Abstract
Considerable debate surrounds the numerous avian-like traits in core maniraptorans (oviraptorosaurs, troodontids, and dromaeosaurs), especially in the Chinese Early Cretaceous oviraptorosaur Caudipteryx, which preserves modern avian pennaceous primary remiges attached to the manus, as is the case in modern birds. Was Caudipteryx derived from earth-bound theropod dinosaurs, which is the predominant view among palaeontologists, or was it secondarily flightless, with volant avians or theropods as ancestors (the neoflightless hypothesis), which is another popular, but minority view. The discovery here of an aerodynamic propatagium in several specimens provides new evidence that Caudipteryx (and hence oviraptorosaurs) represent secondarily derived flightless ground dwellers, whether of theropod or avian affinity, and that their presence and radiation during the Cretaceous may have been a factor in the apparent scarcity of many other large flightless birds during that period.
Zusammenfassung
Die „Neoflightless“-Hypothese im Test: Halsflughaut (Propatagium) offenbart flugfähige Vorfahren der Oviraptorosauria
Es gibt eine ausgiebige Debatte über die zahlreichen vogelähnlichen Eigenheiten der Maniraptora (Oviraptosaurus, Troodontidae, Dromaeosaurus), vor allem des (gefiederten) Oviraptorosauria Caudipteryx aus der frühen chinesischen Kreidezeit, der genau wie rezente Vögel Handschwingen hatte, die an den Handknochen ansetzen. Stammt Caudipteryx von den nur am Erdboden lebenden Theropoda ab - die unter den Paläontologen vorherrschende Meinung -, oder war er sekundär flugunfähig und stammte von flugfähigen Theropoden ab - die „Neoflightless“-Hypothese, eine alternative, wenn auch nur von Wenigen unterstützte These. Die hier berichtete Entdeckung einer aerodynamischen Halsflughaut bei einigen Exemplaren gibt neue Hinweise darauf, dass Caudipteryx (und damit auch Oviraptorosaurus) einen sekundär flugunfähigen Bodenbewohner darstellte, ganz gleich, ob er näher mit den Theropoden oder den Vögeln verwandt ist. Sein Vorkommen und seine Ausbreitung während der Kreidezeit war möglicherweise ein Faktor im offensichtlichen Mangel an anderen großen, flugunfähigen Vögeln während dieser Periode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the unveiling of the Chinese Lower Cretaceous Jehol Biota has provided invaluable new information on bird origins and opened a heretofore unknown window on a poorly known period of geologic time (Barrett and Hilton 2006). New fossil discoveries have led to massive reinterpretation of the entire field of both flight origins and the actual avian ancestors. The concept of Maniraptora, bird-like theropods, has dramatically changed in recent years to include both volant and flightless forms of dromaeosaurs, and most palaeontologists active in this field of research agree on the numerous apomorphies in common between birds and maniraptorans. There is also general agreement that the most bird-like maniraptorans, the so-called “core maniraptorans” [James and Pourtless 2009; the “Pennaraptora” of Foth et al. 2014), can be grouped in a clade comprising the oviraptorosaurs, troodontids, and dromaeosaurs. The existence of these very bird-like “theropods” is generally considered by palaeontologists to be the strongest possible evidence of a dinosaurian origin of birds. However, others contend that these taxa may be avians at all stages of flight and flightlessness (Fig. 1) and therefore represent the hidden birds of China, misidentified as dinosaurs (James and Pourtless 2009; Feduccia 2012). Following their discovery, dromaeosaurs were initially thought to be flightless non-avian dinosaurs ancestral to actual birds which eventually evolved the ability to fly; hence, according to this hypothesis, avian aerodynamic adaptations evolved by exaptations in earth-bound forms (Sereno 1999). The dromaeosaurs therefore appeared to represent feathered dinosaurs which were wingless ancestors of birds that were not yet capable of flight. However, with the discovery of fully volant basal dromaeosaurs, the microraptors, with a four-winged, tetrapteryx bauplan and avian pennaceous, asymmetric flight remiges, the concept of a dinosaurian trees-down model was introduced (Zhou and Zhang 2006; Chatterjee and Templin 2012). Yet, the large number of highly sophisticated avian characters in the oviraptorosaurs has been difficult to explain if they were derived from earth-bound dinosaurs.
Two major hypothesis for the origin of birds. Left the standard BMT (birds are maniraptoran theropods) hypothesis, which is the unchallengeable orthodoxy of today’s field of palaeontology. By this scenario birds are maniraptoran theropod dinosaurs, and the sister group of birds is the Deinonychosauria (or Dromaeosauridae). Right the hypothesis that core maniraptorans are birds, at varied stages of gliding, flight, and flightlessness. By this topology at least three clades of maniraptorans (Dromaeosauridae, Troodontidae, and Oviraptorosauria) were radiations within Aves, with members at varying stages of flight loss or flight. This scenario differs from the dinosaurian neoflightless hypothesis of Paul (2002), who advocated that these groups were secondarily flightless but derived from Theropoda. (Adapted from James and Pourtless 2009: Fig. 3)
Caudipteryx
Specimens of the two species of the turkey-size Caudipteryx come from the Yixian Formation, Lower Cretaceous (early Aptian age) of China, approximately 124.5 million years ago (Ma), specifically from the Jianshangou beds, near Zhangjiakou (Zhou and Wang 2000). Caudipteryx appears to have been common and lived sympatrically with another more primitive oviraptorosaur, Protarchaeopteryx, and two other feathered maniraptorans, Dilong and Sinornithosaurus (Xu and Norell 2006).
Caudipteryx (meaning tail feather) was featured as the first true “feathered dinosaur” on the cover of Nature (Ji et al. 1998). These “feathered dinosaurs” were spectacularly featured in scientific journals and the popular press, and afterwards Nature editor Henry Gee proclaimed: “The debate is over…” (Gee 1998). Since then some analyses have supported this view; however, various detailed cladistic and other analyses have argued for avian status of this enigmatic group (Elzanowski 1999; Jones et al. 2000; Maryańska et al. 2002; Lü et al. 2002; Feduccia 2012). Caudipteryx possesses a lengthy list of avian characters (Table 1) present in modern birds, including—but not limited to—an advanced avian phalangeal formula (2-3-2) and avian-like digital morphology and semilunate carpal (Zhou et al. 2000); a pygostyle and modern avian tail molt (Prum 2010); avian-like endocranial volume (Conchoraptor, and the basal Incisivosaurus) within the range of modern birds (Kundrát 2007; Balanoff et al. 2009); avian auditory anatomy (Kundrát and Janáček 2007); parental care as in living ratites (Kavanau 2010); pennaceous primary feathers attached to the manus as in modern volant birds. Elzanowski (1999) discovered four cranial synapomorphies shared by oviratorosaurs (Oviraptor) and ornithurine birds, but absent in the urvogel Archaeopteryx, which led him to suggest that oviraptorosaurs may have branched off after Archaeopteryx and therefore represent the earliest known flightless birds.
In addition, Caudipteryx (as well as Archaeopteryx) shows no definitive evidence of either a theropod supra-acetabular crest or an avian antitrochanter (Hertel and Campbell 2007). As with dromaeosaurs, the question to be addressed is whether a case of mistaken identity has been made in attributing Caudipteryx as a non-avian dinosaur when it is just as likely, if not more so, to be a secondarily flightless bird (Maryańska et al. 2002; James and Pourtless 2009; Feduccia 2012). Still others believe that maniraptorans evolved and lost flight independently of birds (Paul 2002; Mayr et al. 2005; Xu et al. 2011), so the question of avian versus theropodan affinity remains in debate.
We focus here on the discovery of an anatomical feature, the propatagium, which argues that Caudipteryx supports the neoflightless hypothesis—that is, it is derived from a flighted ancestry (Paul 2002)—and therefore its highly derived avian anatomy was selected for in an aerodynamic context.
Caudipteryx propatagium discovered
The presence of numerous flight features reveal that Caudipteryx, like the extant flightless ratites, originated from volant ancestors (de Beer 1956; Feduccia 2012, 2013), most likely via the evolutionary process of heterochrony, specifically paedomorphosis (arrested development), by which the adult retains the morphology of a younger stage of development (Livesey 1995). While a propatagium is ubiquitous in all extant volant avians, not all extant flightless birds have retained this feature. For example, ratites have far fewer wing muscles than carinates and the adult does not exhibit a propatagium, possibly due to the long length of time since they acquired the flightless feature; volant, closely allied paleognathous tinamous do possess a propatagium (McGowan 2009; Lowe 1928a). Ostrich (Struthio) does however have a patagial skin flap between the wrist and humerus, but lacks the normal propatagial tendons, so there is not a bona fide propatagium. However, all other studied extant flightless species do retain a propatagium, and the pectoral musculature of the New Zealand rail, the Weka (Gallirallus australis) is almost identical to living volant relatives (e.g., the coot, Fulica americana), including details of the propatagial complex (McGowan 2009). The same is true of other flightless carinates, from rails to ducks and cormorants (Lowe 1928b, 1934; authors’ personal observations).
In the avian propatagium the patagialis longus tendon forms the leading edge of the wing. This cambered membrane provides a large area of adjunct flight surface to the wing and is a very important aerodynamic feature of modern birds (Brown and Cogley 1996). These authors carried out experiments involving flight feather removal in living birds and computer modeling, both of which defined the contribution of the propatagium as a very significant aerodynamic component of the wing. The removal of secondary feathers, leaving six distal primaries and an intact propatagium, did not noticeably affect flight in house sparrows (Passer domesticus), and computer modeling revealed that the propatagium produced the majority of the lift. The results of their study led Brown and Cogley (1996) to conclude that the cambered propatagium is the major lift-generating component of the wing proximal to the wrist. The presence of a propatagium is inexplicable except as a flight adaptation, and its presence is considered a highly reliable, if not unequivocal, indicator of flight, as in the classic urvogel Archaeopteryx (Fig. 2) (Martin and Lim 2005), basal birds (Confuciusornis, Fig. 3; authors’ personal observations), volant maniraptorans (including the basal dromaeosaurid four-winged glider Microraptor; (Fig. 2; Xu et al. 2003; Hone et al. 2010), and the less derived but still volant putative troodontid, Anchiornis (Hu et al. 2009). The propatagium has also been documented in some Enantiornithes, such as Noguerornis (Chiappe and Ruiz 2002), and Sinornithosaurus, Anchiornis, Jeholornis, and Confuciusornis all exhibit a well-developed extensor process of the carpometacarpus (Paul 2002), which is an osteological correlate of the site of insertion of the propatagial tendons (Vasquez 1994), and most likely indicates a propatagium and a modern wing design in all these forms (Agnolín and Novas 2013).
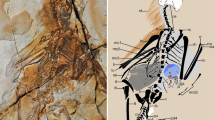
Top drawings of the protopatagium, shown by arrows (reconstructed from a new cast) of the right hand of the Berlin specimen of Archaeopteryx (left), compared to that of a modern duck Anas (right; drawn to same scale). Note that the propatagium constrains the extension of the forearm so the junction between the humerus and radius/ulna normally reflects the pattern of avian wing folding in avian fossils. Bottom left UV image of the left wing of Microraptor (IVPP no. 13354) showing the propatagium, preserved in much the same manner as in Caudipteryx; scale bar: 5 cm. Lower right right wing of the secondarily flightless New Zealand Weka (Gallirallus) showing internal muscular and tendonal anatomy related to the propatagium, especially the ligamentum propatagialis, which defines the area of the propatagium and forms much of the leading edge of the wing. (Top images adapted from Martin and Lim 2005; lower left image is from Hone et al. 2010; Weka wing is modified from McGowan 2009)
As noted, there is a very close adherence of the outer and middle fingers, providing support for anchoring primary feathers, which is also true for Microraptor and Confuciusornis. Although these forms as well as Caudipteryx are often restored with separated fingers engaged in some type of presumed theropodan predatory behavior (Ji et al. 1998; Hu et al. 2009; Padian and Chiappe 1998), such activity would not have been possible.
Another feature of the propatagium is that it constrains the extension of the forearm and, consequently, the junction between the humerus and the radius/ulna usually reflects the pattern of wing folding in fossil birds (Fig. 2; Martin and Lim 2005). It is significant to note that all specimens of Archaeopteryx preserve an avian folding pattern in at least one wing. The same is true for Caudipteryx specimens.
The skeletal flight anatomy in volant dromaeosaurs and the putative troodontid Anchiornis exhibits a mosaic variety of advanced flight characters, some more derived than equivalent traits in Archaeopteryx. The flight hand anatomy in Caudipteryx is much more derived, but as with modern flightless birds, the former has reduced the size of the forelimb in a heterochronic reversal which creates a false impression that it might be a non-avian dinosaur in the process of becoming more avian. Heterochrony in birds, and presumably dinosaurs, results in a disto-proximal attenuation of the forelimbs, producing shortened forelimbs with small reduced hands. To the contrary, the specimen of the Lower Cretaceous, basal Protarchaeopteryx does not preserve significant soft tissue—rather it exhibits wing proportions closely approximating those of modern volant birds such as the seriamids, which during the Cenozoic radiated as a group of large, flightless South American carnivores, the phorusrhacids. There is no compelling reason to assume Protarchaeopteryx was flightless.
Evidence from skeletal anatomy alone in many maniraptorans suggests avian affinity, and a propatagium has recently been discovered in the non-dinosaurian Scansoriopteryx, considered variously as a basal avian (Czerkas and Feduccia 2014) or a theropod (Zhang et al. 2002; Agnolin and Novas 2013). As interpreted here, the phylogenetic implications based on the propatagium in Scansoriopteryx suggests that the presence of propatagia should be expected in volant forms and to be very likely in secondarily flightless forms, such as Caudipteryx.
Our anatomical study of Caudipteryx revealed a derived manus which is remarkably similar to that of extant birds, with a visible outline of a propatagium, extending as in modern birds as a fibrous sheet of tissue from the shoulder to the wrist carpal elements (Fig. 4). While the physical evidence of a propatagium based on soft tissue anatomy may appear inconclusive, in Microraptor, Confuciusornis and Caudipteryx, and other taxa, the position and proximal and distal attachments of the observed structures are totally concordant with the conclusion that they are indeed patagial membranes, the only reasonable explanation. As seen with other fossil birds, in which some have varying degrees of feathers preserved, from highly detailed and unequivocal, to completely missing, the quality of preservation is always a factor to consider. However, despite various factors which may obscure the presence of patagia in fossils, the phyogenetic implication presented by Scansoriopteryx is that propatagia contributed to the avian wing from when the earliest known ancestors of birds first took to the air.
Upper photographs: left complete Caudipteryx specimen (LPM0005), with anterior pectoral region and wing with propatagium (box), right area enclosed in box magnified, with propatagium shaded in red. Lower photographs As for upper photographs but of a specimen from Beijing’s IVPP (V12340). The same area showing the propatagium is repeatedly preserved from the wrist and over the distal part of the radius (dark red) in at least three specimens (color figure online)
Discussion
The importance of a propatagium to the evolution of the avian wing is significant, as it has no apparent function other than contributing to the aerodynamics of the animal. Therefore, its presence in flightless forms lends support to the neoflightless hypothesis (Olshevsky 1992; Paul 2002; Feduccia 2012). The discovery of a propatagium in members of all clades of core maniraptorans, including Caudipteryx (oviraptorosaurs), Microraptor (dromaeosaurs), Anchiornis (a putative troodontid; Chatterjee and Templin 2012), Archaeopteryx (a basal urvogel; Martin and Lim 2005), and the basal avian Scansoriopteryx (Czerkas and Feduccia 2014), is additional evidence that flight was basal in Aves. Similarly, four-winged tetrapteryx wings can best be interpreted in a flight context. As Gong et al. (2012, p. 81) noted of the basal dromaeosaur Microraptor, “Microraptor hangingi is the key to understanding the evolutionary significance of hindlimb wings. A fourwinged structure present on an organism sharing an evolutionary lineage leading to modern birds implies that gliding was a stage in the development of avian flight.”
The recognition of avian characters in oviraptorosaurs goes back to Elzanowski (1999, p. 311) who concluded that: “cranial similarities between oviraptorosaurs and ornithurine birds raise the possibility that oviraptorosaurs are the earliest known flightless birds.” We believe evidence now supports the view that many maniraptorans look avian, despite their inability to fly, because they were derived from basal volant birds and had become secondarily flightless (Fig. 1). Highly derived avian characteristics, such as the reduction of the manual digits in Caudipteryx, are so strikingly similar to that in extant birds that to conclude that this resemblance is only an exaptation defies the logical simplicity that it might look avian because it is a bird.
The presence of a vestigial flight hand and a preserved propatagium in the Early Cretaceous flightless oviraptorosaur Caudipteryx parallels the same phenomenon in living ratites, ostrich, and allies (Palaeognathae). The radiation of large, non-volant oviraptorosaurs during the Cretaceous, as exemplified by such giant Late Cretaceous forms as Gigantoraptor (Mongolia) and Anzu (North America), may help explain the near absence of other large flightless birds, such as ratites, in the Mesozoic. The absence in the Cretaceous fossil record of numerous large secondarily flightless birds has been considered a complex unsolved mystery (Feduccia 2012). Aside from a handful of Late Cretaceous flightless land birds, such as the Patagonian Patagopteryx and the giant flightless non-ornithurine Gargantuavis (Buffetaut and Le Loeuff 1998; Feduccia 2012), there is little else. The highly specialized Late Cretaceous alvarezsaurids have variously been classified as birds (Altangerel et al. 1993) or theropods, but most recently the suggestion that they are primitive maniraptorans has appeared. The belief by Thomas Huxley that ratites were ancient “waifs and strays” of an ancient radiation cannot be substantiated, and there is no evidence for pre-Paleogene ratites (Feduccia 2014). The loss of flight is of such common occurrence within Aves that it should be expected to have occurred any time after flight was initially achieved.
The revelation that Maniraptora consists of volant and neoflightless types of birds resolves many of the problematic issues confronting the evolution of Aves and presents more viable alternative interpretations to answer the complexities of how dinosaurs are related. Recognizing that flightless members of Maniraptora are neoflightless may answer the question of where the secondarily flightless birds of the Mesozoic have been hiding. They have been there but have been invisible to our eyes because they have been misidentified for what they actually are.
References
Agnolín FL, Novas FE (2013) Avian ancestors: a review of the phylogenetic relationships of the theropods Unenlagiidae, Microraptoria, Anchiornis and Scansoriopterygidae. Springer Briefs in Earth Systems Sciences, Springer, Dordrecht, Heidelberg, Springer SBM
Altangerel P, Norell MA, Chiappe LM, Clark JM (1993) Flightless bird from the Cretaceous of Mangolia. Nature 262:623–626
Balanoff AM, Xu X, Kobayashi Y, Matsufune Y, Norell MA (2009) Cranial osteology of the theropod dinosaur Incisivosaurus gauthieri (Theropoda: Oviraptorosauria). Am Mus Novitates 3651:1–36
Barrett PM, Hilton JM (2006) The Jehol Biota (Lower Cretaceous, China): new discoveries and future prospects. Integr Zool 1:11–17
Brown RE, Cogley AC (1996) Contributions of the propatagium to avian flight. J Exp Zool 276:112–124
Buffetaut E, Le Loeuff J (1998) A new giant ground bird from the Upper Cretaceous of southern France. J Geol Soc London 155:1–4
Chatterjee S, Templin RJ (2012) Palaeoecology, aerodynamics, and the origin of avian flight. In: Talent JA (ed) Earth and life, international year of planet earth. Springer, New York, pp 585–612
Chiappe L, Lacasa-Ruiz J (2002) Noguerornis gonzalezi (Aves: Ornithothoraces) from the Early Cretaceous of Spain. In: Chiappe LM, Witmer L (eds) Mesozoic birds: above the heads of dinosaurs. University of California Press, Berkeley, pp 230–239
Czerkas SA, Feduccia A (2014) Jurassic archosaur is a non-dinosaurian bird. J Ornithol 155:841–851
de Beer G (1956) The evolution of ratites. Bull Brit Mus (Nat Hist) 4:59–70
Elzanowski A (1999) A comparison of the jaw skeleton in theropods and birds, with a description of the palate in the Oviraptoridae. Smithsonian Contr Paleobiol 89:311–323
Feduccia A (2012) Riddle of the feathered dragons. Yale University Press, New Haven
Feduccia A (2013) Bird origins anew. Auk 130:1–12
Feduccia A (2014) Avian extinction at the end of the Cretaceous: Assessing the magnitude and subsequent explosive radiation. Cret Res 50:1–15
Foth C, Tischlinger H, Rauhut OWM (2014) New specimen of Archaeopteryx provides insights into the evolution of pennaceous feathers. Nature 511(7507):79–82
Gee H (1998) Birds and dinosaurs—the debate is over. Nature News (online). doi:10.1038/news980702-8
Gong E-P, Martin LD, Burnham DA, Falk AF, L-h Hou (2012) A new species of Microraptor from the Jehol Biota of northeastern China. Palaeoworld 21:81–91
Hertel F, Campbell KE (2007) The antitrochanter or birds: form and function in balance. Auk 124:789–805
Hone DWE, Tischlinger H, Xing X, Zhang F (2010) The extent of the preserved feathers on the four-winged dinosaur Microraptor gui under ultraviolet light. PLoS One 5(2):e9223. doi:10.1371/journal.pone.0009223
Hu D, L-h Hou, Zhang L, Xu X (2009) A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature 461:640–643
James FC, Pourtless JA IV (2009) Cladistics and the origin of birds: a review and two new analyses. Orn Monogr 66:1–78
Ji Q, Currie PJ, Norell MA, Ji S-A (1998) Two feathered dinosaurs from northeastern China. Nature 393:753–761
Jones TD, Farlow JO, Ruben JA, Henderson DM, Hillenius WJ (2000) Cursoriality in bipedal archosaurs. Nature 406(6797):716–718
Kavanau JL (2010) Secondarily flightless birds or Cretaceous non-avian theropods. Med Hypotheses 74(2):275–276
Kundrát M (2007) Avian-like attributes of a virtual brain model of the oviraptorid theropod Conchoraptor gracilis. Naturwissenschaften 994:499–504
Kundrát M, Janáček J (2007) Cranial pneumatization and auditory perceptions of the oviraptorid dinosaur Conchoraptor gracilis (Theropoda, Maniraptora) from the Late Cretaceous of Mongolia. Naturwissenschaften 94:769–778
Livesey BC (1995) Heterochrony and the evolution of avian flightlessness. In: McNamara EJ (ed) Evolutionary change and heterochrony. John Wiley & Sons, New York, pp 169–193
Lowe PR (1928a) Studies and observations bearing on the phylogeny of the Ostrich and its allies. Proc Zool Soc London 1928:185–247
Lowe PR (1928b) A description of Atlantisia rogersi, the diminutive and flightless rail of Inaccessible Island (Southern Atlantic) with some notes on flightless rails. Ibis 1928:99–131
Lowe PR (1934) On the evidence of the existence of two species of Steamer Ducks (Tachyeres), and primary and secondary flightlessness in birds. Ibis 1934:467–495
Lü J, Dong Z, Azuma Y, Barsbold R, Tomida Y (2002) Oviraptorosaurs compared to birds. In: Zhou Z, Zhang F (eds) Proc 5th Symp soc avian paleontol evol. Science Press, Beijing, pp 175–189
Martin LD, Lim JD (2005) Soft body impression of the hand of Archaeopteryx. Curr Sci 89(7):1089–1090
Maryańska T, Osmólska H, Wolsan M (2002) Avialan status for Oviraptorosauria. Acta Palaeontol Polonica 47(1):97–116
Mayr G, Pohl B, Peters DS (2005) A well-preserved Archaeopteryx specimen with theropod features. Science 310:1483–1486
McGowan C (2009) The wing musculature of the Weka (Gallirallus australis) a flightless rail endemic to New Zealand. J Zool 210(3):305–346
Olshevsky G (1992) A revision of the parainfraclass Archosauria Cope, 1869, excluding the advanced Crocodylia. Mesozoic Meanderings 2:1–268
Padian K, Chiappe LM (1998) The origin of birds and their flight. Sci Am 278:38–47
Paul GS (2002) Dinosaurs of the air: The evolution and loss of flight in dinosaurs and birds. Johns Hopkins University Press, Baltimore
Prum RO (2010) Moulting tail feathers in a juvenile ovraptorsaur. Nature Rapid Commun 468:E1
Sereno PC (1999) The evolution of dinosaurs. Science 284:2137–2147
Vasquez RJ (1994) Functional osteology of avian wrist and evolution of flapping flight. J Morph 211:259–268
Xu X, Norell MA (2006) Non-avian dinosaur fossils from the Lower Cretaceous Jehol Group of western Liaoning, China. Geol J 41:419–437
Xu X, Zhou Z, Wang X, Kuang X, Zhang F, Du X (2003) Four-winged dinosaurs from China. Nature 421:335–340
Xu X, You H, Du K, Han F (2011) An Archaeopteryx-like theropod from China and the origin of Avialae. Nature 475:465–470
Zhang F, Zhou Z, Xu X, Wang X (2002) A juvenile coelurosaurian theropod from China indicates arboreal habits. Naturwissenschaften 89(9):394–398
Zhou Z, Wang X (2000) A new species of Caudipteryx from the Yixian Formation of Liaoning, northeast China. Vert Palasiatica 38(2):113–130
Zhou Z, Zhang F (2006) Mesozoic birds of China. Vert PalAsia 44(1):74–98
Zhou Z, Wang X, Zhang F, Xu X (2000) Important features of Caudipteryx—evidence from two nearly complete new specimens. Vert Palasiatica 38(4):241–254
Acknowledgments
We thank Sylvia J. Czerkas, Director of the Dinosaur Museum, for review and other important discussion. Zhonghe Zhou, Institute for Vertebrate Paleontology and Paleoanthropology, Beijing, identified a propatagium in a specimen of Caudipteryx in the collections of the IVPP and kindly provided useful discussion and photos of specimens under his care. We thank Ji Qiang of the Institute of Geology, Chinese Academy of Geological Sciences for help and discussion. The specimen on loan from the Institute of Geology has been returned to China. Susan Whitfield rendered the figures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Rights and permissions
About this article
Cite this article
Feduccia, A., Czerkas, S.A. Testing the neoflightless hypothesis: propatagium reveals flying ancestry of oviraptorosaurs. J Ornithol 156, 1067–1074 (2015). https://doi.org/10.1007/s10336-015-1190-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1190-9