 10, 20, 30, 40, or 50 mg mL−1 of PEI-Mal (structure A, B, or C) dissolved in 20 mM phosphate buffer solution (pH 7), 50 mM acetate buffer solution (pH 4), and 5 mM NaOH (pH 12) for 10, 30, or 60 min. After flushing with polymer solution, the capillary was rinsed with water for 10 min, and then with the corresponding background electrolyte (BGE). The stability of the developed coating was evaluated based on the EOF. DMFA dissolved in the corresponding BGE was used as an EOF marker.
Cu2+-supported coatings were developed by flushing the capillary with PEI-Mal–Cu2+ complexes for 30 min. Solutions of PEI-Mal–Cu2+ were prepared by dissolving 300 mg of the polymer [A (28.6 kDa), B (21.5 kDa), C (13.5 kDa)] in 0.5, 1.5, 2.4, 2.5, 2.6, or 3.0 mM CuSO4 solution. After modification, the resulting capillaries were flushed with water and the appropriate BGE to remove excess hb polymer. The EOF migration time was evaluated to confirm capillary modification and assess the stability of the developed coating.
Electrophoretic Separation Conditions
The analysis conditions were as follows: the water-bath temperature control for the capillary was set at 20 °C; hydrodynamic sample injection at 30 mbar × 2 s; UV detection (210 nm for proteins, 254 nm for catecholamines); voltage at +20 kV, −20 kV. Before each injection, the capillary was flushed with BGE for 3 min. The results were processed with the Elforan software package.
Electrophoretic separation of catecholamines was carried out in a 50 mM acetate buffer solution (0.192 g NH4Ac dissolved in 40 mL of deionized water and adjusted to pH 4 with acetic acid), detection was performed at 254 nm, and hydrodynamic and electrokinetic sample injection were implemented, as described in “Online Preconcentration of Catecholamines with Modified Capillaries.” Protein separations were carried out in 100 mM phosphate buffer solution (0.59 g NaH2PO4 dissolved in 50 mL of deionized water and adjusted to pH 2 with phosphoric acid).
Online Preconcentration of Catecholamines with Modified Capillaries
All analyses were performed at normal polarity with polymer-modified capillaries.
Field-Amplified Sample Stacking (FASS)
Catecholamines were dissolved in 0.001 M HCl solution and diluted with water to 25 μg mL−1. Then the sample was hydrodynamically (30 mbar × 2 s) injected into a capillary filled with 50 mM acetate buffer solution (pH 4).
Head-Column Field-Amplified Sample Stacking (HC FASS)
A water plug followed by the sample dissolved in 0.001 M HCl were hydrodynamically injected into a capillary filled with 50 mM acetate buffer solution. The conditions for sample injection were 30 mbar × 2 s and the duration of water plug injection was varied (30 mbar × 5, 30, 60, 90, 100, and 110 s).
Large-Volume Sample Stacking (LVSS)
The sample was dissolved in 0.01 M HCl and diluted with water to 25.00, 2.50, or 0.25 μg mL−1, and was then hydrodynamically injected into the capillary (30 mbar × 100, 150, 200, 250, or 300 s).
Online Preconcentration of Proteins with Modified Capillaries
All analyses were performed at normal polarity with the polymer-modified capillaries.
Field-Amplified Sample Stacking (FASS)
The proteins were dissolved in water to 1.0 mg mL−1. Then the sample was hydrodynamically injected (60 mbar × 2 s) into a capillary filled with 100 mM phosphate buffer solution (pH 4).
Head-Column Field-Amplified Sample Stacking (HC FASS)
The water plug was hydrodynamically injected into a capillary filled with 100 mM phosphate buffer solution, followed by the sample dissolved in distilled water. The sample injection conditions were 30 mbar × 2 s, and the duration of water plug injection was varied (30 mbar × 10, 30, 60, 90, 100, or 110 s).
Large-Volume Sample Stacking (LVSS)
The sample was dissolved in distilled water to 0.1–0.001 mg mL−1 and then hydrodynamically injected into the capillary (30 mbar × 20, 40, 60 s).
Sample Preparation of Biological Fluids for Analysis, Recovery Studies, and Quantitation
For the analysis of lysozyme, saliva was centrifuged (3000 rpm for 10 min) and diluted five times with deionized water.
Human urine was collected in a container for 24 h. The container was filled with 15 mL of 6 M HCl as a preservative. The urine was kept at 4 °C until assayed. The urine samples for the recovery experiments and the quantitation results were collected on different days. Sample preparation included SPE on alumina sorbent that had previously been washed with 2 M HCl and heated in an oven at 200 °C for 3 h, as described in [28]. Four hundred microliters of 50 mM EDTA were added to 4 mL of urine to prevent oxidation. After that, the pH was adjusted to 8.5 with 1 M Na2CO3. Activated alumina (10 mg) was added to the sample, which was stirred for 2 min and filtered. The urinary free catecholamines were retained on sorbent. They were eluted into a glass vial using 200 μl of 0.1 M acetic acid. Thus, the analytes were concentrated twentyfold.
To evaluate the catecholamine recoveries, human urine samples supplemented with catecholamine standards but with no added standards were extracted by SPE and analyzed by CE. The recoveries were calculated by comparing the difference between spiked and unspiked samples to the signal from standards of the corresponding concentrations. The recovery of lysozyme was evaluated in the same way.
The quantitation of catecholamines was performed by internal standard calibration. 3,4-Dihydroxylbenzylamine (DHBA) was used as an internal standard. Samples of human urine supplemented with catecholamines at various concentrations (0.4–3.0 μg mL−1) and the internal standard at a constant concentration (1.0 μg mL−1) were analyzed. The calibration dependences were determined as the relationships between the area ratios and the concentration ratios of the analytes to the internal standard.
Results and Discussion
The development of a positively charged coating on the internal surfaces of fused-silica capillary walls is one of the most common approaches used to achieve high efficiency, separation selectivity, and migration time reproducibility for the main molecules examined in capillary electrophoresis. Examples of carbohydrates that are used to develop efficient physically adsorbed coatings include chitin and chitosan. Combinations of “anchor” and “functional” components in one material, as described in [15], are also of interest. For example, spermine-graft-dextran, in which spermine acts as an anchor component and dextran as a functional component, has been employed in capillary wall coatings. The anchor part provides the adhesion with the capillary surface, while the functional part behaves as an EOF suppressor.
In this work, the polymers studied are core–shell types, consisting of a hb polyethyleneimine core surrounded by a maltose shell that can vary in density (leading to structures A, B, and C), and are bifunctional macromolecules. The PEI core in such structures can act as an anchor component that interacts with the negatively charged quartz surface. The maltose residues in the polymer shell can act as the functional component. It was assumed that, depending on the maltose content in the shell (i.e., whether structure A, B, or C of the hb polymer was used), the EOF could be suppressed or reverse flow could be attained.
Development of Capillary Wall Coatings with PEI-Mal
In order to investigate the above hypotheses, capillaries were rinsed with PEI-Mal solutions (structures A, B, and C) in 20 mM phosphate buffer, pH 7; the polymer and capillary walls were oppositely charged. The EOF migration time with the unmodified fused silica capillary, using the same BGE, was taken as the reference. Due to adsorption on the fused silica capillary walls, polymer structure A provided a significant decrease (3–5 times) in EOF migration time that depended on the concentration of the polymer solution used (see “Coating Development”). The PEI core of polymer A is hard to reach due to the high-density maltose shell around it.
Flushing the capillary with polymers B and C and then measuring the EOF migration time of the corresponding BGE (without any polymer) resulted in reverse EOF. This is due to the creation of positively charged capillary walls following the protonation of the PEI amino groups, which—unlike in polymer A—are not shielded by a maltose shell and are therefore easier to reach.
The time for which the capillary was flushed with the polymer solution was varied (see “Analysis of Catecholamines Using Capillaries Modified with PEI-Mal” for the polymer concentrations and the pH of the BGE), and the optimal conditions were found to be 30 min, 30 mg mL−1, and 20 mM phosphate buffer solution, pH 7. After modification, each capillary was washed with water for 20 min to remove excess polymer. The stability of each capillary coating was evaluated based on the stability of the EOF and the catecholamine migration time (Table 1).
These capillaries were tested for use in the electrophoretic separation of catecholamines (DA, E, NM, NE), and they showed increased efficiency (up to 220 × 103 t.p./m) compared to unmodified capillaries (around 60 × 103 t.p./m). However, the catecholamine migration times and separation selectivities decreased notably after 3–5 analyses. This indicated that the developed coating was unstable and washed away with the BGE during analysis and capillary flushing. The use of water-soluble polymers as coating materials led to poor coating stability because they dissolved in the BGE [23].
In order to develop more stable coatings, we decided to add Cu2+ to the polymer solution as a supporting additive. Due to the presence of diol fragments in maltose residues, PEI-Mal associates with Cu2+ ions and forms Cu2+–PEI-Mal complexes. The formation of complexes between PEI-Mal and Cu2+ was demonstrated by the appearance of a new absorption band in the UV spectrum originating from the Cu2+–PEI-Mal A complex (311 nm); this band was absent from the individual spectra of both components of the complex. For Cu2+–PEI-Mal B and C, the absorption bands were at 285 and 274 nm, respectively. These complexes are thought to be adsorbed on the negatively charged capillary walls more effectively than PEI-Mal alone.
The modification process included washing the capillary with Cu2+–PEI-Mal dissolved in 5 mM NaOH for 30 min. The capillary was then flushed with water and the BGE for 20 min, and the EOF was measured at pH 7. This pH was chosen because of the strong interactions between the Cu2+–PEI-Mal complexes and the negatively charged capillary walls at this pH.
An important factor is the ratio of Cu2+ to PEI-Mal in the complexes. According to an accepted approximation, the complexes form due to interactions between Cu2+ ions and the diol fragments of the maltose residues in the shell. The optimal ratio of Cu2+ to PEI-Mal depends on the content of maltose residues in the shell, which also influences the stability of the resulting coating. Thus, the optimal molar ratios of Cu2+ to PEI-Mal in the complexes (i.e., those that provided the most stable coatings) were found to be 6:1, 4:1, and 3:1 for polymers A, B, and C, respectively. The durability of the coating increased with increasing maltose content in the shell. The stability of the capillary coating was determined by investigating the stability of EOF migration as well as the stability of the catecholamine migration time.
The separation of catecholamines was performed with a 50 mM acetate buffer solution, pH 4. Table 1 shows the analysis-to-analysis and day-to-day RSDs of the catecholamine migration time and the number of analyses with the corresponding RSD for the capillaries modified with PEI-Mal and PEI-Mal–Cu2+. The acceptable statistical values obtained suggest that the coatings are suitable for CE analysis.
The coatings developed in this work possess much higher stability than coatings based on polymer solutions only. The influence on the EOF, efficiency, and separation selectivity being the same in both cases. The catecholamine migration time and the EOF did not change even after the modified capillaries were lushed with 0.01 M HCl, water, and 0.01 M NaOH each for 5 min between analyses. When the coating deteriorates it can be renewed by flushing the capillary with the modifier under the same conditions as employed when the coating was first generated.
Catecholamines and proteins with various molecular weights and isoelectric points (pI values: lysozyme, 11.0; transferrin, 5.8; myoglobin, 7.0; albumin, 4.7) were chosen as model systems to evaluate the performance of the capillaries with PEI-Mal–Cu2+ coatings.
Analysis of Catecholamines Using Capillaries Modified with PEI-Mal
Small-molecule neurotransmitters are important chemical messengers that mediate signaling between neuronal cells in the brain. While numerous normal neuronal functions lead to fluctuations in the concentrations of these neurotransmitters, they can also be associated with pathological changes in neurological disorders such as Parkinson’s disease [24]. This is why it is important to monitor the concentrations of neurotransmitters in blood. HPLC and CE are usually used to separate these solutes. In the present work, we applied the capillaries coated with PEI-Mal–Cu2+ in our technique for the separation and online concentration of catecholamines.
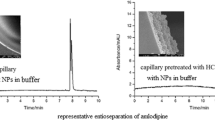
Due to the silanol groups covering the capillary walls, these capillaries provide higher efficiency and separation selectivity for catecholamines than unmodified capillaries do (Fig. 2). Also, the highest efficiencies (up to 480 × 103 t.p./m) were achieved using capillaries modified with polymer C (Fig. 3), which had the most accessible positively charged core. The polymers adsorbed on the capillary surface create a barrier to the sorption of analytes. Moreover, reverse EOF in these capillaries causes the preconcentration of analytes through the expulsion of the sample matrix during sample injection and the movement of the analytes in the opposite direction to the EOF. This is why the catecholamine separation efficiencies of the capillaries treated with polymer structures B and C (in which reverse EOF was observed to occur) were much higher than the corresponding efficiencies of the capillaries modified with polymer A (Fig. 3). The peak resolution was also improved when the PEI-Mal-modified capillaries were used (Table 2).
Electropherograms of catecholamine separation using capillaries modified (A) or unmodified (B) with PEI-Mal (structure C). Experimental conditions: BGE: 50 mM acetate buffer solution, pH 4.0; separation voltage +20 kV; 20 °C; λ = 224 nm; sample injection: 2 s, 30 mbar; concentration of each catecholamine in the sample: 2.5 μg mL−1 (A) and 10.0 µg mL−1 (B)
Despite the increased efficiencies and separation selectivities achieved with the PEI-Mal-modified capillaries, the resulting LODs were still not low enough to permit the analysis of catecholamines in biological fluids (~5 μg mL−1). In view of this, various online concentration techniques were applied in conjunction with the modified capillaries in order to preconcentrate the catecholamines. Given that reverse EOF occurred in the capillaries modified with PEI-Mal B and C, we chose to use the FASS, HC FASS, and LVSS techniques. The preconcentration efficiency was estimated from the stacking efficiency factor (SEFh), which was calculated by dividing the peak height obtained following online concentration by the peak height obtained with conventional injection (hydrodynamic injection for 2 s), after correcting for the dilution factor.
HC FASS was carried out by varying the duration of water plug injection prior to sample injection. However, the resulting increase in efficiency, SEFh, and decrease in the limit of detection were found to be insufficient. Much better SEFh and LOD values were achieved using LVSS in conjunction with the capillary modified with polymer C (Table 3). The SEFh was calculated via \( {\text{SEF}}_{\text{h}} = \frac{{{h}_{ 2} }}{{{h}_{ 1} }} \Delta \). This preconcentration technique is best suited for use in combination with the capillaries modified with polymers B and C, which showed reverse EOF.
SPE-CE of Catecholamines in Urine
The high sensitivity and separation selectivity obtained by applying the LVSS method in conjunction with the capillary modified with polymer C allowed us to determine the catecholamines in human urine after SPE of the analytes using activated alumina. The electropherogram of the urine sample is shown in Fig. 5a. The peak assignments as well as the recoveries were confirmed by spiking human urine with different standard reference solutions of analytes and 3,4-dihydroxylbenzylamine (DHBA), which was chosen for use as the internal standard. The concentrations of the standard reference catecholamines and DHBA in the human urine were 30 and 50 ng mL−1, respectively. After that, the urine sample was subjected to SPE and CE analyses. Recoveries were ranged between 82 and 87% (Table 4), wherein the recoveries based on the spiked amounts were 57–64%.
The concentrations of the catecholamines in the urine were determined using internal standard calibrations, which were obtained using the urine samples. For the experiment, we used urine samples collected on different days. The linear range studied for the catecholamines was 0.25–50 μg mL−1. However, the real concentrations of catecholamines in human urine are below this range. SPE of the urine samples led to the twentyfold preconcentration of all the analytes. This allowed them to be detected using the method developed here. The catecholamine concentrations in the urine were 68 ± 2 ng mL−1 for DA, 38 ± 3 ng mL−1 for NE, and 27 ± 2 ng mL−1 for E, which corresponded to the normal levels of these catecholamines in the urine of healthy patients [25]. The weak reverse EOF caused by the use of the capillary modified with PEI-Mal structure C in conjunction with the LVSS method led to the expulsion of the sample matrix and the complete separation of the catecholamines from the matrix components. These results demonstrate that, when used with the solid-phase extraction procedure, the proposed method is suitable for the determination of catecholamines in human urine.
Analysis of Proteins with Capillaries Modified with PEI-Mal
The same experiments were carried out to determine the proteins lysozyme, myoglobin, transferrin, and albumin. The concentrations of these proteins in biological liquids are important diagnostic criteria for human diseases [26, 27]. The analysis of these analytes using the PEI-Mal-modified capillaries led to substantial increases in efficiency (up to 21-fold for lysozyme) and separation selectivity (Table 5). The highest protein separation efficiencies were observed with the capillaries modified with polymers B and C (Fig. 4).
Electropherograms of the separation of proteins using a capillary modified with PEI-Mal (structure C) (A) and an unmodified capillary (B). Experimental conditions: BGE: 100 mM phosphate buffer solution, pH 2.0; separation voltage +20 kV; 20 °C; λ = 214 nm; sample injection: 2 s, 30 mbar; concentration of each protein in the sample: 50 μg mL−1 (A) or 500 µg mL−1 (B)
Applying the LVSS online concentration technique led to further increases in efficiency and decreases in LOD (3- to 30-fold; see Table 6). This can be explained by the reverse EOF that occurred under these conditions. The other concentration techniques (FASS, HC FASS) proved to be much less effective at promoting the efficiency and lowering the LOD.
The LODs achieved for the proteins allowed us to carry out analyses of lysozyme in saliva (Fig. 5b). These analyses showed high reproducibility of the analyte migration time (RSD <1.5%). The decrease in LOD obtained by using the PEI-Mal-modified capillaries enabled the determination of lysozyme in saliva in the range 20–40 μg mL−1 (Fig. 5b). The recovery of lysozyme was 98 ± 4% as determined by the standard addition (25 μg mL−1) of lysozyme to the saliva before dilution. The determined lysozyme content was 20 ± 1 μg mL−1, which is in good accord with relevant literature data [29]. The linear range for lysozyme was 0.5–45.0 μg mL−1.
Electropherograms of a human urine and b human saliva samples obtained using LVSS in conjunction with a capillary modified with PEI-Mal structure C. Experimental conditions: a BGE: 50 mM acetate buffer solution, pH 4.0; separation voltage: +20 kV; 20 °C; λ = 224 nm; sample injection 300 s, 30 mbar; b BGE: 100 mM phosphate buffer solution, pH 2.0; separation voltage: +20 kV; 20 °C; λ = 224 nm; sample injection: 40 s, 30 mbar (P = 0.95, n = 3)
Concluding Remarks
A new, simple, fast, and reproducible method of modifying capillary walls using the physical adsorption of core–shell-type hb polymers with Cu2+ support was developed. It was shown that the most durable PEI-Mal-based coating was that with the highest amount of maltose in its structure. However, the highest efficiencies and LODs were achieved for protein and catecholamine determination when the PEI-Mal with the lowest maltose residue content was applied, as it facilitated the development of a reverse EOF and positively charged analytes. The use of the LVSS concentration technique in conjunction with this modified capillary yielded the lowest LODs for proteins (down to 0.5 μg mL−1) in the analysis of biological fluids (saliva and blood serum) and for catecholamines in human urine.
References
Bonoli M, Varjo SJ, Wiedmer SK, Riekkola ML (2006) Cationic lipid vesicles as coating precursors in capillary electrochromatography: separation of basic proteins and neutral steroids. J Chromatogr A 1119:163–169
Moseley MA, Deterding LJ, Tomer KB, Jorgenson JW (1991) Determination of bioactive peptides using capillary zone electrophoresis/mass spectrometry. Anal Chem 63:109–114
Hjerten S (1985) High-performance electrophoresis: elimination of electroendosmosis and solute adsorption. J Chromatogr 347:191–198
Aguilar C, Hofte AJP, Tjaden UR, Greef J (2001) Analysis of histones by on-line capillary zone electrophoresis–electrospray ionization mass spectrometry. J Chromatogr A 926:57–67
Guo XF, Guo XM, Wang H, Zhang HS (2015) One step physically adsorbed coating of silica capillary with excellent stability for the separation of basic proteins by capillary zone electrophoresis. Talanta 144:110–114
Bernal J, Rodríguez-Meizosoa I, Elvira C, Ibáneza E, Cifuentes A (2008) Fast and easy coating for capillary electrophoresis based on a physically adsorbed cationic copolymer. J Chromatogr A 1204:104–109
Puetra A, Axen J, Soderberg L, Bergquist J (2006) Novel adsorptive polyamine coating for enhanced capillary electrophoresis of basic proteins and peptides. J Chromatogr B 838:113–121
Cretich M, Chiari M, Pirri G, Crippa A (2005) Electroosmotic flow suppression in capillary electrophoresis: chemisorption of trimethoxy silane modified polydimethylacrylamide. Electrophoresis 26:1913–1919
Yu B, Chi M, Han Y, Cong H, Tang J, Peng Q (2016) Self-assembled and covalently linked capillary coating of diazoresin and cyclodextrin-derived dendrimer for analysis of proteins by capillary electrophoresis. Talanta 152:76–81
Yu B, Shu X, Cong H, Chen X, Liu H, Yuann H, Chi M (2016) Self-assembled covalent capillary coating of diazoresin/carboxylfullerene for analysis of proteins by capillary electrophoresis and a comparison with diazoresin/graphene oxide coating. J Chromatogr A 1437:226–233
Danel C, Melnyk P, Azaroual N, Larchanché P, Goossens J, Vaccher C (2016) Evaluation of three neutral capillary coatings for the determination of analyte-cyclodextrin binding constants by affinity capillary electrophoresis. Application to N,N′-disubstituted piperazine derivatives. J Chromatogr A 1455:163–171
Acunha T, Simó C, Ibanez C, Gallardo A, Cifuentes A (2016) Anionic metabolite profiling by capillary electrophoresis–mass spectrometry using a noncovalent polymeric coating. Orange juice and wine as case studies. J Chromatogr A 1428:326–335
Yao YJ, Li SFY (1994) Capillary zone electrophoresis of basic proteins with chitosan as a capillary modifier. J Chromatogr A 663:97–104
Ullsten S, Soderberg L, Folestad S, Markides KE (2004) Protein-doped monolithic silica columns for capillary liquid chromatography prepared by the sol-gel method: applications to frontal affinity chromatography. Anal Chem 76:2780–2790
Co´rdova E, Gao J, Whitesides GM (1997) Noncovalent polycationic coatings for capillaries in capillary electrophoresis of proteins. Anal Chem 69:1370–1379
Wang AJ, Feng JJ, Dong WJ, Lu YH, Li ZH, Riekkola ML (2010) Spermine-graft-dextran non-covalent copolymer as coating material in separation of basic proteins and neurotransmitters by capillary electrophoresis. J Chromatogr A 1217:5130–5136
Mourey TH, Turner SR, Rubinstein M, Frechet JMJ, Hawker CJ, Wooley KL (1992) Unique behavior of dendritic macromolecules: intrinsic viscosity of polyether dendrimers. Macromolecules 25:2401–2406
Chong-Qi S, Xi-Xue X, Kie-Fen K, Zhi-Liang Z, Nan-Jing S, Pan S (2008) Carbosilane dendrimer for the coated capillary electrophoresis column. Chin J Anal Chem 36:167–171
Yu B, Chi M, Han Y, Cong H, Tang J, Peng Q (2016) Self-assembled and covalently linked capillary coating of diazoresin and cyclodextrin-derived dendrimer for analysis of proteins by capillary electrophoresis. Talanta 152:76–81
Sedlakova P, Svobodova J, Miksık I, Tomas H (2006) Separation of poly(amidoamine)(PAMAM) dendrimer generations by dynamic coating capillary electrophoresis. J Chromatogr B 841:135–139
Appelhans D, Komber H, Quadir MA, Richter S, Schwarz S, Vlist J, Aigner A, M Müller, Loos K, Seidel J, Arndt KF, Haag R, Voit B (2009) Hyperbranched PEI with various oligosaccharide architectures: synthesis, characterization, ATP complexation, and cellular uptake properties. Biomacromolecules 10:1114–1124
Polikarpov N, Potolytsyna V, Bessonova E, Tripp S, Appelhans D, Voit B, Kartsova L (2015) Dendritic glycopolymers as dynamic and covalent coating in capillary electrophoresis: view on protein separation processes and detection of nanogram-scaled albumin in biological samples. J Chromatogr A 1378:65–73
Verzola B, Gelfi C, Righetti PG (2000) Quantitative studies on the adsorption of proteins to the bare silica wall in capillary electrophoresis: II. Effects of adsorbed, neutral polymers on quenching the interaction. J Chromatogr A 874:293–303
Shariatgorji M, Strittmatter N, Nilsson A, Källback P, Alvarsson A, Zhang X, Vallianatou T, Svenningsson P, Goodwin RJA, Andren PE (2016) Simultaneous imaging of multiple neurotransmitters and neuroactive substances in the brain by desorption electrospray ionization mass spectrometry. NeuroImage 136:129–138
Liu L, Li Q, Li N, Ling J, Liu R, Wang Y, Sun L, Chen X, Bi K (2011) Simultaneous determination of catecholamines and their metabolites related to Alzheimer’s disease in human urine. J Sep Sci 34:1198–1204
Artigas A, Wernerman J, Arroyo V, Vincent JL, Levy M (2016) Role of albumin in diseases associated with severe systemic inflammation: pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care 33:62–70
Zheng L, Wan Y, Yu L, Zhang D (2016) Lysozyme as a recognition element for monitoring of bacterial population. Talanta 146:299–302
Kartsova L, Sidorova A, Ganga O (2008) The chromatographic and electrophoretic determination of catecholamines, metanephrines and 3,4-dihydroxyhyphenylalanine in urine and human plasma. Sorpt Chromatogr Process 8:75–82
Yang MW, Wu WH, Ruan Y, Huang LM, Wu Z (2014) Ultra-sensitive quantification of lysozyme based on element chelate labeling and capillary-electrophoresis-inductively coupled plasma mass spectrometry. Anal Chim Acta 812:12–17
Acknowledgements
The authors are thankful for the financial support provided by the Russian Foundation for Basic Research (Project Nos. 14-03-00735-a and 16-03-00791-a). We are also grateful to the Chemistry Educational Centre of the Research Park of Saint-Petersburg State University for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Russian Foundation for Basic Research (Project Nos. 14-03-00735-a and 16-03-00791-a).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Dzema, D., Kartsova, L., Kapizova, D. et al. New Approach to the Formation of Physically Adsorbed Capillary Coatings Consisting of Hyperbranched Poly(Ethylene Imine) with a Maltose Shell to Enhance the Separation of Catecholamines and Proteins in CE. Chromatographia 80, 1683–1693 (2017). https://doi.org/10.1007/s10337-017-3390-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3390-3