Abstract
Recent research has increasingly focused on female ornamentation, with several studies having investigated female ornaments in relation to reproduction. However, most previous studies have focused on single female ornaments, while females of numerous species, particularly birds, possess multiple ornaments. It is still unclear whether multiple female ornaments are linked to reproductive performance, though this information is crucial for understanding how these ornaments have been maintained. In this study, we examined the signaling function of multiple female ornaments in the Asian Barn Swallow Hirundo rustica gutturalis in Japan. First, females with previous breeding experience in the study population had longer tails and more colorful throat patches than other females. This indicates that these ornaments can provide information about the breeding experience of females to conspecifics. In contrast to males, the size of white tail spots was not significantly related to breeding experience in females, partly because females with larger white spots were less likely to return to breed after a failed clutch. Second, females with longer tails and more colorful throats started breeding earlier than others, even after controlling for confounding factors, and they paired with attractive males (thereby obtaining their direct and indirect benefits too), suggesting a mating advantage for females with such ornaments. In addition, males paired with long-tailed females invested more in paternal care. These observed patterns differed from those of males, for whom the throat coloration and the size of white tail spots, rather than tail length, were significant predictors. In fact, a sex-combined analysis of breeding date demonstrated significant interactions of sex in relation to tail length and the size of white tail spots, indicating differential selection between the sexes. Our data suggest that selection on females may facilitate the evolution and maintenance of some female ornaments, partially independently of male ornaments.
Zusammenfassung
Fortpflanzungsvorteile multipler weiblicher Ornamente bei der asiatischen Unterart der Rauchschwalbe ( Hirundo rustica gutturalis )
In letzter Zeit hat sich die Forschung zunehmend auf Ornamentierung bei Weibchen konzentriert, und mehrere Studien haben weibliche Ornamente in Bezug auf die Fortpflanzung untersucht. Die meisten vorherigen Studien haben sich jedoch auf einzelne Weibchenornamente konzentriert, während die Weibchen vieler Arten, insbesondere bei Vögeln, mehrere Ornamente besitzen. Nach wie vor ist unklar, ob multiple Weibchenornamente mit der Fortpflanzungsleistung in Verbindung stehen, obwohl diese Information entscheidend ist, um zu verstehen, wie diese Ornamente aufrechterhalten worden sind. In dieser Studie haben wir die Signalfunktion multipler Weibchenornamente bei der asiatischen Unterart der Rauchschwalbe (Hirundo rustica gutturalis) in Japan untersucht. Erstens hatten Weibchen mit vorheriger Bruterfahrung im Untersuchungsgebiet längere Schwänze und buntere Kehlflecken als andere Weibchen. Dies deutet darauf hin, dass die Ornamente Artgenossen über die Bruterfahrung von Weibchen informieren können. Anders als bei Männchen hing die Größe der weißen Schwanzflecken bei Weibchen nicht mit der Bruterfahrung zusammen, z.T. weil Weibchen mit größeren weißen Flecken nach einer erfolglosen Brut mit geringerer Wahrscheinlichkeit zum Brüten zurückkehrten. Zweitens begannen Weibchen mit längeren Schwänzen und bunteren Kehlflecken früher mit der Brut als andere, selbst wenn die statistische Analyse Störfaktoren berücksichtigte. Zudem waren diese Weibchen mit attraktiven Männchen verpaart (und erlangten auf diese Weise auch noch direkte und indirekte Fitnessvorteile), was darauf hindeutet, dass Weibchen mit solchen Ornamenten einen Paarungsvorteil besitzen. Des Weiteren investierten Männchen, die mit langschwänzigen Weibchen verpaart waren, mehr in die Brutpflege. Diese beobachteten Muster unterschieden sich von denen für Männchen, bei denen die Kehlfärbung und die Größe der weißen Schwanzflecken (und nicht die Schwanzlänge) als signifikante erklärende Variablen fungierten. In der Tat zeigte eine beide Geschlechter berücksichtigende Analyse des Brutdatums signifikante Interaktionen von Geschlecht in Bezug auf Schwanzlänge und Größe der weißen Schwanzflecken, was auf unterschiedliche Selektionsdrücke bei den Geschlechtern hindeutet. Unsere Daten lassen darauf schließen, dass auf Weibchen wirkender Selektionsdruck die Evolution und Aufrechterhaltung einiger Weibchenornamente fördern könnte, und zwar z.T. unabhängig von männlichen Ornamenten.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent research has increasingly focused on female ornamentation [i.e., exaggerated traits that seem to have rather negative effects on survivorship (reviewed by Amundsen and Pärn 2006; Tobias et al. 2012)]. Although female ornamentation is classically viewed as a nonfunctional copy of functional male ornamentation [or a “genetically correlated response to selection on male ornamentation” (Lande 1980)], several recent studies have supported sexual selection, or more broadly social selection, acting on female ornamentation [e.g., male mate choice, differential allocation, differential access or female–female combat for mates or resources (reviewed in Clutton-Brock 2009; Tobias et al. 2012; also see Odom et al. 2014; Dale et al. 2015)], thereby indicating a functional role of female ornaments. While previous studies have focused mainly on single ornaments, females of various species, particularly birds, possess multiple ornaments [e.g., the red–orange bill, red underwing feathers, head crest and blackish face mask of female Northern Cardinals, Cardinalis cardinalis (Jawor et al. 2003; reviewed in Kraaijeveld et al. 2007)]. It is still unclear whether (and why) multiple female ornaments are related to reproductive parameters, maintained by sexual selection (reviewed in Møller and Pomiankowski 1993; van Doorn and Weissing 2004; Amundsen and Pärn 2006).
Sexual selection on (multiple) female ornaments can be predicted when the corresponding male ornaments are sexually selected because of an intersexual genetic correlation [e.g., male mate choice for colorful females may have evolved as a correlated response to selection on females to choose colorful males (Hill 1993)]. This argument suggests that the function of female ornaments can be predicted by the function of the corresponding male ornaments, and the same ornament should be used in each sexual selection mechanism (e.g., mate choice at pairing). However, this may not always be the case. Even within each selection mechanism, different traits would be selected in each sex if males and females signal different qualities with different ornaments. For example, using the Barn Owl, Tyto alba, Roulin et al. (2001a, b) showed that plumage spottiness signals parasite resistance in females but not in males, whereas male plumage coloration, but not female plumage coloration, signals other qualities, such as feeding rate. This corresponds with the finding that sexual selection appears to be exerted on spottiness in females rather than males (Roulin 1999), with the inference that the main targets of sexual selection differ between the sexes [i.e., male coloration and female spottiness (see the discussion in Roulin and Altwegg 2007)]. In addition, the differential detectability of ornaments between sexes may change the targets of sexual selection. Because females rarely exhibit courtship displays, the detectability of certain “hidden” ornaments in females should be low, which can potentially change the relative importance of ornaments in sexual selection (Schluter and Price 1993; Tazzyman et al. 2014; see also Jawor and Breitwisch 2003). In these cases, from an adaptive perspective, there should be sex differences in the targets (and intensity) of sexual selection (i.e., sex-specific signals) in which female ornamentation evolves, at least partly independently of male ornamentation. Of course, no sexual selection on female ornaments is plausible if ornaments do not include valuable information or if they have very low detectability.
The Barn Swallow Hirundo rustica is a socially monogamous songbird with biparental care (Turner 2006). They have long outermost tail feathers, which are suggested to be sexually selected in males (e.g., Møller 1988; reviewed in Møller 1994) and females in populations of the European subspecies Hirundo rustica rustica (Møller 1993, p. 429); though subsequent experiment failed to validate this (Cuervo et al. 1996). However, in addition to their long tails, male and female Barn Swallows have several other ornaments, such as white tail spots and ventral plumage coloration, including a red throat patch. The relative importance of these male traits (and perhaps those of females; see below) in sexual selection differs across the species range [e.g., reddish ventral plumage rather than tail length is important in populations of the North American subspecies Hirundo rustica erythrogaster (Safran and McGraw 2004; for a recent review see Scordato and Safran 2014; see also Vortman et al. 2011, 2013, which demonstrates the signaling roles of both traits in populations of the East Mediterranean subspecies Hirundo rustica transitiva)]. Unfortunately, it is still unclear whether sexual selection on females actually differs from that on males in each population (i.e., whether the target and intensity of selection differ between the sexes, at least in some populations). Although Vortman et al. (2011) refer the differences in sexual selection, deduced from the annual reproductive success of males and females, the mechanism (i.e., breeding date, extra-pair paternity, or others) remains unclear. In addition, male ornaments, including red throat patches (more precisely, their size and coloration), tail length, and white tail spots, were reported to be important in sexual selection (e.g., Møller 1988; Kose and Møller 1999; Kose et al. 1999; Ninni 2003; Safran and McGraw 2004; Hasegawa and Arai 2013a), but the corresponding female ornaments, except for tail length (and sometimes plumage coloration), were not studied in the same population.
Asian Barn Swallows Hirundo rustica gutturalis have short tails and a large red throat patch compared with other subspecies, particularly the nominate H. rustica rustica, with whitish ventral plumage coloration (Fig. 1; see also Cramp 1988; Hasegawa et al. 2010a). In Japanese populations of this subspecies, male throat coloration and white tail spots, but not tail length, would be the most important plumage ornaments in sexual selection, because the former two, but not tail length, have been related to several reproductive parameters [e.g., laying date of the first egg of the first clutch, multiple broods, within-pair paternity, and differential parental investment (Kojima et al. 2009; Hasegawa et al. 2010a; Hasegawa 2011; Hasegawa et al. 2012a; Hasegawa and Arai 2013a; reviewed in Arai et al. 2015)]. In contrast to the European populations of the nominate subspecies, the Japanese populations of Barn Swallows breed sparsely [<1 % breed in colonies (Ministry of Environment 1997); note that 47 % breed in colonies comprising from seven to 22 pairs in Europe, where only 1–2 % of nests were depredated (e.g., Møller 1987; reviewed in Turner 2006)], thus they experience higher nest predation rates [approximately 50 % experience nest failure—see the "Results"; reviewed in Hasegawa et al. (2012b); see Fujita (1993) for the relationship between coloniality and nest predation; also, see Suzuki (1998) and Ringhorfer and Hasegawa (2014) for other non-colonial populations], indicating highly variable territory quality within populations of Swallows that breed outdoors. This situation may contribute to the large red throat patches in these populations because males with colorful throats hold territories with lower nest predation rates and thus are preferred by females (Hasegawa et al. 2012b, 2014a). Nevertheless, it is still unclear how these population characteristics (high nest predation in sparse populations) affect the ecology, morphology, and sexual selection of Barn Swallows, particularly in females.
Red throat patch (upper panel; the male mate is on the left) and long tails (lower panel) of female Asian Barn Swallow Hirundo rustica gutturalis. Note that the white tail spots can only be seen when Swallows spread their tails (e.g., when stretching, as seen in the lower right insert). The upper and lower panels show images of different individuals
We studied sexual selection on all three ornaments (tail length, white tail spots size, and red throat patch, i.e., size and coloration; see Supplementary material 1 for short description of these ornaments) in the female Barn Swallows H. rustica gutturalis. First, to study the information content of ornaments, we investigated female ornaments in relation to breeding experience (i.e., those that experienced one or more breeding seasons or not), a well-known measure of female quality [particularly in Barn Swallows, which are short-lived birds (Turner 2006)]. Subsequently, we studied the two potential causes of the association between female ornaments and breeding experience, i.e., differential return rate to the study site and age-related changes in ornamentation (e.g., Garamszegi et al. 2005). We also studied two other measures of female quality: body condition and arrival date (Turner 2006). These measures of female quality, rather than reproductive parameters (e.g., clutch size, parental care), were chosen because reproductive parameters are greatly affected by environmental factors, including nest and territory quality (e.g., Møller 1982; Hasegawa et al. 2012b; also see Soler et al. 1998) and male quality [i.e., differential parental allocation (reviewed in Sheldon 2000; Møller 1994; also see Hasegawa et al. 2012a)]. Furthermore, we investigated the associations between breeding date and ornaments because these are important fitness components linked to sexual selection in this species [i.e., “date selection” (sensu Møller et al. 2006)]. It should be noted that this link has also been used to assess sexual selection on females in other bird species (e.g., Siefferman and Hill 2005; reviewed in Amundsen and Pärn 2006) and on males in this population of Barn Swallows (Hasegawa et al. 2010a). Because early breeding is associated with high reproductive success via increases in the quality and quantity of offspring [sensu Grüebler and Naef-Daenzer (2010); e.g., high probability of second broods, high survivorship of fledglings due to the benign environment in the early spring, and sufficient post-fledging periods before migration (reviewed in Møller 1994; Turner 2006; see also the “Results”)], female traits linked to early breeding should be selected for. From an adaptive perspective, we predicted that female ornaments that efficiently signal their quality, based on signal content and detectability, should be correlated with the breeding date, even after controlling for female quality (i.e., the signal, rather than quality, should be the target of date selection). We also investigated their mating patterns. Well-ornamented females may not only breed earlier but also pair with males with sexually selected traits, as predicted by differential access to mates, i.e., the sexual selection mechanism in which well-ornamented individuals acquire more profitable mates (Burley 1986). In addition, as in male Barn Swallows (Hasegawa et al. 2012a), we studied male contribution to incubation to investigate differential parental investment, i.e., the sexual selection mechanism in which individuals that have acquired an “attractive” mate invest relatively more in the offspring than individuals that acquired an unattractive mate (sensu Møller and Thornhill 1998). Male Barn Swallows lack a brood patch and thus cannot incubate efficiently (Turner 2006). Nevertheless, males occasionally incubate the clutch in H. rustica gutturalis and H. rustica erythrogaster [6 % in H. rustica gutturalis (Hasegawa et al. 2012a)], which slows egg cooling and thus may play a significant role enabling females to forage longer away from their nests (Voss et al. 2008). Based on these correlational approaches, we discuss the sexual selection on multiple female ornaments and their adaptiveness, also considering sex differences in the function of ornaments, together with other possible explanations for the observed patterns.
Methods
Study site
The capture survey and reproductive performance recording were conducted from March to August during 2005 and 2006 in a residential area of Joetsu city, Niigata Prefecture, Japan (37°07′N, 138°15′E; 10 m a.s.l.). We captured a total of 120 and 89 females in 2005 and 2006, respectively, at the study site, though we included females that were captured at their first breeding attempts in the analyses [see Hasegawa et al. (2010a) for a detailed explanation]. We recaptured a total of 27 females with previous breeding experience in 2006 (see below). We also confirmed the identities of females that returned to the study site between March and August 2007 (Arai et al. 2009; Hasegawa and Arai 2013b), although we captured only a few females in 2007 (i.e., we stopped capturing females to focus our efforts elsewhere). The same study periods and population were used to study sexual selection in males (Hasegawa et al. 2010a). The Barn Swallows nested under the eaves of covered sidewalks along streets (see Tajima and Nakamura 2003).
Measurements
Adult Barn Swallows were captured in sweep nets while roosting at night. Captures were mainly (>80 %) done shortly after clutch completion. This was to avoid variation in mass due to the presence/absence of eggs. Each bird was provided with a standard, numbered aluminum ring and an individually recognizable combination of two or three half-sized plastic colored rings (Hughes, Middlesex). The sex of an individual was determined by the presence (female) or absence (male) of an incubation patch. Similar to male Barn Swallows (Hasegawa et al. 2010a), the females were classified into two groups based on ringing records in 2006: females with previous breeding experience in the study population (i.e., birds ringed during breeding in previous seasons), and other females (i.e., birds captured for the first time in 2006). It should be noted that we classified females based on their previous breeding experience rather than age (although both are positively correlated) because of high breeding dispersal, particularly after failed reproduction (Arai et al. 2009), which indicates that several old females were dispersed across populations (note that, for this reason, most of the females with previous breeding experience were successful in the previous season). We captured ca. 70 % of birds by the end of the breeding season (Hasegawa et al. 2010a). Although females with previous breeding experience belonged to several age classes in principle (i.e., those that experienced one or more breeding seasons), higher age class (i.e., those that experienced two or more breeding seasons, i.e., ≥3 years old) should be rare in this migratory bird with low annual survivorship [e.g., Møller and de Lope (1999) reported that only 10 % birds reached ≥3 years old; reviewed in Turner (2006)]. In our population, among 77 females with known breeding records captured in 2005, only seven (9.1 %) returned to the study site in 2007 (Arai et al. 2009), thereby indicating that few females were present at the study site for more than 2 years. In addition, morphology, breeding date, and reproductive success mainly change from yearlings to 2-year-old individuals (Møller and de Lope 1999; sensu Møller et al. 2006; also see Bradley et al. 2014). Thus, further subdivision is regarded as unnecessary when studying the functions of ornaments in Barn Swallows (Møller et al. 2006). Nest ownership was subsequently determined using binoculars.
At capture, we measured body mass (to the nearest 0.1 g), wing length, tarsus length, tail length, and the size of the white tail spots (to the nearest 0.01 mm), and we collected several throat feathers. The throat patch area was defined as the area of the Swallow’s red throat patch and was measured by placing a transparent plastic sheet over the throat region, ensuring that the feathers were lying flat in their natural position, and tracing the edges of the patch onto the sheet using a marker pen (Hasegawa et al. 2010a). We scanned the sheet and measured the area of the patch (to the nearest 0.1 mm2) using Scion Image software (Scion, Frederick, MD). Each bird’s throat patch was traced twice, and the mean of the two measurements was used. The repeatability of the two throat patch area measurements was highly significant in females [current sample, 2005, repeatability = 0.81, F 71,72 = 9.44, p < 0.0001; 2006, repeatability = 0.86, F 64,65 = 13.66, p < 0.0001 (Lessells and Boag 1987); see also Hasegawa et al. (2010a) for a larger sample size and those of males].
In the laboratory, we placed five throat feathers, which we collected at capture, on a piece of white paper such that their edges were in contact (i.e., five feathers on top of each other). The feather samples were scanned at 800-dpi resolution using a scanner (GT 9300 UF; Epson, Tokyo) and the images obtained were imported into Photoshop Elements 3.0 (Adobe Systems, San José, CA). Our method is not uncommon, i.e., it is one of the four methods introduced in Montgomerie (2006). This method uses consistent lighting conditions and is thus superior to a digital camera, which requires color calibration to control for variation in lighting conditions (e.g., Hasegawa et al. 2014b; also see Montgomerie 2006). We measured the mean red–green–blue values for a 30 × 30-pixel square near the distal end of the feather sample. The mean red–green–blue values were converted into hue (H)–saturation (S)–brightness (B) values using the algorithm described by Foley and van Dam (1984). Throat feathers lack reflectance in ultraviolet regions, so this method captured most of the variation in throat coloration (Safran and McGraw 2004; E. Arai, unpublished data). The saturation value (range 0–255 using our methods) (Yabusaki et al. 2014) was the only color measure used in this study because it does not fade with time, whereas the other two color measurements fade linearly with time during the breeding season (Hasegawa et al. 2008). The repeatability of the saturation values, calculated using two independent feather samples from a total of ten feathers, was significant in females [2005, repeatability = 0.67, F 25,26 = 5.01, p < 0.001; 2006, repeatability = 0.65, F 28,29 = 4.69, p < 0.001 (Lessells and Boag 1987; see Hasegawa et al. 2010a for males)]. The positive correlations between color variables (the current sample, 2005, n = 72; H-S, r = 0.33; S-B, r = 0.43; B-H, r = 0.86; n = 65; 2006, H-S, r = 0.60; S-B, r = 0.56; B-H, r = 0.96; all, p < 0.01) imply that throat plumage with a lower saturation value is generally redder (i.e., a lower hue value) and darker (i.e., a lower brightness value). These relationships could also be predicted from the feather pigmentation, where pheomelanin (reddish) and eumelanin (black) were positively inter-correlated and negatively related to the saturation value [i.e., the throat feathers contained more pheomelanin and eumelanin pigments, which produce less saturated coloration (and produce redder and darker feathers) (Arai et al. 2015)]. Thus, our measures of coloration adequately quantify the coloration on which selection acts (Arai et al. 2015). A less-saturated throat patch denotes a pigment-rich throat patch (or, simply, a “colorful” throat patch). Although previous studies of other subspecies in North American and Mediterranean populations used ventral plumage coloration in addition to throat coloration (e.g., Safran and McGraw 2004; Vortman et al. 2011), we focused only on throat coloration in the current study. This was because, in contrast to the subspecies mentioned above, the Japanese subspecies has a whitish ventral plumage coloration [e.g., see Hasegawa et al. (2016a) for coloration and Arai et al. (2015) for pigmentation]. Thus it seems unlikely that ventral coloration is important in sexual selection in this subspecies (i.e., these traits are not “ornaments”; see the “Introduction”). In fact, our previous analysis based on breast and vent plumage coloration found no significant relationship between male ventral plumage coloration and reproductive parameters, including laying date and annual reproductive success (Hasegawa 2005). These variables were not significantly related to breeding experience or laying date in the current data sets either (see Supplementary material 2).
Observations
We recorded all of the birds that returned to the study site during daily observations in 2006 and 2007 (Arai et al. 2009; Hasegawa and Arai 2013b). Each bird was identified by observing the attached color rings.
We inspected nests every other day to record the breeding date, which was defined as the date the first egg of the first clutch was laid (Hasegawa et al. 2010a). The nests were inspected every day around the estimated hatching date (from approximately 10 days after the laying date) and fledging date. To exclude females that failed to raise their first clutch and subsequently immigrated to the study site, we only used females that arrived at the study site before the first breeding date of the population in the analysis of females with previous breeding experience in the population. We also studied the number of fledglings reared during the season to confirm date effect in our population (Grüebler and Naef-Daenzer 2010; reviewed in Turner 2006) and to assess its relationship with female ornamentation. Extra-pair paternity and intraspecific brood parasitism were virtually absent from our sparse population [<3 % of all nestlings (Hasegawa et al. 2010b)], so we regarded the number of fledglings reared during the season as a measure of annual reproductive success.
In 2006, nests were watched only when the birds were easy to identify. Video-camera recorders (SONY CCDTRV92) were set up circa 3 m from the nests and did not seem to disturb the birds. We only studied first nesting attempts. Each nest was observed for 120 min only once at 4, 8 or 12 days into the incubation period. As Swallows incubate for approximately 14 days to hatch their nestlings (Turner 2006), our video recordings were made during the early (4 days), middle (8 days), and late periods of incubation (12 days after the start of incubation). We recorded male, female, and total nest attentiveness (i.e., the total time each bird incubated the clutch per 120 min). All observations were made during the morning (0530–0800 hours). We used the same sample in the previous study (n = 24; Hasegawa et al. 2012a), though we could not obtain female measurements from four nests, and one female lacked the left outermost tail feather (thus, the final sample size was 19).
Statistical procedures
All data were analyzed using the R (version 2.14.1) statistical package (R Development Core Team 2011). We used parametric tests to examine the relationship between female ornamentation and female quality (i.e., body condition and arrival date) because these did not significantly deviate from a normal distribution in either study year (Shapiro–Wilk test, W > 0.93, p > 0.07; see “Results” for sample size). As in our previous study using male Barn Swallows (Hasegawa et al. 2010a), we defined the body condition as the residual based on a regression of body mass against tarsus length. An alternative approach, using the scaled mass index (Peig and Green 2009), was not applied, because log transformation, which is required for the scaled mass index, did not improve the relationship between body mass and tarsus length (raw, r 2005 = 0.027, r 2005 = 0.099; log transformed, r 2005 = 0.025; r 2006 = 0.097; all, p > 0.40). As predicted by the weak relationship between body mass and tarsus length, qualitatively similar results were found when raw body mass was used instead of residual body mass.
To study female ornaments in relation to the probability of returning to the study site the following year, we built univariable generalized linear mixed models (GLMMs) using a binomial error structure and a logit link function with the glmer function (package lme4) in R. To analyze female ornaments in relation to breeding date and annual reproductive success, we used linear mixed effect models (LMEs), as described previously [lmer function in package lme4 (Hasegawa et al. 2010a)]. In these models, bird identity was entered as a random factor to control for data similarity within individuals [see Gelman and Hill (2007) for background logic]. The annual reproductive success of females with some failed breeding attempts is unclear because females often disperse after failed breeding attempts within seasons and consequently cannot be followed-up (e.g., Shields 1984; also see Arai et al. 2009). Thus, in this analysis, we focused only on females with no failed breeding attempts. This approach would be problematic if failed breeding attempts were related to breeding date. However, this might not be the case because failed or successful breeding attempts were not significantly related to breeding date, i.e., the laying date of the first egg in the first clutch [GLMM with binomial distribution using failed or successful breeding attempts as a response variable, n = 131 (n 2005 = 70, n 2006 = 61, eleven individuals included in both years, χ 2 = 0.51, p = 0.48) or to any female ornaments (χ 2 ≤ 2.78, p ≥ 0.10)]. Similar results were obtained even when we included the significant effect of year (χ 2 = 7.33, p < 0.01) as a covariate (χ 2 ≤ 2.62, p ≥ 0.11).
In the analysis of breeding date, we also conducted an analysis with a general linear model (function, lm) using only females with previous breeding experience to control for the effects of any differences in previous breeding experience among females [i.e., experienced females should have high reproductive performance (Hasegawa et al. 2010a; reviewed in Turner 2006)], which might have confounded the relationship between female ornaments and breeding date. In this analysis, we added arrival date as an extra predictor. Our data included an outlier (>2 SD units from the population mean), which might cause an overestimation of the relationship between female ornamentation and breeding date (see Fig. 3). Thus, we established a robust linear model (lmrob in the R package robustbase), which identified and corrected for the variation caused by outliers in the data (see also Bennett and Ellison 2009). This method was used instead of simply excluding the outlier, considering our small sample size (i.e., n = 18). A short summary of the dependent variables and sample sizes is provided in Supplementary material 1.
We fitted a full model that contained all of the explanatory variables in the multivariable models [i.e., LME and general linear model (Whittingham et al. 2006; Forstmeier and Schielzeth 2011)], although stepwise model selection by progressively eliminating non-significant (p > 0.05) terms yields qualitatively similar results. Multicollinearity should not matter in the current data sets [i.e., low correlation coefficients among predictor variables and low variance inflation factor (VIF); Supplementary material 3]. The VIF values increased when we added the interactions between female traits and study year in a full LME analysis (maximum VIF = 2.56); thus, we did not include these interaction terms in the full model. However, when we added each interaction term separately in the full model (all, VIF < 2.5), we confirmed that neither interaction term was significant, indicating a similar relationship between the laying date and ornamentation across the 2 study years.
Results
Female ornaments and breeding experience
When comparing ornamentation with available information in 2006, females with previous breeding experience in the study population had less saturated (i.e., more colorful; see the "Methods") throat patches and longer tails than other females (Table 1). These relationships remained significant in a multivariable analysis that controlled for each other [generalized linear model with a binomial error distribution using the two female groups (females with breeding experience as 1 and others as 0) as a dependent variable; tail, coefficient = 0.23, χ 2 = 12.82, p < 0.001; throat, coefficient = −0.17, χ 2 = 8.09, p < 0.01; overall, χ 2 = 18.90, p < 0.001], indicating the partial independence of these relationships. There were no significant differences in the other measurements between the two groups (Table 1).
Among females without failed breeding attempts, the probability of a female returning to the study site was marginally positively correlated with her body condition in the previous year (Table 2). However, the probability of females with failed breeding attempts returning to the study site was negatively correlated with the size of white tail spots (Table 2; Fig. 2). No other female ornament significantly predicted the probability of a female Swallow returning to the study site in either group (Table 2).
Relationship between the size of white spots on female tails in the previous year and on females returning to the study site (1 = returned) after failed breeding attempts in Asian Barn Swallow H. rustica gutturalis. The mean ± SD in addition to each point is depicted (n all = 65; n returned = 14, n not-returned = 51)
On the other hand, after controlling for the yearly difference in mean ornament expression between 2005 and 2006 (Hasegawa et al. 2010a), the tail length of each female increased significantly across years (within-individual analysis, yearly changes (mm), mean ± SD = 1.35 ± 2.30, paired t-test, n = 17, t = 1.35, p = 0.03; note that the sample size differed from those of other analyses because we sometimes lacked measurements or reproductive parameters in either year; among these samples, 15 identical females are included in Table 1 and this analysis), whereas throat saturation did not differ significantly (yearly changes in the saturation value, mean ± SD = −2.09 ± 5.43, n = 17, t = −1.59, p = 0.13). Yearly changes in other ornaments were far from significant (n = 17, p ≥ 0.22).
Female ornaments, body condition, and arrival date
Female body condition was not significantly related to any of the four female ornaments in 2005 (|r| ≤ 0.09, p ≥ 0.45, n = 72) or 2006 (|r| ≤ 0.21, p ≥ 0.09, n = 65), except for a significant positive relationship between tail length and body condition in 2005 (r = 0.29, p = 0.01, n = 72). This was perhaps due to outliers (in fact, the robust linear regression, lmrob, yielded non-significant relationships, p ≥ 0.10, in all of these analyses; statistics not shown). Female body condition was not significantly related to any female ornament when we studied females with previous breeding experience alone (|r| ≤ 0.20, p ≥ 0.31, n = 18).
Among females with previous breeding experience (i.e., those that had been ringed previously; note that we could not determine the arrival date of females without rings), the arrival date was not significantly related to any female ornament (|r| ≤ 0.42, p ≥ 0.08), although the sample sizes were relatively small (n = 18).
Female ornaments and breeding date
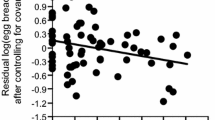
Breeding date was significantly related to two female ornaments: throat saturation and tail length (Table 3). Females with less saturated (i.e., more colorful) throats and longer tails started breeding earlier than others. No other female trait was significant (Table 3). Because females with breeding experience started breeding earlier than other females (mean ± SD with 1 April = 1; former, 30.85 ± 5.23; latter, 47.34 ± 14.34; Welch’s t-test, n = 20, 42, t = 6.60, p < 0.001), previous breeding experience could have confounded the results, but this was not the case. In females with breeding experience, the breeding date was significantly related to body condition, throat saturation, and tail length (Table 4). Females started breeding earlier when they had a better body condition, less saturated (more colorful) throat patches (Fig. 3a), and longer tails (Fig. 3b).
Relationships between female ornaments (x-axis) and breeding date (y-axis) after controlling for significant covariates in female Asian Barn Swallow H. rustica gutturalis with previous breeding experience in the study population: a throat saturation (note that smaller values indicate more colorful throats) and b tail length. The x-axis shows the standardized values for each female ornament, and the y-axis shows the residuals after controlling for the significant terms (Table 4). Simple linear regression lines are shown
In the analysis of the relationship between breeding date and annual reproductive success among females without failed breeding attempts, early breeding females had higher reproductive success than others [LME; n = 79 (n 2005 = 47, n 2006 = 32, where four individuals were duplicated), coefficient = −0.87, χ 2 = 15.47, p < 0.001] because early breeding females had a higher probability of rearing two broods than others [GLMM with binomial distribution; n = 79 (n 2005 = 47, n 2006 = 32, where four individuals were duplicated), coefficient = −1.33, χ 2 = 17.51, p < 0.001]. When female morphological traits were separately added to the analysis (i.e., multivariable LME), breeding date, but none of the female traits, was significant (n = 73, breeding date, χ 2 = 16.41, p < 0.001; female traits, χ 2 ≤ 1.11, p ≥ 0.29), indicating that breeding date, rather than female traits, mainly determined annual reproductive success. This was also the case when we studied the probability of rearing two broods (n = 73, breeding date, χ 2 = 17.55, p < 0.001; female traits, χ 2 ≤ 0.51, p ≥ 0.47). The analysis excluding breeding date demonstrated that, among all the female ornaments, only tail length increased significantly with reproductive success (tail length, coefficient = 0.56, χ 2 = 5.45, p = 0.02; others, χ 2 ≤ 1.07, p ≥ 0.30), but not with the probability of rearing two broods (all, χ 2 ≤ 2.11, p ≥ 0.15), which indicates that female ornaments were only weakly related to reproductive success, although early breeding would increase fledgling survivorship (Grüebler and Naef-Daenzer 2010). The small sample size (n = 7) precluded any meaningful analyses among females with breeding experience alone.
Sex-specific relationship between breeding date and ornaments
A direct approach for studying sex-specific relationships between breeding date and ornaments is to compare male and female selection directly in the same analysis (i.e., including both male and female data to analyze the interaction terms between each ornament and sex, which statistically confers sex differences in the relationships, rather than to analyze the relationship in each sex separately). We found significant interactions of sex with both tail length and the size of white tail spots (Table 5), demonstrating that a longer tail was a more important predictor in females, and, conversely, that larger size of white tail spots was a more important predictor in males. Both sexes bred early when they had colorful throats (Table 5).
Within-pair mating patterns
Based on the above findings, we hypothesized that females with longer tails and more colorful throats that bred earlier than others should be paired with males with larger white tail spots and more colorful throats because these males also bred earlier than others (Hasegawa et al. 2010a). To test this prediction, we focused on pairs in which the females had previous breeding experience, using principal component analysis to combine the size of white tail spots and throat coloration in males and tail length and throat coloration in females. The high scores for principal component 1 (PC1; the prcomp function in R software) for males represented individuals with large tail spots and less saturated (more colorful) throats (PC1male; loadings 0.87 and −0.87, respectively; proportion of variance explained = 0.76). The high scores for PC1 for females represented individuals with long tails and less saturated (more colorful) throats (PC1female; loadings 0.72 and −0.72, respectively; proportion of variance explained = 0.51). Among females with breeding experience, there was a marginal positive correlation between PC1male and PC1female within pairs [n = 18, r = 0.47, p = 0.048; Fig. 4; note that similar significant positive relationships were found when we focused on all females and their mates (2005, n = 49, r = 0.34, p = 0.02; 2006, n = 67, r = 0.38, p < 0.01; detailed analyses not shown)].
Relationship between male and female ornamentation measured by principal component for males (PC1male) and females (PC1female), respectively. A simple linear regression line is shown (see the text for detailed information). The high PC1male indicates males with larger white tail spots and less saturated (i.e., more colorful) throat patches, whereas the high PC1female indicates females with longer tails and less saturated (i.e., more colorful) throat patches
Differential parental investment at incubation
The probability of male participation in incubation was predicted by female tail length (Table 6). Males paired with long-tailed females participated in incubation at a higher probability than other males [Fig. 5; note that male and female age did not predict male participation in incubation (Hasegawa et al. 2012a)]. Among males that participated in incubation (n = 12), the time they spent incubating eggs was not significantly explained by their mates’ ornaments (LME; p > 0.09; data not shown).
Discussion
Our findings, though based on small sample sizes, indicate a reproductive advantage of females that possess colorful throats and long tails for early breeding because early breeding would increase the quantity and quality of offspring due to date effects [e.g., a high probability of second broods and high survivorship of fledglings in Barn Swallows (Møller 1994; Turner 2006; Grüebler and Naef-Daenzer 2010; also see the “Results”]. This suggests that it would be adaptive for conspecifics to pay attention to these ornaments (i.e., males in male mate choice and females in female–female aggression) because they convey partially independent information about previous breeding experience and age, which are factors associated with better parenting and competitive ability (Amundsen and Pärn 2006; Turner 2006; Balbontín et al. 2007). Although the observed pattern (Fig. 3) would be, in part, due to the early breeding onset of high-quality females, which should accompany better ornamentation (Amundsen and Pärn 2006), this confounding effect should be small because we statistically controlled for three measures of female quality: previous breeding experience (and age; see the “Methods”), arrival date, and body condition. Moreover, highly ornamented females paired with males that possessed sexually selected ornaments (Fig. 4; also see the "Introduction"), indicating a sexual selection mechanism, “differential access” (Burley 1986). Highly ornamented females may have differential access to these highly ornamented males, and indeed male Swallows discriminately court females based on plumage characteristics (Hasegawa et al. 2016a). Such a mating pattern was found in previous correlative studies (e.g., Møller 1993; Safran and McGraw 2004) and even in an experiment that manipulated female tail length (Cuervo et al. 1996), in which female ornamentation after manipulation, but not before, was positively correlated with male ornamentation (although a female’s tail manipulation had no apparent effect on her breeding date). Whatever the reasons [e.g., mutual mate choice, intrasexual competition for territory in both sexes and “prudent” mate or territory choice (e.g., Arnqvist et al. 1996; Jawor et al. 2003; Härdling and Kokko 2005; reviewed in Jiang et al. 2013)], females that pair with males possessing colorful throats and large white spots should obtain additional reproductive advantages. The benefits could be direct [e.g., throat coloration, territory quality (Hasegawa et al. 2014a, Wilkins et al. 2015); white tail spots, parasite avoidance (Kose and Møller 1999; Kose et al. 1999; also see Saino et al. 2015)]. Or, females paired with these well-ornamented males could simply obtain offspring with high reproductive value due to the high survivorship or reproductive success of these descendants [see Vortman et al. (2015) for high father-son heritability (reviewed in Kokko et al. 2002; Griffith and Pryke 2006)]. The observed patterns are consistent with the idea that tail length and throat coloration are under sexual selection in female Barn Swallows, which predict early breeding onset of well-ornamented females and that such ornaments provide information on female quality. The finding of the differential parental investment pattern during incubation (Table 6; see the “Introduction”) further supports sexual selection on female tail length.
An alternative, but not mutually exclusive, explanation is that, even after controlling for measurable female qualities in terms of breeding experience, age, arrival date, and body condition, there remains some unmeasured female quality, which might explain the reproductive advantages of females with multiple ornaments. For example, because tail length and throat coloration both increased with age (see the “Results”), age and experience might explain the observed pattern even in females with previous breeding experience, which includes ≥2-year-old females (see “Methods”; sensu Lifjeld et al. 2011). We cannot completely reject this possibility from the current correlational study. However, life history theory predicts an increased investment in current reproduction, including costly sexual traits, with increasing age because of reduced residual reproductive value (Kokko 1997); thus, finding an age dependency of ornaments does not contradict sexual selection for the ornament. Moreover, age and unmeasured quality alone could not explain the sex difference in the relationship between breeding date and ornaments (see below), suggesting that this is not a self-contained explanation.
The pattern between ornamentation and breeding date observed in females differed from that in males. Breeding date, a major fitness component in this species [which is linked to sexual selection (Møller 1994; Møller et al. 2006)], was associated with large white tail spots, rather than tail length, along with colorful throats in males (Kojima et al. 2009; Hasegawa et al. 2010a, 2012a; reviewed in Hasegawa 2011). Because females lay eggs, the relative importance of individual quality versus ornamentation may be higher for females than males at the onset of breeding (i.e., females start breeding earlier when they are in better condition), and thus some quantitative differences would be expected between the sexes. This explains why female body condition, which can predict quality in terms of viability (Table 2), was related to breeding date (Table 4; also see Hasegawa et al. 2010a). However, differential parental investment patterns also differed between the sexes because male white tail spots, rather than tail length, predicted the mate’s incubation investment (Hasegawa et al. 2012a; also see Table 6), indicating that the confounding effect of female internal condition is insufficient to explain the overall pattern. Also, even when confined to the laying date alone, the possibility of a confounding effect of female condition does not explain the reversed signs in the relationships regarding tail and white tail spots between sexes [Table 5 in this study; also see Table 3 in Hasegawa et al. (2010a) for the relationship between laying date and male ornaments]. In fact, strong genetic correlations should lead to quantitatively similar relationships between the sexes in either sexual selection, i.e., linkage between ornaments and each sexual selection episode, or quality indicator mechanisms, i.e., linkage between the ornament and quality of the owner (e.g., Hill 1993; reviewed in Amundsen and Pärn 2006). Thus, sex differences in sexual selection or quality indicator mechanisms including differential age dependency must be involved in the explanation of why tail length, rather than white tail spots, was related to the breeding date in Asian female Barn Swallows (see below).
Tail length and white tail spots are both targets of intersexual, rather than intrasexual, selection, at least in males (e.g., Møller 1994; Kose and Møller 1999; Kose et al. 1999; Hasegawa et al. 2012a, 2014a; reviewed in Hasegawa 2011). Thus, sex differences in the relative importance of intersexual and intrasexual selection (e.g., the greater importance of intrasexual selection in males) could not explain why tail length, but not white tail spots, was related to breeding date in Asian females. A plausible explanation is that sex differences in the detectability of traits affect the sexual selection pressure on the focal trait subsequently (Schluter and Price 1993). Male, but not female, Barn Swallows use courtship displays to reveal their white tail spots (Møller 1994; Turner 2006), and thus female white tail spots would have low detectability (i.e., they are covered by other tail feathers; Fig. 1). Low detectability necessitates a high searching cost due to longer inspection times, by males, causing the preference (and thus intersexual selection) to remain at a low level (Schluter and Price 1993). A similar argument has been posited for the Northern Cardinal C. cardinalis, for which the targets of mate choice differ between sexes, possibly due to sex differences in courtship displays (Jawor and Breitwisch 2003; Jawor et al. 2003).
At the same time, it appears that the sexes differ in the degree to which they reliably signal information, which should affect the relationship between breeding date and ornamentation. High-quality individuals with greater ornamentation commence breeding earlier. This might be because of their higher quality or their sexually selected ornamental signals (or both). Consistent with this idea, males but not females, exhibited a positive link between breeding experience and white tail spots [Table 1; also see Table 1 in Hasegawa et al. (2010a)], indicating that white tail spots may be used as a sex-specific quality indicator. Surprisingly, the observed sex-specific relationship between the size of white tail spots and breeding experience was site specific, because previous studies conducted in a European population of Barn Swallows showed that the size of white tail spots was age dependent (i.e., closely correlated with breeding experience) in both sexes (Kose and Møller 1999; also see Kose et al. 1999; Saino et al. 2015).
Further analyses indicated that both age dependency and differences in returning to the study site after failed breeding attempts perhaps due to breeding dispersal (Arai et al. 2009; reviewed in Turner 2006) may form a link between female morphology and breeding experience. In particular, the low probability of females with large white tail spots returning after failed breeding attempts is intriguing given that females disperse more than males, particularly after failed reproduction (Arai et al. 2009). In fact, failed reproduction is a frequent event due to high nest predation in sparse, outdoor Japanese populations such as ours, but not in Europe, where Swallows breed in aggregations at indoor sites (reviewed in the Introduction). The cause of the low return rates of females with large tail spots has yet to be determined [e.g., differential philopatry or matching habitat choice (Dreiss et al. 2011)], but regardless of the cause, male preferences for this trait in females should be low. The preference for females with large tail spots would decrease the re-mating opportunities of males both within and across breeding seasons, and finding new mates requires extra time and energy (Shields 1984; e.g., Saino et al. 2002; Turner 2006; see also Arai et al. 2009). Thus, there should be decreased intersexual selection for large white spots on the tails of females, particularly in sparsely breeding populations such as ours, thereby resulting in reduced age dependency of this ornament (Kokko 1997).
Consistent with this scenario, when we calculated Cohen’s d-value, a measure of effect size (see Eq. 10 in Nakagawa and Cuthill 2007), the sexual dimorphism in the size of white tail spots was greater in our study population [2005, mean ± SE = 1.79 ± 0.12; 2006, 1.54 ± 0.15 (Hasegawa et al. 2010a)] compared with the European population [1.07 ± 0.11 (Kose and Møller 1999)]. The extent of this dimorphism is interesting given that sexual selection favors large white tail spots among males in both populations (Kose and Møller 1999; Hasegawa et al. 2010a; see also Hasegawa et al. 2012a). No such population difference was found in the sexual dimorphism of tail length between our study population [2005, 2.21 ± 0.12; 2006, 2.03 ± 0.15 (Hasegawa et al. 2010a)] and the European population [2.20 ± 0.11 (Kose and Møller 1999)], although predicting sexual dimorphism in tail length may be complicated by natural selection [(dis)advantages due to its aerodynamic function (e.g., Norberg 1994; Cuervo et al. 2003; reviewed in Hasegawa et al. 2016b); also see Kose and Møller (1999) and Kose et al. (1999) regarding the depigmentation costs of white tail spots]. Sexually antagonistic selection via sexual and natural selection would facilitate sexual dimorphism in ornamentation and its population differentiation.
In this study, though the sample sizes were limited, we showed that multiple female ornaments were associated with differential reproductive advantages (i.e., early breeding onset and access to attractive males); thus they are likely subject to sexual selection in part, though the effects of age and unmeasured qualities cannot be ruled out. Moreover, the female traits related to breeding date and mates’ parental investment appeared to be partially independent of those in males (Tables 3, 4, 5, 6; see above). These sex differences would have affected the evolution of multiple ornaments in a non-parallel fashion among males and females. Together with previous studies conducted in European and North American populations of Barn Swallows, which suggest sexual selection for long tails and colorful plumage in both sexes, respectively (Møller 1994; Safran and McGraw 2004; also see Vortman et al. 2011 for east Mediterranean populations), the current study suggests an evolutionarily labile, adaptive function of female ornaments within species. Because the current study was based on correlational data, further experiments, possibly based on the manipulation of ornaments and behavioral observations, are needed to confirm the roles of plumage ornaments in mate preferences and in intrasexual combat for each sex in each population.
References
Amundsen T, Pärn H (2006) Female coloration: review of functional and nonfunctional hypotheses. In: Hill GE, McGraw KJ (eds) Bird coloration, vol II. Function and evolution. Harvard University Press, Cambridge
Arai E, Hasegawa M, Nakamura M (2009) Divorce and asynchronous arrival in Barn Swallows Hirundo rustica. Bird Study 56:411–413
Arai E, Hasegawa M, Nakamura M, Wakamatsu K (2015) Male pheomelanin pigmentation and the breeding onset in Barn Swallow Hirundo rustica gutturalis. J Ornithol 156:419–427
Arnqvist G, Rowe L, Krupa JJ, Sih A (1996) Assortative mating by size: a meta-analysis of mating patterns in water striders. Evol Ecol 10:265–284
Balbontín J, Hermosell IG, Marzal A, Reviriego M, de Lope F, Møller AP (2007) Age-related change in breeding performance in early life is associated with an increase in competence in the migratory Barn Swallow Hirundo rustica. J Anim Ecol 76:915–925
Bennett KF, Ellison AM (2009) Nectar, not colour, may lure insects to their death. Biol Lett 5:469–472
Bradley RJ, Hubbard JK, Jenkins BR, Safran RJ (2014) Patterns and ecological predictors of age-related performance in female North American Barn Swallows, Hirundo rustica erythrogaster. Behav Ecol Sociobiol 68:1883–1892
Burley N (1986) Sexual selection for aesthetic traits in species with biparental care. Am Nat 127:415–445
Clutton-Brock T (2009) Sexual selection in females. Anim Behav 77:3–11
Cramp S (1988) The birds of the western Palearctic, vol 5. Oxford University Press, Oxford
Cuervo JJ, de Lope F, Møller AP (1996) The function of long tails in female Barn Swallows (Hirundo rustica): an experimental study. Behav Ecol 7:132–136
Cuervo JJ, Møller AP, de Lope F (2003) Experimental manipulation of tail length in female Barn Swallows (Hirundo rustica) affects their future reproductive success. Behav Ecol 14:451–456
Dale J, Dey CJ, Delhey K, Kempenaers B, Valcu M (2015) The effects of life history and sexual selection on male and female plumage colouration. Nature 527:367–370
Dreiss AN, Antoniazza S, Burri R, Fumagalli L, Sonnay C, Frey C, Goudet J, Roulin A (2011) Local adaptation and matching habitat choice in female Barn Owls with respect to melanic coloration. J Evol Biol 25:103–114
Foley JD, van Dam A (1984) Intensity and color. In: Foley JD, van Dam A (eds) Fundamentals of interactive computer graphics. Addison-Wesley, Philippines
Forstmeier W, Schielzeth H (2011) Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol 65:47–55
Fujita G (1993) Nest site selection and reproductive success in Barn Swallows—preliminary report. Strix 12:35–39 (Japanese with English summary)
Garamszegi LZ, Heylen D, Møller AP, Eens M, de Lope F (2005) Age-dependent health status and song characteristics in the Barn Swallow. Behav Ecol 16:580–591
Gelman A, Hill J (2007) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, New York
Griffith SC, Pryke SR (2006) Benefits to females of assessing color displays. In: Hill GE, McGraw KJ (eds) Bird coloration, vol II. Function and evolution. Harvard University Press, Cambridge
Grüebler MU, Naef-Daenzer B (2010) Fitness consequences of timing of breeding in birds: data effects in the course of a reproductive episode. J Avian Biol 41:282–291
Härdling R, Kokko H (2005) The evolution of prudent choice. Evol Ecol Res 7:697–715
Hasegawa M (2005) Nihonno tsubame ni okeru seisentaku keisitu. M.D. thesis, University of Tsukuba (in Japanese)
Hasegawa M (2011) Sexual selection on multiple ornaments in the Barn Swallow Hirundo rustica gutturalis. Ph.D. thesis, University of Tsukuba
Hasegawa M, Arai E (2013a) Divergent tail and throat ornamentation in the Barn Swallow across the Japanese islands. J Ethol 31:79–83
Hasegawa M, Arai E (2013b) Differential female access to males with large throat patches in the Asian Barn Swallow Hirundo rustica gutturalis. Zool Sci 30:913–918
Hasegawa M, Arai E, Watanabe M, Nakamura M (2008) Methods for correcting plumage color fading in the Barn Swallow. Ornithol Sci 7:117–122
Hasegawa M, Arai E, Watanabe M, Nakamura M (2010a) Mating advantage of multiple male ornaments in the Barn Swallow Hirundo rustica gutturalis. Ornithol Sci 9:141–148
Hasegawa M, Arai E, Kojima W, Kitamura W, Fujita G, Higuchi H, Watanabe M, Nakamura M (2010b) Low level of extra-pair paternity in a population of the Barn Swallow Hirundo rustica gutturalis. Ornithol Sci 9:161–164
Hasegawa M, Arai E, Watanabe M, Nakamura M (2012a) High incubation investment of females paired to attractive males in Barn Swallows. Ornithol Sci 11:1–8
Hasegawa M, Arai E, Watanabe M, Nakamura M (2012b) Female mate choice based on territory quality in Barn Swallows. J Ethol 30:143–150
Hasegawa M, Arai E, Watanabe M, Nakamura M (2014a) Colourful males hold high quality territories but reduce paternal care in Barn Swallows. Behaviour 151:591–612
Hasegawa M, Ligon RA, Giraudeau M, Watanabe M, McGraw KJ (2014b) Urban and colorful male house finches are less aggressive. Behav Ecol 25:641–649
Hasegawa M, Watanabe M, Nakamura M (2016a) Promiscuous copulation attempts and discriminate pairing displays in male Barn Swallows as revealed by model presentation. Ethol Ecol Evol 28:163–174
Hasegawa M, Arai E, Kutsukake N (2016b) Evolution of tail fork depth in genus Hirundo. Ecol Evol 6:851–858
Hill GE (1993) Male mate choice and the evolution of female plumage coloration in the house finch. Evolution 47:1515–1525
Jawor JM, Breitwisch R (2003) A unique ornament display in female northern cardinals. Wilson Bull 115:464–467
Jawor JM, Linville SU, Beall SM, Breitwisch R (2003) Assortative mating by multiple ornaments in Northern Cardinals (Cardinalis cardinalis). Behav Ecol 14:515–520
Jiang Y, Bolnick DI, Kirkpatrick M (2013) Assortative mating in animals. Am Nat 181:E125–E138
Kojima W, Kitamura W, Kitajima S, Ito Y, Ueda K, Fujita G, Higuchi H (2009) Female Barn Swallows gain indirect but not direct benefits through social mate choice. Ethology 115:939–947
Kokko H (1997) Evolutionarily stable strategies of age-dependent sexual advertisement. Behav Ecol Sociobiol 41:99–107
Kokko H, Brooks R, McNamara JM, Houston AI (2002) The sexual selection continuum. Proc R Soc Lond B 269:1333–1340
Kose M, Møller AP (1999) Sexual selection, feather breakage and parasites: the importance of white spots in the tail of the Barn Swallow. Behav Ecol Sociobiol 45:430–436
Kose M, Mänd R, Møller AP (1999) Sexual selection for white tail spots in the Barn Swallow in relation to habitat choice by feather lice. Anim Behav 58:1201–1205
Kraaijeveld K, Kraaijeveld-Smit FJL, Komdeur J (2007) The evolution of mutual ornamentation. Anim Behav 74:657–677
Lande R (1980) Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34:292–305
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lifjeld JT, Kleven O, Jacobesen F, McGraw KJ, Safran RJ, Robertson RJ (2011) Age before beauty? Relationships between fertilization success and age-dependent ornaments in Barn Swallows. Behav Ecol Sociobiol 65:1687–1697
Ministry of Environment (1997) http://www.biodic.go.jp/reports/5-4/p000.html. Accessed 6 Mar 2016
Møller AP (1982) Clutch size in relation to nest size in the Swallow Hirundo rustica. Ibis 124:339–343
Møller AP (1987) Advantages and disadvantages of coloniality in the Swallow, Hirundo rustica. Anim Behav 35:819–832
Møller AP (1988) Female choice selects for male sexual tail ornaments in the monogamous Swallow. Nature 332:640–642
Møller AP (1993) Sexual selection in the Barn Swallow (Hirundo rustica). III. Female tail ornaments. Evolution 47:417–431
Møller AP (1994) Sexual selection and the Barn Swallow. Oxford University Press, Oxford
Møller AP, de Lope F (1999) Senescence in a short-lived migratory bird: age-dependent morphology, migration, reproduction and parasitism. J Anim Ecol 68:163–171
Møller AP, Pomiankowski A (1993) Why have birds got multiple sexual ornaments? Behav Ecol Sociobiol 32:167–176
Møller AP, Szép T (2005) Rapid evolutionary change in a secondary sexual character linked to climatic change. J Evol Biol 18:481–495
Møller AP, Thornhill R (1998) Male parental care, differential parental investment by females and sexual selection. Anim Behav 55:1507–1515
Møller AP, Chabi Y, Cuervo JJ, de Lope F, Kilpimaa J, Kose M, Matyjasiak P, Pap PJ, Saino N, Sakraoui R, Schifferli L, von Hirschheydt J (2006) An analysis of continent-wide patterns of sexual selection in a passerine bird. Evolution 60:856–868
Montgomerie R (2006) Analyzing colors. In: Hill GE, McGraw KJ (eds) Bird coloration, vol I. Mechanisms and measurements. Harvard University Press, Cambridge
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605
Ninni P (2003) Carotenoid signals in Barn Swallows. Ph.D. thesis, Université Pierre et Marie Curie
Norberg RA (1994) Swallow tail streamer is a mechanical device for self deflection of tail leading edge, enhancing aerodynamic efficiency and flight manoeuvrability. Proc R Soc Lond B 257:227–233
Odom KJ, Hall ML, Riebel K, Omland KE, Langmore NE (2014) Female song is widespread and ancestral in songbirds. Nat Commun 5:3379. doi:10.1038/ncomms4379
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. http://www.R-project.org/. Accessed 6 Mar 2016
Ringhorfer M, Hasegawa T (2014) Social cues are preferred over resource cues for breeding-site selection in Barn Swallows. J Ornithol 155:531–538
Roulin A (1999) Nonrandom pairing by male Barn Owls Tyto alba with respect to a female plumage trait. Behav Ecol 10:688–695
Roulin A, Altwegg R (2007) Breeding rate is associated with pheomelanism in male and with eumelanism in female Barn Owls. Behav Ecol 18:563–570
Roulin A, Riols C, Dijkstra C, Ducrest A-L (2001a) Female- and male-specific signals of quality in the Barn Owl. J Evol Biol 14:255–267
Roulin A, Riols C, Dijkstra C, Ducrest A-L (2001b) Female plumage spottiness and parasite resistance in the Barn Owl (Tyto alba). Behav Ecol 12:103–110
Ruxton GD (2006) The unequal variance t-test is an underused alternative to Student’s t-test and the Mann–Whitney U test. Behav Ecol 17:688–690
Safran RJ, McGraw KJ (2004) Plumage coloration, not length or symmetry of tail-streamers, is a sexually selected trait in North American Barn Swallows. Behav Ecol 15:455–461
Saino N, Ambrosini R, Martinelli R, Møller AP (2002) Mate fidelity, senescence in breeding performance and reproductive trade-offs in the Barn Swallow. J Anim Ecol 71:309–319
Saino N, Romano M, Romano A, Rubolini D, Ambrosini R, Caprioli M, Parolini M, Scandolara C, Bazzi G, Constanzo A (2015) White tail spots in breeding Barn Swallows Hirundo rustica signal body condition during winter moult. Ibis 157:722–730
Schluter D, Price T (1993) Honesty, perception and population divergence in sexually selected traits. Proc R Soc Lond B 253:117–122
Scordato ESA, Safran RJ (2014) Geographic variation in sexual selection and implications for speciation in the Barn Swallow. Avian Res 5:8
Sheldon BC (2000) Differential allocation: tests, mechanisms and implications. Trends Ecol Evol 15:397–402
Shields WM (1984) Factors affecting nest and site fidelity in Adirondack Barn Swallows (Hirundo rustica). Auk 101:780–789
Siefferman L, Hill GE (2005) Evidence for sexual selection on structural plumage coloration in female Eastern Bluebirds (Sialia sialis). Evolution 59:1819–1828
Soler JJ, Cuervo JJ, Møller AP, de Lope F (1998) Nest building is asexually selected behaviour in the Barn Swallow. Anim Behav 56:1435–1442
Suzuki H (1998) The breeding status of the Barn Swallow Hirundo rustica at Yahata River mouth Hiroshima. Strix 16:99–108 (in Japanese with English summary)
Tajima K, Nakamura M (2003) Response to manipulation of partner contribution: a handicapping experiment in the Barn Swallow. Ornithol Sci 2:65–72
Tazzyman SJ, Iwasa Y, Pomiankowski A (2014) Signaling efficacy drives the evolution of larger sexual ornaments by sexual selection. Evolution 68:216–229
Tobias JA, Montgomerie RD, Lyon BE (2012) The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Philos Trans R Soc B 367:2274–2293
Turner AK (2006) The Barn Swallow. Poyser, London
van Doorn GS, Weissing FJ (2004) The evolution of female preferences for multiple indicators of quality. Am Nat 164:173–186
Vortman Y, Lotem A, Dor R, Lovette IJ, Safran RJ (2011) The sexual signals of the East-Mediterranean Barn Swallow: a different Swallow tale. Behav Ecol 22:1344–1352
Vortman Y, Lotem A, Dor R, Lovette I, Safran RJ (2013) Multiple sexual signals and behavioral reproductive isolation in a diverging population. Am Nat 182:514–523
Vortman Y, Safran RJ, Reiner Brodetzki T, Dor R, Lotem A (2015) Expression of multiple sexual signals by fathers and sons in the East-Mediterranean Barn Swallow: are advertising strategies heritable? PLoS One 10:e0118054. doi:10.1371/journal.pone.0118054
Voss MA, Rutter MA, Zimmerman NG, Moll KM (2008) Adaptive value of thermally inefficient male incubation in Barn Swallows (Hirundo rustica). Auk 125:637–642
Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP (2006) Why do we still use stepwise modelling in ecology and behaviour? J Anim Ecol 75:1182–1189
Wilkins MR, Shizuka D, Joseph MB, Hubbard HK, Safran RJ (2015) Multimodal signalling in the North American Barn Swallow: a phenotype network approach. Proc R Soc Lond B 282:20151574
Yabusaki K, Faits T, McMullen E, Figueiredo JL, Aikawa M, Aikawa E (2014) A novel quantitative approach for eliminating sample-to-sample variation using a hue saturation value analysis software. PLoS One 9:e89627. doi:10.1371/journal.pone.0089627
Acknowledgments
We are grateful to the residents of Joetsu city for their kind support and assistance. We also thank the members of the Laboratory of Animal Ecology of Joetsu University of Education, and the Laboratory of Conservation Ecology of the University of Tsukuba, and anonymous referees. M. H. was supported by a Research Fellowship of the Japan Society for the Promotion of Science (15J10000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hasegawa, M., Arai, E., Watanabe, M. et al. Reproductive advantages of multiple female ornaments in the Asian Barn Swallow Hirundo rustica gutturalis . J Ornithol 158, 517–532 (2017). https://doi.org/10.1007/s10336-016-1401-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-016-1401-z