Abstract
Foraging behavior of Brown Rats (Rattus norvegicus) is commonly thought to be guided by olfactory cues. Here we tested the hypothesis that foraging Brown Rats eavesdrop on bird vocalizations to locate prey. We recorded calls of nestling Starlings (Sturnus vulgaris) with microphones sensitive in the sonic and ultrasonic range, respectively, and compiled clear begging calls into a single sound file (2-min long) which included the entire recorded sound range (0–100 kHz; Audio File 1). Sound analyses revealed a fundamental two-tone modulated sound centered around 3 kHz, a first and second harmonic at, respectively, 9 and 16 kHz, and bands of ultrasonic frequency components at 20, 25, and 37 kHz. We subjected Audio File 1 to low- and highpass-filtering, thereby producing a sonic-range only file (< 20 kHz; Audio File 2), and an ultrasonic-range only file (20–100 kHz; Audio File 3). In binary-choice large arena bioassays, we then tested the behavioral responses of single Brown Rats to paired trap boxes each fitted with a speaker, one of which emitting a white noise control and the other playing back Audio File 1 (Exp. 1), Audio File 2 (Exp. 2), or Audio File 3. In each of experiments 1–3, female and male rats (i) significantly more often entered first the trap box broadcasting an Audio File, and (ii) spent significantly more time in the arena quadrant with an Audio File trap box. Our data support the conclusion that foraging Brown Rats, as opportunistic omnivores, exploit begging calls of nestling Starlings, and possibly other birds, as cues to obtain a proteinaceous meal.
Zusammenfassung
Phonotaktische Reaktionen von Wanderratten ( Rattus norvegicus ) auf Bettelrufe von Nestlingen des Stars ( Sturnus vulgaris )
Es wird allgemein angenommen, dass das Nahrungssuchverhalten von Wanderratten (Rattus norvegicus) durch Geruchsreize gesteuert wird. Hier testeten wir die Hypothese, dass Wanderratten bei der Nahrungssuche Vogellaute nutzen, um Beute zu finden. Wir nahmen Rufe von Nestlingen des Stars (Sturnus vulgaris) mit Mikrofonen auf, die Töne im Schall- und Ultraschallbereich empfangen, und fassten deutliche Bettelrufe zu einer einzigen Klangdatei (zwei Minuten lang) zusammen, die den gesamten aufgezeichneten Klangbereich (0–100 kHz; Audiodatei 1) umfasste. Klanganalysen ergaben ein grundlegendes, moduliertes Zwei-Ton Element, das um 3 kHz zentriert war, eine erste und zweite Oberschwingung bei 9 bzw. 16 kHz sowie Frequenzbänder mit Ultraschallkomponenten bei 20, 25 und 37 kHz. Wir setzten die Audiodatei 1 einer Tief- und Hochpassfilterung aus, wodurch eine Datei nur für den Schallbereich (< 20 kHz; Audiodatei 2) und eine nur für den Ultraschallbereich (20–100 kHz; Audiodatei 3) erstellt wurde. In einer großen Wahlarena testeten wir die Verhaltensreaktion einzelner Wanderratten gegenüber zwei Entscheidungsmöglichkeiten (engl. binary-choice large arena bioassays) mit der Wahl zwischen zwei gegenüberstehenden Versuchsboxen, welche jeweils mit einem Lautsprecher ausgestattet waren: ein Lautsprecher spielte ein Kontrollrauschen ab, der andere die Audiodatei 1, 2 oder 3 (Experiment 1–3). In jedem dieser Experimente betraten weibliche und männliche Wanderratten (i) signifikant häufiger zuerst die Versuchsbox, in der eine Audiodatei abgespielt wurde, und (ii) verbrachten signifikant mehr Zeit in dem Quadranten mit einer Audiodatei abspielenden Versuchsbox. Unsere Daten stützen die Schlussfolgerung, dass Nahrung suchende Wanderratten als opportunistische Allesfresser die Bettelrufe von Nestlingen des Stars und möglicherweise auch die von anderen Vogelarten als Anhaltspunkte nutzen, um eine proteinreiche Mahlzeit zu erhalten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the late Pleistocene, murine rodents in the genus Rattus have followed humans all over the globe becoming highly adaptable and prolific pests worldwide (Buckle and Smith 1994; Aplin et al. 2003; Stenseth et al. 2003; Capizzi et al. 2014). Adopting a generalist diet is one of the key adaptations that has contributed to the success of rats (Aplin et al. 2003), allowing them to inhabit almost every ecosystem (Himsworth et al. 2013). Rats even prey on other vertebrates such as birds (Ringler et al. 2015). On islands, rats are particularly problematic for small ground-nesting terrestrial and sea birds, especially if they prey on all of the birds’ life stages (Jones et al. 2008; Croxall et al. 2012). Rats hunting and killing avian prey on the island of Norderoog in the North Sea is a behavior that has been known for decades (Steiniger 1950). On the Hawaiian Islands, Brown Rats (R. norvegicus) prey on arboreal nesting birds, and in New Zealand, both Brown Rats and Black Rats (R. rattus) greatly harm the indigenous fauna (Bellingham et al. 2010; Brooke et al. 2010; Simeone and Luna-Jorquera 2012). Worldwide, the semi-arboreal Black Rat is considered the most impactful murine predator of bird nests (Harper and Bunbury 2015) but on some islands of British Columbia (BC, Canada) (e.g., Langara Island) it is the semi-fossorial Brown Rat that is a more prominent nest predator of ground- and burrow-nesting birds (Rodway et al. 1983; Hobson et al. 1999; Taylor et al. 2000).
Rats have excellent hearing and can detect sound in the range of 0.2–90 kHz (Turner et al. 2005). Vocalizations of rats are both sonic (< 20 kHz) and ultrasonic (> 20 kHz) and play a role in their rivalry and reproductive behavior such as aggression, courtship and maternal pup retrieval (Noirot 1972; Takeuchi and Kawashima 1986; White et al. 1992; Brunelli et al.1994; Burgdorf et al. 2008; Takács et al. 2016). Foraging behavior of rats is thought to be informed primarily by olfactory cues (Slotnick 2001; Vander Wall et al. 2003; Price and Banks 2012) but vocalizations of prospective prey could serve as a sound cue, literally guiding rats to the micro-location of their next meal. Indeed, begging vocalizations of avian nestlings are often so conspicuous that they could become an acoustic beacon to foraging rats. Here, we tested the hypothesis that Brown Rats phonotactically respond to begging vocalizations of nestling Starlings (Sturnus vulgaris) to locate prey.
Materials and methods
Experimental rats
Brown Rats (Rattus norvegicus; strain: BN; 20 males, 25 females) were obtained from Charles River Laboratories Ltd. (Sherbrooke, Québec J1E 0B5, Canada) and housed in the Animal Research Centre of Simon Fraser University (SFU) in accordance with the Canadian Council on Animal Care guidelines and experimental protocols approved by SFU’s Animal Care Committee (UACC protocol number 1134B-14). All bioassay rats had not experienced any avian-produced sound of any kind and were considered naïve in this regard.
Recordings of begging calls of Starling nestlings
Microphones (see below) were positioned 0.5 m from a Starling nest (with 3–4 nestlings) located between the roof rafters of a rural cabin in southern BC, Canada. The begging calls of these nestlings were recorded continuously for 2 h (10:00–12:00) nine and seven days before fledging.
Recordings of nestling vocalizations in the sonic range were obtained using an AKG CK 61-ULS condenser microphone (flat peak response: 0.2–15 kHz; sensitivity: 0.06–19 kHz ± 10 dB; AKG Acoustics, Nashville, TN 37217, USA), whereas recordings of vocalizations in the ultrasonic range were obtained using a “Mini” SiSonic™ Ultrasonic Acoustic Sensor (SPM0404UD5 Knowles®, Itasca, IL 60143, USA; peak response: 20–65 kHz, sensitivity: 10–100 kHz ± 10 dB). Prior to digitizing at 250 kHz per channel via the DAQ card, the signal-to-noise ratio of recordings was improved by pre-amplifying sounds (SC-2040 amplifier; National Instruments (NI), Austin, TX 78682, USA). Recordings were saved to a desktop computer (Dell Inspiron i5, Round Rock, TX, USA) equipped with a 16-bit National Instruments data acquisition card (NI PCIe-6259) and programmed with LabVIEW 7.1 (NI). Recorded sounds were analyzed for the duration, frequency, intermittency and relative intensity using LabView’s Joint Time Frequency Analyzer.
After sound analyses, segments with at least two clear distant begging calls (when adult parents are absent from the nest) (Chaiken 1990) were spliced into a 2-min long sound file (Audio File 1) which included the entire recorded sound range (0–100 kHz). Audio File 1 was then digitally lowpass-filtered to produce a sonic-range only file (< 20 kHz; Audio File 2) and highpass-filtered to produce an ultrasonic-range only file (20–100 kHz; Audio File 3). During behavioral bioassays with rats (see below), Audio Files 1, 2 or 3 were looped (automatically rerun) and continuously played back for 50 min through a Sennheiser 70 dielectric headphone speaker (frequency response: 10–41,000 Hz, < 0.05% total harmonic distortion; Sennheiser Electronic Co., Old Lyme, CT 06371, USA).
Open arena experiments
The experimental set-up consisted of a circular galvanized steel arena (2 m diam × 60 cm high) that offered each bioassay rat a choice between two test stimuli (‘binary choice bioassay’; Fig. 1). The arena was illuminated from above by a 7.5-W red bulb (Halco Lighting Technologies, Norcross, GA 30071, USA) to realize a ‘crepuscular’ light intensity while facilitating recordings of the rat’s behavior and position by an observer situated behind a cardboard barrier with a small viewing port. For each binary choice 30-min bioassay, two metal trap boxes (25 × 20 × 12 cm; T. Eaton & Co. Inc, Twinsburg, OH 44087, USA) containing 1 g of a food bait (attractive food odorants mixed into grain-based feeding stimulants; Takács et al. 2018) were placed in opposite quadrants of the arena, 10 cm from the wall, and fitted with a Sennheiser headphone speaker playing back by random assignment (i) Audio File 1 or white noise (Exp. 1), (ii) Audio File 2 or white noise (Exp. 2), or (iii) Audio File 3 or white noise (Exp. 3). During playbacks, the peak sound pressure level at 2 cm from the speaker was set to 75 dB. Replicates of each of parallel experiments 1–3 were run on the same day on each of several days, invariably testing the responses of rats during their crepuscular activity period (1 h before and after the onset of the scotophase). Each rat was tested only once in each experiment but was tested in all three experiments, with the order of experiments randomly assigned to each rat, and bioassays separated by at least seven days.
Illustration (not to scale) of the bioassay arena (modified from Takács et al. 2016) used to test behavioral responses of Brown Rats (Rattus norvegicus) in experiments 1–3 to playback begging calls of nestling Starlings (Sturnus vulgaris). Numbers refer to components of the experimental design: (1) open arena; (2) bioassay rat transport container positioned equidistant to each of two metal trap boxes (3a, 3b) placed in opposite quadrants of the arena, 10 cm from the wall; Inset: (4) food bait in watch glass; (5) Sennheiser headphone speaker
To initiate an experimental replicate, the headphone speakers in both trap boxes were turned on and a single adult rat (40- to 52-weeks-old; food-deprived for 4 h prior to the bioassay) was transferred from its “home” cage to an empty gated mesh and sheet metal box (25 × 15 × 15 cm) which was placed in the arena equidistant to both trap boxes. After 15 min of acclimation, the gate was raised, allowing the rat to leave the box on its own accord and to explore the arena and the trap boxes.
For each rat, we recorded (i) the trap box it entered first with all four paws (“first choice”), and (ii) the percent time it spent in arena quadrants associated with a trap box. The latter data were obtained by recording the rat’s position in any one of the four arena quadrants at each of 30 1-min intervals. Following each replicate, the speakers were wiped with a pet urine odor remover (Nature’s Miracle®), and the bioassay arena and trap boxes were cleaned with Percept™ disinfectant detergent (Virox Technologies Inc., Mississaugua, ON L5N 5M4, Canada) and wiped with the urine odor remover.
Data analyses
First-choice data and the percent time spent in quadrants with a trap box were analyzed using a χ2-test with Yate’s correction for continuity (α = 0.05) and the Student’s t-test (α = 0.05), respectively. Replicates with a rat not leaving the transfer box were excluded from analyses.
Results
Analyses of distant begging calls of Starling nestlings
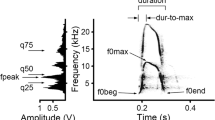
Begging calls of Starling nestlings show a fundamental two-tone modulated sound centered around 3 kHz with a first harmonic at 9 kHz and a second harmonic at 16 kHz (Fig. 2). Begging calls also show 3- to 5-kHz-wide bands of ultrasonic frequency components at 20, 25, and 37 kHz, with additional faint-signal bands in the 40- and 50-kHz range (Fig. 2). Beyond 54 kHz, no discernible frequency components were detectable (Fig. 2).
Analysis of (1) waveform, (2) frequency, and (3) time–frequency sound intensity (sonogram) of a representative begging call of a nestling Starling (Sturnus vulgaris) 9 days prior to leaving the nest. The darker shades in the sonogram indicate more intense frequency components. Note the distinct ultrasonic frequency components
Behavioral experiments
In binary-choice experiments 1–3 (Fig. 3), both female and male rats significantly more often entered first the trap box which emitted Audio File 1 (0–100 kHz; Exp. 1), Audio File 2 (< 20 kHz; Exp. 2), or Audio File 3 (20–100 kHz; Exp. 3) than the corresponding control box which emitted white noise (Exp. 1: females: χ2 = 7.20, p < 0.014, df = 19; males: χ2 = 7.36, p < 0.016, df = 15; Exp. 2: females: χ2 = 5.26, p < 0.04, df = 23; males: χ2 = 7.36, p < 0.016, df = 15; Exp. 3: females: χ2 = 13.76, p < 0.001, df = 20; males: χ2 = 6.00, p < 0.05, df = 19). Similarly, in each of experiments 1–3 (Fig. 4), both male and female rats spent significantly more time in the quadrant with the trap box which emitted Audio File 1 (0–100 kHz; Exp. 1), Audio File 2 (< 20 kHz; Exp. 2), or Audio File 3 (20–100 kHz; Exp. 3) than in the corresponding quadrant with the control box which emitted white noise (Exp. 1: females: t-statistic = 7.78, p < 0.001; males: t = 3.51, p < 0.002; Exp. 2: females: t = 7.11, p < 0.001; males: t = 3.46, p < 0.002; Exp. 3: females: t = 5.44, p < 0.001; males: t = 4.29, p < 0.001).
Effect of sound stimuli randomly assigned to one of two trap boxes in a large bioassay arena on decisions which box to enter first by Brown Rat (Rattus norvegicus) males or females. In each replicate, the randomly assigned control box emitted white noise, whereas the corresponding treatment box emitted playback begging calls of nestling Starlings (Sturnus vulgaris). The audio file of the treatment sound entailed (1) the complete recorded sound range (sonic and ultrasonic frequency components; 0–100 kHz; Exp. 1), (2) the sonic range only (lowpass-filtered; Exp. 2), or (3) the ultrasonic range only (highpass-filtered; Exp. 3). For each experiment, an asterisk (*) denotes a significant preference (p < 0.05) for the treatment stimulus and n indicates the number of single female or male rats tested
Effect of sound stimuli randomly assigned to one of two trap boxes in a large bioassay arena on decisions by Brown Rat (Rattus norvegicus) males or females whether to spend more time in the arena quadrant fitted with the treatment box or the control box. In each replicate, the randomly assigned control box emitted white noise, whereas the corresponding treatment box emitted playback begging calls of nestling Starlings (Sturnus vulgaris). The audio file of the treatment sound entailed (1) the complete recorded sound range (sonic and ultrasonic frequency components; Exp. 1), (2) the sonic range only (lowpass-filtered; Exp. 2), or (3) the ultrasonic range only (highpass-filtered; Exp. 3). For each experiment, the asterisk (*) denotes the quadrant in which the rats spent most of their time (Student’s t-test; p < 0.05)
Discussion
Our data show that begging calls of Starling nestlings are remarkably complex comprising both sonic and ultrasonic components and that Brown Rat females and males phonotactically respond to playback recordings of these calls. The begging calls of Starling nestlings, as shown here (Fig. 2), resemble those reported by Chaiken (1990, 1992). With their 3- to 5-kHz-wide bands of ultrasonic frequency components, the sonic (< 20 kHz) characteristics of these calls also exhibit a type of pattern that was noted in the ‘chirps' and 'screams' of Starling nestlings (Chaiken 1990, 1992).
While it is long accepted that bird calls are within the range of human hearing (0–20 kHz) (Weismana et al. 2014), there is also evidence that some avian species echolocate and some produce songs with ultrasonic frequency components. For example, nocturnal oilbirds (Steatornis caripensis) and cave-dwelling swiftlets (Collocalia spp.`) produce broadband echolocation clicks up to 50 kHz (Suthers and Hector 1982, 1985), and the songs of some parrots (Psittaculidae), warblers (Parulidae) and hummingbirds (Trochilidae) comprise both sonic and ultrasonic frequency components (Narins et al. 2004; Pytte et al. 2004). Begging calls of Starling nestlings also have significant ultrasonic frequency components which could be exploited by foraging predators such as cats and rats that have an excellent hearing in the ultrasonic range (Turner et al. 2005).
To determine whether foraging Brown Rats indeed exploit sound cues from prospective bird prey, we tested phonotactic responses of male and female rats to playback recordings of compiled begging calls produced by Starling nestlings. To also determine the frequency components of these begging calls that may mediate the rats’ behavioral responses, we ran three parallel binary-choice experiments, subjecting rats to playback audio files which covered the entire recorded sound range (0–100 kHz; Exp. 1), the sonic range (< 20 kHz; Exp. 2), and the ultrasonic range (> 20 kHz; Exp. 3). As predicted, the begging call audio file covering the entire sound range attracted both female and male rats and prompted them to stay near the sound source most of the time (Figs. 3, 4, top). These data imply that foraging rats eavesdrop on vocal communications of prospective bird prey and that they co-opt the birds’ communication signals as sound cues to locate prey. Remarkably, the audio files covering either just the sonic range (< 20 kHz) or the ultrasonic range (> 20 kHz), were also effective in attracting and arresting rats (Figs. 3, 4, middle and bottom). Moreover, bioassay data obtained with female rats seem to reveal that the ultrasonic audio file elicited the relatively strongest behavioral responses.
The excellent hearing ability of rats in the ultrasonic range apparently plays a role in the context of both intraspecific communication and prey location. It is well known that male rats impress their mates with ultrasonic courtship songs (Sewell 1970; Brudzynski 2005; Costantini and D’Amato 2006; Sales 2010) and that nursing female rats respond to ultrasonic discomfort calls of their pups (Allin and Banks 1971; Noirot 1972; Brouette-Lahlou et al. 1992; Brunelli et al. 1994). Here we show that foraging male and female rats exploit ultrasonic frequency components in begging calls of Starling nestlings. These results underline previous findings that foraging juvenile, female and male Brown Rats respond to playback recordings of rat pup vocalizations (Takács et al. 2016), with male rats being known predators of rat pups (Hrdy 1979; Mennella and Moltz 1988; Shapira et al. 2013).
It would be interesting to bioassay the responses of Brown Rats to playback recordings of burrow-nesting seabirds such as ancient murrelets (Synthliboramphus antiquus), Cassin’s auklets (Ptychoramphus aleuticus), and Leach’s storm-petrels (Oceanodroma leucorhoa), the nestlings of which engage in begging calls and the adults produce greet, aggression and defensive calls in and near burrows (Buxton et al. 2013). It is conceivable that Brown Rats use the calls of these seabird nestlings to locate burrows and thus ultimately encounter nestling prey. If so, this would explain, in part, the devastating impact of invasive Brown Rats on seabird communities. However, bioassaying the responses of Brown Rats to vocalization recordings from these seabirds is challenging in that currently available sound files (Macaulay Library at the Cornell Lab of Ornithology) fall only within the range of human hearing (0.2–20 kHz) (Thorpe and Griffin 1962; Pytte et al. 2004). Similarly, online sound libraries are in wav format and were obtained with a 44.1 kHz digital sampling rate capable of reproducing sound up to only 22 kHz. Therefore, these recordings do not represent the full range and potential complexity of the birds’ vocalizations, thereby possibly altering the rats’ behavioral responses, as we have experienced in pre-screening rat bioassays with some online audio files of birds (data not shown). To address this challenge, we decided not to rely on online sound files of Starling nestlings in bioassays but to obtain our own recordings that included both the sonic and ultrasonic range.
Field-testing our audio file of Starling vocalizations for the responses of wild Brown Rats proved too challenging. First and possibly most importantly, the Starlings’ vocalizations are most complex (Fig. 2), making it almost impossible to produce “synthetic” replica that could be broadcast by electronic devices (see Takács et al. 2016). These devices are advantageous for broadcasting sound because they can be driven by an algorithm that modulates critical sound characteristics such as intensity and frequency of occurrence. Exposure of wild Brown Rats to playback recordings closely resembling the calls of live birds is paramount to triggering “natural” foraging responses of rats. Lacking a “synthetic” audio file, we were left with the option of playing back our compiled audio file in repetitive and unmodulated form from a laptop computer in multiple field settings with a low rat infestation. With this option, the prospect of luring notoriously cautious (neophobic) wild Brown Rats (Inglis et al. 1996) into traps was very low, and as the likelihood of losing laptop computers to theft was exceedingly high, we chose not to field test our Starling audio file for the response of wild Brown Rats. Although domesticated rodents can behave differently than their wild counterparts (Wolff 2003; Calisi and Bentley 2009; Kondrakiewicz et al. 2019), we have laboratory and field data showing that both laboratory-strain Brown Rats and wild Brown Rats respond to ‘synthetic’ vocalizations from rat pups (Takács et al. 2016, 2021) which—like bird nestlings—fall into the prey spectrum of foraging adult rats. Based on these data, we predict that both wild and domesticated rats respond similarly to vocalizations of prospective bird prey.
Conclusions
Omnivorous Brown Rats not only feed on grains, seeds, nuts and fruits, they also prey upon insects and smaller animals such as mice, rat pups, and bird nestlings. The rats’ excellent hearing in the sonic and ultrasonic range mediates rivalry and reproductive behavior but could also play a role during predation. Currently, foraging rats are thought to be guided primarily by olfactory cues but they may also respond to vocalizations of prospective prey. Two studies have already shown that prey-seeking rats respond to rat pup vocalizations, suggesting they may also respond to vocalizations from avian prey. Here we tested the hypothesis that foraging Brown Rats eavesdrop on bird vocalizations to locate bird prey. Using the European Starling as a model species, we show that begging calls of nestlings contain frequency components in both the sonic and ultrasonic range. We further show that playback recordings of nestling begging calls trigger phonotactic responses of male and female rats seeking the micro-location of potential prey. Our data support the conclusion that foraging Brown Rats exploit begging calls of nestling Starlings, and likely other birds, to locate prey. If this concept were to be proven correct for burrow-nesting seabirds, it would explain the devastating impact invasive Brown Rats have on the populations of these birds.
References
Allin JT, Banks EM (1971) Effect of temperature on ultrasound production by infant albino rats. Dev Psychobiol 4:149–156. https://doi.org/10.1002/dev.420040206
Aplin KP, Chesser T, Have JT (2003) Evolutionary biology of the genus Rattus: profile of an archetypal rodent pest. In: Singleton GR, Hinds LA, Krebs CJ, Spratt DM (eds) Rats, mice and people: rodent biology and management. ACIAR, Canberra, pp 487–498
Bellingham PJ, Towns DR, Cameron EK, Davis JJ, Wardle DA, Wilmshurst JM, Mulder CPH (2010) New Zealand island restoration: seabirds, predators, and the importance of history. New Zealand J Ecol 34:115–136
Brooke ML, O’Connell TC, Wingate D, Madeiros J, Hilton GM, Ratcliffe N (2010) Potential for rat predation to cause decline of the globally threatened Henderson petrel Pterodroma atrata: evidence from the field, stable isotopes and population modelling. Endang Species Res 11:47–59. https://doi.org/10.3354/esr00249
Brouette-Lahlou I, Vernet-Maury E, Vigouroux M (1992) Role of pups’ ultrasonic calls in a particular maternal behavior in Wistar rat: pups’ anogenital licking. Behav Brain Res 50:147–154. https://doi.org/10.1016/S0166-4328(05)80296-7
Brudzynski SM (2005) Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav Genet 35:85–92. https://doi.org/10.1007/s10519-004-0858-3
Brunelli SA, Shair HN, Hofer MA (1994) Hypothermic vocalizations of rat pups (Rattus norvegicus) elicit and direct maternal search behavior. J Comp Psychol 108:298–303. https://doi.org/10.1037/0735-7036.108.3.298
Buckle AP, Smith RH (1994) Rodent pests and their control. CAB International, Wallingford, Oxfordshire, UK, p 403
Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J (2008) Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol 122:357–367. https://doi.org/10.1037/a0012889
Buxton RT, Major HL, Jones IL, Williams JC (2013) Examining patterns in nocturnal seabird activity and recovery across the Western Aleutian Islands, Alaska, using automated acoustic recording. Auk 130:331–341. https://doi.org/10.1525/auk.2013.12134
Calisi RM, Bentley GE (2009) Lab and field experiments: are they the same animal? Horm Behav 56:1–10
Capizzi D, Bertolino S, Mortelliti A (2014) Rating the rat: global patterns and research priorities in impacts and management of rodent pests. Mammal Rev 44:148–162. https://doi.org/10.1111/mam.12019
Chaiken M (1990) The ontogeny of antiphonal calling in European starlings. Dev Psychobiol 23:233–246. https://doi.org/10.1002/dev.420230304
Chaiken M (1992) Individual recognition of nestling distress screams by European starlings (Sturnus vulgaris). Behaviour 120:139–150. https://doi.org/10.1163/156853992X00255
Costantini F, D’Amato FR (2006) Ultrasonic vocalizations in mice and rats: social contexts and functions. Acta Zool Sinn 52:619–633
Croxall JP, Stuart H, Butchart M, Lascelles B, Stattersfield AJ, Sullivan B, Symes A, Taylor P (2012) Seabird conservation status, threats and priority actions: a global assessment. Bird Conserv Int 22:1–34. https://doi.org/10.1017/S0959270912000020
Harper GA, Bunbury N (2015) Invasive rats on tropical islands: their population biology and impacts on native species. Glob Ecol Conserv 3:607–627. https://doi.org/10.1016/j.gecco.2015.02.010
Himsworth CG, Parsons KL, Jardine C, Patrick DM (2013) Rats, cities, people, and pathogens: a systematic review and narrative synthesis of literature regarding the epidemiology of rat-associated zoonoses in urban centers. Vector Borne Zoonotic Dis 13:349–359. https://doi.org/10.1089/vbz.2012.1195
Hobson KA, Drever MC, Kaiser GW (1999) Norway rats as predators of burrow-nesting seabirds: insights from stable isotope analyses. J Wildl Manag 63:14–25
Hrdy SB (1979) Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol Sociobiol 1:13–40. https://doi.org/10.1016/0162-3095(79)90004-9
Inglis IR, Shepherd DS, Smith P, Haynes PS, Bull DS, Cowan DP, Whitehead D (1996) Foraging behaviour of wild rats (Rattus norvegicus) towards new foods and bait containers. Appl Anim Behav Sci 47:175–190. https://doi.org/10.1016/0168-1591(95)00674-5
Jones HP, Tershy BR, Zavaleta ES, Croll DA, Keitt BS, Finkelstein ME, Howald GR (2008) Severity of the effects of invasive rats on seabirds: a global review. Conserv Biol 22:16–26. https://doi.org/10.1111/j.1523-1739.2007.00859.x
Kondrakiewicz K, Kostecki M, Szadzińska W, Knapska E (2019) Ecological validity of social interaction tests in rats and mice. Genes Brain Behav 18:1–14
Mennella JA, Moltz H (1988) Infanticide in rats: male strategy and female counter-strategy. Physiol Behav 42:19–28. https://doi.org/10.1016/0031-9384(88)90254-5
Narins P, Feng A, Lin W, Schnitzler H, Denzinger A, Suthers R, Xu C (2004) Old world frog and bird vocalizations contain prominent ultrasonic harmonics. J Acoust Soc Am 115:910–913. https://doi.org/10.1121/1.1636851
Noirot E (1972) Ultrasounds and maternal behavior in small rodents. Dev Psychobiol 5:371–387. https://doi.org/10.1002/dev.420050410
Price CJ, Banks PB (2012) Exploiting olfactory learning in alien rats to protect birds’ eggs. PNAS 109:19304–19309. https://doi.org/10.1073/pnas.1210981109
Pytte CL, Ficken MS, Moisef A (2004) Ultrasonic singing by the blue-throated hummingbird: a comparison between production and perception. J Comp Physiol A 190:665–673. https://doi.org/10.1007/s00359-004-0525-4
Ringler D, Russell JC, Le Corre M (2015) Trophic roles of black rats and seabird impacts on tropical islands: mesopredator release or hyperpredation? Biol Conserv 185:75–84. https://doi.org/10.1016/j.biocon.2014.12.014
Rodway MN, Hillis N, Langley L (1983) Nesting population of ancient murrelets on Langara Island, British Columbia. Canadian Wildlife Service Technical Report, Delta, British Columbia, Canada
Sales G (2010) Ultrasonic calls of wild and wild-type rodents. In: Brudzynski SM (ed) Handbook of mammalian vocalization: an integrative neuroscience approach, pp 77–88. https://doi.org/10.1016/B978-0-12-374593-4.00009-7
Sarmento R, Brito D, Ladle RJ, da Rosa LG, Efe MA (2014) Invasive house (Rattus rattus) and brown rats (Rattus norvegicus) threaten the viability of red-billed tropicbird (Phaethon aethereus) in Abrolhos National Park, Brazil. Trop Conserv Sci 7:614–627. https://doi.org/10.1177/194008291400700403
Sewell GD (1970) Ultrasonic communication in rodents. Nature 227:410. https://doi.org/10.1038/227410a0
Shapira I, Shanas U, Raubenheimer D, Brunton DH (2013) Laboratory rats as trap lures for invasive Norway rats: field trial and recommendations. N Z J Ecol 37:240–245
Simeone A, Luna-Jorquera G (2012) Estimating rat predation on Humboldt Penguin colonies in north-central Chile. J Ornithol 153:1079–1085. https://doi.org/10.1007/s10336-012-0837-z
Slotnick B (2001) Animal cognition and the rat olfactory system. Trends Cognt Sci 5:216–222. https://doi.org/10.1016/S1364-6613(00)01625-9
Steiniger F (1950) Beiträge zur Soziologie und sonstigen Biologie der Wanderratte. Z Tierpsychol 7:356–379. https://doi.org/10.1111/j.1439-0310.1950.tb01630.x
Stenseth NC, Leirs H, Skonhoft A, Davis SA, Pech RP, Andreassen HP, Singleton GR, Lima M, Machang’u RS, Makundi RH, Zhang Z, Brown PR, Shi D, Wan X (2003) Mice, rats, and people: the bio-economics of agricultural rodent pests. Front Ecol Environ 1:367–375. https://doi.org/10.1890/1540-9295(2003)001[0367:MRAPTB]2.0.CO;2
Suthers RA, Hector DH (1982) Mechanism for the production of echolocating clicks by the grey swiftlet, Collocalia spodiopygia. J Comp Physiol A 148:457–470. https://doi.org/10.1007/BF00619784
Suthers RA, Hector DH (1985) The physiology of vocalization by the echolocating oilbird, Steatornis caripensis. J Comp Physiol A 156:243–266. https://doi.org/10.1007/BF00610867
Takács S, Kowalski P, Gries G (2016) Natural and synthetic vocalizations of brown rat pups, Rattus norvegicus, enhance attractiveness of bait boxes in laboratory and field experiments. Pest Manag Sci 72:1873–1882. https://doi.org/10.1002/ps.4219
Takács S, Musso AE, Gries R, Rozenberg E, Borden JH, Brodie BS, Gries G (2018) New food baits for trapping house mice, black rats and brown rats. Appl Anim Behav Sci 200:130–135. https://doi.org/10.1016/j.applanim.2017.11.011
Takács S, Kowalski P, Gries G (2021) Vocalizations of infant brown rats, but not infant house mice, enhance rodent captures in sex pheromone-baited traps. Appl Anim Behav Sci 36:105267. https://doi.org/10.1016/j.applanim.2021.105267
Takeuchi H, Kawashima S (1986) Ultrasonic vocalizations and aggressive behaviour in male rats. Physiol Behav 38:545–550. https://doi.org/10.1016/0031-9384(86)90423-3
Taylor RH, Kaiser GW, Drever MC (2000) Eradication of Norway rats for recovery of seabird habitat on Langara Island, British Columbia. Restor Ecol 8:151–160. https://doi.org/10.1046/j.1526-100x.2000.80022.x
Thorpe WH, Griffin DR (1962) Ultrasonic frequencies in bird song. Nature 193:595. https://doi.org/10.1038/193595a0
Turner JG, Parrish JL, Hughes LF, Toth LA, Caspary DM (2005) Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp Med 55:12–23
Vander Wall WSB, Beck MJ, Briggs JS, Roth JK, Thayer T, Hollander JL, Armstrong JM (2003) Interspecific variation in the olfactory abilities of granivorous rodents. J Mammal 84:487–496
VanderWerf EA (2001) Rodent control decreases predation on artificial nests in O’ahu Elepaio habitat. J Field Ornithol 72:448–457. https://doi.org/10.1648/0273-8570-72.3.448
Weismana R, Hoeschele M, Sturdy CB (2014) A comparative analysis of auditory perception in humans and songbirds: a modular approach. Behav Process 104:35–43. https://doi.org/10.1016/j.beproc.2014.02.006
White NR, Adox R, Reddy A, Barfield RJ (1992) Regulation of rat maternal behavior by broadband pup vocalizations. Behav Neural Biol 58:131–137. https://doi.org/10.1016/0163-1047(92)90363-9
Wolff JO (2003) Laboratory studies with rodents: facts or artifacts? Bioscience 53:421
Acknowledgements
We thank two anonymous reviewers for constructive comments, the staff of the Animal Care Center at Simon Fraser University for their care of rats, and Sharon Oliver for word processing and comments. The research was funded by a Natural Sciences and Engineering Research Council of Canada—Industrial Research Chair to G.G., with BASF Canada Inc. and Scotts Canada Ltd. as the industrial partners. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All experiments comply with the current laws of Canada. Specifically, rats were housed in the Animal Research Centre of Simon Fraser University (SFU) in accordance with the Canadian Council on Animal Care guidelines and experimental protocols approved by SFU’s Animal Care Committee (UACC protocol number 1134B-14).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. G. Guglielmo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takács, S., Gries, G. Phonotactic responses of Brown Rats (Rattus norvegicus) to begging calls of Starling nestlings (Sturnus vulgaris). J Ornithol 162, 1173–1181 (2021). https://doi.org/10.1007/s10336-021-01895-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-021-01895-9