Abstract
A simultaneous analytical method was developed and validated to quantify three lipophilic compounds; namely glabridin (an isoflavonoid isolated from crude licorice), (−)-α-bisabolol (a sesquiterpene alcohol obtained from plant extracts), and ascorbyl tetraisopalmitate (a fat-soluble molecule derived from vitamin C) in functional cosmetic cream using high-performance liquid chromatography (HPLC) coupled with photodiode array detection (PAD). Cosmetic cream samples were extracted with a mixture of acetonitrile and isopropyl alcohol (45:55, v/v) and the target compounds were separated on a C18 column with a gradient mobile phase consisting of deionized water, acetonitrile, and isopropyl alcohol. The detector wavelengths were 228, 202, and 221 nm, for glabridin, (−)-α-bisabolol, and ascorbyl tetraisopalmitate, respectively. The calibration curves showed good linearity with determination coefficients (R 2) ≥ 0.999. The mean recoveries were ranged between 89.8 and 103.9 % with relative standard deviations (RSDs) <5 %. The intra- and inter-day precision was <2 %. The limits of detection (LODs) were 0.03, 0.4, and 4.02 μg mL−1 for glabridin, (−)-α-bisabolol, and ascorbyl tetraisopalmitate, respectively. The method was successfully applied for monitoring 11 market samples, in which glabridin was quantified in the range of 17.5–25 mg 100 g−1, (−)-α-bisabolol in the range of 25.1–677 mg 100 g−1, and 140.6–291.5 mg 100 g−1 for ascorbyl tetraisopalmitate. The proposed analytical method is simple, sensitive, and versatile and can be used for the quantification of lipophilic compounds in cosmetics in a single chromatographic run.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Korean Cosmetic Act defines cosmetics as safe goods used via rubbing, spraying or in similar ways for cleaning and beautifying the human body, brightening appearance, maintaining or improving the health of skin and hair [1]. To be used, the cosmeceuticals (functional cosmetics in Republic of Korea) are regulated by The Korean Cosmetic Act and Korean Functional Cosmetics Codex. Cosmeceuticals are intended to carry out their functions as whitening, tanning, anti-wrinkle, antiaging, and nail and hair care [2]. Pharmaceuticals are agents intended to alter, change, or protect skin from abnormal or pathological conditions, whereas cosmeceuticals represent a category of products placed between non-prescribed and prescribed [3].
There were several cosmeceutical ingredients that have been shown to be effective in skin whitening, such as hydroxyacetic acid, kojic acid, azelaic acid, hydroquinone, resorcinol, arbutin, niacinamide, vitamin C and its derivatives, (−)-α-bisabolol, and glabridin [2, 4–8]. However, for safety reason, some of them have been banned for uses in cosmetics [9, 10]. In Republic of Korea, ascorbyl tetraisopalmitate, glabridin, (−)-α-bisabolol, arbutin, niacinamide, ascorbyl glucoside, and ethyl ascorbyl ether are the main ingredients used in whitening functional cosmetics [11]. Glabridin, (−)-α-bisabolol, and ascorbyl tetraisopalmitate are lipophilic substances, whereas the arbutin, niacinamide, ascorbyl glucoside, and ethyl ascorbyl ether are water-soluble compounds. In our previous research studies, we have developed methodologies to determine hydrophilic substances in whitening functional cosmetics [4, 12].
Glabridin is an isoflavonoid originally isolated from crude licorice (Glycyrrhiza glabra L.). Glabridin has been associated with numerous biological properties such as anticancer, antioxidant, anti-inflammatory, antibacterial, and skin-whitening activities [13–15]. Because it is a potent tyrosinase inhibitor, the skin-whitening effect was due to the inhibition of melanin [16]. (−)-α-Bisabolol is sesquiterpene alcohol obtained from several plant extracts, such as Chamomilla recutita, Plinia cerrocampanensis Barrie, Pogostemon speciosus Benth and others. It has been used in cosmetic products, fine fragrances, toilet soaps and other toiletries as well as in non-cosmetic products [17–21]. (−)-α-Bisabolol inhibits the cAMP response element (CRE) induced by α-melanocyte-stimulating hormone (α-MSH), thereby reducing the melanin content. Additionally, it alters the gene expression of microphthalmia-associated transcription factor (MITF) and tyrosinase; implying that it inhibits the melanogenesis by reducing the intra cellular cAMP levels [22]. Vitamin C, or L-ascorbic acid, is the most plentiful antioxidant in human skin and has an ability to inhibit the activity of tyrosinase [23, 24]. Due to oxidation, vitamin C is easily degraded and unstable when exposed to air, light, etc. To overcome this defect, ascorbyl tetraisopalmitate and other vitamin C’s derivatives have been introduced [24]. The ascorbyl tetraisopalmitate is a fat-soluble substance derived from vitamin C [25]; exhibiting a good percutaneous absorption and a strong antioxidant activity in vitro in a lipid system [26]. Analytical methods for determination of ascorbyl tetraisopalmitate in cosmetic formulations using HPLC were reported in the literatures [25, 27]. Pedro et al. validated a HPLC method for the determination of (−)-α-bisabolol in chitosan milispheres and liposomes [28]. Meanwhile, there were a very few literatures for determination of (−)-α-bisabolol in human blood and chamomile flowers, but not cosmetics [29–31]. The HPLC method for quantitation of glabridin in polyherbal preparations and crude extracts was evaluated by Kamal et al. [15]. Similar to (−)-α-bisabolol, there was no report for detection glabridin in cosmetics, however, it was detected in crude drugs and human plasma [15, 32]. So far, no such method is described in the literature neither for simultaneous determination of the three lipophilic compounds (glabridin, (−)-α-bisabolol, and ascorbyl tetraisopalmitate) nor in whitening cosmetic creams.
Thus, the aim of this study was to develop and validate a simple and sensitive method to quantify glabridin, (−)-α-bisabolol, and ascorbyl tetraisopalmitate in whitening creams using a single HPLC-PAD chromatographic run.
Experimental
Chemicals and Reagents
Commercial functional cosmetics containing glabridin, (−)-α-bisabolol, and ascorbyl tetraisopalmitate were bought from internet markets and cosmetic shops located in Suwon City, Republic of Korea. An analytical standard of glabridin (purity: 97.0 %) and ascorbyl tetraisopalmitate (purity: 88.8 %) were obtained from Wako Chemicals (Tokyo, Japan). (−)-α-Bisabolol (purity: 95.0 %) was supplied by Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade methanol (purity: 99.99 %) and isopropyl alcohol (purity: 99.99 %) were provided by J. T. Baker (Griesheim, Germany). A 0.4 μm nylon membrane filter and 0.20 μm polytetrafluorethylene syringe filter (Advantec, Tokyo, Japan) was used to filter the mobile phase and sample solutions, before using. A Barnstead Nano pure Diamond (Dubuque, IA, USA) was used to produce purified deionized water.
Standard Preparation
Stock standard solutions of glabridin (5.0 μg mL−1), (−)-α-bisabolol (60.0 μg mL−1), and ascorbyl tetraisopalmitate (200.0 μg mL−1) were prepared by dissolving in a mixture of acetonitrile and isopropyl alcohol (45:55, v/v). Working standard solutions were obtained by diluting the stock solutions with the same mixture solution. The concentration ranges of each standard calibration curve are presented in Table 1. The standard solutions were stable for 1 month when stored at 4 °C in refrigerator.
Sample Preparation
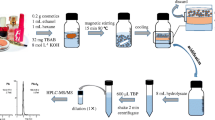
Commercial creams (0.1 g) was accurately weighed (XSE 205DU Analytical Balance, Mettler Toledo, Greifensee, Switzerland) in 15 mL polypropylene centrifuge tubes (SPL Life Sciences, Gyeonggi-do, Republic of Korea) to which a mixture of acetonitrile and isopropyl alcohol (45:55, v/v, 10 mL) was added and vortex-mixed (Vortex-Genie 2, Scientific Industries, NY, USA) for 2 min. Subsequently, the solution was sonicated (Branson 8510 sonicator, CT, USA) for 30 min, then centrifuged (Sigma 2–6 centrifuge, Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany) at 3500 rpm for 20 min. The supernatant was filtered through a 0.20 μm polytetrafluorethylene (PTFE) syringe filter before injection onto the HPLC system.
Analytical Method
HPLC-PAD system (Dionex, UltiMate 3000, Sunnyvale, CA, USA) consisting of a degasser, a quaternary pump, an auto sampler, and column compartment was used for detection. The Chromeleon software (Dionex, Sunnyvale, CA, USA) was used for data acquisition. An Eclipse Plus C18 column (250 × 4.6 mm, 5 μm, Agilent Technologies, CA, USA) maintained at 25 °C was used to separate the analytes. Capcell Pak C18 MG (250 × 4.6 mm, 5 μm, Shiseido Co, Ltd., Tokyo, Japan) was used to evaluate the chromatographic robustness test. The target compounds were separated using a stepwise gradient mobile phase consisting of deionized water (A), acetonitrile (B), and isopropyl alcohol (C), as following: 0–5 min, 30:70:0, v/v/v (A:B:C); 5–12 min, 0:100:0, v/v/v (A:B:C); 12–15 min, 0:0:100, v/v/v (A:B:C); and 15–25 min, 0:0:100, v/v/v (A:B:C), 25–32 min, 0:100:0, v/v/v (A:B:C); 32–37 min, 0:100:0, v/v/v (A:B:C); and 37–45 min, 30:70:0, v/v/v (A:B:C). The flow rate was 1.0 mL min−1 and the injection volume was 10 μL. The detection wavelengths were 228 nm for glabridin, 202 nm for (−)-α-bisabolol, and 221 nm for ascorbyl tetraisopalmitate.
Method Validation
For the quantification of the target compounds, the determination coefficient (R 2) was calculated and evaluated as linearity. The limit of detection and limit of quantitation were determined using the signal-to-noise (S/N) ratios of 3.3 and 10, respectively. The recovery and precision of the developed method were validated according to US Pharmacopeia [33]. Recovery (expressed as accuracy) was determined via spiking three different concentration levels of each compound into blank cream samples (n = 3). The method precision (expressed as intra- and inter-day variations) was evaluated by the repeated analysis of cream samples (n = 3) spiked with various compounds during 1 day and was repeated for another couple of days, respectively. The robustness evaluation of the chromatographic method was tested by the introduction of minor changes in the separation techniques of sample containing (−)-α-bisabolol (the compound with low detection wavelength value = 202 nm) by means of Youden’s test. The slope of mobile phase gradient, column temperature, flow rate, detection wavelength, injection volume, column manufacturer, and the initial mobile phase composition were chosen as the seven variables for Youden’s robustness test. As shown in Table 2, eight experiments were conducted to evaluate the selected factors [34]. For each variable, the calculated difference was indicated as D i . The standard deviation of the differences, \( S_{{D_{i} }} \), was calculated by the formula:
When \( S_{{D_{i} }} \) is significantly higher than the standard deviation of the method, it means that all the chosen factors together have an effect on the result [35].
Additionally, with t test, it is possible to evaluate the influence of each investigated factor. The experimental t value is given by the equation:
where n is the number of experiments carried out at each level for each parameter (n = 4) The standard deviation was obtained from the analysis of (−)-α-bisabolol at 50 μg mL−1 during the inter-day precision test. For all seven variables, the obtained t value was compared with the 2-tailed t critical value (t crit) at n − 1 degree of freedom, where n is the number of determinations used in the estimation of S.D. at 95 % confidence level. If t value is greater than t crit, the investigated variable shows a significant influence, and the method is not sufficiently robust against the chosen modification [36].
Results and Discussion
Extraction Procedure
The extraction efficiency of the tested compounds from commercially available cream samples was investigated using acetonitrile, isopropyl alcohol, or a combination of them as following: (A) 100 % acetonitrile, (B) 100 % isopropyl alcohol, (C) acetonitrile and isopropyl alcohol (30:70 %, v/v), (D) acetonitrile and isopropyl alcohol (40:60 %, v/v), (E) acetonitrile and isopropyl alcohol (45:55 %, v/v), (F) acetonitrile and isopropyl alcohol (50:50 %, v/v), (G) acetonitrile and isopropyl alcohol (55:45 %, v/v), (H) acetonitrile and isopropyl alcohol (60:40 %, v/v), and (I) acetonitrile and isopropyl alcohol (70:30 %, v/v). It has to be noted that a mixture of acetonitrile and isopropyl alcohol (45:55 %, v/v) efficiently extracted the three analytes compared to others. From previous studies, for instance, Almeida et al. used isopropyl alcohol as an extraction solvent to determine ascorbyl tetraisopalmitate in cosmetic cream [25] and n-hexane was used as an extraction solvent for determination of (−)-α-bisabolol in particulate systems by Pedro et al. [28]. Kamal et al. [15] extracted glabridin from polyherbal preparations using 30 % aqueous ethanol.
Optimization of Chromatographic Conditions
Because, the λ max value of (−)-α-bisabolol was 192 nm under the chromatographic conditions, therefore, methanol cannot be used as a mobile phase, as its UV cutoff value = 205 nm. First, acetonitrile and deionized water (gradient condition: 10 % acetonitrile → 90 % acetonitrile → 10 % acetonitrile) was tested as a mobile phase (data not shown); however, the ascorbyl tetraisopalmitate peak was not detected. Afterwards, isopropyl alcohol was added to acetonitrile and deionized water. In this context, Almeida et al. used isocratic elution with methanol and isopropyl alcohol (25:75, v/v) to separate tocopheryl acetate and ascorbyl tetraisopalmitate in cosmetic formulations using HPLC [25]. Kamal et al. used acetonitrile and deionized water in gradient elution method [15]. In this study, the mobile phase gradient condition described in “Analytical method” was selected to separate the three compounds, as shown in Fig. 1A. The chromatograms of (B), (C), and (D) were built with mobile phases without deionized water; resulting in poor resolution of glabridin and (−)-α-bisabolol compared to (A). The resolution values of (−)-α-bisabolol and glabridin were 38.30, 12.19, 6.55, and 7.80, in mobile phase A, B, C, and D, respectively (Fig. 1).
HPLC-PAD chromatograms of standard solution using various mobile phase composition. Initial mobile phase compositions; A deionized water:acetonitrile (30:70, v:v), B 100 % acetonitrile, C acetonitrile:isopropyl alcohol (70:30, v:v), D acetonitrile:isopropyl alcohol (80:20, v:v). Entire mobile phase compositions are stated in Table 3. Standard solutions; glabridin (5.0 μg mL−1), (−)-α-bisabolol (60.0 μg mL−1), and ascorbyl tetraisopalmitate (200.0 μg mL−1)
The enlarged chromatograms of dotted zone in Fig. 1 are presented in Fig. 2 at 202 nm. The mobile phase composition is shown in Table 3. Finally, mobile phase containing deionized water, acetonitrile, and isopropyl alcohol was used for separation and detection of the tested compounds.
Method Performance
The linearity, expressed as determination coefficient (R 2), was calculated by external standard calibration curves as shown in Table 1. The R 2 values of the three compounds were ≥0.999. The method was specific since there is no overlap or interference peak around the retention time of the tested compounds (Figs. 1, 2, 3). The LOD values of 0.03 μg mL−1 (glabridin), 0.4 μg mL−1 ((−)-α-bisabolol), and 4.02 μg mL−1 (ascorbyl tetraisopalmitate) were satisfactory for analysis. The current LODs were considerably lower than those reported for ascorbyl tetraisopalmitate in cosmetic products (15.05 μg mL−1 [15]), glabridin in crude drug (0.35 μg mL−1 [25]), and (−)-α-bisabolol in particulate systems (0.5 μg mL−1 [28]). Recoveries at three fortification levels were ranged from 89.8 to 103.9 % with RSD < 5 % (Table 4). The precision values using standard solutions shown in Table 4 were <2 % for both inter- and intra-day variation. System suitability testing was investigated using mixed standard solutions [37] (Table 5). As shown in Fig. 3, although the capacity factor of glabridin was lower than 2.0, the instrumental analysis was not affected. The abovementioned chromatographic parameters were deemed acceptable [38], indicating that the validated method is accurate for analysis. \( S_{{D_{i} }} \) value in robustness test (=0.07) was lower than the estimated method precision value (=0.21, standard deviation value of (−)-α-bisabolol at 50 μg mL−1). All selected variables for Youden’s robustness test have no effect on the results. The experimental t values are lower than that of the 2-tailed t critical value for all seven factors: t crit = 4.30 for 2 degrees of freedom at 95 % confidence level (Table 6). Regarding the variable “column supplier” all standard deviation values obtained in the eight robustness experiments (Table 2) are reported in Table 7. This factor was confirmed as the most critical (t = 0.63) with a change of column supplier. The alteration of column supplier did not present significant variations in the standard deviation value of (−)-α-bisabolol (n = 3) The tested procedure proved to be robust, since minor fluctuations in the operative parameters that can occur during the routine application of the method are significantly affecting its performance characteristics.
Analysis of Commercial Products
The proposed method was applied for the quantitation of glabridin, (−)-α-bisabolol, and ascorbyl tetraisopalmitate in 11 functional cosmetic products. The three compounds were not labeled as major functional ingredients, just general components. As shown in Table 8, the detected amounts were in the range of 25.1–677.0 mg 100 g−1 for (−)-α-bisabolol, 17.5–25 mg 100 g−1 for glabridin, and 140.6–291.5 mg 100 g−1 for ascorbyl tetraisopalmitate. According to the Korean Ministry of Food and Drug safety, the content of (−)-α-bisabolol should be higher than 0.5 % (w/w) to enhance its functional effect. From the above reported data, (−)-α-bisabolol was considered as a major functional ingredient (except for sample no. 1) and the other couples were considered as minor ingredients. Figure 3 shows the typical HPLC chromatograms of market samples.
Conclusions
A simple and sensitive analytical method using an HPLC-PAD was developed for simultaneous quantification of glabridin, (−)-α-bisabolol, and ascorbyl tetraisopalmitate in functional cosmetic products. The validated method exhibited good linearity, sensitivity, recovery, precision, and robustness and can be used for detection of lipophilic compounds in cosmetic creams. Up to the author knowledge, this is the first report for simultaneous detection of the three lipophilic compounds in functional cosmetics.
References
Korea Ministry of drug and food safety (2013) Cosmetic act, Seoul, Republic of Korea
Gao XH, Zhang L, Wei H, Chen HD (2008) Efficacy and safety of innovative cosmeceuticals. Clin Dermatol 26:367–374
Amer M, Maged M (2009) Cosmeceuticals versus pharmaceuticals. Clin Dermatol 27:428–430
Jeon JS, Kim HT, Kim MG, Oh MS, Hong SR, Yoon MH, Cho SM, Shin HC, Shim JH, Ramadan A, Abd El-Aty AM (2016) Simultaneous determination of water-soluble whitening ingredients and adenosine in different cosmetic formulations by high-performance liquid chromatography coupled with photodiode array detection. Int J Cosmet Sci 38:286–293
Chang ML, Chang CM (2003) Simultaneous HPLC determination of hydrophilic whitening agents in cosmetic products. J Pharm Biomed Anal 33:617–626
Jain P, Sonti S, Garruto J, Mehta R, Banga AK (2012) Formulation optimization, skin irritation, and efficacy characterization of a novel skin-lightening agent. J Cosmet Dermatol 11:101–110
Rendon M, Berneburg M, Arellano I, Picardo M (2006) Treatment of melasma. J Am Acad Dermatol 54:272–281
Huang SC, Lin CC, Huang MC, Wen KC (2004) Simultaneous determination of magnesium ascorbyl phosphate, ascorbyl glucoside, kojic acid, arbutin and hydroquinone in skin whitening cosmetics by HPLC. J Food Drug Anal 12:13–18
Jin W, Wang WY, Zhang YL, Yang YJ, Chu QC, Ye JN (2013) Determination of phenolic whitening agents in cosmetics by micellar electrokinetic capillary chromatography with amperometric detection. Chin Chem Lett 24:636–638
Jeon JS, Kim BH, Lee SH, Kwon HJ, Bae HJ, Kim SK, Park JA, Shim JH, Abd El-Aty AM, Shin HC (2015) Simultaneous determination of arbutin and its decomposed product hydroquinone in whitening creams using high-performance liquid chromatography with photodiode array detection: effect of temperature and pH on decomposition. Int J Cosmet Sci 37:567–573
Korea Ministry of drug and food safety (2013) Korean Functional Cosmetics Codex, Seoul, Republic of Korea
Jeon JS, Lee MJ, Yoon MH, Park JA, Yi H, Cho HJ, Shin HC (2014) Determination of arbutin, niacinamide, and adenosine in functional cosmetic products by high-performance liquid chromatography. Anal Lett 47:1650–1660
Simmler C, Pauli GF, Chen S-N (2013) Phytochemistry and biological properties of glabridin. Fitoterapia 90:160–184
Yokota T, Nishio H, Kubota Y, Mizoguchi M (1998) The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res 11:355–361
Kamal YT, Singh M, Tamboli ET, Parveen R, Zaidi SM, Ahmad S (2012) Rapid RP-HPLC method for the quantification of glabridin in crude drug and in polyherbal formulation. J Chromatogr Sci 50:779–784
Rosenblat M, Belinky P, Vaya J, Levy R, Hayek T, Coleman R, Merchav S, Aviram M (1999) Macrophage enrichment with the isoflavan glabridin inhibits NADPH oxidase-induced cell-mediated oxidation of low density lipoprotein a possible role for protein kinase c. J Biol Chem 274:13790–13799
Waleczek KJ, Marques HMC, Hempel B, Schmidt PC (2003) Phase solubility studies of pure (−)-α-bisabolol and camomile essential oil with β-cyclodextrin. Eur J Pharm Biopharm 55:247–251
Murugan R, Mallavarapu GR (2013) α-Bisabolol, the main constituent of the essential oil of Pogostemon speciosus. Ind Crops Prod 49:237–239
Bhatia SP, McGinty D, Letizia CS, Api AM (2008) Fragrance material review on α-bisabolol. Food Chem Toxicol 46:72–76
Vila R, Santana AI, Pérez-Rosés R, Valderrama A, Castelli MV, Mendonca S, Zacchino S, Gupta MP, Cañigueral S (2010) Composition and biological activity of the essential oil from leaves of Plinia cerrocampanensis, a new source of α-bisabolol. Bioresour Technol 101:2510–2514
Kamatou GP, Viljoen A (2010) A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J Am Oil Chem Soc 87:1–7
Kim S, Lee J, Jung E, Huh S, Park J-O, J-w Lee, Byun SY, Park D (2008) Mechanisms of depigmentation by α-bisabolol. J Dermatol Sci 52:219–222
Hakozaki T, Takiwaki H, Miyamoto K, Sato Y, Arase S (2006) Ultrasound enhanced skin-lightening effect of vitamin C and niacinamide. Skin Res Technol 12:105–113
Manela-Azulay M, Bagatin E (2009) Cosmeceuticals vitamins. Clin Dermatol 27:469–474
Almeida MM, Alves JMP, Patto DCS, Lima CRRC, Quenca-Guillen JS, Santoro MIRM, Kedor-Hackmann ERM (2009) Determination of tocopheryl acetate and ascorbyl tetraisopalmitate in cosmetic formulations by HPLC. Int J Cosmet Sci 31:445–450
Maia Campos PMBG, Gonçalves GMS, Gaspar LR (2008) In vitro antioxidant activity and in vivo efficacy of topical formulations containing vitamin C and its derivatives studied by non-invasive methods. Skin Res Technol 14:376–380
Gaspar LR, Campos PMBGM (2007) Photostability and efficacy studies of topical formulations containing UV-filters combination and vitamins A, C and E. Int J Pharm 343:181–189
Pedro AS, Detoni C, Ferreira D, Cabral-Albuquerque E, Sarmento B (2009) Validation of a high-performance liquid chromatography method for the determination of (−)-α-bisabolol from particulate systems. Biomed Chromatogr 23:966–972
Perbellini L, Gottardo R, Caprini A, Bortolotti F, Mariotto S, Tagliaro F (2004) Determination of alpha-bisabolol in human blood by micro-HPLC–ion trap MS and head space-GC–MS methods. J Chromatogr B 812:373–377
Scalia S, Giuffreda L, Pallado P (1999) Analytical and preparative supercritical fluid extraction of chamomile flowers and its comparison with conventional methods. J Pharm Biomed Anal 21:549–558
Weber B, Herrmann M, Hartmann B, Joppe H, Schmidt CO, Bertram H-J (2008) HPLC/MS and HPLC/NMR as hyphenated techniques for accelerated characterization of the main constituents in Chamomile (Chamomilla recutita [L.] Rauschert). Eur Food Res Technol 226:755–760
Aoki F, Nakagawa K, Tanaka A, Matsuzaki K, Arai N, Mae T (2005) Determination of glabridin in human plasma by solid-phase extraction and LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 828:70–74
US Pharmacopeia (2009) General Chapters <1225> Validation of compendial methods. United States Pharmacopeal Convention, Inc., Rockville, Maryland, USA
Karageorgou E, Samanidou V (2014) Youden test application in robustness assays during method validation. J Chromatogr A 1353:131–139
Commission Decision 2002/657/EC implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Commun L221 8
Scortichini G, Annunziata L, Haouet MN, Benedetti F, Krusteva I, Galarini R (2005) ELISA qualitative screening of chloramphenicol in muscle, eggs, honey and milk: method validation according to the Commission Decision 2002/657/EC criteria. Anal Chim Acta 535:43–48
Furman WB, Dorsey JG, Snyder LR (1998) System suitability tests in regulatory liquid and gas chromatographic methods: adjustments versus modifications. Pharm Technol 22:58–65
US Food and Drug Administration (1994) Reviewer Guidance, validation of chromatographic methods. Centre for Drug Evaluation and Research, Washington
Acknowledgments
The authors gratefully acknowledge the financial support from the Gyeonggi Province Institute of Health and Environment (Suwon city, Republic of Korea).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Rights and permissions
About this article
Cite this article
Jeon, JS., Kim, HT., Kim, MG. et al. Simultaneous Detection of Glabridin, (−)-α-Bisabolol, and Ascorbyl Tetraisopalmitate in Whitening Cosmetic Creams Using HPLC-PAD. Chromatographia 79, 851–860 (2016). https://doi.org/10.1007/s10337-016-3104-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3104-2