Abstract
The Scytalopus superciliaris complex consists of three recognized taxa: the Zimmer´s Tapaculo Scytalopus zimmeri in Bolivia and Argentina, and the Argentina endemic White-browed Tapaculo S. superciliaris with subspecies superciliaris and santabarbarae, while its southernmost population has a distinctive plumage but remains vocally unknown. Scytalopus zimmeri has been considered as an intermediate taxon between Puna Tapaculo S. simonsi and S. superciliaris based on plumage characters and vocalizations; and potential hybrids between S. zimmeri and S. simonsi were reported from two localities in Bolivia. We characterized the geographic variation in plumages and vocalizations of the S. superciliaris complex and S. simonsi, and describe a new pale and large subspecies, S. superciliaris ambatensis from the Ambato and Velasco ranges. All studied forms were allopatric. The three subspecies of S. superciliaris inhabit different sub-Andean mountain ranges. Two allopatric plumage forms of S. simonsi (northern, Peru and Bolivia; and southern, Bolivia) and two of S. zimmeri (northern, Bolivia; and southern, Bolivia and Argentina), occur on the eastern slope of the Andes. We reject the existence of hybridization between the allopatric S. zimmeri and S. simonsi; presumed hybrids pertain to normal southern S. simonsi based on plumage and vocalizations. All S. superciliaris taxa had similar songs and calls over 600 km. Songs and calls of S. superciliaris, southern S. zimmeri and both S. simonsi populations are diagnostic and support their recognition as different species, while northern zimmeri remains vocally unknown. The southern and northern populations of S. simonsi differ vocally, the latter possibly being an undescribed species.
Zusammenfassung
Artgrenzen und Biogeographie des Weißbrauentapaculo-Komplexes (Scytalopus superciliaris) und des Punatatapaculo (S. simonsi)
Der Scytalopus superciliaris-Komplex besteht aus drei anerkannten Taxa: dem Graukehltapaculo Scytalopus zimmeri in Bolivien und Argentinien und dem in Argentinien endemischen Weißbrauentapaculo S. superciliaris mit den Unterarten superciliaris und santabarbarae, wobei die südlichste Population ein charakteristisches Gefieder hat, aber von den Lautäußerungen her unbestimmt bleibt. Aufgrund von Gefiedermerkmalen und Lautäußerungen wird Scytalopus zimmeri als Zwischentaxon zwischen dem Punatatapaculo S. simonsi und S. superciliaris angesehen; aus zwei Gegenden in Bolivien wurden mögliche Hybriden zwischen S. zimmeri und S. simonsi gemeldet. Wir untersuchten die geographischen Unterschiede in Gefieder und Lautäußerungen des S. superciliaris-Komplexes und von S. simonsi charakterisiert und beschreiben hier eine neue farblose und große Unterart, S. superciliaris ambatensis aus den Gegenden um Ambato und Velasco. Alle untersuchten Formen sind allopatrisch. Die drei Unterarten von S. superciliaris leben in verschiedenen Gebirgszügen der Sub-Anden. Zwei allopatrische Gefiederformen von S. simonsi (nördlich, Peru und Bolivien; und südlich, Bolivien) und zwei von S. zimmeri (nördlich, Bolivien; und südlich, Bolivien und Argentinien) kommen am Osthang der Anden vor. Eine Hybridisierung zwischen den allopatrischen S. zimmeri und S. simonsi halten wir für unrealistisch; die vermeintlichen Hybriden gehören aufgrund des Gefieders und der Lautäußerungen zu den normalen S. simonsi im Süden. Alle S. superciliaris-Taxa hatten über 600 km hinweg ähnliche Gesänge und Rufe. Die Gesänge und Rufe von S. superciliaris, den südlichen S. zimmeri und den beiden S. simonsi-Populationen sind sehr charakteristisch und sprechen für ihre Anerkennung als jeweils eigene Art, während der nördliche zimmeri von den Lautäußerungen her unbestimmt bleibt. Die südlichen und nördlichen Populationen von S. simonsi unterscheiden sich in ihren Lautäußerungen, wobei die letztere möglicherweise eine bisher nicht beschriebene Art ist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Scytalopus (Gould 1837) is one of the taxonomically most complex groups of Neotropical birds. Only 10 species were recognized in the mid-1960s, whereas around 50 species are currently recognized within the genus (Meyer de Schauensee 1966; Remsen et al. 2021), and more species await formal description (Krabbe et al. 2020; Cadena et al. 2020; this work). Scytalopus are small blackish birds with particularly reclusive behavior. Most species are distributed along the Andes and mountains of southeast Brazil where they inhabit the low and dense strata of forests, scrub and grasslands, often associated with wet ravines and streams (Fjeldså and Krabbe 1990; Ridgely and Tudor 2009). They are partially terrestrial birds with short and rounded wings and rarely fly, resulting in limited dispersal capabilities. Geographic ranges are typically small and species tend to replace each other altitudinally within each mountain range. These particular characteristics give them high chances of becoming geographically isolated leading to evolutionary differentiation, with a different species or subspecies of Scytalopus in each relatively isolated mountain range (Krabbe and Schulenberg 1997; Whitney 1994; Cadena and Céspedes 2020).
Historically, Scytalopus taxa were described based on specimens held in museums, where they were examined and classified by ornithologists who knew little about their natural history, behavior and ecology. For this reason, many similar species were lumped under single species with wide distributions. We now know that plumage and measurements can be misleading to classify group members, since some species with very similar plumage and morphology are genetically, ecologically and vocally very distinct (Arctander and Fjeldså 1994; Krabbe and Schulenberg 1997). The traditional taxonomy was based for almost fifty years on the lists and monographs by Zimmer (1939) and Peters (1951), until the first bioacoustic works in the 1990s in southern Brazil and in the Andes began to question these well entrenched taxonomies (Fjeldså and Krabbe 1990; Riveros and Villegas 1994; Vielliard 1990; Whitney 1994). However, the taxonomy of Scytalopus was massively rearranged by the monumental work of Krabbe and Schulenberg (1997), which provided an exhaustive study of spectrograms and the consequent recognition of most populations whose vocalizations were diagnostic as species-level entities. Following this method, many subsequent investigations have clarified other taxonomic questions and at the same time discovered new species (Avendaño et al. 2015; Bornschein et al. 1998, Coopmans et al. 2001, Cuervo et al. 2005; Hosner 2013, 2015; Krabbe et al. 2005, Krabbe and Cadena 2010; Maurício 2005, Maurício et al. 2014; Raposo et al. 2006; Whitney et al. 2010).
The Scytalopus superciliaris complex consists of three currently recognized taxa (Figs. 1, 2; Remsen et al. 2021). In Bolivia and Argentina, the monotypic Zimmer´s Tapaculo Scytalopus zimmeri (Bond and Meyer de Schauensee 1940) extends from Chuquisaca to Salta; while in Argentina, the White-browed Tapaculo Scytalopus superciliaris superciliaris (Cabanis 1883) inhabits the Pampean and Sub-Andean Sierras of Tucumán, Catamarca and La Rioja and Scytalopus superciliaris santabarbarae (Nores 1986) is exclusive to the Sierra de Santa Bárbara complex in Jujuy and Salta (Fjeldså and Krabbe 1990; de la Peña 1989). During surveys in the Sierra de Velasco, the southernmost population of S. superciliaris was reported to have a distinctive plumage, suggesting subspecific treatment but without presenting specimens, photographs or recordings (Nores and Cerana 1990).
Photographs of the members of the Scytalopus superciliaris complex from Argentina. A Scytalopus zimmeri, PN Los Cardones (Sierra del Candado, Salta) B S. superciliaris santabarbarae, El Amancayal (Sierra de Santa Barbara, Jujuy). C S. superciliaris superciliaris, Cuesta del Clavillo/El Cochuna (Sierra de Aconquija, Catamarca). D S. superciliaris superciliaris, El Infiernillo (Sierra de Aconquija, Tucumán). E S. superciliaris ambatensis subsp. nov., Mutquin (Sierra de Ambato, Catamarca). F S. superciliaris ambatensis subsp. nov., El Cantadero (Sierra de Velasco, La Rioja). All pictures by Juan I. Areta, except B by Rosana Ursino
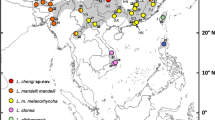
Distribution of the Scytalopus superciliaris complex and S. simonsi. Solid lines and letters A–F indicate main presumed barriers in the S. superciliaris complex and S. simonsi from north to south: A-Río La Paz, La Paz; B-Río Grande, Cochabamba; C-Río Pilcomayo, Chuquisaca; D-Río Juramento, Salta; E-Campo de Pucará, Catamarca; F-Río Salado, La Rioja. Dashed lines and numbers (1–4) indicate western geographic barriers separating extra-Andean population in the Scytalopus superciliaris complex: 1-Río San Francisco, 2-Calchaquí Valley, 3-Pipanaco salt-flat, 4-dry valleys west of Sierra de Velasco. H letters indicate localities where alleged hybrids between southern S. simonsi and northern S. zimmeri were collected (north: Colomi, south: Khasa Punta Pampa). Black arrows and “type” texts indicate the type localities, from north to south: Choquecamate (simonsi), 25 km E of Padilla (zimmeri), El Fuerte (santabarbarae), Casa de Piedra de los Cajones (ambatensis subsp. nov.), and west of Sauciyaca (possibly Sauce Yaco) (superciliaris). All the mountain ranges inhabited by Scytalopus superciliaris are named
Species limits within the superciliaris complex have changed from the late nineteenth century to the present and the status of different populations has not been adequately clarified. The song of nominate S. superciliaris is the only vocalization in the complex that has been described in some detail (Whitney 1994). The few recordings available in sound archives belong to the nominate subspecies and to subspecies santabarabarae, but no formal taxonomic studies based on vocalizations of the latter subspecies have been published (Nores 1986). No geographic variation in plumage has been described for S. zimmeri; however, our examination of museum specimens suggests the existence of two phenotypically diagnostic disjunct clusters in northern and southern populations. While the plumage descriptions of S. zimmeri are based largely on the specimens of the type series that belongs to the northern population, the only known recordings come from the southern population (Fjeldså and Krabbe 1990; Mayer 2000; Whitney 1994; this study). In addition, S. zimmeri has been considered as an intermediate taxon between Puna Tapaculo S. simonsi (Chubb 1917) and S. superciliaris based on plumage characters and vocalizations (Krabbe and Schulenberg 1997; N. Krabbe fide Whitney 1994); and potential hybrids between S. zimmeri and S. simonsi were reported from two localities in Bolivia (Whitney 1994), although this was subsequently rejected (Krabbe and Schulenberg 1997). During our research we noticed the existence of two geographically segregated song types in S. simonsi, one in southern Peru and northern Bolivia (northern simonsi) and another one in central Bolivia (southern simonsi) (see also Fjeldså and Krabbe 1990). More recently, mitochondrial (ND2) phylogenetic data suggested that S. superciliaris is sister (with low support) to a clade including (northern) S. simonsi and (southern) S. zimmeri (Cadena et al. 2020). Additionally, an expanded UCE + nuclear exons dataset showed that (northern) S. zimmeri was either sister to S. superciliaris or distantly related to it within the Southern Andes clade depending on the phylogenetic method applied (but note that no S. simonsi samples were included in these UCE analyses) (Cadena et al. 2020). Similarly, a phylogenomic study lacking S. simonsi samples found (northern) S. zimmeri as sister to S. superciliaris (Harvey et al. 2020). Clearly, further genetic work is warranted.
Our objectives in the present work are to (1) evaluate the taxonomy of all the known populations of the superciliaris complex and the related S. simonsi through comparative studies of morphology, vocalizations, distribution and habitat use, (2) describe a new subspecies of Scytalopus superciliaris from the Sierras de Ambato and Velasco, (3) assess the existence of hybrids between S. zimmeri and S. simonsi, and (4) discuss biogeographic patterns and ecomorphological variation within the superciliaris complex and biogeographic patterns in S. simonsi.
Methods
Plumage and morphology
To understand the geographic variation of plumage in the superciliaris complex and S. simonsi, we examined and took notes on the plumage of 132 museum specimens (8 S. zimmeri [3 northern zimmeri and 5 southern zimmeri], 13 S. superciliaris santabarbarae, 47 S. s. superciliaris, 8 S. superciliaris subsp. nov. and 56 S. simonsi [19 northern simonsi and 37 southern simonsi]) (Supplementary Appendix 1). In addition, we studied another 12 specimens of particular importance from photographs obtained in museums or from private collections (5 Scytalopus zimmeri [1 northern zimmeri and 4 southern zimmeri], 1 S. s. superciliaris, 1 S. superciliaris subsp. nov. and 5 southern simonsi) (Supplementary Appendix 1). Type specimens of all taxa were studied from photographs (zimmeri, superciliaris and simonsi) or in person (santabarbarae) (Supplementary Appendix 1). For color nomenclature we used names in Ridgway (1912). Most of the specimens were photographed for later comparison between individuals and populations. In addition, photographs of live birds of the superciliaris complex were taken at several study sites, documenting all plumage types recognized from the examination of museum specimens, allowing for the association of plumage types with vocalizations (except for the northern population of S. zimmeri that was not photographed in nature and which remains vocally unknown).
To characterize the morphology of each plumage type in the superciliaris complex and in S. simonsi, we measured six morphometric variables in museum specimens: length of culmen from the nostril, height and width of bill at the base and length of the tarsus with a dial caliper (to the nearest 0.01 mm); and wing chord and tail length with a metallic ruler (to the nearest 0.5 mm). Juvenile and presumptive “immature” individuals of unknown age were excluded from statistical analyses. A total of 94 museum specimens were measured, corresponding to 7 S. zimmeri (2 northern zimmeri and 5 southern zimmeri), 13 S. superciliaris santabarbarae, 41 S. s. superciliaris, 5 S. superciliaris subsp. nov., and 28 S. simonsi (16 northern simonsi and 12 southern simonsi). Despite the plumage differences in the S. zimmeri populations, the small sample size of the northern population (N = 2 adults) forced us to group it with the southern population (N = 5 adults) as a single statistically analyzable unit.
Vocalizations
We recorded vocalizations of members of the superciliaris complex and northern simonsi using a Marantz PMD-661 digital recorder with Sennheiser ME-62 and Telinga Pro-6 microphones mounted on a Telinga Universal parabola. All our recordings were deposited in the Macaulay Library of Natural Sounds (MLNS, Cornell Laboratory of Ornithology, Ithaca). We compiled a total of 174 recordings belonging to ca. 147 individuals: 63 southern zimmeri (50 individuals), 29 S. superciliaris santabarbarae (25 individuals), 21 S. s. superciliaris (20 individuals), 14 S. superciliaris subsp. nov. (12 individuals), 32 northern simonsi (ca. 27 individuals) and 17 southern simonsi (ca. 14 individuals) (Supplementary Appendix 2).
Vocalizations were grouped in two basic types: calls and songs. Calls are typically simple and isolated notes, although they can sometimes be repeated in series, and are usually emitted when alarmed or for contact between individuals. The song usually has more than one element, is repeated at high rates early in the morning and late in the afternoon either hidden on grasslands, perched on rocks or less often in shrubs, and is associated with mating or territorial defense. We described the structure (i.e., the graphical representation in a spectrogram, including number of notes and duration) of all vocalizations and identified possibly homologous structures for adequate comparisons between the taxa/populations and attempted to find homologue call types across taxa based on similarities in structure and context of emission. Vocalizations thought to be homologous were given the same name across taxa.
We obtained bioacoustic measurements of songs in Raven Pro 1.5 (www.birds.cornell.edu/brp/raven) using standard parameters [window type: Hann, window size: 256 samples (= 5.33 ms), 3 dB Filter Bandwidth: 270 Hz, Time grid-Overlap: 50%, Hop Size: 128 samples (= 2.67 ms), Frequency grid-DFT: 256 samples, Grid spacing: 188 Hz]. We considered the last two notes of the song of southern S. zimmeri and the complete song of southern and northern S. simonsi as structurally homologous to the typical song of two notes of S. superciliaris (i.e., several single-noted songs of S. simonsi were composed by a first short sound and a second trilled sound that were considered to be homologous to the first and second note of the song of S. superciliaris, respectively). We obtained bioacoustic measurements from high quality recordings (signal-to-noise ratio greater than 20 dB) originally available in PCM format, except for 7 individuals of northern S. simonsi and 6 individuals of southern S. simonsi that were transformed from mp3 to PCM and resampled to 48 kHz so that spectrogram parameters were equal to those of originally uncompressed PCM files. We filtered sounds below 500 Hz and above 8000 Hz using a band-pass filter in Raven 1.5, to avoid unwanted noise that could interfere with measurements. We delimited selection borders in the oscillogram covering the entire bandwidth and exceeding the beginning and end of each note. Within each selection we obtained the following robust measurements: interquartile bandwidth (IQR BW), interquartile duration (IQR Dur), 90% bandwidth (BW 90%), duration 90%, and Frequency (Peak Freq). We measured three songs of each of 51 individuals (12 southern S. zimmeri, 22 S. superciliaris, 9 northern S. simonsi and 8 southern S. simonsi, Supplementary Appendix 2). To obtain representative quantitative data and given our moderate sample sizes, we discarded the extreme high and low values of the three measurements of each variable per individual, and kept the middle value for statistical comparisons among taxa.
Statistical analyses
Morphometric and acoustic variables of note 2 did not pass a normality test (Shapiro–Wilk test). Thus we performed nonparametric Kruskal Wallis tests to test for significant differences between taxa/populations at alpha = 0.05, while acoustic variables of note 1 were normally distributed and were compared through one-way ANOVA at alpha = 0.05. Non-overlapping means ± sd were considered as statistically different. Statistical tests were carried out with Statistica (Stat Soft, version 10), InfoStat (Universidad Nacional de Córdoba, 2015 version), and GraphPad Prism version 6 (GraphPad Software, La Jolla California USA).
Distribution
We compiled a distributional database of all members of the superciliaris complex and S. simonsi from museum specimens, vocalizations, eBird data (ebird.org), third party records, own unpublished data, and data from our surveys (Supplementary Appendix 3). Localities from specimen labels were georeferenced using the ornithological gazetteers of Peru, Bolivia and Argentina (Paynter 1992, 1995; Stephens and Traylor 1983). When information was confusing we consulted the authors of the records directly, and when no reliable information was obtained, the records were discarded. Altitudinal data were taken from museum labels, GPS data or information provided by observers. Measurements in kilometers were taken using Google Earth. Maps were made using QGIS 2.12 (http://qgis.org/en/site).
Fieldwork
We visited eight localities in Argentina covering most of the range of the superciliaris complex, and one locality in Peru where northern S. simonsi was studied. Each site was explored on foot following water-courses or trails, prospecting potentially suitable habitats. Individuals were most frequently detected naturally through their vocalizations; however, we also performed playback to find silent birds, especially at localities where their presence was unknown. We surveyed the following sites (see Supplementary Appendix 3 for geographical coordinates). Sierra de Santa Bárbara, Jujuy (5–7 April 2013/1700–1900 masl) with S. superciliaris santabarbarae: upper montane forest with predominance of Alnus acuminata and Podocarpus parlatorei, humid ravines with ferns, bamboos and great diversity of smaller shrubs and trees. Cuesta del Clavillo/El Cochuna, Tucumán and Catamarca (8–9 April 2013/1750 masl) with S. s. superciliaris: pure Alnus acuminata upper montane forest with dense undergrowth composed of generally sparse shrubs, and densely packed grasses and ferns. El Infiernillo, Tucumán (9–10 April 2013/2500–2800 masl) with S. s. superciliaris: deep shrubby creeks surrounded by rocky grasslands. Mutquin, Catamarca (4–5 October 2013/2100–2200 masl) with S. superciliaris subsp. nov.: shrubs and xerophytic grasslands growing on steep slopes that incline toward fast flowing streams. Las Juntas, Catamarca (7–8 October 2013/1970–1990 msnm): upper montane forest of Podocarpus parlatorei surrounded by grasslands and Andean scrub. El Cantadero, La Rioja (6–7 October 2013/1100–1400 masl) with S. superciliaris subsp. nov.: Yungas forest relicts with Acacia visco, Bougainvillea stipitata, and Fagara coco, restricted to thin linear strips on the bottom of deep valleys and steep slopes in quebradas. Parque Nacional Los Cardones and Cuesta del Obispo, Salta (26–29 October 2012 and 29 March 2013/3100 masl) with southern S. zimmeri: Andean rocky grasslands with large boulders generally along water-courses. Sierra de Medina, Tucumán (5–6 June 2015/1400–1500 masl): upper montane forest of Podocarpus parlatorei growing mostly along wet ravines, but also spanning slopes. Abra Málaga, Cusco, Peru (15 November 2011/4000 masl) with northern S. simonsi: Puna grassland interspersed with patches of Polylepis forest.
Results
Description of a new subspecies of Scytalopus superciliaris
Given the existence of diagnostic plumages in populations of Scytalopus superciliaris of isolated mountain ranges and the constancy of their vocalizations throughout the species´ geographical distribution, we propose to recognize the southernmost populations as a new subspecies that we call:
Scytalopus superciliaris ambatensis subsp. nov. Areta and Monteleone
Holotype
Unsexed adult, held in the collection of the Fundación Miguel Lillo, Tucumán, Argentina (FML 14,845; Fig. 3, Figures S1–S2). Collected by C. Laredo and A. Budin on 30 October 1985, at Casa de Piedra de Los Cajones, El Manchao, Ambato, Catamarca, Argentina (4300 masl).
Specimens showing geographic variation in plumage in the Scytalopus superciliaris complex and S. simonsi. A Type specimen of (southern) Scytalopus simonsi from Choquecamate, Bolivia (BMNH-1902.3.13.1258). B Type specimen of (northern) Scytalopus zimmeri from Padilla, Bolivia (ANSP-146342). Note the general gray coloration and the restricted ventral barring. C Some specimens of southern Scytalopus zimmeri from Jujuy province, Argentina (FML-13215, 13,214, 10,050). Note the difference with the type specimen of northern zimmeri from Padilla and the great similarity with Scytalopus superciliaris superciliaris from Tucumán province, Argentina. D Comparison of plumage of different populations of the Scytalopus superciliaris complex from Argentina. From left to right: southern Scytalopus zimmeri (FML-13215), type specimen of S. superciliaris ambatensis subsp. nov. (FML-14845), S. superciliaris superciliaris (FML-17043) and S. superciliaris santabarbarae (MLP-12193). Note the dark (north) to light (south) color gradient in Scytalopus superciliaris. E: Part of the series of (northern) Scytalopus simonsi collected in Cuzco and Puno, Peru (LSU-78581, 98,397, 98,398). F: Part of the series of (southern) Scytalopus simonsi collected in Cochabamba, Bolivia (LSU-124234, 124,233, 36,132). G: One of the specimens collected in Colomi, Cochabamba, Bolivia (FMNH-180580). H: One of the specimens collected in Khasa Punta Pampa, Cochabamba, Bolivia (ZMUC-80031)
Diagnosis
Buffy Brown upperparts. Belly between Light Mouse and Mouse Gray much paler than S. superciliaris superciliaris and S. superciliaris santabarbarae. Center of belly with a noticeable white longitudinal band. Tail longer than S. superciliaris superciliaris and S. superciliaris santabarbarae (Figs. 3–4, Table 1).
Description of the holotype
Forehead Mouse Gray. White eyebrow starting in the loral and passing behind the eye. Cheeks Mouse Gray, darker than the belly. White throat. Sides of the neck and upper flanks between Light Mouse Gray and Mouse Gray, but closer to the latter. Underparts between Light Neutral Gray and Neutral Gray, with a whitish longitudinal band that broadens on the lower belly. Crown and upperparts Buffy Brown, with sparse Mummy Brown dotting on crown and nape. Lower back, rump, uppertail coverts, undertail coverts and lower flanks barred, with light bars ranging from Pale Ochraceus Buff to Light Ochraceous Buff; and dark bars between Prout's Brown and Mummy Brown. Tertial feathers Buffy Brown irregularly spotted of Mummy Brown. Tertials exhibit a subapical dark brown band tipped white. Wing coverts concolorous with the belly. Underwing coverts with small white dots on the trailing edge. The outer portion of the outer vane of all the flight feathers is very similar to the dorsal color; and each successive feather (outwards) becomes darker than the preceding (i.e., the outermost primary is the darkest flight feather). Tail Buffy Brown with Mummy Brown barring. Weight: no data. Wing chord: 55 mm, tail: 39 mm, exposed culmen: 11.58 mm, tarsus: 19.6 mm. Iris: no data. Dark brown beak with paler tip. Pale orange or dark yellow legs.
Paratypes
We designate three specimens collected in the type locality and its surroundings as paratypes, all deposited in the Fundación Miguel Lillo collection. An adult male collected on 30 October 1985 (FML-14844) and a sub-adult female collected on 31 October 1985 (FML-14846), from Casa de Piedra de Los Cajones, Sierra de Ambato are topotypical and were collected the same and the next day of the collection of the type specimen; and an adult male collected on 20 October 1986 in Cerro La Muñuda, Sierra de Ambato (FML-14966).
Plumage variation
The four specimens of the type series are very similar in plumage. The most notable variations correspond to the irregular pattern of the underparts and the extension of the lower barring (the sub-adult individuals are presumably more barred). The length of the eyebrow is variable and in at least one individual encompasses the loral region, while in the others it begins closer to the eye. The gray of the forehead is variable, being barely visible in FML-14846 while extending almost across the whole crown in FML-14844. This last specimen also has plain crown and nape without dotting. Two other specimens, one from west of Chumbicha (MACN-unnumbered) and one from El Cantadero (photograph of Sergio A. Salvador) show hardly any white in the lower belly. There are no known differences between sexes.
Description of sub-adult
The only non-adult specimen is included in the type series (FML-14846). It corresponds to a sub-adult female of unknown age whose coloration is very similar to adults, with the exception of having dark transverse bars in the wing coverts and greater extension of dorsal barring.
Vocalizations
All known vocalizations of the new taxon coincide with those described for the two currently recognized subspecies of Scytalopus superciliaris. The holotype was not recorded, but recordings were obtained by JIA at lower altitude in the same mountain range (Fig. 3C and G, MLNS-213011, 213,012, 213,021).
Habitat and distribution
Scytalopus superciliaris ambatensis inhabits the high peaks, shrubby creaks, rocky grasslands, and forests of Podocarpus parlatorei of the Sierra de Ambato and the humid valleys (“quebradas húmedas”) of the eastern slope of the eastern range of the Sierra de Velasco. In the Sierra de Ambato it is more frequent in rocky grasslands between 2100 and 4300 m; and in the Sierra de Velasco it inhabits relicts of Sierran Chaco/Austral Yungas forest between 1100 and 1400 m.
Etymology
The name of the new taxon refers to the Sierra de Ambato, where the specimens of the type series were collected.
Conservation
The degree of threat is unknown. Mining activities in the summits of Ambato could affect the quality of the water in the streams where this subspecies lives. An abandoned Wolfram mine exists above El Cantadero in the Sierra de Velasco.
ZooBank registration codes
urn:lsid:zoobank.org:pub:9E061B6E-3B2E-4DA8-904D-4BABA2B1DD8B (work), and urn:lsid:zoobank.org:act:F33F7F20-5409-402A-A09B-43B47CAEDE61 (nomenclatural act).
Plumage
Detailed plumage descriptions of members of the superciliaris complex show some diagnostic differences in plumage (Fig. 3, Figures S1–S3, Table 1, Supplementary Appendix 4). We found two distinct plumage variants in Scytalopus zimmeri (Fig. 3, Table 1, Supplementary Appendix 4). Individuals from the northern population (northern zimmeri; skins from the type locality, Fig. 3A, Figure S3) have almost completely gray underparts with the barring very restricted to the lower belly and undertail, throat barely paler than the belly and grading into it, gray supercilium and brownish upperparts near Buffy Brown. Individuals of the southern population (southern zimmeri; Fig. 3D, Figure S1) are ventrally gray with barring extending into the flanks (though restricted to the lower flanks in some specimens), throat grayish to white contrasting noticeably with the gray belly, white supercilium, and rustier brown upperparts being Cinnamon Brown. The southern S. zimmeri population is virtually indistinguishable from S. s. superciliaris (Fig. 1A vs. 1C, Figure S1), although some specimens of southern S. zimmeri show a slightly grayer (dirty white) throat (Fig. 3D). The color of the throat of recently collected specimens of southern S. zimmeri changed depending on the angle of the head; a bird with an upward pointing head would display a grayer coloration in the throat, while the same bird showed a whiter throat when the neck was flexed forward matching its natural position in the wild (JIA and F. Burgos pers. obs.). However, specimens of northern and southern zimmeri collected at similar times and prepared with similar levels of neck extension demonstrate that the color differences here reported for them do not stem from foxing and do not constitute an artifact due to different neck positions. Photographs of living individuals of southern Scytalopus zimmeri from several localities in Argentina show that eyebrow length and extension of white in the throat are very variable.
We distinguished three plumage variants in Scytalopus superciliaris (Figs. 1 and 3, Figure S1, Table 1, Supplementary Appendix 4). S. superciliaris superciliaris has white throat and supercilium, ventral region between Mouse Gray and Deep Mouse Gray and upperparts Dresden Brown. S. superciliaris santabarbarae has a very similar pattern to the nominal form but is darker overall, with underparts between Dark Mouse and Blackish Mouse and upperparts Prout’s Brown. The specimens of S. superciliaris ambatensis subsp. nov. from the Ambato massif (FML-14844, 14,845, 14,846, 14,966) are noticeably paler than the rest of the specimens examined, with a wide white longitudinal band in the center of the belly, and upperparts Buffy Brown. Our observations and photographs of the birds of Mutquin and El Cantadero correspond to this color pattern (Fig. 1E–F). However, a specimen collected west of Chumbicha (MACN-unnumbered) and another specimen collected from El Cantadero (photograph by Sergio A. Salvador) have a paler coloration only limited to the lower belly. A few specimens of S. superciliaris superciliaris most of which are subadults show a certain amount of white in the lower belly (e.g., MACN-20166, 20,167, 23,037, 23,038, FML-617), while none of the S. superciliaris santabarbarae specimens has a white lower belly.
The overall coloration of Scytalopus simonsi is very similar to the northern population of S. zimmeri (Fig. 3B–C, Table 1, Supplementary Appendix 4). In both populations of S. simonsi, the supercilium, when present, is variable in length and varies from light gray to almost white in some specimens. The ventral region has a uniform grayish tone, becoming darker gradually toward the lower belly. Most individuals show a uniformly gray dorsal coloration, darker than the belly. The rump has the same pattern as the flanks, and sometimes this pattern extends to the lower back. Northern simonsi has apparently overall paler and brighter gray tones, brighter and more orangey flanks with sparser and broader dark barring, while southern simonsi seems to be on average darker and duller, with browner and deeper flanks with more profuse and finer barring. Females of both populations have browner backs (generally gray in males).
Morphology
Specimens of the superciliaris complex and S. simonsi differed slightly in morphometric measurements, with the greatest differences occurring between members of the S. superciliaris complex in comparison to both populations of S. simonsi rather than within members of each group (Fig. 4, Supplementary Appendix 5). Members of the S. superciliaris complex had significantly shorter tarsus and tail (except ambatensis subsp. nov.) than both S. simonsi populations, and overall had longer, higher, and wider bills, and were heavier than members of S. simonsi (Fig. 4, Appendix S1). Within S. superciliaris, ambatensis subsp. nov. differed from both santabarbarae and superciliaris only in its longer tail and from superciliaris by its longer bill, while no morphological differences were found between santabarbarae and superciliaris (note however, that the weight suggests an increase in weight from the smallest santabarbarae to the largest ambatensis subsp. nov.). The two populations of S. simonsi were remarkably similar in morphology with no significant differences in any variable, however, the southern birds tended to show heavier weight and higher bills than the northern ones. Southern S. simonsi had a narrower and lower bill and a longer tarsus than all S. superciliaris complex members (except S. zimmeri), a shorter bill (except superciliaris) and lighter body than ambatensis subsp. nov., and a longer tail than santabarbarae and superciliaris. Northern S. simonsi had a lower bill, and weight than all S. superciliaris complex members, and a shorter bill than ambatensis subsp. nov. Scytalopus zimmeri (northern and southern populations lumped) did not differ from santabarbarae and superciliaris in any measurement, and differed only in its shorter tail from ambatensis subsp. nov., while it had a shorter tail and tarsus than both populations of S. simonsi, a longer and wider bill than southern S. simonsi and a higher bill and heavier weight than northern S. simonsi. Wing chord did not differ between any taxa.
Distribution and habitat
All members of the superciliaris complex diagnosable by plumage have allopatric distributions and do not coexist with any other species of Scytalopus, either in Argentina or Bolivia (Fig. 2).
Scytalopus zimmeri is distributed along the eastern slope of the main Andes from the Chuquisaca department to the province of Salta, on an altitudinal gradient between 2150 (rarely down to 1650) and 3370 masl. The northernmost locality is Padilla, where the type specimens come from and this is the only known locality for what we here call northern zimmeri. The northernmost southern zimmeri skins come from Portillo, Bolivia (Fig. 2). All skins and photographs from Argentina belong to the southern population. In Argentina S. zimmeri inhabits mostly shrubby creeks, grasslands and rocky areas well above the tree-line but below Puna habitat; although it can also be found locally in alder patches of Alnus acuminata and also in patches of Polylepis spp. Its distribution in Argentina comprises the Sierra de Santa Victoria, Sierra del Zenta, Serranía de Calilegua, the eastern slope of the Sierra del Chañi and the Sierra del Candado (Fig. 2).
Scytalopus superciliaris is endemic to NW Argentina in the provinces of Jujuy, Salta, Tucumán, Catamarca and La Rioja (Fig. 2). S. superciliaris santabarbarae is restricted to the mountain range conformed by the Sierra de Santa Bárbara, Serranía del Centinela, Sierra del Maíz Gordo and Sierra de la Cresta del Gallo (1500–2400 masl). It was locally common in humid ravines in upper montane forest, although it also occurs in grasslands and rocky areas above the tree-line. Most records of S. superciliaris superciliaris come from the eastern slope of the Aconquija massif (1400–4000 masl), extending southwards through the Sierra de Graciana, Sierra de Ancasti and most probably the northern end of the Sierra de Ambato where environmental connectivity allows. It inhabits a wide range of environments, from humid montane forests to thickets and Puna grasslands. We recorded several individuals in El Infiernillo and Cuesta del Clavillo/El Cochuna, in Andean scrubland and montane forests, respectively. The records of S. superciliaris ambatensis subsp. nov. are from El Manchao and La Muñuda (Sierra de Ambato, 2720–4300 m), Las Juntas (Sierra de Ambato, 1950–2000 m), Mutquin (Sierra de Ambato, 2100–2200 m) and El Cantadero (Sierra de Velasco, 1100–1400 m). In Mutquin, the birds inhabit a xerophilous environment dominated by shrubby creeks with boulders and grasslands, while at El Cantadero they were found inside Sierran Chaco/Austral Yungas relicts. Our observations at Las Juntas occurred in pure patches of Podocarpus parlatorei in deep gorges surrounded by dense grasslands. We did not record any Scytalopus in a survey of the Sierra de Medina, Tucumán (26°24′ S 65°03′ W), despite the extensive patches of montane forest that seem appropriate for the species.
Northern simonsi ranges from the department of Cusco, Peru to central La Paz, Bolivia (2700–3900 masl), while southern simonsi occurs from central La Paz to Cochabamba (3050–4420 masl) and is endemic to Bolivia. According to our data, neither form of S. simonsi overlap with S. zimmeri. The southernmost locality of southern simonsi is Sacha Loma, located about 230 km to the northwest of the northernmost record of northern S. zimmeri at Padilla (Fig. 1, Supplementary Appendix 3).
Vocalizations
We studied recordings of all the forms of the superciliaris complex recognized by plumage (except for the vocally unknown northern zimmeri) and of both populations of S. simonsi. Here we describe the vocal repertoire of all these forms and make a quantitative comparison of their songs. Vocalizations thought to be homologous were given the same name across taxa.
Vocal repertoire of southern Scytalopus zimmeri
We found 10 types of vocalizations: song and 9 calls (Fig. 5). All the recordings analyzed in this work belong to the southern population. As far as we know, the vocalizations of the northern population were never described in the literature and we did not find any publicly available recording.
Vocalizations of southern Scytalopus zimmeri. A 4-noted song with long trill. PN Los Cardones, Salta, Argentina (J.I. Areta, MLNS-171456). B 5-noted song with long trill. Reserva Tariquia, Tarija, Bolivia (P. Hosner, MLNS-132800). C 6-noted song with short trill. PN Los Cardones, Salta, Argentina (J.I. Areta, MLNS-171440). D ∩ -shaped cuek call. Portillo, Chuquisaca, Bolivia (N. Krabbe, XC-16297). E chuik call. Finca El Candado, Salta, Argentina (N. Krabbe, XC-32118). F chrrr call (alternative song?), Finca El Candado, Salta, Argentina (N. Krabbe, XC-112736). G U-shaped cuek call, Portillo, Chuquisaca, Bolivia (N. Krabbe, XC-16296). H M-shaped cuek call. Portillo, Chuquisaca, Bolivia (N. Krabbe, XC-16302). I chair-shaped cuek call. Portillo, Chuquisaca, Bolivia (N. Krabbe, XC-16291). J chiiu call. Finca El Candado, Salta, Argentina (N. Krabbe, XC-32117). K trill. Finca El Candado, Salta, Argentina (N. Krabbe, XC-32123). L complex call in alarm. Cuesta del Obispo, Salta, Argentina (J. I. Areta, MLNS-212256)
Song (N = 36, Fig. 5A–C): The basic structure consists of two emphatic introductory notes followed by a variable number of 1 to 5 somewhat syncopated notes that become shorter, resembling the decomposition of a trill ("Chek-et krr krr-kr"). In some individuals the final notes merge into a wavy trill. Some songs include just the two introductory notes. The behavior during its emission is like that of S. superciliaris (see below).
∩ -shaped cuek call (N = 1, Fig. 5D): simple and brief notes that are ∩ -shaped spectrogram tracings.
chuik call (N = 6, Fig. 5E): short and emphatic sigmoid call that ascends in frequency in a very short time. Often repeated at a regular pace, most likely in alarm situations.
chrrr call (N = 1, Fig. 5F): brief and low-pitched churring trill repeated in series. Resembles the final part of the song and is probably part of the female song (N. Krabbe in litt.).
U-shaped cuek call (N = 9, Fig. 5G): simple and brief notes that are "U-shaped" in spectrogram tracings. They are emitted either in isolation or as bi-syllables. In some examples this note is combined with another one with sigmoid shape [e.g., XC-98795].
M-shaped cuek call (N = 5, Fig. 5H): simple and brief notes that are "M" shaped in the spectrogram.
Chair-shaped cuek call (N = 1, Fig. 5I): note with a “chair” shape in the spectrogram. It begins with an ascending spike and then descends quickly in an arc. Frequently occurs in series of 2–3 repeated notes.
chiiiu call (N = 1, Fig. 5J): short and high-pitched note that ascends and descends rapidly in frequency, forming an inverted "V" in the spectrogram. It was thought to be part of the female song (N. Krabbe in litt.).
Trill (N = 2, Fig. 5K): consists of several " ∩ " notes (between 14 and 24) that are more or less evenly pitched and spaced. The number of notes varies widely in different renderings within a bout. Performed when alarmed.
Complex call (N = 1, Fig. 5L): syncopated series of bi or tri-syllabic calls "peech-teeW" or "peech-teeW-teeW" with emphasis on the last note. Given as alarm or in response to playback.
Vocal repertoire of Scytalopus superciliaris
We recorded 4 types of vocalizations: song and 3 types of calls (Fig. 6). The three forms recognizable by plumage (santabarbarae, superciliaris and ambatensis subsp. nov.) emitted the same vocalizations.
Vocalizations of Scytalopus superciliaris. A song (santabarbarae). Sierra de Santa Barbara, Jujuy, Argentina (J.I. Areta, MLNS-212285). B song (superciliaris). Cuesta del Clavillo/El Cochuna, Catamarca, Argentina (J.I. Areta, MLNS-212311). C song (ambatensis). Mutquin, Catamarca, Argentina. (J.I. Areta, MLNS-213011). D: ∩ -shaped cuek call (santabarbarae). Sierra de Santa Barbara, Jujuy, Argentina (J.I. Areta, MLNS-212298). E ∩ -shaped cuek call (superciliaris). Cuesta del Clavillo/El Cochuna, Catamarca, Argentina (J.I. Areta, MLNS-212309). F ∩ -shaped cuek call (ambatensis). El Cantadero, La Rioja, Argentina (J.I. Areta, MLNS-213023). G chuik call (ambatensis). Mutquin, Catamarca, Argentina (J.I. Areta, MLNS-213021). H chrrr call (santabarbarae). Sierra de Santa Barbara, Jujuy, Argentina (J.I. Areta MLNS-212323)
Song (N = 45, Fig. 6A–C): consists of two harsh, trilled notes, the first one is short and slightly descending/ascending and the second one is longer and descending ("ch-chrrr"). The song is repeated in long series. This common vocalization is easily heard from a distance and typically performed while perching on hidden rocks or hidden in the vegetation close to the ground. During territorial disputes with neighbors or in the presence of an intruder in the territory, the birds may climb to high branches or jump to exposed boulders to vocalize.
∩ -shaped cuek call (N = 13, Fig. 6D–F): simple ∩ -shaped note, given in isolation or as a fast bisyllabic "cuek-cuek", but also often repeated in long series of up to 40 notes, whence the pace is variable and accelerated in stressful situations. It is the most common voice in alarm or disturbance, although it may also function as a contact between distant individuals.
chuik call (N = 1, Fig. 6G): short and emphatic sigmoid call that ascends in frequency in a very short time.
chrrr call (N = 5, Fig. 6H): brief and low-pitched churring trill. This infrequent call is given once or twice when alarmed, but under severe stress it can be repeated in long series. We did not detect this call in S. superciliaris ambatensis subsp. nov., but we suspect more sampling will eventually show it to occur.
Vocal repertoire of southern Scytalopus simonsi
We found 3–4 types of vocalizations: song and 2–3 calls (Fig. 7).
Vocalizations of Scytalopus simonsi. A song (southern simonsi). Chapare, Cochabamba, Bolivia (T.A. Parker, MLNS-33687). B song (northern simonsi). Abra Málaga, Cusco, Peru (J.I. Areta, MLNS-171137). C main trill (southern simonsi); identification uncertain. Chapare, Cochabamba, Bolivia (T.A. Parker, MLNS-33686). D main trill (northern simonsi). Abra Málaga, Cusco, Peru (T.A. Parker, MLNS-35432). E ∩ -shaped cuek call (southern simonsi). Rio Milluni, La Paz, Bolivia (J. Mayer, XC-2009). F ∩ -shaped cuek call (southern simonsi). Serranía Ayopaya, Cochabamba, Bolivia (N. Krabbe, XC-16117). G chuik call (southern simonsi). Serranía Ayopaya, Cochabamba, Bolivia (N. Krabbe, XC-16118). H chuik call (northern simonsi). Abra Málaga, Cusco, Peru (P. Boesman, XC-229605). I tuit-tuit-tuit call (northern simonsi). Cuyocuyo, Puno, Peru (N. Krabbe, XC-47790)
Song (N = 13, Fig. 7A): single but clearly two-parted note starting with an emphatic sound that descends quickly in frequency and continues into a trill ("Tewgrr").
Main trill (N = 1?, Fig. 7C): fast series of 6–13 sharp elements. It is uncertain whether this call [MLNS-33686] was given by southern simonsi or by S. schulenbergi (both taxa having been sound recorded in Upper Chapare). Whitney (1994:611) underscored that he was perplexed by the fact of not having heard this call or equivalents of this call ("scold") from southern simonsi in Depto. Cochabamba, while having heard it often in the northern populations (see Fig. 7D).
∩ -shaped cuek call (N = 2, Fig. 7E–F): ascending/descending or nearly flat notes that vary little in frequency and are issued alone or in pairs. Probably emitted when alarmed or disturbed.
chuik call (N = 1, Fig. 7G): series of ascending vibrating calls that sometimes morph into ascending-descending notes.
Vocal repertoire of northern Scytalopus simonsi
We found 5 types of vocalizations: song and 4 types of calls (Fig. 7).
Song (N = 16, Fig. 7B): clearly bisyllabic, formed by two notes repeated in long series. The first ∩ -shaped chik most intense and the second a short chirping trill of softer intensity usually rising in tone ("WEEK-brr"). Sometimes a sort of partial song includes only the initial note repeatedly, forming long series [e.g., XC-36120]. Another variant of the song includes a soft " ∩ " note followed by a very short quavering trill that rises in frequency, and it may occur at the beginning of some song bouts [e.g., MLNS-143691], and some notes resembling this soft version can be given as patternless series [e.g., XC-229606].
Main trill (N = 9, Fig. 7D): fast series of 6 to 13 " ∩ " sharp elements. A common call in alarm situation, with a variable number of notes. Some skepticism must be exercised with the identification of these calls, which are difficult to diagnose from those of S. schulenbergi (N. Krabbe in litt.).
chuik call (N = 1, Fig. 7H): simple, vibrated and ascending note.
tuit-tuit-tuit call (N = 1, Fig. 7I): series of 4–5 sharp ∩ -shaped notes, given by females.
Trill with “U” notes (N = 1, not illustrated): monotone fast series of up to 17 U-shaped notes, possibly emitted in alarm situations [XC-47788].
Quantitative comparison of songs in the superciliaris complex and in S. simonsi
The quantitative comparison of the two homologous notes of the songs of S. superciliaris, southern S. zimmeri and northern and southern S. simonsi showed significant differences in several variables depending on the notes and taxa/populations being compared (Table 2). Scytalopus superciliaris was the most distinctive taxon, differing in 6/10 variables from S. zimmeri (three differences in note 1, three in note 2), 7/10 variables from northern S. simonsi (3 in note 1, five in note 2), and 4/10 variables from southern S. simonsi (3 in note 1, one in note 2). Second in degree of differentiation was southern S. zimmeri, which differed in 3/10 variables from northern simonsi (none in note 1, three in note 2) and in 2/10 variables from southern simonsi (none in note 1, two in note 2). Finally, northern and southern S. simonsi differed in 1/10 variables (none in note 1 despite the structural differences, one in note 2). The degree of quantitative differentiation between southern S. zimmeri and southern simonsi was similar to that between northern and southern S. simonsi (note however the marked structural differences among their songs; Figs. 5 and 7). No variable was different between all the four taxa/populations being compared, while IQR BW and BW 90% of Note 1 were identical among all the comparisons. Intraspecific variation within S. superciliaris subspecies was not statistically significant (Supplementary Appendix 6). The quantitative differences coupled to the qualitatively characteristic structure of the vocalizations provide a solid basis for taxonomic discussion in these taxa.
Discussion
We have characterized the geographic variation in plumages and vocalizations of the S. superciliaris complex and S. simonsi, resulting in the description of a new subspecies, S. superciliaris ambatensis. The distributions of all studied forms are allopatric, both in Argentina and Bolivia. The three subspecies of S. superciliaris inhabit different sub-Andean mountain ranges, and two forms of each, S. simonsi and S. zimmeri, do so on the eastern slope of the Andes. The three subspecies of S. superciliaris have similar songs and calls, and a much smaller repertoire of calls than southern S. zimmeri. Songs and calls of S. superciliaris, southern S. zimmeri and S. simonsi are diagnostic and support their recognition as different species, while northern zimmeri remains vocally unknown. In turn, S. simonsi possesses at least two plumage and vocal types that differ diagnosably. Further study of differences is warranted, as S. simonsi as currently defined may be composed of more than one species-level entity (Areta et al. unpubl. data).
Geographic variation in plumage
The data presented in this work show the existence of a geographic pattern of intraspecific morphological change that is repeated in S. zimmeri and S. superciliaris: in both species there is a change from dark forms in the north to paler forms in the south. In the case of S. superciliaris the change in plumage is accompanied by an increase in size towards the south, passing from the small and dark santabarbarae, through the intermediate superciliaris to the large and pale ambatensis. Since taxa of the superciliaris complex are not distributed continuously but in isolated mountain ranges, it is expected that clinal variation would be expressed by relatively discrete leaps in line with different orographic barriers (Areta and Pearman 2013).
The repeated pattern of shifting from darker forms in the north to paler forms in the south in S. superciliaris and S. zimmeri seems consistent with the simple version of Gloger’s rule. This rule proposes that within the same species darker birds occur in humid and warmer environments whereas lighter ones occur in drier and colder habitats (Delhey 2019). The correlation of increased darkness with increase in temperature and humidity awaits formal testing, as does the exploration of the three adaptive causes that may account for this pattern: crypsis via background matching, resistance to keratin-degrading micro-organisms and thermoregulation (Zink and Remsen 1986; Lev-Yadum 2015). The wide geographic and altitudinal ranges of these species, inhabiting forests at lower altitudes and open grasslands at higher altitudes on the same slopes, further offer interesting study cases to understand the relative importance of local adaptation to explain geographic variation in plumages.
The original description and most subsequent mentions of Scytalopus zimmeri mention gray throat and upper chest and short eyebrow, a plumage very similar to that of S. simonsi (Bond and Meyer de Schauensee 1940; Fjeldså and Krabbe 1990). While this description refers to the northern population, all sound recordings come from the southern population (Supplementary Appendix 2). Additional material from Bolivia is required to understand the distribution of this type of plumage and its possible isolation from southern populations (see “Geographical barriers” below). Plumage differences between the northern and southern populations of S. zimmeri are greater than those found between southern zimmeri and the nominal subspecies of S. superciliaris. It is understandable that those examining only skins from the type locality (25 km E of Padilla, northern population) would consider S. zimmeri as an intermediate between S. simonsi and S. superciliaris (Krabbe and Schulenberg 1997; Whitney 1994). On the contrary, our observations indicate that southern zimmeri and nominate superciliaris are often indistinguishable in the field (Fig. 1; see also Pearman and Areta 2020). The similarity between the southern population of S. zimmeri and nominate S. superciliaris is so remarkable, that it generated confusion in Argentina for more than half a century. Only Claës Olrog detected some differences in the plumage of birds from the southern population of S. zimmeri in comparison to nominate S. superciliaris from the Aconquija massif, mentioning that “the plumage of a female collected in Jujuy possesses a paler ventral region, grayer throat and shorter eyebrow than specimens of S. superciliaris from Tucumán” (Olrog 1958). In a later publication, the same author argued that “the population of Jujuy [of S. superciliaris] probably belongs to Scytalopus superciliaris zimmeri” (Olrog 1963). The presence of Scytalopus zimmeri in Argentina was recently confirmed through recordings of vocalizations (unpublished data by Mark Pearman and recordings by Juan Mazar Barnett; Pearman and Areta 2021); however, up until recently, the literature persisted in indicating that S. zimmeri (sensu lato) is endemic to Bolivia and that the populations of the Sierra de Calilegua belong to S. superciliaris (Krabbe and Schulenberg 2003) instead of to southern zimmeri.
The population of S. superciliaris from the Sierra de Velasco (presently known only from El Cantadero) was considered as a possible new subspecies by Nores and Cerana (1990). However, these authors neither presented detailed descriptions nor proposed a formal name. They characterized these birds from Sierra de Velasco as having dull cinnamon brown upperparts, throat with white area much broader, extended on to the chest and ashy gray upperparts as differing from Sierra de Ambato birds (from Las Juntas) that had light ochraceus brown upperparts and white area smaller, not extended onto the chest. The specimens of ambatensis from the Ambato range summits, here described for the first time, are remarkably similar to the description given by Nores and Cerana (1990) for the birds from Velasco range. Current evidence suggests that birds from Sierra de Velasco are better included in subspecies ambatensis, but future assessments should clarify this issue.
Geographical barriers
All the forms treated in this work are distributed in different mountain ranges and are allopatric to each other. The geographical barrier that separates the populations of northern zimmeri and southern simonsi would be the dry valley of the Río Grande and the arid regions of western and central Chuquisaca (Krabbe and Schulenberg 1997; Fig. 2). Our compilation of localities shows an important geographical separation between the northern and southern populations of S. zimmeri. The most relevant geographic feature that seems to separate these two populations is the Río Pilcomayo valley at 20°S (Fig. 2). In Argentina, southern zimmeri has a more or less continuous distribution along the humid eastern slope of the Andes. The southernmost known locality of southern zimmeri is located at 25°S, well north of the distribution of subspecies superciliaris and ambatensis. However the southernmost section of the distribution of southern zimmeri runs north–south and sub-parallel, but to the west of, the populations of subspecies santabarbarae. The separation between these populations is relatively small in geographic terms (about 55 km) and corresponds to the low areas located between the Sierra de Santa Bárbara and the Serranía de Calilegua where the San Francisco and Lavayén rivers flow (25°52′ S 64°36 ′W, 400 m) (Fig. 2). The pattern of geographic replacement of southern S. zimmeri and S. superciliaris is very similar to that observed in two sister species of Atlapetes brushfinches of the southern Yungas that share the same habitat type and whose distributions are disjunct. While Fulvous-headed Brushfinch (A. fulviceps) has a restricted distribution along the eastern slope of the Andes of Bolivia and Argentina, Yellow-striped Brushfinch (A. citrinellus) inhabits exclusively the extra-Andean sierras like Santa Bárbara and Aconquija being consequently endemic to Argentina (Ridgely and Tudor 1994, 2009; records attributed to A. citrinellus in the main Andes by Capllonch et al. 2014 and Sánchez-González et al. 2015 are in error, pers. obs.).
Three important gaps can be recognized in the distribution of S. superciliaris. The northernmost discontinuity is formed by the low areas of xerophytic vegetation crossed by the Río Juramento (25°13′ S 64°55′ W, 700 masl) that separate the Santa Barbara/Cresta del Gallo ranges from the Aconquija massif (Fig. 2). The populations of santabarbarae are in complete isolation, both from the other subspecies of S. superciliaris in the Pampean and Sub-Andean Sierras and from the populations of southern zimmeri in the Andes. The distance between the southernmost locality of santabarbarae (PN El Rey) and the northernmost superciliaris (San Pedro de Colalao) is approximately 190 km in a straight line. Between both localities is the Sierra de Metán range, which most likely shelters populations of the nominal form due to its continuity with the Aconquija massif and the presence of montane birds restricted to the extra-Andean mountains such as Yellow-striped Brushfinch.
The second discontinuity, the separation between Aconquija and Ambato, is somewhat blurry (Fig. 2). The Campo de Pucará (27°36′ S 66°04′ W, 1700 masl) was regarded as an important geographic barrier between both mountain ranges (Nores 1986). However, this geographical accident would not impede the north–south connection of populations of S. superciliaris. Nores et al. (2000) remark that “the occurrence [of forest birds] in the summits of Narváez, de los Pinos, Balcosna and Potrerillo, la Silleta de la Higuera, Aconquija, Graciana, Guayamba and Ancasti, would be simply by dispersion trough humid forest of Tucumán, since these forests are continuous”. This assumption makes us assume that the Scytalopus found in these ranges and, perhaps also the northern end of Ambato belong to nominate superciliaris. Thus, only the partially isolated populations from the Ambato summits, and the central and southern expanses of montane forests and gullies of Ambato and the relictual forests of Velasco would correspond to the subspecies ambatensis. It is important to examine more specimens in the northern transition between the Sierra de Ambato and the Aconquija massif to accurately understand the distribution of ambatensis and superciliaris, and to rigorously assess whether these forms grade into each other locally. Intriguingly, the absence of some species from the central and southern Ambato montane forests, such as Mountain Wren (Troglodytes solstitialis) and Pale-legged Warbler (Myiothlypis signata) (JIA and F. Burgos pers. obs.) may either suggest currently unsuitable ecological conditions and/or indicate the existence of former barriers not evident nowadays.
The third gap is much more drastic and consists of a desert area located between the Ambato and Velasco mountain ranges (Fig. 2), where the Río Salado flows (28°58′ S 66° 37′, 600 masl). However, this gap occurs within the distribution of ambatensis and apparently does not separate populations diagnosable by plumage. It is therefore evident that the Sierra de Velasco is completely separated from Ambato and represents the southernmost and isolated population of the superciliaris complex. The geographical barriers that separate the populations of both, superciliaris and ambatensis, from the Eastern Cordillera of the Andes consist of numerous arid zones that include the Valles Calchaquíes, Campo del Arenal, Campo de Belén, Salar de Pipanaco and the valleys between the Sierra de Velasco and the Sierra de Famatina (Fig. 2).
The altitudinal range occupied by S. superciliaris varies geographically throughout its distribution. The southern population of El Cantadero occurs at lower altitudes than other populations and this seems to be directly related to the appearance of humid forest at lower altitudes in those latitudes and to the extreme aridity of the Serranía de Velasco at higher altitudes. The wide altitudinal range of many populations of the superciliaris complex contrasts sharply with the Scytalopus of northern South America, where two or more sympatric species live in the same mountain range and each occupy a relatively narrow altitudinal fringe (Cadena and Céspedes 2020).
The Pampean and Sub-Andean Sierras of Argentina constitute two important orographic systems where several populations of birds have differentiated, many of which were described as subspecies based on plumage differences in comparison to populations in the Andes or in different mountain ranges (Nores and Yzurieta 1983; Nores 1986, 1995; Nores and Cerana 1990). The geographic barriers between extra-Andean mountains have allowed the differentiation of apparently diagnostic populations in several species of birds. For the Sierra de Ambato, differences at the subspecies level were suggested for Cinclodes cf. albiventris and Mecocerculus leucophrys; while in the Sierra de Velasco presumably new subspecies may await formal description in Phacellodomus maculipectus, Mecocerculus leucophrys, Arremon dorbignii and Microspingus erythrophrys (Nores and Cerana 1990, F. Burgos and JIA unpubl. data). The only endemic subspecies described for the Sierra de Santa Bárbara is S. superciliaris santabarbarae (Nores 1986), but the existence of a color variant of Amazona aestiva from the western sector of the Sierra de Santa Bárbara, reinforces the idea of differentiation of plumage and isolation in these ecological islands (Areta 2007).
Northern and southern simonsi are seemingly narrowly allopatric in central La Paz, Bolivia. The southernmost record of an individual attributable to northern simonsi on the basis of vocalizations comes from Pongo, while the northernmost records of southern simonsi based on voice come from Río Milluni. Specimens attributable to northern simonsi extend south to Unduavi and Hichuloma, and those presumably of southern simonsi to the area around Viloco and just to the south of the Río La Paz valley. This deep valley might mark the division between the two vocal and plumage types, but further studies are needed to understand their geographic distribution in central and northern La Paz.
Possible hybrids
Possible hybrids between S. zimmeri and S. simonsi were reported from the locality of Colomi, Bolivia (LSU-37781) by Whitney (1994), while two other skins from Khasa Punta Pampa, Bolivia (ZMUC-80030, 80,031) were also considered as intermediates between them (J. Fjeldså pers. comm. in Whitney 1994). Our examination of the Colomi specimen and six other specimens from the same locality (FMNH-180579, 180,580, 180,581, 180,582, 180,583, 180,584), and of pictures of the Khasa Punta Pampa specimens, allows us to conclude that the possible hybrids are not such but instead correspond to southern simonsi. All these specimens exhibit the uniform gray ventral coloration and barred undertail coverts that are typical of this species. Additionally, at least one of the Khasa Punta Pampa specimens gave typical southern simonsi songs (Krabbe and Schulenberg 1997, J. Fjeldså in litt. 2016). No genetic studies have attempted to solve the identity of specimens from these locations (Krabbe and Schulenberg 1997; Cadena et al. 2020). We conclude that there are no hybrids of S. zimmeri and S. simonsi, and moreover, current data indicate that they are allopatric and therefore cannot hybridize in nature.
Vocalizations and species limits
The study of vocalizations constitutes a simple but powerful tool to analyze species limits among birds, and this is especially facilitated in taxa that do not learn their songs. Vocalizations in Suboscine Passerines are essentially innate and therefore possess a predominantly genetic basis (Arctander and Fjeldså 1994; Kroodsma 1984, 1989; Kroodsma and Konishi 1991), with the notable exception of bellbirds (Procnias spp.) whose songs are learned (Kroodsma et al. 2013). Marked differences in vocalizations are considered as sufficient to identify species limits in Scytalopus (Krabbe and Schulenberg 1997; Maurício et al. 2014; Stiles et al. 2017; Krabbe et al. 2020). Similarly many recent works have modified the taxonomic status of species and subspecies in Thamnophilidae, Furnariidae and Tyrannidae based on differences or similarities in vocalizations among taxa (e.g., Abalos and Areta 2009; Alonso and Whitney 2001; Areta and Pearman 2009, 2013; Isler et al., 1997; Whitney et al., 2000; Zimmer 2000; Zimmer and Whittaker 2000). We base the following taxonomic conclusions on the recognition concept of species (Paterson 1985). While the same species and subspecies that we recognized would be delimited by the application of the biological species concept (Mayr 1963; “isolation concept” fide Paterson 1985), it is likely that proponents of the phylogenetic concept of species (Cracraft 1983; Nelson and Platnick 1981) may recognize the subspecies of S. superciliaris as separate species.
The southern population of S. zimmeri possesses a set of very distinctive vocalizations, which differ clearly from the rest of the populations of the superciliaris complex and from both populations of S. simonsi. This reinforces its recognition at the species level as proposed by Krabbe and Schulenberg (1997). The song of southern S. zimmeri is composed of multiple notes being very different in structure from songs of S. simonsi and S. superciliaris. Thus, as far as vocalizations are concerned, S. zimmeri does not seem to be an “intermediate” between S. superciliaris and S. simonsi (see Whitney 1994). A key piece missing in this puzzle is the recording of vocalizations of northern zimmeri (from where the type material comes from). This would allow solving the taxonomic treatment of southern populations, tentatively assigned here to zimmeri.
Both the song and the calls of the three forms of Scytalopus superciliaris treated in the present work are sufficiently similar to consider these diagnostic populations as subspecies of the same species. Temporal separation among these allopatric populations would not have been long enough for them to lose the ability to recognize themselves as members of the same reproductive community. Thus, we recognize a polytypic S. superciliaris with subspecies superciliaris (Cabanis 1883), santabarbarae (Nores 1986), and ambatensis (Areta and Monteleone, this work). The consistency in the vocalizations of S. superciliaris throughout its extensive geographic distribution (> 600 km long) is notable and contrasts strongly with the obvious vocal differentiation between southern S. zimmeri and the Santa Barbara population of S. superciliaris, separated by a relatively small (ca. 55 km) geographic gap.
The remarkable vocal differences in song here reported between the southern Peru and northern Bolivia populations (northern simonsi) and the central Bolivia populations (southern simonsi), provide strong evidence on the existence of two species within what is now considered as S. simonsi. Further studies are needed to evaluate the biological and taxonomic implications of these vocal variants that appear to also differ in plumage. Our preliminary data indicates that northern simonsi represents an undescribed species, but we refrain from formally describing this taxon until our ongoing work clarifies the geographic limits, phylogenetic affinities, and further vocal distinctions in the calls of these populations. Molecular phylogenetic and field studies will shed light on the history of differentiation in the understudied simonsi and superciliaris complexes.
Data availability
See Supplementary Appendices 1 and 2 for specimens and sound recordings.
Code availability
Not applicable.
References
Abalos R, Areta JI (2009) Historia natural y vocalizaciones del doradito limón (Pseudocolopteryx cf. citreola). Ornitol Neotrop 20:215–230
Alonso J, Whitney BM (2001) A new Zimmerius tyrannulet (Aves: Tyrannidae) from white sand forests of northern Amazonian Peru. Wilson Bull 113:1–9
Arctander P, Fjeldså J (1994) Andean tapaculos of the genus Scytalopus (Aves, Rhinocryptidae): a study of modes of differentiation, using DNA sequence data. In: Loeschcke V, Tomiuk J, Jain SK (eds) Conservation genetics. Birkhäuser Verlag
Areta JI (2007) A green-shouldered variant of the Turquoise-fronted Amazon Amazona aestiva from the Sierra de Santa Barbara, northwest Argentina. Cotinga 27:71–73
Areta JI, Pearman M (2009) Natural history, morphology, evolution, and taxonomic status of the earthcreeper Upucerthia saturatior (Furnariidae) from the Patagonian forests of South America. Condor 111:135–149
Areta JI, Pearman M (2013) Species limits and clinal variation in a widespread high Andean furnariid: the Buff-breasted Earthcreeper (Upucerthia validirostris). Condor 115:131–142
Avendaño JE, Cuervo AM, López-O JP, Gutiérrez-Pinto N, Cortés-Diago A, Cadena CD (2015) A new species of tapaculo (Rhinocryptidae: Scytalopus) from the Serranía de Perijá of Colombia and Venezuela. Auk 132:450–466
Bond J, Meyer de Schauensee R (1940) Descriptions of new birds from Bolivia. Part III. Notula Naturae 44. Academy of natural sciences of Philadelphia
Bornschein MR, Reinert BL, Pichorim M (1998) Descrição, ecologia e conservaçao de um novo Scytalopus (Rhinocryptidae) do sul do Brasil, com comentários sobre a morfologia da familia. Ararajuba 6:3–36
Bornschein MR, Mauricio GN, Belmonte-Lopes R, Mata H, Bonatto SL (2007) Diamantina tapaculo, a new Scytalopus endemic to the Chapada Diamantina, northeast Brazil (Passeriformes: Rhinocryptidae). Rev Brasil Ornitol 15:151–174
Cabanis J (1883) Bericht über die December-Sitzung. J Ornithol 31:104–106
Cadena CD, Céspedes LN (2020) Origin of elevational replacements in a clade of nearly flightless birds: most diversity in tropical mountains accumulates via secondary ontact following allopatric speciation. In: Rull V, Carnaval AC (eds) Neotropical diversification: patterns and processes. Springer Nature
Cadena CD, Cuervo AM, Céspedes LN, Bravo GA, Krabbe NK, Schulenberg TS, Derryberry GE, Silveira LF, Derryberry EP, Brumfield RT, Fjeldså J (2020) Systematics, biogeography and diversification of Scytalopus tapaculos (Rhinocryptidae), an enigmatic radiation of Neotropical montane birds. Auk. https://doi.org/10.1093/auk/ukz077
Capllonch P, Ortiz D, NúñezMontellano MG, Blendinger PG (2014) Aportes sobre la distribución, comportamiento y biología del cerquero amarillo, Atlapetes citrinellus (Aves: Emberizidae). Acta Zool Lilloana 58:222–240
Chubb C (1917) Scytalopus simonsi; sp. nov. Bulletin of the British Ornithologists’ Club 38: 17
Coopmans P, Krabbe N, Schulenberg TS (2001) Vocal evidence of species rank for nominate Unicolored Tapaculo Scytalopus unicolor. Bull Br Ornithol Club 121:208–213
Cracraft J (1983) Species concept and speciation analysis. Curr Ornithol 1:159–187
Cuervo A, Cadena CD, Krabbe N, Renjifo LM (2005) Scytalopus stilesi, a new species of tapaculo (Rhinocryptidae) from the cordillera central of Colombia. Auk 122:445–463
Meyer de Schauensee R (1966) The species of birds of South America and their distribution. The academy of Natural Sciences of Philadelphia. Livingston Publishing Company, Pennsylvania
de la Peña M (1989) Guia de aves argentinas. LOLA, Buenos Aires
Delhey K (2019) A review of Gloger’s rule, an ecogeographical rule of colour: definitions, interpretations and evidence. Biol Rev. https://doi.org/10.1111/brv.12503
Fjeldså J, Krabbe N (1990) Birds of the high andes. Zoological Museum University of Copenhagen and Apollo Books, Svendborg
Gould J (1837) Exhibition of birds allied to the European Wren, with characters of new species. Proc Zool Soc London Part 4:88–90
Harvey MG, Bravo GA, Claramunt S, Cuervo AM, Derryberry GE, Battilana J, Seeholzer GF, McKay JS, O’Meara BC, Faircloth BC, Edwards SV, Pérez-Emán J et al (2020) The evolution of a tropical biodiversity hotspot. Science 370:1343–1348
Hosner P, Robbins M, Valqui T, Peterson T (2013) A new species of Scytalopus tapaculo (Aves: Passeriformes: Rhinocryptidae) from the Andes of central Peru. Wilson J Ornithol 125:233–242
Hosner P, Andersen M, Robbins M, Urbay-Tello A, Cueto-Aparicio L, Verde-Guerra K, Sánchez-González L, Navarro-Sigüenza A, Boyd R, Núñez J, Tiravanti J, Combe M, Owens H, Peterson T (2015) Avifaunal surveys of the upper Apurímac river valley, Ayacucho and Cuzco departments, Peru: new distributional records and biogeographic, taxonomic, and conservation implications. Wilson J Ornithol 127:563–581
Isler M, Isler P, Whitney BM (1997) Biogeography and systematic of the Thamnophilus punctatus (Thamnophilidae) complex. Ornithol Monogr 48:355–381
Krabbe NK, Cadena CD (2010) A taxonomic revision of the Paramo Tapaculo Scytalopus canus Chapman (Aves: Rhinocryptidae), with description of a new subspecies from Ecuador and Peru. Zootaxa 2354:56–66
Krabbe NK, Schulenberg TS (1997) Species limits and natural history of Scytalopus tapaculos (Rhinocryptidae), with descriptions of the Ecuadorian taxa, including three new species. Ornithol Monogr 48:47–88
Krabbe NK, Schulenberg TS (2003) Family Rhinocryptidae (tapaculos). In: del Hoyo J, Elliot A, Christie D (eds) Handbook of the birds of the World. Broadbills to tapaculos, vol 8. Lynx Editions, Barcelona
Krabbe NK, Salaman P, Cortez A, Quevedo A, Ortega LA, Cadena CD (2005) A new species of Scytalopus tapaculo from the upper Magdalena Valley, Colombia. Bull Br Ornithol Club 125:93–108
Krabbe NK, Schulenberg TS, Hosner PA, Rosenberg KV, Davis TJ, Rosenberg GH, Lane DF, Andersen MJ, Robbins MB, Cadena CD, Valqui T, Salter JF, Spencer AJ, Angulo F, Fjeldså J (2020) Untangling cryptic diversity in the High Andes: revision of the Scytalopus [magellanicus] complex (Rhinocryptidae) in Peru reveals three new species. Auk. https://doi.org/10.1093/auk/ukaa003
Kroodsma D (1984) Songs of the Alder Flycatcher (Empidonax alnorum) and Willow Flycatcher (Empidonax traillii) are innate. Auk 101:13–24
Kroodsma D (1989) Male eastern phoebes (Sayornis phoebe; Tyrannidae, Passeriformes) fail to imitate songs. J Comp Psychol 103:227–232
Kroodsma D, Konishi M (1991) A suboscine bird (eastern phoebe, Sayornis phoebe) develops normal song without auditory feedback. Anim Behav 42:477–487
Kroodsma D, Hamilton D, Sánchez JE, Byers BE, Fandiño-Mariño H, Stemple DW, Trainer JM, Powell GVN (2013) Behavioral evidence for song learning in the Suboscine bellbirds (Procnias spp.; Cotingidae). Wilson J Ornithol 125:1–14
Lev-Yadum S (2015) Gloger’s rule in plants: the species and ecosystem levels. Plant Signal Behav 10:12
Maurício GN (2005) Taxonomy of southern populations in the Scytalopus speluncae group, with description of a new species and remarks on the systematics and biogeography of the complex (Passeriformes: Rhinocryptidae). Ararajuba 13:7–28
Maurício GN, Belmonte-Lopes R, Pacheco JF, Silveira LF, Whitney BM, Bornschein MR (2014) Taxonomy of ‘“Mouse-colored Tapaculos”’ (II): an endangered new species from the montane Atlantic Forest of southern Bahia, Brazil (Passeriformes: Rhinocryptidae: Scytalopus). Auk 131:643–659
Mayer S (2000) Bird songs of bolivia/sonidos de aves de Bolivia. version 2.0. Bird Songs International, Enschede
Mayr E (1963) Animal species and evolution. Harvard University Press, Cambridge
Nelson G, Platnick NI (1981) Systematics and biogeography. Columbia University Press, New York
Nores M (1986) Diez nuevas subespecies de aves provenientes de islas ecológicas Argentinas. Hornero 12:262–273
Nores M (1995) Insular biogeography of birds on mountain-tops in north western Argentina. J Biogeogr 22:61–70
Nores M, Cerana M (1990) Biogeography of relicts forests in the mountains of northwestern Argentina. Rev Chil Hist Nat 63:37–46
Nores M, Yzurieta D (1983) Especiación en las sierras pampeanas de Cordoba y San Luis (Argentina), con descripción de siete nuevas subespecies de aves. Hornero 12:88–102
Nores M, Salvador S, Yzurieta D (2000) Registros de aves de selva en Catamarca, Argentina. Hornero 15:111–115
Olrog CC (1958) Notas ornitológicas sobre la colección del Instituto Miguel Lillo (Tucumán) III. Acta Zool Lilloana 15:5–18
Olrog CC (1963) Lista y distribucion de las aves Argentinas. Opera Lilloana 9:1–377
Paterson HEH (1985) The recognition concept of species. In: Vrba ES (ed) Species and speciation. Transvaal Museum Monograph, Pretoria
Paynter R (1992) Ornithological Gazetteer of Bolivia. Museum of Comparative Zoology, Cambridge
Paynter R (1995) Ornithological gazetteer of Argentina. Museum of Comparative Zoology, Cambridge
Pearman M, Areta JI (2020) Birds of Argentina and the South-west Atlantic. Field guide. Helm, London
Pearman M, Areta JI (2021). Species lists of birds for South American countries and territories: Argentina. http://www.museum.lsu.edu/~Remsen/SACCCountryLists.html
Peters JL (1951) Check-list of birds of the world, vol 7. Museum of Comparative Zoology, Cambridge
Raposo MA, Stopiglia R, Loskot V, Kirwan G (2006) The correct use of the name Scytalopus speluncae (Ménétriés, 1835), and the description of a new species of Brazilian tapaculo (Aves: Passeriformes: Rhinocryptidae). Zootaxa 1271:37–56
Remsen JV, Areta JI, Bonaccorso E, Claramunt S, Jaramillo A, Pacheco JF, Robbins MB, Stiles FG, Stotz DF, Zimmer KJ (2021) A classification of the bird species of South America. American Ornithologists’ Union. http://www.museum.lsu.edu/Remsen/SACCBaseline.html
Ridgely RS, Tudor G (1994) The birds of South America. The Suboscine Passerines. University of Texas Press
Ridgely RS, Tudor G (2009) Field guide to the Songbirds of South America. The Passerines. University of Texas Press
Ridgway R (1912) Color standards and color nomenclature. A. Hoen and Co., Baltimore
Riveros G, Villegas N (1994) Análisis taxonómico de las subespecies chilenas de Scytalopus magellanicus (Fam. Rhinocryptidae, Aves) a través de sus cantos. An Mus Hist Nat Valparaiso 22:91–101
Sánchez-González LA, Navarro-Sigüenza AG, Krabbe MK, Fjeldså J, García-Moreno J (2015) Diversification in the Andes: the Atlapetes brush-finches. Zoolog Scr 44:135–152
Stephens L, Traylor M (1983) Ornithological Gazetteer of Peru. Museum of Comparative Zoology, Cambridge
Stiles FG, Laverde-R O, Cadena CD (2017) A new species of tapaculo (Rhinocryptidae: Scytalopus) from the Western Andes of Colombia. Auk 134:377–392
Vielliard J (1990) Estudo bioacústico das aves do Brasil: o gênero Scytalopus. Ararajuba 1:5–18
Whitney BM (1994) A new Scytalopus tapaculo (Rhinocryptidae) from Bolivia, with notes on the other Bolivian members of the genus and the magellanicus complex. Wilson Bull 106:585–612
Whitney BM, Pacheco JF, Buzzetti D, Parrini R (2000) Systematic revision and biogeography of the Herpsilochmus pileatus complex, with description of a new species from northeastern Brazil. Auk 117:869–891
Whitney BM, Vasconcelos MF, Silveira LF, Pacheco JF (2010) Scytalopus petrophilus (Rock Tapaculo): a new species from Minas Gerais, Brazil. Reva Brasil Ornitol 18:73–88
Zimmer JT (1939) Studies of Peruvian birds. Nº 32. Amer. Mus. Novitates Nº 1044
Zimmer K (2000) The rufous Cacholote (Furnariidae: Pseudoseisura) is two species. Condor 102:409–422
Zimmer K, Whittaker A (2000) Species limits in Pale-tipped Tyrannulets (Inezia: Tyrannidae). Wilson Bull 112:51–66
Zink R, Remsen JV (1986) Evolutionary processes and patterns of geographic variation in birds. In: Johnston RF (ed) Current ornithology, vol 4. Plenum Press, New York, pp 1–69
Acknowledgements
This work was enriched with the direct and indirect collaboration of many people. The patience and ability of Cristina Pita were essential in making the distribution maps, and she also had to endure hours of work and talks about the Scytalopus. Several photographers sent images for possible inclusion in this work, among them E. Peña, P. Pagano, A. Terán, S. Vitale, F. Burgos and R. Ursino. F. Uriona (Aves Argentinas) helped with bibliographic searches. During our surveys in Sierra de Santa Bárbara we were lucky to have the company of our friend José “Podo” Segovia; while the Cuñado family provided us with lodging in its spectacular mountain refuge. We would like to express our gratitude to the staff of museums and ornithological collections who granted access to skins and photographs of specimens: S. Cardiff (LSUMZ), N. Rice (ANSP), P. Sweet (AMNH), D. Ardia (CLO), J. Bates (FMNH), K. Thorup (ZMUC), I. Gomez (CBF), Y. Davies (MACN), A. Echevarría (FML), and C. Darrieu (MLP). S.A. Salvador kindly sent data and photographs of the birds collected in El Cantadero. We appreciate key information on some Bolivian specimens by J. Fjeldså and N. Krabbe, and thank S. Cardiff for providing detailed descriptions of key specimens. M. Medler and M. Young allowed granted access to recordings held at the Macaulay Library. The ECOSON lab team offered support and affection, especially I. Holzmann, G. Mangini, F. Gandoy, E. Depino, and G. Núñez. Many local people helped in various localities such as Las Juntas, El Cantadero, Tafí del Valle, Chumbicha, Mutquin, Río Nío and Yunka Suma. Special thanks are due to Freddy Burgos for sharing field trips and unpublished weight data of S. zimmeri and S. superciliaris. The manuscript benefited from the constructive comments of Niels K. Krabbe and an anonymous reviewer.
Funding
JIA’s research was supported by CONICET.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts or competing interests to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by A. Aleixo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Areta, J.I., Monteleone, D.L. Species limits and biogeography of the White-browed Tapaculo (Scytalopus superciliaris) complex and the Puna Tapaculo (S. simonsi). J Ornithol 164, 13–35 (2023). https://doi.org/10.1007/s10336-022-02012-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-022-02012-0