Abstract
Large herbivores directly and indirectly influence ecosystem function, positively and negatively affecting diversity of plants and animals, including birds. Such cascading effects are clearly important, particularly given ongoing global declines in large herbivores and many avian communities. We examined relationships between bird diversity (species richness and Shannon-Weiner Index, at a species and functional group level) at three similarly vegetated and flooded sites in northern Botswana. We explored the role that herbivore presence plays in ecosystem functioning considering bird species richness was significantly higher at the site of intermediate presence, followed by the high presence site. At a functional group level, the site of highest presence consistently had the greatest functional group richness. Also, at a functional group level we identified higher species richness and diversity in the two sites where herbivores were present at high levels. This was particularly pronounced for the avian aquatic carnivore, terrestrial herbivore, and aerial invertivore functional groupings. Large herbivores probably increased habitat complexity through their browsing and grazing, altering habitat structure, alongside other benefits such as faeces deposition and mutualistic relationships, creating more niches for avian communities. Fencing out large herbivores to reduce their grazing and browsing may therefore reduce bird diversity, and correspondingly, allowing large herbivores to increase in abundance through protected areas may indirectly increase bird diversity, acknowledging over abundance may be detrimental.
Zusammenfassung
Quantifizierung von Vogelarten an drei Standorten mit unterschiedlicher Pflanzenfresser-Präsenz
Große Pflanzenfresser beeinflussen die Funktion von Ökosystemen direkt und indirekt durch ihre positiven und negativen Auswirkungen auf die vorkommenden Pflanzen und Tiere. Solche Kaskadeneffekte sind ohne Frage wichtig, besonders in Hinblick auf den derzeitigen globalen Rückgang der großen Pflanzenfresser und vieler Vogelpopulationen. Wir untersuchten den Zusammenhang zwischen Vogelvielfalt (Artenreichtum und Shannon-Weiner-Index auf Art-Ebene und der Ebene funktionaler Gruppen) an drei ähnlich bewachsenen und überfluteten Standorten im Norden Botswanas. Wir betrachteten die Rolle der Präsenz von Pflanzenfressern für das Funktionieren des Ökosystems, wobei der Artenreichtum der Vögel an Stellen mit mittlerer Pflanzenfresser-Präsenz signifikant höher war, gefolgt von Stellen mit hoher Präsenz. Auf der Ebene funktionaler Gruppen zeigte der Standort mit höchster Präsenz den größten Reichtum an solchen Gruppen. Ebenso auf der funktionalen Ebene fanden wir eine höhere Artenvielfalt an den beiden Standorten, an denen Pflanzenfresser in hohem Maß anwesend waren. Besonders ausgeprägt war dies für die carnivoren Wasservögel, die terrestrischen Pflanzenfresser und die funktionalen Gruppen der Fluginsektenfresser. Wahrscheinlich vergrößern große Pflanzenfresser durch ihr Herumwandern und Grasen den Komplexitätsgrad des Habitats. Sie verändern die Habitatstruktur, bieten Vorteile durch Kotablagerungen und ihre mutualistischen Interaktionen und schaffen mehr ökologische Nischen für die Vögel. Die großen Pflanzenfresser durch Zäune auszusperren, um ihr Herumwandern und Grasen zu verringern, könnte auch die Vogelvielfalt reduzieren. Dementsprechend könnte es die Artenvielfalt erhöhen, ließe man mehr große Pflanzenfresser in Schutzgebieten zu, wobei zu viele von ihnen natürlich auch von Schaden sein könnten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Given the current global loss of large herbivores (Ripple et al. 2015), understanding their functional role in ecosystems is critical to tracking long-term ecosystem change and developing effective management strategies. Large herbivores (body mass ≥ 100 kg) consume and alter vegetation, sometimes not only increasing (Rutina et al. 2005; Kerley and Landman 2006; Landman and Kerley 2014; Ripple et al. 2015), but also reducing vegetation available to other animals (Landman et al. 2013). They also disperse seeds (Blake et al. 2009), deposit nutrient-rich urine and faeces (van der Waal et al. 2011), and decrease predator numbers (Tambling et al. 2013) whilst also providing predators with a food source when they die (Loarie et al. 2013; Clements et al. 2014). Such alterations can cause cascading effects through ecosystems (Owen-Smith 1992), with complex and wide-ranging ramifications.
In particular, the presence of large herbivores can affect bird community diversity (Ogada et al. 2008; Gregory and van Strien 2010). In Kenya, for example, elephants Loxodonta africana thinned the understory through browsing, which consequently decreased akalat abundance (insectivorous tree dwelling birds Sheppardia sp.)(Banks et al. 2010). Further, high densities of elephants and giraffe Giraffa camelopardalis decreased the diversity of granivorous and insectivorous birds, and when large herbivores were excluded, bird diversity increased by 30% (Ogada et al. 2008). Large herbivores can have significant impacts on areas near rivers when congregating during migration, particularly in the dry season (Dipotso and Skarpe 2006; Rutina and Moe 2014) as seen in the Okavango Delta where high elephant numbers disrupted and reduced the size of waterbird colonies (Muller 2013). Waterbird breeding colonies are particularly vulnerable because they usually nest close to water, given their breeding cycles are closely tied to flooding regimes (Kingsford and Auld 2005; Desgranges et al. 2006; Arthur et al. 2012; Bino et al. 2014).
Large herbivore effects on ecosystems and human communities are sometimes mitigated by fence exclusion (Hayward and Kerley 2009) and the culling of animals (Gordon et al. 2004). Contrastingly, protected areas can increase large herbivore densities, albeit varying in success (Craigie et al. 2010), with inevitable restrictions on migration and dispersal (Cushman et al. 2010; Naidoo et al. 2012). In Botswana, about 20–30% of the land is considered as protected areas (Index Mundi 2019) and it has possibly the highest density of large herbivores in Africa, including a third of Africa’s remaining elephants (Chase et al. 2016; Schlossberg et al. 2019) and high densities of buffalo Syncerus caffer (Alexander et al. 2012). Given the country’s rich avifauna including nearly 600 bird species (Hancock and Weiersbye 2015), understanding how large herbivores affect avian populations is important, particularly along rivers where large herbivore pressures can be highest (Smit et al. 2007; Ogutu et al. 2014).
We investigated bird diversity in three equally sized survey sites with similar vegetation types, coverage, and flooding regimes, equidistant to the Chobe River in northern Botswana. Importantly, these three sites had a known differential level of herbivore presence. We relate herbivore presence to the amount of possible environmental disturbance, which we define as processes that temporally disrupt ecosystems, communities or population structures, changing resource availability (Connell 1978). Generally, despite site similarities, we predicted to see differences in species richness and diversity, with both highest at the site with intermediate presence (and therefore disturbance), because intermediate levels of disturbance provide niches for species who prefer minimal and maximum disturbance (Connell 1978; Wilkinson 1999). We predicted waterbird diversity would decrease over time, with decreasing proportions of sites flooded, given their dependence on aquatic habitats. We also predicted that arboreal species would be lowest in diversity at the site with the highest herbivore presence, due to the role of large herbivores in altering canopy cover (Ogada et al. 2008). Finally, bird species in symbiotic relationships with herbivores were predicted to be most diverse in the site of high herbivore presence, due to the direct reliance of these species on large herbivores for food (Ndlovu and Combrink 2015).
Materials and methods
Study sites
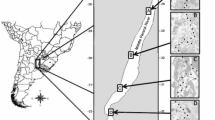
We surveyed avian communities in three equally sized sites (1000 m × 500 m, separated by at least 500 m, Fig. 1), chosen for their similarities in vegetation types, coverage, and flooding regimes. Sites were located on seasonally inundated floodplain equidistant from the Chobe River. These sites differed in levels of large herbivore presence, including elephant, buffalo, giraffe, hippopotamus Hippopotamus amphibius, zebra Equus quagga and greater kudu Tragelaphus strepsiceros (Chase 2011; Chase et al. 2015, 2018) (Online Appendix S1). The “Fenced” site was enclosed in September 2017, and experienced low levels of large herbivore presence, with only the occasional herbivore bypassing the fences giving it a large herbivore density of roughly 0. The “Unfenced” site was an unprotected site experiencing intermediate herbivore density of 2.2–4.3 herbivores km−2, and the “National Park” site had the highest herbivore presence with a density of 5.0–8.8 herbivores km−2. Herbivore density measures were calculated from 2010, 2014 and 2018 aerial survey data covering our intermediate site (in the Kasane Forest Reserve survey stratum) and our high presence site (covered within the Chobe River survey stratum) (Online Appendix S1) (Chase 2011; Chase et al. 2015, 2018).
Satellite images (Copernicus Sentinel 2018) of our three study sites Fenced (middle), Unfenced (right) and National Park (left, in Chobe National Park), along the Chobe River (north eastern Botswana, red dot), where we surveyed bird diversity, using transect counts, every week for 10 weeks, 19 June 2018–22 August 2018 (colour figure online)
Bird surveys
Within each site, we surveyed birds 10 times (weekly over 10 weeks) (Robbins et al. 1989; Hostetler and Knowles-Yanez 2003), 19 June 2018–22 August 2018, within a 1 km long transect, 30–50 m parallel to the river’s edge (Fig. 1). Surveys were within 2 h of sunrise over 35–45 min for each transect, with the day of the week randomised; we did not survey in inclement weather. Observations were from within the vehicle (< 5 km h−1), due to the presence of dangerous animals, using the double observer method (Nichols et al. 2000), where one observer stood through the sunroof, allowing for a wide field of vision, and the second acted as driver and recorder, identifying and counting all birds seen and/or heard up to 50 m on each side of the transect. Birds observed as fly-overs were excluded from analyses (Hostler and Martin 2006). Bird nomenclature followed the International Ornithological Congress World Bird List (Gill and Donsker 2019), with functional/dietary guilds following Sundstrom et al. (2012) (Online Appendix S2).
Environmental variables
To track effects of receding flood levels and test for differences in the normalised difference vegetation index (NDVI) amongst sites, we used Google Earth Engine (Gorelick et al. 2017) and images from the Sentinel-2 satellite (Copernicus Sentinel data 2018). We used the threshold function (Wolski et al. 2017) to calculate percent inundation, filling data gaps with Landsat-8 images (Inman and Lyons 2020) where Sentinel images were of low quality, or did not exist for survey dates (often due to cloud cover, < 10% of data).
The sites (Fenced, Unfenced and Chobe National Park) had similar dominant vegetation types, including Woolly Caper Bush Capparis tomentosa, Large Fever-berry Croton Croton megalobotrys, Blackthorn Acacia Senegalia mellifera and Wild Sage Pechuel-loeschea leubnitziae, all seasonally inundated by the Chobe River (peak flows in April–May). To explore further potential differences in vegetation amongst sites, we extracted NDVI values at 10 m resolution pixels, across sites for images available in September 2018, coinciding with our surveys. NDVI values were classified; values approaching < 0.1 water or barren areas (free of vegetation), and values > 0.1 grass, shrubland or forest (Sentinel Hub 2018). These groups were separated into relative percent coverage by summing the shrub/grassland and forest values, divided by the total pixel number in the images. Vegetation indices were similar during 2018 surveys, with sites dominated by shrubs and grasses across the three sites: the Fenced was 51.21% vegetated (± 0.13), with 2.40% forest; Unfenced was 46.70% vegetated (± 0.50), with 4.12% classified as forest and finally; Chobe National Park was 50.56% vegetated (± 0.24), with 1.33% forest. Given the limited differences in vegetation across the three sites, NDVI was not used in the herbivore presence or flood modelling.
We also collected data on three environmental variables during each survey: wind speed, temperature and cloud cover. Plots of environmental variables against avian species richness and diversity highlighted potential relationships with cloud cover and wind speed (Online Appendix S3). However, only wind speed was important in explaining avian species richness and diversity and was included in subsequent modelling, based on the Akaike Information Criterion of the models.
Statistical analyses
We first investigated differences in broad biodiversity metrics for the avian community (i.e., species richness and Shannon–Wiener Diversity Index, referred hereafter as ‘diversity’) amongst sites (Magurran 2013; Morris et al. 2014). We then analysed community-level differences amongst sites, using non-metric multidimensional scaling (NMDS), with Bray–Curtis similarity of abundance-weighted matrices. Data were square-root transformed and standardised, according to the Wisconsin double standardisation method to satisfy assumptions of normality and homogeneity of variance (Oksanen et al. 2013). We then tested for statistical differences in community structure amongst sites using an analysis of similarities (ANOSIM), followed by a similarity percentages procedure (SIMPER) to investigate which species contributed most to differentiation amongst sites. We used the ‘adonis’ and ‘simper’ functions for analyses, respectively, from the vegan package (Oksanen et al. 2013).
We investigated species-level metrics, with species richness and the Shannon–Wiener Index, as the response variables, using generalised least square models, specifically the ‘gls’ function from the nlme package (Pinheiro et al. 2014). We included temporal autocorrelation in the modelling (week of survey), given likely non-independence of surveys over time; exploratory data analysis revealed some seasonal changes in the bird diversity over our survey period. Wind speed was also included in the models as a covariate. The parametric predictors were changes in percent inundation and site.
Each bird species was then allocated to a functional group, reflecting a combination of dietary and foraging strategies (Online Appendix S2). Dietary information was taken from Roberts bird guide (Chittenden et al. 2007) and available advice (Francey 2018). To explore community differences at a functional group level (Sundstrom et al. 2012), we first tested for differences in the number of functional bird groups amongst sites. We then tested for differences in species richness and Shannon–Wiener Index, within each functional group amongst sites, using generalised least square models with temporal autocorrelation, with percent inundation and site as predictor variables. An individual model was also run for within each functional bird group over time, to test for changes in the species richness and Shannon–Wiener diversity of that functional group, in relation to percent inundation and site. The terrestrial carnivores bird functional group was removed from the Shannon–Wiener Index modelling, given White-browed Coucal was the only species.
All analyses were done in the R computing environment (R Core Team 2014), relying on the tidy verse workflow (Wickham 2017) and ggplot2 package (Wickham 2016). Significance of statistical tests was concluded at α < 0.05.
Results
A total of 128 bird species were identified across the three sites (Online Appendix S2), with the highest total species richness in Unfenced (intermediate presence, 96), followed by the National Park (high presence, 78) and Fenced (low presence, 71) (Online Appendix S4). Average species richness differed significantly amongst sites (P = 0.02, F26 = 4.91, Table 1), highest in Unfenced (33.80 ± 6.32), followed by National Park (29.05 ± 4.78) and finally Fenced (23.20 ± 3.93). Total Shannon–Wiener diversity did not differ significantly amongst sites (P = 0.07, F26 = 2.99, Table 1, Online Appendix S4).
There was a significant difference in avian community composition amongst the three sites, varying in large herbivore presence, with relatively little overlap in the non-metric dimensional plot (P = 0.001, F27 = 7.2, Fig. 2). There was a 74% difference between the National Park and the Fenced, the sites with high and low herbivore presence; a 69% difference between Unfenced and Fenced (intermediate and low presence) and; a 51% difference between the National Park and Unfenced (high and intermediate). Differences amongst sites were mostly explained by variation in the presence and abundance of Ring-necked Doves, accounting for 27–29% of community differences between the low large herbivore presence site Fenced (where they were considerably less abundant) and the two other sites (Unfenced, and National Park). Crowned Lapwings and Red-billed Quelea also explained community-level differences amongst sites (Online Appendix S5), most often observed in sites with herbivore presence.
Non-metric multidimensional plot showing separation of avian communities amongst three sites in Botswana varying in large herbivore presence, Fenced (low presence, triangles), Unfenced (intermediate presence, squares) and National Park (high presence, circles) (see Fig. 1), where each point represents a unique weekly survey (N = 10 for each location, 19 June 2018–22 August 2018)
In total 15 avian functional groups occurred, and whilst each site had 14 avian functional groups, the weekly number differed significantly amongst sites (F26 = 3.47, P = 0.04, Online Appendix S4); the National Park regularly had the highest number of functional groups, followed by Unfenced and then Fenced (high, intermediate and then low). Within avian functional groups, avian species’ richness differed significantly amongst sites in 11 out of the 15 avian functional groups (Fig. 3a). 73% of functional groups were significantly richer in the National Park with aquatic carnivores the richest group (F26 = 2.13, P = 0.04). Of those that were significantly different in Fenced, 3 out of the 4 groups were significantly less rich (Table 2). The only functional group richest at Fenced was the aerial carnivores (F26 = 2.25, P = 0.03). Average species’ richness over the 10 weeks was highest in aquatic carnivores and terrestrial herbivores (5.9 species per group).
Modelled estimates of avian functional groups amongst three sites varying in large herbivore presence (fenced (low, mid grey triangles), unfenced (intermediate, light grey squares) and National Park (high, black circles) measured in species richness (a) and Shannon–Wiener diversity Index (b) in northern Botswana, surveyed weekly (19 June 2018–22 August 2018). Statistically significant and insignificant differences marked respectively by large and small symbols, with lines showing the confidence intervals. Symbols < 0 and > 0 on the x-axis respectively represent negative and positive differences amongst sites
Also, the Shannon–Wiener Index differed within functional groups amongst sites for seven avian functional groups (Fig. 3b). Six of 14 functional avian groups were most diverse in the National Park or Unfenced (Table 2). For the one group showing a significant difference in Fenced, it was significantly lower in diversity. National Park was the only site with terrestrial carrion feeders (vultures, Online Appendix S2). The most diverse functional groups were the aquatic carnivores (1.46) and arboreal omnivores (1.25).
At Fenced, the functional groups with the highest species’ richness and the most diverse were the aquatic carnivores (5, 0.90) and terrestrial herbivores (4.6, 0.91). At Unfenced, the most species’ rich group was the terrestrial herbivores (5.9), followed by the arboreal omnivores (4.8). At National Park, the functional groups with the highest species’ richness were the aquatic carnivores (5.9) followed by the terrestrial herbivores (4.8).
Flooding responses
At a species level, neither bird species’ richness nor the Shannon–Wiener index differed significantly, with changes in the proportion of each site flooded (F27 = 2.6, P > 0.05). At a functional group level, three groups responded significantly to flooding, when modelling for species’ richness; arboreal invertivores showed the strongest response (F27 = 4.77, P < 0.001, Fig. 4a), followed by terrestrial omnivores (F27 = 2.08, P = 0.05) and finally terrestrial carnivores (F27 = 2.62, P = 0.01), with species’ richness decreasing with less relative area flooded. Diversity within two functional groups decreased significantly with decreased percentage of site flooded (Fig. 4b); terrestrial omnivores (F27 = 2.31, P = 0.03) and arboreal invertivores (F27 = 5.28, P < 0.001).
Modelled estimates of avian functional groups to changes in flooding (percent inundation) measured in species’ richness (a) and Shannon–Wiener diversity Index (b), across the three sites varying presence of large herbivores (Fenced (low), Unfenced (intermediate) and National Park (high)) in northern Botswana, surveyed weekly (19 June 2018–22 August 2018). Statistically significant and insignificant responses marked respectively by triangles and circles, with lines showing confidence intervals. Symbols < 0 and > 0 on the x-axis respectively represent negative and positive responses to inundation
Discussion
There is increasing evidence that some animals are functionally important for ecosystems, directly or indirectly driving patterns of diversity and abundance (Sundstrom et al. 2012; Mouillot et al. 2013; Baker et al. 2016; Rolo et al. 2017). The sites of intermediate or high levels of herbivore presence in our study in Botswana were consistently associated with higher avian richness and diversity at species and functional group levels (Fig. 3). This is supported by other evidence that large African herbivores are functional drivers of ecosystems (Ogada et al. 2008; Banks et al. 2010), including within Botswana (Herremans 1995).
The higher diversity and richness of 11 avian functional groups at sites with medium or high levels of herbivore presence were likely due to a mix of direct effects and indirect benefits provided by large herbivores. These include variation in vegetation condition, structure, dispersal and germination (Herremans 1995; Campos-Arceiz and Blake 2011; Baker et al. 2016), faeces and urine deposition (van der Waal et al. 2011), and the creation of opportunities for other species including frogs (Nasseri et al. 2011), small herbivorous mammals (Valeix et al. 2011) and birds (Banks et al. 2010), increasing food availability for the avian community.
Avian aquatic carnivores, parasitic carnivores, and terrestrial carrion functional groups likely directly benefitted (Fig. 3, Online Appendix S2). The Red and Yellow-billed Oxpeckers, which feed mutualistically on parasites of large herbivores (Ndlovu and Combrink 2015), occurred where there was high herbivore presence. Also, the carrion feeders (e.g., White-backed Vultures) only occurred where there was high herbivore presence, reflecting a common pattern in Africa, where populations are generally higher in protected areas than outside, given the presence of large herbivores and the relative availability of carcasses (Rushworth et al. 2007; Murn et al. 2013). Further, large herbivores leave abundant seeds in their dung for avian terrestrial herbivores, such as spurfowl and francolins (Maclean et al. 2011), which may also explain the high abundance of Ring-necked Doves (Campos-Arceiz and Blake 2011; Maclean et al. 2011). Finally, avian aerial invertivores probably also directly benefitted from large herbivores which attracted insects, providing an abundant food supply (Møller 1983; Pryke et al. 2016).
There were likely a range of indirect benefits from large herbivore presence, related to vegetation, and we would expect to see differences between sites increase the longer large herbivores are excluded from Fenced. Large herbivores create space in shrub communities, remove grass biomass, and create open patches and areas of bare ground (Onyeanusi 1989; Waldram et al. 2008; Kohi 2013), which likely increased feeding opportunities for the terrestrial invertivores, herbivores, and omnivores. This probably favoured babblers (terrestrial omnivores, Online Appendix S2) which forage in and around leaf litter, whilst the bare ground patches probably favoured the foraging of waxbills and finches in open areas (terrestrial herbivores, Online Appendix S2) (Maclean et al. 2011). Crowned Lapwings, another major driver of community differences (terrestrial invertivores) also prefer open areas, where the grass is kept short, for both foraging and breeding (Maclean et al. 2011). Further, alteration of canopy heights and increased habitat complexity caused by large herbivores (Kohi 2013) provide habitat for a range of nesting birds, lizards and insects (Whitmore et al. 2002; Tews et al. 2004), the prey for arboreal carnivores, invertivores, omnivores and aerial invertivores (such as the weavers, rollers, bee-eaters and martins, Online Appendix S2). Aquatic species such as aquatic carnivores and invertivores may benefit from the stirring of sediment by herbivores disturbing fish, insects and freshwater molluscs (Dinsmore 1973; Wolanski and Gereta 1999; Bakker et al. 2016), assisting the foraging of African Spoonbills and African Openbills (Maclean et al. 2011).
The effects of flooding were not reflected in changes in aquatic functional groups as predicted (Fig. 4). Contrastingly, the terrestrial carnivore, terrestrial omnivore and arboreal invertivore groups increased in richness with more flooded area, which could have reflected increased insect populations or seed germination with the flood (Linhoss et al. 2012; Mackay et al. 2012; Pricope 2013).
Our ‘high’ levels of herbivore presence were not likely to exceed natural or historical densities (Chase et al. 2015, 2018; Schlossberg et al. 2019), and although the aerial survey data support the local knowledge of herbivore presence at the three sites (Francey 2018), we recognise further work should look to elaborate on these patterns with fine-scale herbivore measurements. We acknowledge that we only included three sites, one corresponding to the relative level of presence, but highlight that we deliberately chose sites of similar vegetation type and coverage to limit such inherent differences affecting modelling. Nevertheless, other factors may contribute to the differences in bird diversity at the three sites, and future work should look to further replicate these results across a greater number of sites and longer timeframe to capture temporal and climatic change. Importantly, the 10-week time frame of this study is only a small insight into the long-term functioning of these sites. One external factor to be considered is human presence, which could also have affected bird species’ richness and diversity. However, considering the wealth of information describing the negative effects of human disturbance on bird diversity (Lepczyk et al. 2008; Kang et al. 2015; Vollstädt et al. 2017), we think it unlikely human presence (both on foot and in vehicles) is the major driving factor controlling diversity at these three sites as we would expect to see the opposite pattern; reduced diversity in the site with the most traffic, the National Park.
Herbivores have been shown to play an important functional role directly and indirectly altering biotic communities (Waldram et al. 2008; Prugh and Brashares 2012; Parsons et al. 2013; Howland et al. 2014). The high avian diversity in the sites with high herbivore presence in our study probably reflected these dependencies. Declining large herbivore populations through fencing exclusion, habitat loss and degradation and illegal harvesting will likely detrimentally affect species richness or diversity of different bird functional groups. Therefore, future efforts in the conservation of large herbivores and their designated protected areas may also directly contribute to the conservation of bird populations across Africa, particularly if we move away from a single species approach, and implement connected ecosystem management (Schultz et al. 2015).
Conclusions
Avian species richness was higher in sites with intermediate to high presence of large herbivores, likely due to both the direct effects of herbivores on birds (e.g., a food source to carrion feeders, dung deposition) and indirect effects (altered vegetation structure). Both species richness and diversity were higher in the sites of intermediate to high herbivore presence at a functional group level, particularly for the avian aquatic carnivores, terrestrial herbivores and aerial invertivores. Future conservation efforts should consider the importance of the relationship between birds and large herbivores, with herbivore protected areas therefore likely also benefiting avian diversity.
References
Alexander KA, Blackburn JK, Vandewalle ME, Pesapane R, Baipoledi EK, Elzer PH (2012) Buffalo, bush meat, and the zoonotic threat of brucellosis in Botswana. PLoS One 7:e32842
Arthur AD, Reid JR, Kingsford RT, McGinness HM, Ward KA, Harper MJ (2012) Breeding flow thresholds of colonial breeding waterbirds in the Murray-Darling Basin, Australia. Wetlands 32:257–265
Baker AG, Cornelissen P, Bhagwat SA, Vera FW, Willis KJ (2016) Quantification of population sizes of large herbivores and their long-term functional role in ecosystems using dung fungal spores. Methods Ecol Evol 7:1273–1281
Bakker ES, Pagès JF, Arthur R, Alcoverro T (2016) Assessing the role of large herbivores in the structuring and functioning of freshwater and marine angiosperm ecosystems. Ecography 39:162–179
Banks JE, Jackson C, Hannon LM, Thomas CM, Baya A, Njoroge L (2010) The cascading effects of elephant presence/absence on arthropods and an Afrotropical thrush in Arabuko-Sokoke Forest, Kenya. Afr J Ecol 48:1030–1038
Bino G, Steinfeld C, Kingsford RT (2014) Maximizing colonial waterbirds’ breeding events using identified ecological thresholds and environmental flow management. Ecol Appl 24:142–157
Blake S, Deem SL, Mossimbo E, Maisels F, Walsh P (2009) Forest elephants: tree planters of the Congo. Biotropica 41:459–468
Campos-Arceiz A, Blake S (2011) Megagardeners of the forest—the role of elephants in seed dispersal. Acta Oecologica 37:542–553
Chase M (2011) Dry season fixed-wing aerial survey of elephants and wildlife in northern Botswana, September–November 2010. Botswana documents
Chase M, Schlossberg S, Landen K, Sutcliffe R, Seonyatseng E, Keitsile A, Flyman M (2015) Dry season aerial survey of elephants and wildlife in northern Botswana, July–October 2014. Elephants Without Borders, Kasane
Chase MJ, Schlossberg S, Griffin CR, Bouché PJ, Djene SW, Elkan PW, Ferreira S, Grossman F, Kohi EM, Landen K (2016) Continent-wide survey reveals massive decline in African savannah elephants. PeerJ 4:e2354
Chase M, Schlossberg S, Sutcliffe R, Seonyatseng E (2018) Dry season aerial survey of elephants and wildlife in northern Botswana, July–October 2018. Elephants Without Borders, Kasane
Chittenden H, Dean WRJ, Gibbon G, Upfold G (2007) Roberts bird guide: a comprehensive field guide to over 950 bird species in southern Africa. John Voelcker Bird Book Fund
Clements HS, Tambling CJ, Hayward MW, Kerley GI (2014) An objective approach to determining the weight ranges of prey preferred by and accessible to the five large African carnivores. PLoS One 9:e101054
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310
Copernicus Sentinel (2018) Copernicus sentinel satellite imagery. Retrieved from Google Earth Engine
Craigie ID, Baillie JE, Balmford A, Carbone C, Collen B, Green RE, Hutton JM (2010) Large mammal population declines in Africa’s protected areas. Biol Conserv 143:2221–2228
Cushman SA, Chase M, Griffin C (2010) Mapping landscape resistance to identify corridors and barriers for elephant movement in southern Africa. Spatial complexity, informatics, and wildlife conservation. Springer, pp 349–367
Desgranges J-L, Ingram J, Drolet B, Morin J, Savage C, Borcard D (2006) Modelling wetland bird response to water level changes in the Lake Ontario–St. Lawrence River hydrosystem. Environ Monit Assess 113:329–365
Dinsmore JJ (1973) Foraging success of cattle egrets, Bubulcus ibis. Am Midland Nat 89(1):242–246
Dipotso F, Skarpe C (2006) Population status and distribution of puku in a changing riverfront habitat in northern Botswana. South Afr J Wildl Res 36:89–97 (24-month delayed open access)
Francey L (2018) Large herbivore disturbance and bird dietary descriptions. Kasane, Botswana
Gill F, Donsker D (2019) IOC world bird list (v9.2). In: International Ornithological Congress
Gordon IJ, Hester AJ, Festa-Bianchet M (2004) The management of wild large herbivores to meet economic, conservation and environmental objectives. J Appl Ecol 41:1021–1031
Gorelick N, Hancher M, Dixon M, Ilyushchenko S, Thau D, Moore R (2017) Google Earth Engine: planetary-scale geospatial analysis for everyone. Remote Sens Environ 202:18–27
Gregory RD, van Strien A (2010) Wild bird indicators: using composite population trends of birds as measures of environmental health. Ornithol Sci 9:3–22
Hancock P, Weiersbye I (2015) Birds of Botswana
Hayward MW, Kerley GI (2009) Fencing for conservation: restriction of evolutionary potential or a riposte to threatening processes? Biol Conserv 142:1–13
Herremans M (1995) Effects of woodland modification by African elephant Loxodonta africana on bird diversity in northern Botswana. Ecography 18:440–454
Hostetler M, Knowles-Yanez K (2003) Land use, scale, and bird distributions in the Phoenix metropolitan area. Landscape Urban Plann 62(2):55–68
Hostler M, Martin M (2006) Florida monitoring program: transect method for surveying avians. University of Florida, Gainesville, Florida
Howland B, Stojanovic D, Gordon IJ, Manning AD, Fletcher D, Lindenmayer DB (2014) Eaten out of house and home: impacts of grazing on ground-dwelling reptiles in Australian grasslands and grassy woodlands. PLoS One 9:e105966
Index Mundi (2019) Botswana—terrestrial protected areas (% of total land area). https://www.indexmundi.com/facts/botswana/indicator/ER.LND.PTLD.ZS. Accessed 28 Mar 2019
Inman VL, Lyons MB (2020) Automated inundation mapping over large areas using landsat data and google earth engine. Remote Sens 12:1348
Kang W, Minor ES, Park C-R, Lee D (2015) Effects of habitat structure, human disturbance, and habitat connectivity on urban forest bird communities. Urban Ecosyst 18:857–870
Kerley GI, Landman M (2006) The impacts of elephants on biodiversity in the Eastern Cape Subtropical Thickets: elephant conservation. S Afr J Sci 102:395–402
Kingsford RT, Auld KM (2005) Waterbird breeding and environmental flow management in the Macquarie Marshes, arid Australia. River Res Appl 21:187–200
Kohi E (2013) The bulldozer herbivore: how animals benefit from elephant modifying an African savanna. In: Wageningen University, editor
Landman M, Kerley GI (2014) Elephant both increase and decrease availability of browse resources for black rhinoceros. Biotropica 46:42–49
Landman M, Schoeman DS, Kerley GI (2013) Shift in black rhinoceros diet in the presence of elephant: evidence for competition? PLoS One 8:e69771
Lepczyk CA, Flather CH, Radeloff VC, Pidgeon AM, Hammer RB, Liu J (2008) Human impacts on regional avian diversity and abundance. Conserv Biol 22:405–416
Linhoss AC, Muñoz-Carpena R, Allen MS, Kiker G, Mosepele K (2012) A flood pulse driven fish population model for the Okavango Delta, Botswana. Ecol Model 228:27–38
Loarie SR, Tambling CJ, Asner GP (2013) Lion hunting behaviour and vegetation structure in an African savanna. Anim Behav 85:899–906
Mackay AW, Davidson T, Wolski P, Woodward S, Mazebedi R, Masamba WR, Todd M (2012) Diatom sensitivity to hydrological and nutrient variability in a subtropical, flood-pulse wetland. Ecohydrology 5:491–502
Maclean GL, Roberts A, Newman K, Lockwood G (2011) Roberts VII multimedia birds of Southern Africa, New Holland
Magurran AE (2013) Measuring biological diversity. Wiley
Møller AP (1983) Breeding habitat selection in the swallow Hirundo rustica. Bird Study 30(2):134–142
Morris EK, Caruso T, Buscot F, Fischer M, Hancock C, Maier TS, Meiners T, Müller C, Obermaier E, Prati D, Socher SA, Sonnemann I, Wäschke N, Wubet T, Wurst S, Rillig MC (2014) Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecol Evol 4(18):3514–3524
Mouillot D, Graham NA, Villéger S, Mason NW, Bellwood DR (2013) A functional approach reveals community responses to disturbances. Trends Ecol Evol 28:167–177
Muller MFA (2013) A survey of the Kanana Heronry, 20–22 October 2012
Murn C, Combrink L, Ronaldson GS, Thompson C, Botha A (2013) Population estimates of three vulture species in Kruger National Park, South Africa. Ostrich 84:1–9
Naidoo R, Du Preez P, Stuart-Hill G, Jago M, Wegmann M (2012) Home on the range: factors explaining partial migration of African buffalo in a tropical environment. PLoS One 7:e36527
Nasseri NA, McBrayer LD, Schulte BA (2011) The impact of tree modification by African elephant (Loxodonta africana) on herpetofaunal species richness in northern Tanzania. Afr J Ecol 49:133–140
Ndlovu M, Combrink L (2015) Feeding preferences of Oxpeckers in Kruger National Park, South Africa. Koedoe 57:1–6
Nichols JD, Hines JE, Sauer JR, Fallon FW, Fallon JE, Heglund PJ (2000) A double-observer approach for estimating detection probability and abundance from point counts. Auk 117(2):393–408
Ogada DL, Gadd ME, Ostfeld RS, Young TP, Keesing F (2008) Impacts of large herbivorous mammals on bird diversity and abundance in an African savanna. Oecologia 156:387
Ogutu JO, Reid RS, Piepho H-P, Hobbs NT, Rainy ME, Kruska RL, Worden JS, Nyabenge M (2014) Large herbivore responses to surface water and land use in an East African savanna: implications for conservation and human-wildlife conflicts. Biodivers Conserv 23:573–596
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Package ‘vegan’. Community ecology package, version 2(9)
Onyeanusi A (1989) Large herbivore grass offtake in Masai Mara National Reserve: implications for the Serengeti–Mara migrants. J Arid Environ 16:203–209
Owen-Smith RN (1992) Megaherbivores: the influence of very large body size on ecology. Cambridge University Press, Cambridge
Parsons EW, Maron JL, Martin TE (2013) Elk herbivory alters small mammal assemblages in high-elevation drainages. J Anim Ecol 82:459–467
Pinheiro J, Bates D, DebRoy S, Sarkar D (2014) R Core Team (2014) nlme: linear and nonlinear mixed effects models. R package version 3.1-117. Available at https://CRAN.R-project.org/package=nlme
Pricope NG (2013) Variable-source flood pulsing in a semi-arid transboundary watershed: the Chobe River, Botswana and Namibia. Environ Monit Assess 185:1883–1906
Prugh LR, Brashares JS (2012) Partitioning the effects of an ecosystem engineer: kangaroo rats control community structure via multiple pathways. J Anim Ecol 81:667–678
Pryke JS, Roets F, Samways MJ (2016) Wild herbivore grazing enhances insect diversity over livestock grazing in an African grassland system. PLoS One 11:e0164198
R Core Team (2014) R: a language and environment for statistical computing. In: R foundation for statistical computing, Vienna
Ripple WJ, Newsome TM, Wolf C, Dirzo R, Everatt KT, Galetti M, Hayward MW, Kerley GI, Levi T, Lindsey PA (2015) Collapse of the world’s largest herbivores. Sci Adv 1:e1400103
Robbins CS, Sauer JR, Greenberg RS, Droege S (1989) Population declines in North American birds that migrate to the Neotropics. Proc Nat Acad Sci 86(19):7658–7662
Rolo V, Olivier PI, van Aarde R (2017) Tree and bird functional groups as indicators of recovery of regenerating subtropical coastal dune forests. Restor Ecol 25:788–797
Rushworth I, Wakelin J, Bawden G (2007) Lappet-faced Vulture (Torgos tracheliotos) breeding in Ithala Game Reserve, KwaZulu-Natal, South Africa. Vulture News 57:65–67
Rutina LP, Moe SR (2014) Elephant (Loxodonta africana) disturbance to riparian woodland: effects on tree-species richness, diversity and functional redundancy. Ecosystems 17:1384–1396
Rutina LP, Moe SR, Swenson JE (2005) Elephant Loxodonta africana driven woodland conversion to shrubland improves dry-season browse availability for impalas Aepyceros melampus. Wildl Biol 11:207–214
Schlossberg S, Chase MJ, Sutcliffe R (2019) Evidence of a growing elephant poaching problem in Botswana. Curr Biol 29(13):2222–2228 (e2224)
Schultz L, Folke C, Österblom H, Olsson P (2015) Adaptive governance, ecosystem management, and natural capital. Proc Natl Acad Sci 112:7369–7374
Sentinel Hub (2018) NDVI (Normalized Difference Vegetation Index). https://www.sentinel-hub.com/eoproducts/ndvi-normalized-difference-vegetation-index. Accessed 15 Nov 2018
Smit IP, Grant CC, Devereux BJ (2007) Do artificial waterholes influence the way herbivores use the landscape? Herbivore distribution patterns around rivers and artificial surface water sources in a large African savanna park. Biol Conserv 136:85–99
Sundstrom SM, Allen CR, Barichievy C (2012) Species, functional groups, and thresholds in ecological resilience. Conserv Biol 26:305–314
Tambling CJ, Minnie L, Adendorff J, Kerley GI (2013) Elephants facilitate impact of large predators on small ungulate prey species. Basic Appl Ecol 14:694–701
Tews J, Brose U, Grimm V, Tielbörger K, Wichmann M, Schwager M, Jeltsch F (2004) Animal species diversity driven by habitat heterogeneity/diversity: the importance of keystone structures. J Biogeogr 31:79–92
Valeix M, Fritz H, Sabatier R, Murindagomo F, Cumming D, Duncan P (2011) Elephant-induced structural changes in the vegetation and habitat selection by large herbivores in an African savanna. Biol Conserv 144:902–912
van der Waal C, Kool A, Meijer SS, Kohi E, Heitkönig IM, de Boer WF, van Langevelde F, Grant RC, Peel MJ, Slotow R (2011) Large herbivores may alter vegetation structure of semi-arid savannas through soil nutrient mediation. Oecologia 165:1095–1107
Vollstädt MG, Ferger SW, Hemp A, Howell KM, Töpfer T, Böhning-Gaese K, Schleuning M (2017) Direct and indirect effects of climate, human disturbance and plant traits on avian functional diversity. Glob Ecol Biogeogr 26:963–972
Waldram MS, Bond WJ, Stock WD (2008) Ecological engineering by a mega-grazer: white rhino impacts on a South African savanna. Ecosystems 11:101–112
Whitmore C, Slotow R, Crouch TE, Dippenaar-Schoeman AS (2002) Diversity of spiders (Araneae) in a savanna reserve, Northern Province, South Africa. The Journal of Arachnology 30:344–357
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer
Wickham H (2017) Tidyverse: easily install and load ‘tidyverse’ packages. R package version 1
Wilkinson DM (1999) The disturbing history of intermediate disturbance. Oikos 84(1):145–147
Wolanski E, Gereta E (1999) Oxygen cycle in a hippo pool, Serengeti National Park, Tanzania. Afr J Ecol 37:419–423
Wolski P, Murray-Hudson M, Thito K, Cassidy L (2017) Keeping it simple: monitoring flood extent in large data-poor wetlands using MODIS SWIR data. Int J Appl Earth Obs Geoinf 57:224–234
Acknowledgements
We thank Elephants Without Borders, Taronga Conservation Society, the Australian Government, the University of New South Wales and the Centre for Ecosystem Science for their financial contributions to this study. We are very grateful to Elephants WithoutBorders and the Government of Botswana for access to research permits EWT 8/36/4 XXIV (179). A huge thank you to Lyn Francey for her exceptional knowledge of the area and her passion and dedication to the wildlife of southern Africa. Lastly, we thank three anonymous reviewers for their comments and suggestions which helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data availability statement
Raw data will be provided openly.
Additional information
Communicated by T. Gottschalk.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Francis, R.J., Brandis, K.J., Kingsford, R.T. et al. Quantifying bird diversity at three sites of differing herbivore presence. J Ornithol 161, 1117–1127 (2020). https://doi.org/10.1007/s10336-020-01804-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-020-01804-6