Abstract
Aza-15-crown-5-capped (3-(C-methylcalix[4]resorcinarene)-2-hydroxypropoxy)-propylsilyl (MCR-HP)-appended silica particles (15C5-MCR-HPS), a new type of substituted C-methylcalix[4]resorcinarene (MCR)-bonded chiral stationary phase (CSP) for high-performance liquid chromatography (HPLC), have been synthesized by reaction of bromoacetate-substituted MCR-HPS with excessive aza-15-crown-5 in anhydrous acetonitrile in the presence of potassium carbonate. The bonded phase 15C5-MCR-HPS is characterized by elemental analysis and further evaluated by separating several disubstituted benzenes and some chiral drug compounds in HPLC. The new CSP has a chiral selector with two recognition sites: 15-crown-5 and MCR-HP, which can provide cooperative multiple interactions with solutes to enhance chiral recognition and to improve chromatographic separation. The chromatographic evaluation results show that 15C5-MCR-HPS has excellent selectivity for the separation of aromatic positional isomers and enantiomers of some chiral compounds under multiple-mode mobile phase conditions including normal phase, reversed phase, and polar organic mobile phase conditions.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Regardless of drugs obtained from natural sources or from synthetic means, a large number of the commercially available drugs are chiral and may exist in different enantiomeric forms [1,2,3]. Enantiomers of the drug may have differences in their potency, toxicity and behaviors in the biological systems [3,4,5]. For instance, one enantiomer of dopamine is used to treat Parkinson’s disease, while the other is actually toxic to the nerve cells [3]. Therefore, there has been growing concern and awareness of the impact brought about by chirality. The consideration of chirality of drug compounds has become a vital part in drug discovery and research [1,2,3]. Apart from this, there are also other potential benefits of having enantiopure drug such as reduction of drug dosage and new opportunities for a previously marketed racemate drug to be re-introduced as an enantiomerically pure medicine [2, 3]. Currently, there are still a large number of drug compounds which are being marketed as racemic mixtures and pharmaceutical companies are keen to explore efficient and economic ways to develop these chiral drugs as pure enantiomers [2,3,4].
Besides asymmetric synthesis of the desired enantiomeric drug, chiral separation is yet another effective method that has gained importance to obtain enantiopure drug compounds [4,5,6,7]. Among the various chiral separation methods available, the use of liquid chromatographic separation of enantiomers on effective chiral stationary phases (CSPs) has been one of the most convenient and economical ways for both analytical and preparative purposes [7, 8]. It is also useful in detection of the respective enantiomeric composition of the chiral drugs [3]. Consequently, various efforts have been devoted to prepare effective CSPs and a great number of CSPs materials were developed [2,3,4]. At present, many successful chiral separations have been achieved in liquid chromatography using different types of effective CSPs containing various chiral selectors including cyclodextrins [4,5,6,7,8], crown ethers [2], calixarenes [9], and calixresorcinarenes [10].
Crown ethers are macrocyclic polyethers with specific cavity sizes [11]. Due to the cavity structure, crown ethers can form host–guest complexes with many amine-containing compounds and metal ions [5, 11]. Excellent enantiomeric discriminating ability of a novel type of chiral selectors calix[4]azacrowns [12, 13], which are made up of calix[4]arenes capped with aza-crown ethers, was achieved toward the enantiomers of phenylalanine and alanine methyl ester [12]. Another chiral selector that has gained our interest is C-methylcalix[4]resorcinarene (MCR), which is a derivative of calix[4]resorcinarene. MCRs are reported to be able to form stable host–guest complexes with various guest molecules [14,15,16]. Our group previously reported two types of MCR-bonded CSPs MCR-HPS and bromoacetate-substituted MCR-HPS (BAMCR-HPS) [10]. Excellent enantioselectivity for some chiral drug compounds was achieved on these MCR-bonded phases. Therefore, it is our interest to prepare and evaluate a new type of aza-crown ether-capped MCR-bonded phase for use as CSP in liquid chromatography.
In this article, we describe the first synthetic method to prepare aza-15-crown-5-capped (3-(C-methylcalix[4]resorcinarene)-2-hydroxypropoxy)-propylsilyl-appended silica particles (15C5-MCR-HPS) via reaction of BAMCR-HPS with excessive aza-15-crown-5 in anhydrous acetonitrile in the presence of potassium carbonate. The chromatographic performance of the new bonded phase 15C5-MCR-HPS is evaluated with several disubstituted benzenes and some chiral drug compounds in HPLC under normal phase, reversed phase, and polar organic mobile phase conditions.
Experimental
Instrumentation
HPLC was performed on an Agilent Technologies (Waldbronn, Germany) Model 1100 system consisting of an autosampler, a binary pump, a degasser, and a multiple wavelength UV detector. A Chemstation software (Rev. A.09.03) was applied to record and process the obtained chromatographic data. A Perkin Elmer (Norwalk, CT, USA) 2400 elemental analyzer was used for elemental analysis of the synthetic silica particles.
Chemicals
Aza-15-crown-5 (15C5) and C-methylcalix[4]resorcinarene were purchased from Sigma-Aldrich (St. Louis, MO, USA) and were dried in 0.1-mmHg (1 mmHg = 133.322 Pa) vacuum at 80 °C for 12 h before chemical reaction. Premium R f spherical silica gel (3 μm, 100Å) was obtained from Sorbent Technologies (Atlanta, GA, USA). The disubstituted benzene derivatives were purchased from Fluka (Buchs, Switzerland). Analytical-grade acetic acid (AA), phosphoric acid, triethylamine (TEA), and chiral compounds including benzyl mandelate, furoin, indapamide, indoprofen, α-methylbenzylamine, methyl mandelate, metoprolol, 1-(1-naphthyl)ethylamine, 1-phenyl-1-propanol, pindolol, promethazine, propanolol, proglumide and warfarin were obtained from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade solvents acetonitrile (ACN), hexane, isopropanol (IPA), and methanol (MeOH) were purchased from Merck (Darmstadt, Germany). Ultrapure water was obtained using a Milli-Q water purification system (Bedford, MA, USA).
Preparation of Bonded Silica Particles 15C5-MCR-HPS
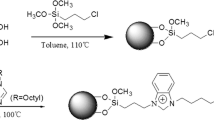
The 15C5-MCR-HPS was synthesized using BAMCR-HPS as starting materials. The BAMCR-HPS was prepared according to our previously reported procedure [10]. Briefly, MCR was first anchored onto silica particles, and then treated with bromoacetyl chloride in the presence of aluminum chloride to prepare BAMCR-HPS. The amount of anchored MCR and substituted bromoacetate moieties in BAMCR-HPS were 93.8 and 140.1 μmol g−1, respectively. Accordingly, the degree of substitution of bromoacetate in BAMCR-HPS was calculated as 1.5. The 15C5-MCR-HPS particles were finally prepared by reaction of the obtained BAMCR-HPS with excess aza-15-crown-5 in anhydrous acetonitrile in the presence of potassium carbonate. Figure 1 shows the synthetic routine. Typically, after 0.4 g (1.82 mol) of aza-15-crown-5 was resolved in 50 mL anhydrous acetonitrile containing 8 mg of potassium carbonate, 2 g of dry BAMCR-HPS was immediately added to the reaction mixture. Then, the mixture was refluxed for 24 h under the protection of dry nitrogen gas. The obtained 15C5-MCR-HPS was filtered with G4 glass filter, successively washed with acetonitrile, acetone, water, and methanol, and then purified by Soxhlet extraction with acetone overnight. After drying for 6 h under vacuum at 60 °C, elemental analysis was performed for the bonded phase15C5-MCR-HPS. The result of C, 5.68%; H, 1.08%; indicated the amount of 15-crown-5 moieties in the new bonded phase as 82.8 μmol g−1. Accordingly, the degree of substitution of 15-crown-5 was calculated to be around 0.9 in the 15C5-MCR-HPS phase.
Chromatographic Procedures
The new phase 15C5-MCR-HPS was packed into a 150 mm × 2.0 mm I.D. stainless steel column (Phenomenex, Torrance, CA, USA) to use as CSP using a previously reported procedure [4]. The mixtures of hexane/isopropanol, methanol/water, acetonitrile/water, acetonitrile/1% triethylamine-acetate (TEAA) buffer solution, methanol/phosphate buffer solution, acetonitrile/phosphate buffer solution, and methanol/acetonitrile/acetic acid/triethylamine by different volume ratios were used as mobile phases for the HPLC separations. The TEAA buffer was obtained by adding proper amount of acetic acid into 1% triethylamine (TEA) water solution to achieve the required pH [17]. Similarly, the phosphate buffers were prepared via our previously established procedure [5] by adding sodium hydroxide solution into 5 mM phosphoric acid to achieve the proper pH. The mobile phase flow rate was set as 0.2 mL/min. The samples (0.5–5 mmol/L) were resolved in the mobile phases and filtered through 0.2 µm membrane before injection. The injection volume is about 0.5–10 µL. The void volume marker (t 0) was determined by the baseline perturbation resulting from injection of the mobile phase. The HPLC separation was performed at room temperature. Confirming evidences for chiral separation were supplied by at least duplicate separations with UV detection at different wavelengths from 210 to 280 nm.
Results and Discussion
Separation of the Positional Isomers of the Disubstituted Benzenes
The 15C5-MCR-HPS-packed column was first evaluated by separating positional isomers of nitroaniline and nitrophenol under both normal and reversed phase conditions. Table 1 lists the retention factor of the solutes under two reversed phase conditions using mixtures of methanol/water and acetonitrile/water as mobile phases. It was observed that the retention factors of all the solutes generally increased when the content of methanol or acetonitrile decreased. This implied that the 15C5-MCR-HPS had hydrophobic interactions with the solutes. These observations were similar to the retention trend of the disubstituted benzenes on the BAMCR-HPS-packed column [10]. Interestingly, compared to the starting material BAMCR-HPS [10], the new bonded phase 15C5-MCR-HPS exhibited generally higher retention for the same solutes under similar conditions using mixture of methanol/water as mobile phases (except for 100% methanol conditions). The elution order of the solutes was also different between the BAMCR-HPS-packed column and the 15C5-MCR-HPS-packed column using the mixture of methanol/water as mobile phases. For example, the p-nitrophenol was eluted first among the isomers of nitrophenol on the BAMCR-HPS-packed column; however, m-nitrophenol was eluted first on the 15C5-MCR-HPS-packed column. This suggests the introduction of aza-15-crown-5 moieties in the new bonded particles 15C5-MCR-HPS produced other interactions such as extra host–guest interaction and hydrogen interaction with the solutes and exhibited different retention characteristics from the starting synthetic material BAMCR-HPS.
As shown in the Table 1, the elution order of the solutes on the 15C5-MCR-HPS-packed column was not the same under the two reversed phase conditions with the same water contents in the mobile phases. For example, the m-nitroaniline was eluted last among the isomers of nitroaniline from 40 to 5% acetonitrile. However, o-nitroaniline was eluted last from 40 to 5% methanol. This implies that the 15C5-MCR-HPS is a versatile bonded phase which can easily provide different separation and retention under the reversed phase conditions when switching different organic modifiers for the mobile phases.
Interestingly, we found that the o-nitroaniline was always eluted first among the isomers of nitroaniline under normal phase conditions using mixture of isopropanol/hexane as mobile phases. This elution order is completely different from that under the two revered phase conditions. Baseline separations of all the positional isomers of the nitrophenols and nitroanilines were easily achieved on the 15C5-MCR-HPS-packed column. Typical chromatogram depicting the separation of the isomers of o-, m-, p-nitroaniline is shown in Fig. 2. As shown in Fig. 2, the elution order of the nitroaniline isomers on 15C5-MCR-HPS-packed column was o < p < m when using mixture of isopropanol/hexane (5:95, v/v) as mobile phase. However, the elution order (o < m < p) was different on the BAMCR-HPS-packed column [10]. This confirms that the aza-15-crown-5 moieties in 15C5-MCR-HPS play an important role in the retention and separation process.
Evaluation of the 15C5-MCR-HPS-Packed Column by Separation of the Chiral Drug Compounds
As previously reported [10], after the MCR was linked with 3-glycidoxypropyltrimethoxysilane, a chiral ligand (3-(C-methylcalix[4]resorcinarene)-2-hydroxypropoxy)-propyltrimethoxysilane (MCR-HP) was formed and successfully used as a chiral selector in the bonded-phases MCR-HPS and BAMCR-HPS. As shown in Fig. 1, a new complex chiral ligand aza-15-crown-5-capped MCR-HP was used as a novel chiral selector in the new bonded phase 15C5-MCR-HPS. The enantioseparation performance of the 15C5-MCR-HPS-packed column was evaluated using some chiral drug compounds under normal phase, reversed phase, and polar organic mobile phase conditions. Figure 3 shows the progressive separation of the enantiomers of warfarin on 15C5-MCR-HPS-packed column as mobile phase composition of acetonitrile/water was varied. As shown in Fig. 3, decreasing the acetonitrile content resulted in longer retention, higher enantioselectivity, and higher enantioseparation resolution. Hence, the main enantiomeric recognition mechanism appears to be the formation of host–guest complex in which the hydrophobic portion of the solute is included in the cavity of MCR-HP [10, 18], and the crown ether moiety provides further other interactions [19], e.g., hydrogen-bonding interaction and dipolar interaction, etc., with the solute. Complete baseline enantioseparation for warfarin was easily achieved when acetonitrile content was reduced to 30% (or less) on the 15C5-MCR-HPS-packed column.
The bonded phase 15C5-MCR-HPS contains a new type of complex chiral selector aza-15-crown-5-capped MCR-HP with two recognition sites: MCR-HP and 15-crown-5. Due to the multiple interactions available and cooperative functioning of the anchored MCR-HP and aza-15-crown-5 moieties, the new stationary phase 15C5-MCR-HPS has exhibited excellent performance for separation of enantiomers of some chiral drug compounds under kinds of mobile phase conditions. Table 2 lists the retention factor (k), selectivity (α), and separation resolution (R S) for the chiral compounds resolved on the 15C5-MCR-HPS-packed column under polar organic mobile phase, normal phase, and reversed phase conditions, including the addition of TEAA and phosphate buffers into the mobile phases. Typical chromatograms of the enantioseparation of the chiral compounds on the 15C5-MCR-HPS-packed column are shown in Fig. 4. The column efficiency for the 15C5-MCR-HPS-packed column was determined as 22,800 plates m−1 under reversed-phase condition using the second enantiomer (eluted out at second order) of 1-phenyl-1-propanol as solute and methanol/water (20:80, v/v) as the mobile phase. This column efficiency is much better than the previously reported BAMCR-HPS-packed column under the same conditions. The higher column efficiency allows the 15C5-MCR-HPS to be able to achieve better enantioseparation resolution than the BAMCR-HPS phase. Compared to the single-type chiral selector MCR-containing BAMCR-HPS-packed column [10], complex chiral selector aza-15-crown-5-capped MCR-HP-containing 15C5-MCR-HPS-packed column exhibited better enantioseparations for some chiral compounds. For example, under similar mobile phase conditions, better enantioselectivities and enantiomeric resolutions for warfarin (no enantioseparation on BAMCR-HPS [10], however, baseline enantioseparation (α = 1.36, R S = 2.03) on 15C5-MCR-HPS) and indapamide (α = 1.45, RS = 2.09 on BAMCR-HPS [10] and α = 1.76, R S = 2.28 on 15C5-MCR-HPS) and 1-phenyl-1-propanol (α = 1.89, R S = 2.79 on BAMCR-HPS [10] and α = 2.24, R S = 5.78 on 15C5-MCR-HPS) were achieved on the 15C5-MCR-HPS-packed column. This is mainly due to co-operative functioning of the MCR-HP and aza-15-crown-5 moieties in the anchored aza-15-crown-5-capped MCR-HP selector of 15C5-MCR-HPS. This enhanced the host–guest interaction with the solute and resulted in improved chiral recognition and enantioselectivity. Compared to the previously reported complex chiral selector rifamycin-capped-β-cyclodextrin-containing RCD-HPS-packed column [2], the 15C5-MCR-HPS-packed column exhibited better enantioseparations for some chiral compounds. For example, better enantioseparations for propanolol (α = 1.18, R S = 0.87 on RCD-HPS [2] and α = 1.31, R S = 1.27 on 15C5-MCR-HPS) and metoprolol (α = 2.03, RS = 1.21 on RCD-HPS [2] and α = 2.56, R S = 1.36 on 15C5-MCR-HPS) were achieved on the 15C5-MCR-HPS-packed column under the same mobile phase (methanol/water (40:60, v/v) for propanolol and acetonitrile/water (20:80, v/v) for metoprolol) conditions. Compared to the reported crown ether-capped-β-cyclodextrin-containing AQ2D18C6-CD-HPS-packed column [5], the 15C5-MCR-HPS-packed column also exhibited better enantioseparations for some chiral compounds. For example, better enantiomeric separation for 1-pheny1-1,2-ethanediol (α = 1.09, R S = 1.43 on AQ2D18C6-CD-HPS [5] and α = 4.57, R S = 3.64 on 15C5-MCR-HPS) was achieved on the 15C5-MCR-HPS-packed column under the similar mobile phase condition. This suggests that the new stationary phase 15C5-MCR-HPS not only is novel in chemical structure but also has some advantages for use as CSP for chiral separation. There has been no observable change of chromatographic performance of the 15C5-MCR-HPS-packed column for continuous usage over 10 months under various mobile phase conditions including addition of TEAA and phosphate buffer solutions. This demonstrates that the new phase 15C5-MCR-HPS is a fairly robust CSP with potential wide applications for chiral separations in liquid chromatography.
Typical chromatograms for enantioseparations on 15C5-MCR-HPS-packed column. a Promethazine, mobile phase: acetonitrile/water (30:70, v/v), 254 nm UV detection; b 1-phenyl-1-propanol, mobile phase: methanol/water (20:80, v/v), 254 nm UV detection; c α-methylbenzylamine, mobile phase: methanol/phosphate buffer pH 4 (40:60, v/v), 254 nm UV detection
Conclusions
A new type of aza-15-crown-5-capped MCR-bonded silica particles 15C5-MCR-HPS have been successfully synthesized and applied as chiral stationary phase in HPLC. The new phase contains a novel type of complex chiral selector with two recognition moieties: MCR-HP and aza-15-crown-5. Due to cooperative functioning of the anchored MCR-HP and aza-15-crown-5 moieties and the multiple interactions available, the 15C5-MCR-HPS phase has shown excellent selectivity for the separation of aromatic positional isomers and enantiomers of some chiral drug compounds under multiple-mode mobile phase conditions. The new phase has also exhibited fairly robust chromatographic performance in HPLC.
References
Patel DC, Wahab MF, Armstrong DW, Breitbach ZS (2016) J Chromatogr A 1467:2–18
Zhao J, Tan D, Thamarai SK, Yong EL, Lee HK, Gong Y (2010) Talanta 83:286–290
Ali I, Alam SD, Al-Othman ZA, Farooqi JA (2013) J Chromatogr Sci 51:645–654
Thamarai SK, Zhao J, Chen L, Yan S, Yin X, Sun J, Yong EL, Wei Q, Gong Y (2014) J Chromatogr A 1324:104–108
Gong Y, Xiang Y, Yue B, Xue G, Bradshaw JS, Lee HK, Lee ML (2003) J Chromatogr A 1002:63–70
Poon YF, Muderawan IW, Ng SC (2006) J Chromatogr A 1101:185–197
Spudeit DA, Breitbach ZS, Dolzan MD, Micke GA, Armstrong DW (2015) Chirality 27:788–794
Wang Y, Ong TT, Li LS, Tan TY, Ng SC (2009) J Chromatogr A 1216:2388–2393
Krawinkler KH, Maier NM, Sajovic E, Lindner W (2004) J Chromatogr A 1053:119–131
Tan HM, Soh SF, Zhao J, Yong EL, Gong Y (2011) Chirality 23:E91–E97
Liu T, Han L, Yu Z, Zhang D, Liu C (2012) Comput Biol Med 42:480–484
Demirtas HN, Bozkurt S, Durmaz M, Yilmaz M, Sirit A (2009) Tetrahedron 65:3014–3018
He Y, Xiao Y, Meng L, Zeng Z, Wu X, Wu CT (2002) Tetrahedron Lett 43:6249–6253
Faull JD, Gupta VK (2003) Thin Solid Films 440:129–137
Brown PO, Enright GD, Ripmeester JA (2002) J Supramol Chem 2:497–500
Jumina Sarjono RE, Siswanta D, Santosa SJ, Ohto K (2011) J Korean Chem Soc 55:454–462
Gong Y, Lee HK (2003) J Sep Sci 26:515–520
Ruderisch A, Iwanek W, Pfeiffer J, Fischer G, Albert K, Schurig V (2005) J Chromatogr A 1095:40–49
Ali I, Suhail M, Al-Othman ZA, Alwarthan A, Aboul-Enein HY (2016) Biomed Chromatogr 30:683–694
Acknowlegements
The authors thank Xuzhou Medical University for financial support of this work (Grant No.: 531150).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Mingxuan Ma and Qunli Wei are Joint first authors.
Rights and permissions
About this article
Cite this article
Ma, M., Wei, Q., Meng, M. et al. Preparation and Application of Aza-15-crown-5-capped Methylcalix[4]resorcinarene-Bonded Silica Particles for Use as Chiral Stationary Phase in HPLC. Chromatographia 80, 1007–1014 (2017). https://doi.org/10.1007/s10337-017-3312-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-017-3312-4