Abstract
Studies of parental care in tropical birds are still relatively scarce in comparison with northern temperate species, especially regarding to the division of parental tasks, leading to a biased and incomplete knowledge of avian reproductive strategies. Herein, we studied the sexual division of parental care in a sexually monomorphic Neotropical passerine, the Pale-breasted Thrush (Turdus leucomelas). We recorded food provisioning and food quantity rates (e.g. feeding trips/h and food items/h, respectively), nest sanitation rate (e.g. events of faecal sacs removal/h), and the time devoted to nest attendance and brooding behaviours by each parent. Throughout the 2015–2017 breeding seasons, we video-recorded 153.5 h of parental care in 33 nesting attempts by 26 breeding pairs in a suburban area of south-eastern Brazil. We found that males had higher food provisioning rates and delivered more food items to larger broods, while female did not respond to brood size. As expected, brood age positively affected food provisioning and food quantity rates of both sexes. Faecal sacs were removed predominantly by ingestion throughout the nesting stage, and males had higher nest sanitation rates. Nest attendance reached 34 ± 27% of the time and decreased with nestling age following a decrease in brooding behaviour, a female-only task, while males stayed in the nest for only 4 ± 4% of observation time. Although most of the parental activities are performed by both sexes, males and females differed in which tasks they invested the most, with brood size and brood age being important modulating factors.
Zusammenfassung
Geschlechtsspezifische Arbeitsteilung von Fahlbrustdrossel-Eltern (Turdus leucomelas) bei der Brutpflege
Studien über die Brutpflege der Eltern sind bei tropischen Vögeln im Vergleich zu denen aus den nördlichen gemäßigten Zonen immer noch relativ rar, insbesondere was die Arbeitsteilung zwischen den Eltern betrifft. Dies führt zu einem einseitigen und unvollständigen Wissensstand über die Fortpflanzungsstrategien von Vögeln. Wir untersuchten die geschlechtsspezifische Aufteilung der elterlichen Brutpflege bei einem geschlechtsunabhängig einheitlich aussehenden neotropischen Sperlingsvogel, der Fahlbrustdrossel (Turdus leucomelas). Hierfür erfassten wir die Futterbeschaffung und -menge (z. B. Flüge/Stunde bzw. Futterstücke/Stunde), die Nestputzrate (z. B. Entfernung von Kotbeuteln/Stunde) und die Zeit, die die beiden Elterntiere für die Anwesenheit im Nest und für die Brutpflege aufwandten. Während der Brutsaison 2015-2017 machten wir Videoaufnahmen von 153,5 Stunden Brutpflege bei 33 Bruttversuchen von 26 Brutpaaren in einem Stadtrandgebiet im Südosten Brasiliens. Wir stellten fest, dass die Männchen intensiver Futter herbeischafften und bei größeren Bruten mehr Nahrung lieferten, während die Weibchen nicht auf die Brutgröße reagierten. Wie erwartet, wirkte sich bei beiden Gechlechtern das Alter der Brut verstärkend auf das Futtersuchverhalten und die Menge der herbei geschaffften Nahrung aus. Kotbeutel wurden während der gesamten Brutzeit zumeist durch Herunterschlucken entfernt, wobei die Männchen eine höhere Nestputzrate hatten. Die Anwesenheit im Nest betrug 34 ± 27 % der ganzen Zeit und nahm, wie auch das gesamte Brutpflegeverhalten, mit dem Alter der Jungen ab. Gebrütet wurde ausschließlich von den Weibchen, während die Männchen nur 4 ± 4 % der Beobachtungszeit im Nest zu sehen waren. Obwohl die meisten Arbeiten von beiden Geschlechtern ausgeführt wurden, unterschieden sich Männchen und Weibchen darin, in welche Aufgaben sie am meisten investierten, wobei die Größe und das Alter der Brut wichtige Steuerungsfaktoren waren.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parental care is described as any strategy or behaviour adopted by breeding individuals to promote the survival of their offspring, encompassing from energy provision for gametes to food provisioning and protection of offspring (Trivers 1972; Clutton-Brock 1991). Despite the great variety of reproductive strategies and patterns of parental care, social monogamy with biparental care is the most common reproductive strategy in birds (Cockburn 2006). In monomorphic and monogamous species, parents commonly have similar investment in reproductive activities like food provisioning, nest sanitation, and brooding (Gill and Haggerty 2012; Sánchez et al. 2018). However, in some of such species, energy investment and the role performed by parents differ considerably, with each sex performing certain breeding tasks exclusively or more frequently than its mate (Gowaty 1996; Stutchbury and Morton 2001; Kokko and Jennions 2008).
Even in more equitable division of parental tasks, there are asymmetric investments between parents due to anisogamy, physiological and behavioural differences between sexes (Lessels 2012). Although parental investment in monogamous monomorphic birds is considered fairly equitable, higher reproductive costs associated with parental care normally rests upon females due to egg laying and a higher frequency of egg and nestling brooding (Gowaty 1996; Carere and Alleva 1998; Bowers et al. 2014). In contrast, males usually engage in territory and nest defence through vigilance, vocalization, and physical aggression, while also providing food to the incubating and/or brooding female (Gowaty 1996; Winkler 2016). Therefore, differences in parental investment and division of reproductive tasks are expected to be found to varying degrees in monogamous monomorphic birds (Carere and Alleva 1998; Espíndola-Hernández et al. 2017; Sánchez et al. 2018).
Costs of parental investment in offspring increase considerably with brood size and age in altricial birds (Lack 1947; Skutch 1949). This investment increase is often nonlinear, as large broods usually receive less per capita food provisioning, leading to a trade-off between brood size and offspring quality (Silver et al. 1985; Stutchbury and Morton 2001; Bowers et al. 2014). The most frequent form of care used to quantify parental effort in the nesting stage is food provisioning to nestlings, as the time and energy required to supply the energetic demand of nestlings escalates with brood size and age (Silver et al. 1985; Bowers et al. 2014). By being more easily quantified, food provisioning has been used to gauge parental care, sexual and parent–offspring conflicts, as well as to understand breeding strategies of altricial birds (Low et al. 2012; Gill and Haggerty 2012; MacLeod and Brouwer 2018). Nest attendance and nestling brooding (nestling thermoregulation) are also important for the comprehension of sex asymmetry in energy investment, as such tasks may occupy a large part of the daily activities of parents (Carere and Alleva 1998; Hill et al. 1999; Evans and Stutchbury 2012). Lastly, nest sanitation can be a costly and frequent activity for parents, as it requires parents visiting the nest, waiting for and/or prodding nestlings to defecate, and then disposing off the nestlings’ excrements (Hurd et al. 1991; Ibáñez-Álamo et al. 2017).
In many altricial birds, nestling excrements are enclosed in a mucous covering called faecal sacs (Ibáñez-Álamo et al. 2013). Through the removal of faecal sacs, birds avoid the potential negative effects of excrements soiling the nest structure (Ibáñez-Álamo et al. 2014, 2016). The research on nest sanitation has been usually focused on its importance to prevent diseases and parasites from afflicting the parents and offspring through removal of external or unwanted material and faecal sacs, or on the direct benefits of faecal sac ingestion by the parents (i.e. the acquisition of nutrients or water) (Hurd et al. 1991; Ibáñez-Álamo et al. 2016, 2017). The more frequent disposal of faecal sacs by a specific sex could serve as an indication of higher breeding effort, indicating a need and/or benefit to recycle water and nutrients from faecal sacs, possibly providing another way to compare parental expenditure between sexes (Hurd et al. 1991; McKay et al. 2009). The few studies that integrate nest sanitation in the framework of sexual differences in parental care report cases of female-dominant, male-dominant, and equitable nest sanitation (Carere and Alleva 1998; Markman et al. 1995; McKay et al. 2009; Bolopo et al. 2015).
The topic of parental care has traditionally attracted attention from the ornithological scientific community, but severe gaps in our knowledge do remain, especially in the southern hemisphere and regarding tropical birds, where few studies exist and extended parental care occurs in comparison with northern hemisphere species (Russell et al. 2004). We studied a Neotropical passerine, the Pale-breasted Thrush (Turdus leucomelas), to evaluate the parental division of tasks during the nesting stage by investigating different components of parental care (i.e. food provisioning, nest attendance and brooding, and nest sanitation). We hypothesized that the Pale-breasted Thrush would present indistinguishable food provisioning rates between sexes irrespective of brood size and age, but nest attendance and brooding would be carried out by females, akin to other species of the genus (e.g. Hill et al. 1999; Sánchez et al. 2018; Batisteli et al. 2020). Regarding nest sanitation, we expected a pattern similar to food provisioning with which it is usually synchronized (Quan et al. 2015).
Methods
Study area
The study was conducted in the campus of the Universidade Estadual Paulista “Júlio de Mesquita Filho” (UNESP) (22°23′45.7″S 47°32′38.3″W) at Rio Claro, Brazil, between 2015 and 2017 from September to December, which covers most of the species’ breeding season (Batisteli et al. 2021). Rio Claro is at the transition between the semideciduous Atlantic Forest and the Cerrado biomes, though heavily impacted by anthropic land uses as sugarcane farmland, pasture, and Eucalyptus plantations. The campus (111.46 ha) consists of a mix of manmade gardens and small forest patches of native vegetation. The regional climate has two well defined seasons: a dry season from April to September (~ 240 mm rainfall) and a wet season from October to March (~ 1100 mm) (Alvares et al. 2013).
Study species
The Pale-breasted Thrush is a visually monomorphic species with a widespread distribution in South America, from the Guyanas, south of Colombia and Venezuela, almost every state in Brazil (except Acre and Rondônia), Paraguay and north Argentina (Sick 1997). It is an omnivore species, feeding primarily on fruits and invertebrates (mainly arthropods), and rarely small vertebrates, found in forest borders, clearings, savannahs, gallery forests, rural and urban areas (Sick 1997; Collar 2005).
The breeding season at the study region spans from September to January; and the breeding cycle of the Pale-breasted Thrush has an average duration of two months from egg laying and incubation (12–13 days), nestling (14–16 days) to post-fledging stages (30–40 days) (Davanco et al. 2013). At the campus, the species prefers areas with sparse to dense trees and shrubs, nesting in buildings and on the vegetation, mainly on forks of large tree trunks. One to four eggs per clutch are laid (usually two to three) in a cup-shaped nest built by the female with roots, vegetation, and mud (Davanco et al. 2013). Up to two successful clutches are laid in a breeding season, with a replacement clutch occurring if one of the previous clutches was unsuccessful (Batisteli et al. 2021).
Field procedures
Adult thrushes were captured with mist nets and marked with a coded metal ring and coloured rings. We collected a small drop of blood by cutting the tip of the nail from each captured individual for sex identification, which was done by an outsourced laboratory (UNIGEN, São Paulo) through Polymerase Chain Reaction (PCR) amplification of DNA sex-specific gene sequences. We observed only breeding pairs for which at least one of the parents was sexed and colour-ringed to enable sex identification from a distance.
Nests were found by active searching and monitored every 2–3 days with the aid of a pole with an attached mirror to record clutch size and hatching days. We used GoPro Hero 2 cameras positioned 0.5–1.0 m from nests, distances that allowed for quality recordings. Parents quickly returned to the nests and resumed breeding tasks after camera installation, which allows us to assume a minimal interference of this procedure on the parents’ behaviour. Recordings began the day following the hatching of all the eggs in nests found with eggs (n = 24), or immediately for nests discovered already with nestlings (n = 9). For clutches found after eggs had already hatched, nestling age was estimated by comparing feather development to photos of other monitored clutches of known age. We carried out filming sessions of 0.9–2.0 h duration (see Results) every two days either during the morning (07:00–12:30 h) or afternoon (14:00–18:00 h) depending on weather conditions. Recordings ceased when nestlings were 14–16 days old to avoid their premature departure from the nest upon our approximation (Appendix 1, Fig. S1).
Video recordings were then viewed to record for each parent the number of feeding visits per hour (hereafter “food provisioning rate”), the number of food items brought per hour (hereafter “food quantity rate”) (Biermann and Sealy 1982; Gill and Haggerty 2012), nest sanitation visits (i.e. visits in which there was faecal sac removal), nest sanitation rate (i.e. number of faecal sacs removed per hour), and the method of faecal sac removal (i.e. either the removal by carrying the faecal sac away from the nest or the ingestion of it at the nest; Ibáñez-Álamo et al. 2013). We also estimated the percentage of parental attendance to the nest, defined as the time spent inside or perched on the nest rim (Appendix 1, Fig S2), and the percentage of time spent brooding the nestlings, defined as the parent laying on top of the nestlings, both calculated in relation to the duration of each filming session. Since brood parasitism can modify parental care behaviour and sometimes sexual conflict (Hoover and Reetz 2006; Požgayová et al. 2015), nests parasitized by the Shiny Cowbird (Molothrus bonariensis) were excluded from the study. We gathered data from 10 broods in 2015, 12 in 2016 and 11 in 2017.
Statistical analysis
We used Generalized Linear Mixed-Effects Models (GLMMs) with Poisson error distribution to investigate whether there were sex differences in parental care behaviour during the nestling stage, having as response variables food provisioning, food quantity, and nest sanitation rates. In these models, we included parent sex (male or female), brood size (1, 2 or 3), period of day (morning/afternoon), and the interaction between sex × brood size as fixed factors, and brood age as a covariate. We included in all these models the duration of each filming session as an offset variable, i.e. an independent variable with coefficient fixed at 1. As two nests were reused in the same breeding season and other five nests were used in multiple years (i.e. one nest used three times and five nests used twice), nest identification and a brood code made up of brood identification and year were used as random factors.
We ran Tukey pairwise post-hoc tests to evaluate male and female differences when the interaction term was significant, and to identify the differences in parental care behaviours according to brood size when it was a significant factor. For nest attendance, we built Linear Mixed-Effects Model (LMM) using the percentage of time spent on the nest (arc-sine and square-root transformed to achieve residual normality) as a continuous response variable, while sex, brood size and period of day as fixed factors and brood age as a covariate. In this case, the interaction between sex and brood age was included because brooding differs between sexes in this genus, with the females being the major or sole sex responsible for brooding (Hill et al. 1999; Batisteli et al. 2020), which tends to cease as nestlings age. Herein, nest identification and brood code were used as random factors. We used a backward stepwise procedure to select the significant factors of the models. Additionally, we utilised the Akaike Information Criterion (AIC) to confirm the results and best model, and the most relevant factors from our previous procedure. Models were ranked from the lowest AIC score. Only models with ΔAIC < 2 for each response variable were presented, except for cases in which there would be a single model, when the second-best model was presented as well. We visually checked whether the residuals of each model complied with assumptions of normality. All analyses were done in R version 3.6.2 (R Core Team 2019) using the packages ‘car’ (Fox et al. 2012), ‘emmeans’ (Lenth et al. 2020), ‘lme4’ (Bates et al. 2015), ‘lmerTest’ (Kuznetsova et al. 2017), AICcmodavg (Mazerolle 2019). Values are reported as mean ± standard deviation.
Results
We recorded 33 nesting attempts (with initial brood sizes of 1, 2 and 3 nestlings in 9, 13, and 11 nests, respectively) by 26 unique breeding pair combinations. Brood reduction from 3 to 2 and from 2 to 1 nestling occurred in two and three breeding events, respectively. Filming sessions averaged 1.5 ± 0.2 h, ranging from 0.9–2.0 h, totalling 153.5 h. Each nest was filmed on average 3.2 ± 1.4 times (range 1–6 times).
Food provisioning and food quantity
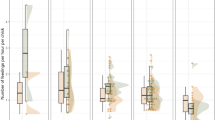
The overall average food provisioning rate was 1.61 ± 1.13 feeding trips/h. The best model for food provisioning rate included the single terms sex, brood size and brood age, whereas the interaction between sex and brood size and the period of day were deemed unimportant and removed (Table 1). Both sexes fed the nestlings, with a significant difference between them in food provisioning (Table 1, males = 1.76 ± 1.12 feeding trips/h/nestling, females = 1.45 ± 1.12 feeding trips/h/nestling). Female provisioning rates were 2.23 ± 1.51, 2.45 ± 1.43, 2.70 ± 1.24 feeding trips/h for broods with one, two and three nestlings, respectively, whereas the corresponding male food provisioning rates were 2.42 ± 1.42, 3.35 ± 2.21, 4.28 ± 1.96 feeding trips/h (Fig. 1A). Overall food provisioning rate increased with brood age (Table 1, Fig. 2A). Post-hoc tests revealed a difference in food provisioning between broods with one and three nestlings (Tukey: z = −3.156, p < 0.005), but no difference between broods with one to two, and two to three nestlings (both p > 0.120).
Food provisioning rate A food quantity rate B and nest sanitation rates C of males and females Pale-breasted Thrushes (Turdus leucomelas) according to brood size. In boxplots horizontal lines represent medians, whiskers represent minimum and maximum values, while dots represent outliers. Asterisk indicates significant difference at α = 0.05
The overall average food quantity rate was 2.08 ± 1.66 food items/h. The best model for food quantity rate included all the factors except period of day (Table S1). Food quantity rate, in contrast to food provisioning, was not affected by brood size, but brood age, sex, and the interaction sex × brood size were all significant factors (Table 1). Food quantity rate increased with brood age (Fig. 2B) and differed between sexes (Table 1, males = 2.33 ± 1.64 food items/h/nestling, females = 1.80 ± 1.64 food items/h/nestling). Post-hoc tests revealed that males showed higher food quantity rates than females in broods with three nestlings (Tukey: z = −1.336, p < 0.001), but not in broods with one or two nestlings (both p > 0.786) (Fig. 1B). Post-hoc tests also revealed that food quantity rate provided by males did not differ between broods with one to two nestlings (z = 2.625, p = 0.091), and two to three nestlings (z = −2.658, p = 0.084), but there was difference between broods of one to three nestlings (z = 4.756, p = < 0.001). The quantity of food delivered by females did not change with brood size (p > 0.786 for all pairwise comparisons) (Fig. 1B).
Nest sanitation
In 91.5% of the nest sanitation visits, a single faecal sac was removed. Therefore, we did not analyse the quantity of faecal sacs removed per nest sanitation visit. In 377 events of nest sanitation, 418 faecal sacs were removed by either ingesting or carrying faecal sacs away in the beak. Both sexes participated in nest sanitation, and faecal sacs were ingested 153 times by females (90.53% of their total faecal sac removal) and 214 times by males (86.29%). Females and males carried away the remaining 16 and 34 faecal sacs, respectively. A female fed 1 faecal sac to a nestling. There was no faecal sac unremoved from the nest, and most of them were removed as soon as nestlings defecated, which often occurred synchronized with food provisioning.
The best model for nest sanitation rate included brood size, sex, and the interaction between these two factors, whereas brood age and period of day were dropped off (Table 1). There was a significant difference between sexes in sanitation rates (Table 1, males = 0.71 ± 0.59 sanitation event/h/nestling, females = 0.59 ± 0.73). Nest sanitation rates were not affected by brood size, whereas the p-value of the interaction sex × brood size was significant but close to the critical level (Table 1). However, pairwise comparisons by the post-hoc test revealed no differences between sexes in nest sanitation rates in broods with one, two or even three nestlings (p > 0.184 for all pairwise comparisons).
Nest attendance
Visits to the nest lasted on average 7.05 ± 8.45 min for females and 0.82 ± 1.7 min for males. Females spent on average 34 ± 27% of the observation time on the nest, and 24 ± 26% brooding nestlings. Males spent 4 ± 4% of the observation time on the nest and did not exhibit any brooding behaviour toward nestlings.
The best model for nest attendance included sex, brood age, the interaction between these two terms, but not brood size and period of day (Table 1). Nest attendance was not influenced by the number of nestlings in the brood, but it decreased with nestling age (Fig. 2C). The interaction between sex and brood age was significant, indicating that females decreased their attendance to nests as nestling grew, while nest attendance by males remained constant and low (Fig. 2C).
Discussion
We found that both males and females of the Pale-breasted Thrush engaged in food provisioning, nest sanitation and nest attendance, but differed in their investment in each of the tasks. Interestingly, for food quantity and nest sanitation rates, we found a significant effect of the interaction between sex and brood size, suggesting that the number of nestlings influences care investment differently by each parent. In this sense, while females keep constant their food quantity rate with increasing brood size, males modulate their effort by increasing the number of food items delivered to the young. Furthermore, brood age was positively related to both food provisioning and food quantity rates, likely due to the gradual changes of nestling energy requirements over their rapid growth and development period. Nest sanitation was performed by both sexes, with males having higher rates of faecal sac removal. Finally, only females brooded nestlings and thus remained on the nest for longer periods than males. This extensive nest attendance by females decreased rapidly through the nesting stage, as nestling development leads to an increased thermoregulation capacity (Andreasson et al. 2016), consequently allowing for a reduction of time spent brooding and attending nests. Males had consistently short nest attendance periods throughout the nesting period, usually only visiting to feed nestlings and remove faecal sacs. Nonetheless, while body masses do not differ between sexes in the non-breeding period in our study population, females tend to have higher body mass than males during the breeding season (Floreste et al. 2021). Therefore, it is possible that the care of nestlings in addition to the other reproductive tasks performed by males (e.g. territory defence, nest sanitation, fledgling care) take a more severe toll on their body condition than to females.
Food provisioning and food quantity
Food provisioning and food quantity, two complementary aspects of nestling provisioning effort, were influenced by different factors. Similar provisioning rates were expected for males and females of the Pale-breasted Thrush as observed in other thrush species (Sánchez et al. 2018), but males showed higher food provisioning and quantity rates, both increasing with nestling age. These intersexual differences cannot be attributed to a specialization in parental roles as both delivered invertebrates and fruits to the nestlings in similar frequencies (Pizo, unpubl. data). A larger brood size (e.g. three nestlings) elicited a higher food provisioning rate (feeding trips/h) irrespective of the parent sex, however, the effect of brood size on food quantity (food items/h) was restricted to males. Brood size and age are factors known to affect positively food provisioning rates in birds (Silver et al. 1985; Gill and Haggerty 2012; Bowers et al. 2014), as both age and the number of nestlings increase energy requirement and thus food demand. Therefore, larger broods require greater parental investment to sustain growth and development, and stable per capita food provisioning across brood sizes benefit the survival chances and quality of the offspring (Stutchbury and Morton 2001; Bowers et al. 2014).
In our study, although total food quantity increased with brood size, the largest broods still received lower per capita provisioning in comparison with single-nestling broods (Fig. 1A, B). Males of the Pale-breasted Thrush fulfil the role of keeping the per capita food quantity rate from declining drastically in the largest broods. In contrast, the number of food items delivered per hour by females are nearly constant across brood sizes, meaning that its per capita food quantity rate decreases in larger broods. This difference is only present in broods with three nestlings, in which males had significantly higher food quantity rates. A similar case of only males adjusting food provisioning rates as a function of brood size was found in the Common Swift (Apus apus), in which larger broods resulted in increased male effort, while smaller and older broods lead to reduced care by males in favour of seeking extra-pair copulations (Carere and Alleva 1998). Indeed, the certainty of paternity has been suggested as an important factor influencing the commitment of male passerines to parental duties (Arnold and Owens 2002), but unfortunately we do not have information on the frequency of extra-pair copulations in our study population to evaluate this possibility.
As larger clutches represent a higher energetic cost for females than smaller clutches (Visser and Lessells 2001), the female tendency to maintain food quantity rates constant across brood sizes could be explained with fitness constraints associated with laying a larger clutch. However, if that was the case, the number of food items per hour delivered by females would have been expected to be higher in smaller clutches, which did not happen. A likely alternative explanation for the constant provisioning effort by females is that they performed at their maximum level (Drent and Daan 1980), but this seems not feasible in our case. Otherwise, we should assume that females are at their maximum effort even in clutches of a single nestling, which provides weak support for this idea considering that the modal clutch size in this species is three (Batisteli et al. 2021).
While males do increase their food quantity for large broods, alone they are not able to keep the food quantity rates as high as in smaller broods, meaning that the combined per capita food quantity will inevitably be reduced for large broods, which may impact nestling quality (i.e. body mass and/or nestling development) and survival in the post-fledging stage (Styrsky et al. 2005; Gill and Haggerty 2012; Bowers et al. 2014; Sofaer et al. 2018; Evans et al. 2020). Thus, a trade-off could be at play, as the higher survival chance of a small clutch might be a breeding strategy as viable as a larger clutch with lower survival chance (Smith et al. 1989). Still, there is some contention on the claim that clutch size directly influences fledgling survival (Bowers et al. 2014; Remeš and Matysiovoká 2016). Given that clutches of three eggs are common in our study population (66% of 129 clutches; M. A. Pizo unpubl.), we assume that despite the possibility of lower post-fledging survival of a larger brood, producing a higher number of fledglings remains advantageous for the Pale-breasted thrush.
The amount of investment provided by males of the Pale-breasted Thrush was unexpected, as there are few cases of higher male food provisioning in passerines with biparental care (Carere and Alleva 1998; Hill et al. 1999; Reed et al. 2007). Additionally, males were responsible for increasing the parental effort in delivering food to broods with three nestlings. For this to happen, a higher brood size needs to be a worthwhile investment considering the potential inverse relationship between the energy invested in current reproduction and future breeding prospects (Parejo and Danchin 2006; Fokkema et al. 2016). In a more equal sexual selection scenario, both sexes will seek to maintain high quality partners over a breeding season or consecutive years. It might be that males perceive the ability to lay clutches of three eggs as a trait that indicates a high-quality female (Gill and Stutchbury 2005; Mahr et al. 2012; Peralta-Sánchez et al. 2020), as the parental investment of an individual has been shown to influence the investment of its mate (Gori 1988; Bebbington and Hatchwell 2016). Possibly, males might also be contributing to avoid decay in female condition, which could enhance the chance of a second nesting attempt (Ritchison et al. 2019). If breeding pairs last through multiple nesting attempts and years, the advantages of great investment in the current partnership increase, as securing high quality and experienced partners is advantageous for reproductive success (Peralta-Sánchez et al. 2020). This is precisely what seems to happen in our study population (A. Batisteli unpubl. data).
Nest sanitation
Both males and females removed faecal sacs, but as with food provisioning rate, nest sanitation rates differed between sexes, being higher for males. The per capita faecal sac removal remained constant in larger broods despite the per capita reduction of food provisioning, which might have led to a decrease in faecal sac removal in larger broods. The synchronization of provisioning and nest sanitation events led us to expect higher nest sanitation rates by males in larger broods, which was not the case. This might simply be explained by the short time that males spent in the nest to feed the nestlings, leaving the nest prior to nestling defecation. As females spent much more time in the nest, they might have removed the faecal sacs that were left unremoved by the males. Furthermore, removal of faecal sacs did not increase with brood age, which suggests that the size of faecal sacs rather than the number of faecal sacs produced increases with nestling age (Weatherhead 1984), akin to what we suggested previously for food provisioning rates. This suspicion is supported by video evidence when comparing the size of faecal sacs with the beak of the parents (Appendix 1, Fig. S3).
As previously mentioned, our results on the method of disposal of faecal sacs might imply that the ingestion of faecal sacs contributes to supply the energetic need of the parents. Hypotheses explaining the putative benefits of faecal sac ingestion are: (1) Parental-Nutrition Hypothesis, which predicts that recycling nutrients from faecal sacs are beneficial, increasing with energetic stress and brood size (McGowan 1995; McKay et al. 2009); the (2) Economic Disposal Hypothesis (Hurd et al. 1991) that proposes that the time saved by ingestion of faecal sacs may be invested in other activities, and the (3) Nest Predation Hypothesis that predicts that ingestion is beneficial by reducing parental movement in and out of the nest, therefore reducing the risk of nest predation and enabling higher nest attendance. In this hypothesis, frequency of faecal sac ingestion increases with larger brood sizes (Ibáñez-Álamo et al. 2013). The first two hypotheses predict higher frequency of ingestion from the most stressed and energy constrained sex, usually the female. In our case, however, males ingested a higher proportion of faecal sacs, which may imply a higher energetic demand, which is supported by the previously mentioned study reporting a reduction in male body condition during the breeding season in our study population (Floreste et al. 2021). Interestingly, the preferential method of faecal sac removal was ingestion throughout the whole nestling stage, contrary to the gradual decrease in faecal sac ingestion with the progress of the nesting stage usually reported in the literature (Darveau et al. 1993). Apart from the energetic demands of parents occasionally supplied by faecal sac ingestion, a lack of mechanical constraint due to the large size of parents in relation to the faecal sacs produced, even at later stages of nestling development, is what makes possible the continued ingestion of faecal sacs throughout the nestling period. Otherwise, faecal sacs would be likely removed rather than ingested (Darveau et al. 1993).
To conclude, several of our initial expectations for the division of reproductive tasks between the sexes in the Pale-breasted Thrush were not met, as the parental investment in our study species differs for most tasks. We have found that only males increased their effort by providing more food items for nestlings according to brood size, while a higher demand from offspring development is also supplied by both sexes with an increase in food provisioning and food quantity. Males also performed faecal sac removal more often than females. Our results then suggest a complex sexual conflict underlying the division of parental tasks in this monomorphic and socially monogamous bird.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alvares CA, Stape JL, Sentelhas PC, Gonçalves JDM, Sparovek G (2013) Köppen’s climate classification map for Brazil. Meteorol Z 22:711–728. https://doi.org/10.1127/0941-2948/2013/0507
Andreasson F, Nord A, Nilsson JÅ (2016) Brood size constrains the development of endothermy in blue tits. J Exp Biol 219:2212–2219. https://doi.org/10.1242/jeb.135350
Arnold KE, Owens IP (2002) Extra-pair paternity and egg dumping in birds: life history, parental care and the risk of retaliation. Proc Royal Soc London B Biol Sci 269:1263–1269. https://doi.org/10.1098/rspb.2002.2013
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Batisteli AF, Costiuc MY, Santieff IZ, Costa RO, Sarmento H, Pizo MA (2020) Breeding biology of the creamy-bellied Thrush (Turdus amaurochalinus) in southeast Brazil. Stud Neotrop Fauna Environ 55:233–241. https://doi.org/10.1080/01650521.2020.1728032
Batisteli AF, Sarmento H, Pizo MA (2021) Nest reuse by Pale-breasted Thrushes reduces the chance of cowbird parasitism and allows earlier initiation of breeding. J Field Ornithol 92:105–114. https://doi.org/10.1111/jofo.12363
Bebbington K, Hatchwell BJ (2016) Coordinated parental provisioning is related to feeding rate and reproductive success in a songbird. Behav Ecol 27:652–659. https://doi.org/10.1093/beheco/arv198
Biermann GC, Sealy SG (1982) Parental feeding of nestling Yellow Warblers in relation to brood size and prey availability. Auk 99:332–341. https://doi.org/10.1093/auk/99.2.332
Bolopo D, Canestrari D, Marcos JM, Baglione V (2015) Nest sanitation in cooperatively breeding carrion crows. Auk 132:604–612. https://doi.org/10.1642/AUK-14-233.1
Bowers EK, Nietz D, Thompson CF, Sakaluk SK (2014) Parental provisioning in house wrens: effects of varying brood size and consequences for offspring. Behav Ecol 25:1485–1493. https://doi.org/10.1093/beheco/aru153
Carere C, Alleva E (1998) Sex differences in parental care in the common swift (Apus apus): effect of brood size and nestling age. Can J Zool 76:1382–1387. https://doi.org/10.1139/z98-073
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Cockburn A (2006) Prevalence of different modes of parental care in birds. Proc Royal Soc B Biol Sci 273:1375–1383. https://doi.org/10.1098/rspb.2005.3458
Collar NJ (2005) Family Turdidae (Thrushes). In: Del Hoyo J, Elliot A, Christie DA (eds) Handbook of the birds of the world, 10th edn. Lynx Edicions, Barcelona, pp 514–807
Darveau M, Gauthier G, Desgranges JL, Mauffette Y (1993) Nesting success, nest sites, and parental care of the least flycatcher in declining maple forests. Can J Zool 71:1592–1601. https://doi.org/10.1139/z93-22
Davanco PV, Oliveira LS, Sousa LM, Francisco MR (2013) Breeding life-history traits of the pale-breasted thrush (Turdus leucomelas) in southeastern Brazil. Ornitol Neotrop 24:401–411
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding 1. Ardea 55:225–252. https://doi.org/10.5253/arde.v68.p225
Espíndola-Hernández P, Castaño-Villa GJ, Vásquez RA, Quirici V (2017) Sex-specific provisioning of nutritious food items in relation to brood sex ratios in a non-dimorphic bird. Behav Ecol Sociobiol 71:65. https://doi.org/10.1007/s00265-017-2294-4
Evans ML, Stutchbury BJ (2012) Nest attendance and reproductive success in the wood thrush. Condor 114:401–406. https://doi.org/10.1525/cond.2012.110112
Evans DR, Hobson KA, Kusack JW, Cadman MD, Falconer CM, Mitchell GW (2020) Individual condition, but not fledging phenology, carries over to affect post-fledging survival in a neotropical migratory songbird. Ibis 162:331–344. https://doi.org/10.1111/ibi.12727
Floreste FR, Batisteli AF, Pizo MA (2021) Sex-specific seasonal body mass variation in the pale-breasted thrush (Turdus leucomelas). Ornithol Res 29:84–88. https://doi.org/10.1007/s43388-021-00055-0
Fokkema RW, Ubels R, Tinbergen JM (2016) Great tits trade off future competitive advantage for current reproduction. Behav Ecol 27:1656–1664. https://doi.org/10.1093/beheco/arw097
Fox J, Weisberg S, Adler D, Bates D et al (2012) Package ‘car.’ R Foundation for Statistical Computing, Vienna, p 16
Gill SA, Haggerty TM (2012) A comparison of life-history and parental care in temperate and tropical wrens. J Avian Biol 43:461–471. https://doi.org/10.1111/j.1600-048X.2012.05637.x
Gill SA, Stutchbury BJ (2005) Nest building is an indicator of parental quality in the monogamous neotropical buff-breasted wren (Thryothorus leucotis). Auk 122:1169–1181. https://doi.org/10.1093/auk/122.4.1169
Gori DF (1988) Colony-facilitated foraging in Yellow-headed Blackbirds: experimental evidence for information transfer. Ornis Scand 19:224–230. https://doi.org/10.2307/3676563
Gowaty PA (1996) Field Studies of Parental Care in Birds: New Data Focus Questions on Variation among Females. In: Snowdon CT (ed) Parental Care: Evolution, Mechanisms, and Adaptive Significance. Academic Press, San Diego, pp 477–531
Hill IF, Cresswell B, Kenward RE (1999) Comparison of brooding patterns between Blackbird Turdus merula and Song Thrush T. philomelos. Bird Study 46:122–126. https://doi.org/10.1080/00063659909461124
Hoover JP, Reetz MJ (2006) Brood parasitism increases provisioning rate, and reduces offspring recruitment and adult return rates, in a cowbird host. Oecologia 149:165–173. https://doi.org/10.1007/s00442-006-0424-1
Hurd PL, Weatherhead PJ, McRae SB (1991) Parental consumption of nestling feces: good food or sound economics? Behav Ecol 2:69–76. https://doi.org/10.1093/beheco/2.1.69
Ibáñez-Álamo JD, Sanllorente O, Arco L, Soler M (2013) Does nest predation risk induce parent birds to eat nestlings’ fecal sacs? An experimental study. Ann Zool Fenn 50:71–78. https://doi.org/10.5735/086.050.0106
Ibáñez-Álamo JD, Ruiz-Raya F, Roncalli G, Soler M (2014) Is nest predation an important selective pressure determining fecal sac removal? The effect of olfactory cues. J Ornithol 155:491–496. https://doi.org/10.1007/s10336-013-1031-7
Ibáñez-Álamo JD, Ruiz-Raya F, Rodríguez L, Soler M (2016) Fecal sacs attract insects to the nest and provoke an activation of the immune system of nestlings. Front Zool 13:1–9. https://doi.org/10.1186/s12983-016-0135-3
Ibáñez-Álamo JD, Rubio E, Soler JJ (2017) Evolution of nestling faeces removal in avian phylogeny. Anim Behav 124:1–5. https://doi.org/10.1016/j.anbehav.2016.11.033
Kokko H, Jennions MD (2008) Parental investment, sexual selection and sex ratios. J Evol Biol 21:919–948. https://doi.org/10.1111/j.1420-9101.2008.01540.x
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:1–26
Lack D (1947) The significance of clutch-size. Ibis 89:302–352. https://doi.org/10.1111/j.1474-919X.1947.tb04155.x
Lenth RV, Buerkner P, Herve M et al (2020) Emmeans: estimated Marginal Means, aka Least- Squares Means. R package version 1.4.8 [Online]. https://CRAN.R-project.org/package=emmeans. Accessed 12 Dec 2020
Lessels CM (2012) Patterns of parental care in vertebrates. In: Royle NJ, Smiseth PT, Kölliker M (eds) The evolution of parental care. Oxford University Press, Oxford, pp 150–168
Low M, Makan T, Castro I (2012) Food availability and offspring demand influence sex-specific patterns and repeatability of parental provisioning. Behav Ecol 23:25–34. https://doi.org/10.1093/beheco/arr145
MacLeod KJ, Brouwer L (2018) Social context-dependent provisioning rules in red-winged fairy-wrens do not vary with signals of increased chick need. Anim Behav 143:105–111. https://doi.org/10.1016/j.anbehav.2018.07.010
Mahr K, Griggio M, Granatiero M, Hoi H (2012) Female attractiveness affects paternal investment: experimental evidence for male differential allocation in blue tits. Front Zool 9:1–8. https://doi.org/10.1186/1742-9994-9-14
Markman S, Yom-Tov Y, Wright J (1995) Male parental care in the orange-tufted sunbird: behavioural adjustments in provisioning and nest guarding effort. Anim Behav 50:655–669. https://doi.org/10.1016/0003-3472(95)80127-8
Mazerolle MJ (2019) AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R package version 2.2–2
McGowan KJ (1995) A test of whether economy or nutrition determines fecal sac ingestion in nesting corvids. Condor 97:50–56. https://doi.org/10.2307/1368982
McKay JE, Murphy MT, Smith SB, Richardson JK (2009) Fecal-sac ingestion by spotted towhees. Condor 111:503–510. https://doi.org/10.1525/cond.2009.080065
Parejo D, Danchin E (2006) Brood size manipulation affects frequency of second clutches in the blue tit. Behav Ecol Sociobiol 60:184–194. https://doi.org/10.1007/s00265-005-0155-z
Peralta-Sánchez JM, Colmenero J, Redondo-Sánchez S, Ontanilla J, Soler M (2020) Females are more determinant than males in reproductive performance in the house sparrow Passer domesticus. J Avian Biol 51:e02240. https://doi.org/10.1111/jav.02240
Požgayová M, Beňo R, Procházka P, Jelínek V, Abraham MM, Honza M (2015) Lazy males and hardworking females? Sexual conflict over parental care in a brood parasite host and its consequences for chick growth. Behav Ecol Sociobiol 69:1053–1061. https://doi.org/10.1007/s00265-015-1918-9
Quan RC, Li H, Wang B, Goodale E (2015) The relationship between defecation and feeding in nestling birds: observational and experimental evidence. Front Zool 12:1–7. https://doi.org/10.1186/s12983-015-0116-y
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 5 July 2019
Reed LP, Vallender R, Robertson RJ (2007) Provisioning rates by golden-winged warblers. Wilson J Ornithol 119:350–355. https://doi.org/10.1676/06-050.1
Remeš V, Matysioková B (2016) Survival to independence in relation to pre-fledging development and latitude in songbirds across the globe. J Avian Biol 47:610–618. https://doi.org/10.1111/jav.00841
Ritchison G, Hawkins JA, Ritchison BC (2019) Brooding and provisioning of nestlings by male and female White-eyed Vireos (Vireo griseus). Avian Biol Res 12:28–32. https://doi.org/10.1177/1758155919832138
Russell EM, Yom-Tov Y, Geffen E (2004) Extended parental care and delayed dispersal: northern, tropical, and southern passerines compared. Behav Ecol 15:831–838. https://doi.org/10.1093/beheco/arh088
Sánchez NV, Vargas-Castro LE, Barrantes G (2018) Nestling feeding, nest success, and notes of parental care in the Clay-colored Thrush (Turdus grayi): the role of females and males. Wilson J Ornithol 130:437–444. https://doi.org/10.1676/17-002.1
Sick H (1997) Ornitologia Brasileira, 3rd edn. Editora Nova Fronteira, Rio de Janeiro
Silver RAE, Andrews H, Ball GF (1985) Parental care in an ecological perspective: a quantitative analysis of avian subfamilies. Am Zool 25:823–840. https://doi.org/10.1093/icb/25.3.823
Skutch AF (1949) Do tropical birds rear as many young as they can nourish? Ibis 91:430–455. https://doi.org/10.1111/j.1474-919X.1949.tb02293.x
Smith HG, Kallander H, Nilsson JA (1989) The trade-off between offspring number and quality in the great tit Parus major. J Anim Ecol 58:383–401. https://doi.org/10.2307/4837
Sofaer HR, Sillett TS, Yoon J, Power ML, Morrison SA, Ghalambor CK (2018) Offspring growth and mobility in response to variation in parental care: a comparison between populations. J Avian Biol 49:jab-01646. https://doi.org/10.1111/jav.01646
Stutchbury JM, Morton ES (2001) Behavioral ecology of tropical birds. Academic Press, London
Styrsky JN, Brawn JD, Robinson SK (2005) Juvenile mortality increases with clutch size in a neotropical bird. Ecology 86:3238–3244. https://doi.org/10.1890/04-1613
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Aldine Press, Chicago, pp 136–179
Visser ME, Lessells CM (2001) The costs of egg production and incubation in great tits (Parus major). Proc Royal Soc London B Biol Sci 268:1271–1277. https://doi.org/10.1098/rspb.2001.1661
Weatherhead PJ (1984) Fecal sac removal by tree swallows: the cost of cleanliness. Condor 86:187–191. https://doi.org/10.2307/1367039
Winkler DW (2016) Breeding biology of birds. In: Lovette IJ, Fitzpatric JW (eds) Handbook of bird biology. John Wiley & Sons, New York, pp 407–450
Acknowledgements
We thank the field assistants for nest searching and monitoring. AFB receives a postdoctoral grant #2020/12211-0, São Paulo Research Foundation (FAPESP). MAP receives a research fellowship from the Brazilian Research Council (CNPq #304742/2019-8).
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Communicated by S. Bouwhuis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Haddad, R.N.M., Batisteli, A.F., Ibáñez-Álamo, J.D. et al. Sexual division of nestling parental care in the Pale-breasted Thrush (Turdus leucomelas). J Ornithol 165, 193–202 (2024). https://doi.org/10.1007/s10336-023-02100-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02100-9