Abstract
In the urbanized world, the diurnal cycle of light and darkness has lost its accuracy due to artificial light at night (LAN). Because light is one of the most important zeitgebers for the synchronization of the endogenous clock, this loss of the night has serious implications for health and activity patterns. Although it is a well-known phenomenon that LAN advances the onset of dawn song of passerines, little is known ABOUT whether birds extend their activity into the evening hours and THUS may benefit from exploiting the night light niche. By observing wild urban Blackbirds (Turdus merula) under different LAN intensities, we found birds exposed to high levels of LAN to forage longer in the evening than their conspecifics in the darker areas. This difference was most pronounced during the short days in March, but decreased steeply towards the summer solstice. However, body condition of the Blackbirds did not correlate with the exposure to LAN, indicating that urban birds extending their activity under LAN might not benefit from the prolonged foraging times. Our findings further indicate that male Blackbirds are more sensitive to LAN than females. This study reveals that LAN plays a considerable role in the activity times of urban Blackbirds but, regarding their body condition, other urban factors may be more important than the influence of LAN.
Zusammenfassung
Nutze die Nacht: Amseln (Turdus merula) verlängern ihre Nahrungssuche unter künstlichem Nachlicht
Der tägliche Wechsel zwischen Hell und Dunkel hat in der urbanisierten Welt durch das künstliche Nachtlicht seine Präzision verloren. Da Licht einer der wichtigsten Zeitgeber der inneren Uhr ist, hat dieser Verlust der Nacht weitreichende Auswirkungen auf Gesundheit und Aktivitätsmuster. Der unter künstlichem Licht früher einsetzende Morgengesang von Singvögeln ist ein gut untersuchtes Phänomen, jedoch ist nur wenig darüber bekannt, ob Vögel ihre Aktivität auch in die Abendstunden ausweiten und so von der Nachlicht-Nische profitieren können. Wir beobachteten frei lebende Amseln unter verschiedenen Lichtintensitäten und fanden, dass sie bei hohen nächtlichen Lichtintensitäten ihre Nahrungssuche stärker in die Abendstunden ausweiteten als Artgenossen im dunkleren Gebieten. Dieser Unterschied war während der kurzen Tage im März besonders ausgeprägt, nahm aber zum Sommer hin stark ab. Einen Zusammenhang zwischen der Körperkondition der Vögel und der nächtlichen Lichtintensität konnten wir nicht finden, was darauf hinweist, dass Vögel mit lichtbedingt längeren Aktivitätszeiten nicht von der verlängerten Nahrungssuche profitieren. Darüber hinaus scheinen Amselhähne sensibler als Amselhennen auf künstliches Nachtlicht zu reagieren. Diese Studie deutet darauf hin, dass künstliches Nachtlicht einen bedeutenden Einfluss auf die Aktivitätszeiten von städtischen Amseln hat, aber hinsichtlich der Körperkondition andere urbane Faktoren eine wichtigere Rolle als künstliches Nachtlicht haben.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life on earth evolved with daily alternation of sunlight and darkness and its fluctuations between the seasons (Bradshaw and Holzapfel 2007). The circadian rhythmic of endogenous biological clocks and their adjustment to the external environment allow organisms to optimally time their activity and biological processes (Navara and Nelson 2007; Dawson et al. 2001). Entrainment of the circadian clock is ensured by synchronizing with environmental cues, so-called zeitgebers, of which light acts as the most important (Aschoff 1954). However, with the rapid proliferation of electric light, the natural light signal has lost its accuracy. Since the world is becoming increasingly urban, we continuously spoil the natural night with artificial illumination far beyond the daytime. Artificial light at night (LAN) scatters through the atmosphere and, in some areas, causes sky brightness to exceed that of full moon nights (Cinzano et al. 2001; Kyba et al. 2011). Consequently, the darkness of night is decreased to a persistent twilight. This loss of the night has serious consequences for almost all groups of animals, including humans (Navara and Nelson 2007). Possible consequences range from misalignment of biological processes such as physiology and behavior, disorientation of organisms, and habitat loss due to avoidance of LAN (Longcore and Rich 2004). On the other hand, only a few species seem to benefit from LAN either by preying upon aggregations of insects at artificial lights (Frank 1988; Garber 1978) or by extending their foraging activity into the night to meet their energy requirements (Byrkjedal et al. 2012; Dwyer et al. 2012; Hötker 1999). However, this temporal or spatial shift in predator activity to exploit the night light niche may lead to changes of competitive communities and disruption of predator–prey interactions (Longcore and Rich 2004; Schwartz and Henderson 1991).
In cities, the areas most affected by LAN, organisms encounter further challenges. Compared to less disturbed habitats, the urban environment challenges urbanites by a modified habitat structure, a different microclimate, and novel food resources. Furthermore, vehicles, guy wires, and glass panes pose collision risks, and urban noise masks acoustic communication (reviewed in Marzluff 2001). To cope with the new environment, urban organisms show traits both in behavior, physiology, and life history which diverge from those of their rural conspecifics. A well-studied example is the European Blackbird (Turdus merula), which has developed in Europe from a previous forest species to a common city dweller in just two centuries (Luniak et al. 1990). Urban Blackbirds are tamer, breed in higher densities, increase their sedentariness, and advance their reproductive period by up to 3 weeks. These adaptations have been developed because of the more favorable conditions in the city, such as the permanent food availability and milder climate (Dominoni et al. 2013a; Luniak and Mulsow 1988; Partecke and Gwinner 2007; Partecke et al. 2005).
Another striking adaptation to city life is the advanced dawn song of urban passerines, which has been attributed to both LAN and anthropogenic noise (Fuller et al. 2007; Miller 2006; Nordt and Klenke 2013; Kempenaers et al. 2010; Arroyo-Solís et al. 2013; Bergen and Abs 1997). Urban Blackbirds start their morning song up to 5 hours before their conspecifics in the forest (Nordt and Klenke 2013). Surprisingly, in another study, Blackbirds cease their activity more or less at the same time in the evening, irrespective of their origin (Dominoni et al. 2013b). If urban conditions drive Blackbirds to extend their morning activity into the night, why is there no effect in the evening, despite, presumably, similar conditions?
Here, we investigate whether urban Blackbirds can extend their foraging activity under LAN in the evening and whether this correlates with differences in the body condition of urban and rural birds.
Methods
Study area and artificial night light
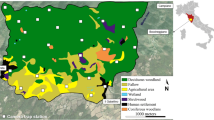
The study was carried out in the city of Leipzig, Germany (51°20′N, 12°25′E). The city is characterized by a densely built-up center and a riparian forest which crosses the center in a north–south direction (Strohbach et al. 2009). The riparian forest and several parks cause natural, semi-natural, and urbanized habitats to occur in close vicinity. The study was conducted along the whole gradient of habitats: Blackbirds were caught in the forest, the parks, and the center, while activity observations took place at four different observation sites in the forest and the center (Fig. 1). Two close-by observation sites were situated at the edge of an urban forest and two further sites in a recreation area in the city center. The sites were chosen so as to maximize the difference in artificial night light but also to resemble each other in habitat characteristics, size, and the amount of human disturbance. All observation sites were between 0.2 and 0.3 ha in size and comprised of an open lawn with few deciduous bushes and trees.
To quantify the artificial night light, we used data of municipal illumination (e.g., street lamps and spotlights of historical buildings) as a proxy, because street lighting is the largest single contributor to urban artificial night light (Kuechly et al. 2012). Light point data were kindly provided by the traffic and works service of Leipzig. We used a kernel density estimation to calculate an index of street lamp density from light point data with a search radius of 50 m and a resolution of 10 m (ArcGIS 10, ESRI). In an earlier study (Nordt and Klenke 2013), the accuracy of this method was validated by measuring the illuminance at 100 randomly chosen points with a luxmeter (resolution 0.01 lx; LM 37, TFA Dostmann). Measured illumination and the calculated indices of the same points correlated significantly (Pearson’s r = 0.629, df = 98, P < 0.001). The index of street lamp density was, thus, regarded as an accurate indicator of artificial night light and is further referred to as lamp density. For every capture site, the value of the lamp density index was extracted and related to the individual bird caught at this site. For every observation site, we obtained a mean value of the lamp density. To facilitate comparability to other studies, we provide the corresponding illuminance values (in lux) calculated from the correlation between the lamp density index and the illuminance measurements.
End of foraging activity
The end of the foraging activity of Blackbirds was investigated on 35 survey days between March and July. Observations started approximately 30 min before sunset. All Blackbirds foraging at the site were counted by sex. If upon arrival at the observation site no Blackbird appeared for 5 min, the close-by alternative site was used. On all occasions, birds were found on at least one of the alternative sites. Following Stephan (1999), handling food items and different modes of behavior were regarded as foraging activities: (1) digging or pecking for food items on the ground, (2) hurling aside foliage, and (3) observing the surface with the head cocked. To account for different bird densities per observation day and site, we counted the maximum number of Blackbirds foraging at the observation site per observation day (bird count). When the last bird stopped foraging and left the site, its sex and the time were noted. The cloud cover in oktas was estimated every 10 min during the observation. As it did not change by more than one okta during the observation, we used the final estimate for further analyses. Foraging activity was considered to have ceased if no Blackbird returned for at least 10 min and observations subsequently ended. The time of the end of the foraging activity (end time) was stated in minutes after sunset with positive values, or negative values if foraging activity ended before sunset, respectively. This relative end time still showed a considerable non-linear relationship with the day of the observation (Julian date). To handle this nonlinearity, we applied a general additive mixed model (GAMM) in the mgcv package (Wood 2004) of the R software system (R Development Core Team 2013). The initial model included the end time as response variable, the habitat (center vs. forest) and the cloud cover as fixed factors, the maximum bird count as linear covariate, and the Julian date as a thin plate regression spline smoother, one for each level of habitat. Furthermore, the full model included the two-way interaction between cloud cover and habitat. To account for the dependency of repeated observations at the same observation sites, we included the site as a random effect.
To determine whether there is a tendency for males or females to forage under lower light conditions, we tested if the probability of males to be the last bird leaving the observation site is different from the sex ratio of Blackbirds foraging at the sites using an exact binomial test of goodness-of-fit.
Body condition
In total, 217 free-living adult Blackbirds were captured with mistnets (Ecotone 719/6) in the different habitats of the city (center, park, forest; Fig. 1). To account for the dependency of recaptures, we included only data of recaptures when capture dates differed by at least 3 months. This reduced the sample size to 240 captures (217 first captures, 20 recaptures). The birds were marked with an individual metal ring on the right leg and an alphanumeric coded color ring (Interrex) on the left leg. Birds were weighed in a weighing cone to the nearest 0.1 g using a digital balance (Kern TCB 200-1), and the tarsus length was measured to the nearest 0.1 mm with a digital measuring slide (Wiha 29422 DigiMax). All measurements were taken by the same individual who carried out the ringing. The birds were released at the site of capture immediately after handling. A body condition index was calculated as residuals from an ordinary least-squares linear regression between body mass and tarsus length for males and females separately (Schulte-Hostedde et al. 2005). We analyzed whether the body condition of Blackbirds varied between capture sites by fitting a General Linear Model (GLM). The initial model included the explanatory variables of year, habitat, sex, and season (capture date) as factors and the time of capture and lamp density as linear covariates. The season was included as a three-level factor to differentiate between pre-breeding (1 February–14 April), breeding (15 April–31 July), and winter (1 October–31 January) because the body weight and consequently the body condition changes non-linearly over the year (Stephan 1999). No birds were captured in August and September in general, or during winter in the urban forest. To separate the influence of capture time from the season, the capture time was divided by the length of day. The length of day was defined to start with morning civil twilight (when the sun is 6° below the horizon) and to end with evening civil twilight when the sun is, again, 6° below the horizon. The full model included the two-way interactions between habitat, lamp density, season, sex, and time of capture, respectively.
Further statistics and model validation
Model selection for both GLMs and GAMMs were based on an Akaike’s Information Criterion corrected for small sample sizes (AICc), an information-theoretical approach (Burnham and Anderson 2002). Furthermore, we assessed standard model validation graphs to verify homogeneity, normality, and independence of residuals (Zuur et al. 2009). All models with ΔAICc < 4 of the respective best model were assumed to have considerably support and Akaike weights (ω) were assigned accordingly (Burnham and Anderson 2002). Detailed results are reported for the best model only. Homogeneity of variances was verified by Levene’s tests (all P > 0.05). Levels of significant factors of the body condition analysis were compared in post hoc tests with Tukey’s comparisons and Bonferroni corrections using the multcomp package (Hothorn et al. 2008). Means are given with standard deviation.
Results
The three parts of the study area differed significantly in their amount of artificial night light (ANOVA, F 2,21670 = 9979.3, P < 0.001). On average, the center, parks, and forest were illuminated by 12.8 ± 11.5, 5.4 ± 3.9, and 0.1 ± 0.8 street lights per ha, respectively. These lamp densities correspond to 0.44 ± 0.36, 0.15 ± 0.12, and 0.07 ± 0.07 lux for the center, park, and forest, respectively. Accordingly, the observation sites differed significantly in their amount of artificial night light (ANOVA, F 1,2 = 377.6, P < 0.001; Fig. 2). The observation sites in the center were illuminated by 5.6 street lights/ha, whereas the sites at the forest were lit by 0.2 street lights/ha, on average, which corresponds to 0.15 and 0.07 lux, respectively.
End of activity
Blackbirds ceased their foraging activity on average 18.7 ± 16.7 min after sunset. Depending on the habitat, the cloud cover, the number of birds, and the season, there were significant differences in the end time (Online Resource; Table 1). Especially, the habitat was correlated with an extension of the foraging activity of Blackbirds into the night. At the center sites, the Blackbirds foraged significantly longer than at the forest sites. This observation can be dedicated to the high amount of artificial night light at the center (Fig. 2), because the observation sites where as similar as possible in other respects. Furthermore, the cloud cover had a significant negative effect on the end time, reducing the time used for foraging by almost 3 min per okta cloud cover. However, forest birds ceased their activity on average 4 min later at a given cloud cover. Apart from the environmental effects, the intraspecific aspect of the quantity of birds further influenced the end time. With every additional bird, the end time was delayed by 3 min. After controlling for other effects, the end of the foraging activity showed significantly different patterns over the study period for Blackbirds at the two habitats (Fig. 3). At the beginning of the observation period, forest birds ended foraging almost 50 min earlier than birds in the center. In the time span of 1 month, this difference shrank to a few minutes. Afterwards, forest birds continued to leave the foraging sites earlier than center birds in general, but the variability was high and differences were marginal. Towards the end of the observation period, birds in both habitats ceased their foraging activity considerably earlier than in May and the beginning of June.
In total, approximately 10 % more males than females foraged at the observation sites. Although the last bird to leave was twice as often a male than a female, this distribution did not significantly deviate from the observed sex ratio of 1:0.81 (males: females, χ 2 = 2.80, P = 0.094). However, in the center, the sex ratio was slightly shifted towards more females (1:1.10), but the last bird leaving the foraging site was significantly more often a male than a female (χ 2 = 4.78, P = 0.029, Fig. 4). In the forest, the probability of the sex of the last bird was equal to the distribution of the sexes among the foraging birds of 1:0.54 (χ 2 = 0.003, P = 0.987).
Body condition analysis
The body condition index of Blackbirds varied significantly over the seasons from −12.8 to 22.7 (0.06 ± 5.75) (F 2,235 = 4.32, P = 0.014) and between the three habitats (F 2,235 = 4.96, P = 0.008; Fig. 5). The best model consisted only of these two explanatory variables to describe the variation in the body condition (Online Resource), but a model further adding the lamp density also received support. However, the body condition did not differ significantly with the lamp density. In general, birds in the city center had a higher body condition score than their conspecifics in the forest (β Center–Forest = 2.8 ± 0.9, t = −3.04, P = 0.007). Body condition scores of birds in the park were intermediate and did not differ significantly from those in the forest (β Park–Forest = 0.8 ± 0.9, t = −0.88, P = 0.653), and only marginally non-significant from those in the center (β Park–Center = −2.0 ± 0.9, t = −2.28, P = 0.060). The comparisons between seasons revealed that, during winter, birds had the highest body condition scores which differed significantly from the pre-breeding and breeding period. The difference in body condition between winter and pre-breeding was −4.56 (t = −2.42, P = 0.040) and −4.51 (t = −2.55, P = 0.028) between winter and breeding, respectively. Body conditions between pre-breeding and breeding periods did not differ significantly (t = 0.05, P = 0.998).
Variation of the body condition of Blackbirds over the seasons in the three different habitats of the study area. Numbers below the boxes indicate the sample size per group. No birds were caught in winter in the forest. Asterisks differences at the 0.05 significance level in the body condition between the connected groups of Blackbirds
Considering the season and habitat together, only the body condition scores of breeding center and forest birds differed significantly (z = 2.71, P = 0.042). Center birds had, on average, a 2.68 ± 0.99 higher body condition score during breeding (Fig. 4). Despite tendencies that body condition of birds varied between habitats during the seasons, no other tested comparison reached statistical significance (pre-breeding: Forest vs. Center: −4.23 ± 2.85, z = 1.48, P = 0.570; Center vs. Park: 2.23 ± 1.73, z = 1.29, P = 0.707; Forest vs. Park: −2.00 ± 2.86, z = 0.70, P = 0.970; breeding: Center vs. Park: 2.10 ± 1.05, z = 2.01, P = 0.241; Forest vs. Park: −0.58 ± 0.96, z = −0.60, P = 0.985; winter: Centre vs. Park: −2.44 ± 3.23, z = −0.75, P = 0.959). As the interaction between season and habitat did not reach statistical significance, it was excluded from the final model.
Discussion
Our study reveals that European Blackbirds, a successful urban species, extend their foraging activity into the night under urban conditions, which is most probably due to the different amount of artificial night light. The difference in the end time of foraging activity between Blackbirds at the center (illuminated) and the forest (dark) is highest in March and decreases considerably by the middle of April. This might be due to the short daytime in winter and early spring which restricts the time available for foraging. This hypothesis is also supported by a comparative study of the time budgets of Great Tits (Parus major) at three different latitudes during early breeding (Mace 1989). Arctic Great Tits had more time available for foraging and ceased their activity at higher light densities. In contrast, Tits at low latitudes spent less time foraging and roosted later with respect to twilight, indicating that they used the full capacity of the shorter day length, which was obviously a limiting factor. Consequently, prolonging the activity time due to artificial night light might be beneficial during seasons of short day lengths. Actually, some passerines overwintering at boreal latitudes were found to extend their daily activity by 4–5 h under artificial night light (Byrkjedal et al. 2012). However, the observed prolongation was achieved by starting activity long before the morning civil twilight, whereas activity ceased relatively homogenously with the evening twilight in all investigated bird species (Byrkjedal et al. 2012). Similarly, center Blackbirds in Leipzig were active during the early morning hours long before daybreak which was dedicated to LAN and anthropogenic noise (Nordt and Klenke 2013). During that study, we frequently observed Blackbirds foraging on lawns illuminated only by LAN after their first peak of dawn song (personal observation) which indicates that the light intensity of LAN is sufficient for Blackbirds to forage.
In contrast to the situation during the early breeding season, in May and June there is only marginally longer evening foraging activity of center Blackbirds which further coincides with a steep decrease of the difference in onset times of activity at dawn between forest and center birds (Nordt and Klenke 2013). At our latitudes, the long days around the summer solstice provide approximately 16 h of sunlight per day which do not restrict the time available for foraging. This is in line with other studies on Blackbirds comparing the activity times of rural and urban birds in southern Germany (Dominoni et al. 2013b, 2014). They found a slightly longer but non-significant evening activity (6 min) of urban birds during the peak of breeding in May and June. However, urban birds were active longer, for 40 min per day, when compared to forest birds, again due to an earlier onset of activity of urban birds. An in-depth examination of the activity times revealed that light intensities and weather conditions influence the onset of dawn activity, but none of these significantly affect the end of dusk activity (Dominoni et al. 2014). Nevertheless, site characteristics other than light intensities were found to explain some of the variation in the end of activity of urban birds, and, later in the season, rural Blackbirds cease their activity earlier with respect to twilight as was also observed in our study.
In the present study, center birds had significantly higher body condition scores than forest birds only during the breeding season, but not during the time when the difference in activity times was most pronounced. Accordingly, we discovered no effect of lamp density on the body condition, only habitat differences and the seasonal variation had a significant influence (Online Resource). That body condition varies over the seasons is well known (Stephan 1999). Macleod et al. (2005), for example, argued that a bird’s body mass is optimized according to the theory of starvation/predation risk trade-off. The theory predicts body mass to be highest in winter to reduce the starvation risk when food resources and environmental factors are less predictable. The starvation risk becomes less severe during breeding but predator avoidance increases, and therefore, body mass declines to enhance maneuverability and allows for a faster escape (Macleod et al. 2005). These predicted variations in body condition coincide with the seasonal variation in our data, but different reasons might trigger this pattern in center and forest Blackbirds. If the predation risk is not equal between the habitats but lower in urban areas (Anderies et al. 2007), the pressure to reduce body weight might be less severe and, hence, explain the higher body condition scores of center birds during breeding. That center birds, nevertheless, lose weight towards the breeding season might either be the consequence of reproductive costs (Stearns 1976) or an adaptation to reduce energetic costs especially during chick provisioning (Freed 1981). Furthermore, the starvation/predation risk trade-off assumes that food availability varies over the season, but this is rarely measured, including in our study. Nevertheless, several authors proposed that the urban environment has a higher food availability and predictability (Batten 1978; Marzluff 2001; Shochat 2004). Therefore, urban factors such as food availability and reduced predation risk may be more important for the bird’s body weight than the influence of LAN, as the Blackbirds seem to take no advantage of the extra amount of time spent foraging. However, we need to admit that our observation is based on a very low sample size in winter.
If the extra amount of time spent foraging under LAN is not advantageous in terms of body condition, why do birds chose to be active during this time? One possible reason may be the role that light plays as physiological zeitgeber. Under natural conditions, the light–dark-cycle gives the signal to be active (light) or rest (dark). In urbanized areas, light or rather LAN is ubiquitous, hence the signal is always set to activity which could result in faster, free-running circadian clocks (Kumar et al. 2000; Dominoni et al. 2013b). The daily sunlight, which is approximately 1,000- to 40,000-fold brighter than LAN, usually re-phases the endogenous clock day by day. Nevertheless, the continuous light in the city may coerce birds to be active during the night. Physical constraints to rest from time to time may explain why they are inactive for a certain amount of time during the night despite the continuous light (Hoffmann 1959). These constraints intensify with decreasing night length, and consequently the time active during the night shortens toward the summer solstice. Birds are more active in the early morning hours due to intraspecific social reasons. Singing early is important for male passerines to defend their territory and to demonstrate their quality (Murphy et al. 2008; Poesel et al. 2006). Therefore, the intensity of this display is high in the early spring but decreases in the course of the breeding period (Cuthill and Macdonald 1990; Glutz von Blotzheim and Bauer 1988).
This hypothesis is also supported by the model indicating the habitat, which differs significantly by the amount of LAN to be an important factor in predicting the end time of Blackbird foraging activity. Furthermore, in 75 % of the observations at the center, male Blackbirds were the last to leave the foraging site despite an almost equal sex ratio. Males are approximately 4 % larger in body size than females (Glutz von Blotzheim and Bauer 1988) and have slightly larger eyes. Because eye size is positively correlated with visual capabilities (Ockendon et al. 2009; Thomas et al. 2002), males should be more sensitive to light, especially at low light intensities. Hence, the longer activity of males may also be caused by the intensity of artificial night light.
Surprisingly, forest birds ended their foraging activity in March before sunset, which indicates that (1) foraging time is not a limiting factor during the relatively short days and can, thus, be used otherwise, in this case probably for singing and indicating quality, or that (2) the length of daytime is limited in the forest by the low incidence angle of the sun during March and causes an even earlier decrease in natural light conditions making foraging less profitable, while in the center the sky brightness is still high due to LAN and permits continued foraging. This is further supported by Davies et al. (2013) who reported that the brightening effect of LAN on the night sky is more pronounced in winter than in summer.
However, there might be confounding effects due to our study design. We observed Blackbirds only at the foraging site, but did not follow them after they left. Information about birds’ behavior after leaving the foraging site is limited, although observations suggest that especially males continued with extensive song before proceeding to the night roost. Nevertheless, we cannot exclude the possibility that foraging was continued out of the observers’ sight in the shrubbery or understory of the forest. Although Blackbirds use visual and acoustic cues to locate food (Stephan 1999) and, thus, depend on a certain amount of light to forage, it seems unlikely that birds continued foraging hidden in dense vegetation, where less light permeates through a vegetation cover instead of using open areas during the retreat of sun light.
In conclusion, our study revealed that artificial light at night plays a considerable role in the activity times of urban Blackbirds. It enables birds to extend their daily activity but this effect diminishes with increasing day lengths. In contrast to former expectations, urban Blackbirds seem not to rely on the extra amount of light and, hence, time available for foraging to improve their body condition. Other urban factors such as food availability and reduced predation risk may be more important than the influence of LAN.
References
Anderies JM, Katti M, Shochat E (2007) Living in the city: resource availability, predation, and bird population dynamics in urban areas. J Theor Biol 247(1):36–49. doi:10.1016/j.jtbi.2007.01.030
Arroyo-Solís A, Castillo JM, Figueroa E, López-Sánchez JL, Slabbekoorn H (2013) Experimental evidence for an impact of anthropogenic noise on dawn chorus timing in urban birds. J Avian Biol 44(3):288–296. doi:10.1111/j.1600-048X.2012.05796.x
Aschoff J (1954) Zeitgeber der tierischen Tagesperiodik. Naturwissenschaften 41(3):49–56. doi:10.1007/BF00634164
Batten LA (1978) Seasonal distribution of recoveries and causes of blackbird mortality. Bird Study 25(1):23–32
Bergen F, Abs M (1997) Verhaltensökologische Studie zur Gesangsaktivität von Blaumeise (Parus caeruleus), Kohlmeise (Parus major) und Buchfink (Fringilla coelebs) in einer Großstadt. J Ornithol 138(4):451–467. doi:10.1007/bf01651380
Bradshaw WE, Holzapfel CM (2007) Evolution of animal photoperiodism. Annu Rev Ecol Evol Syst 38:1–25. doi:10.1146/annurev.ecolsys.37.091305.110115
Burnham KP, Anderson DR (2002) Model selection and multimodel interference: a practical information-theoretic approach, 2nd edn. Springer, New York
Byrkjedal I, Lislevand T, Vogler S (2012) Do passerine birds utilise artificial light to prolong their diurnal activity during winter at northern latitudes? Ornis Norveg 35:37–42
Cinzano P, Falchi F, Elvidge CD (2001) The first World Atlas of the artificial night sky brightness. Mon Not R Astron Soc 328(3):689–707. doi:10.1046/j.1365-8711.2001.04882.x
Cuthill IC, Macdonald WA (1990) Experimental manipulation of the dawn and dusk chorus in the blackbird Turdus merula. Behav Ecol Sociobiol 26(3):209–216
Davies TW, Bennie J, Inger R, Gaston KJ (2013) Artificial light alters natural regimes of night-time sky brightness. Sci Rep 3. doi:10.1038/srep01722
Dawson A, King VM, Bentley GE, Ball GF (2001) Photoperiodic control of seasonality in birds. J Biol Rhythms 16(4):365–380. doi:10.1177/074873001129002079
Dominoni D, Quetting M, Partecke J (2013a) Artificial light at night advances avian reproductive physiology. Proc R Soc Lond B 280 (1756):20123017. doi:10.1098/rspb.2012.3017
Dominoni DM, Helm B, Lehmann M, Dowse HB, Partecke J (2013b) Clocks for the city: circadian differences between forest and city songbirds. Proc R Soc Lond B 280 (1763):20130593. doi:10.1098/rspb.2013.0593
Dominoni DM, Carmona-Wagner EO, Hofmann M, Kranstauber B, Partecke J (2014) Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. J Anim Ecol 83(3):681–692. doi:10.1111/1365-2656.12150
Dwyer RG, Bearhop S, Campbell HA, Bryant DM (2012) Shedding light on light: benefits of anthropogenic illumination to a nocturnally foraging shorebird. J Anim Ecol 82(2):478–485. doi:10.1111/1365-2656.12012
Frank KD (1988) Impact of outdoor lighting on moths: an assessment. J Lepidopterists Soc 42(2):63–93
Freed LA (1981) Loss of mass in breeding wrens-stress or adaptation. Ecology 62(5):1179–1186. doi:10.2307/1937282
Fuller RA, Warren PH, Gaston KJ (2007) Daytime noise predicts nocturnal singing in urban robins. Biol Lett 3(4):368–370. doi:10.1098/rsbl.2007.0134
Garber SD (1978) Opportunistic feeding behavior of Anolis cristatellus (Iguanidae: Reptilia) in Puerto Rico. Trans Kans Acad Sci 81:79–80
Glutz von Blotzheim U, Bauer KM (1988) Handbuch der Vögel Mitteleuropas. Aula, Wiesbaden
Hoffmann K (1959) Über den Tagesrhythmus der Singvögel im arktischen Sommer. J Ornithol 100(1):84–89. doi:10.1007/bf01671316
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50(3):346–363
Hötker H (1999) What determines the time-activity budgets of Avocets (Recurvirostra avosetta)? J Ornithol 140(1):57–71. doi:10.1007/bf02462089
Kempenaers B, Borgström P, Loes P, Schlicht E, Valcu M (2010) Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr Biol 20:1735–1739. doi:10.1016/j.cub.2010.08.028
Kuechly HU, Kyba CCM, Ruhtz T, Lindemann C, Wolter C, Fischer J, Hölker F (2012) Aerial survey and spatial analysis of sources of light pollution in Berlin, Germany. Remote Sens Environ 126:39–50
Kumar V, Gwinner E, Van’t Hof TJ (2000) Circadian rhythms of melatonin in European starlings exposed to different lighting conditions: relationship with locomotor and feeding rhythms. J Comp Physiol A 186(2):205–215. doi:10.1007/s003590050020
Kyba CCM, Ruhtz T, Fischer J, Hölker F (2011) Cloud coverage acts as an amplifier for ecological light pollution in urban ecosystems. PLoS ONE 6(3):e17307. doi:10.1371/journal.pone.0017307
Longcore T, Rich C (2004) Ecological light pollution. Front Ecol Environ 2(4):191–198. doi:10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2
Luniak M, Mulsow R, Walasz K Urbanization of the European Blackbird - expansion and adaptations of urban population. In: Luniak M (ed) Urban ecological studies in Central and Eastern Europe; international symposium, Warsaw, Poland, 1990. Polish Academy of Sciences, Warsaw, pp 187–198
Luniak M, Mulsow R (1988) Ecological parameters in urbanization of the European Blackbird. Acta Congr Int Ornithol 19:1787–1793
Mace R (1989) A comparison of great tits (Parus major) use of time in different daylengths at 3 European sites. J Anim Ecol 58(1):143–151
Macleod R, Barnett P, Clark JA, Cresswell W (2005) Body mass change strategies in blackbirds Turdus merula: the starvation–predation risk trade-off. J Anim Ecol 74(2):292–302. doi:10.1111/j.1365-2656.2005.00923.x
Marzluff JM (2001) Worldwide urbanization and its effect on birds. In: Marzluff JM, Bowman R, Donnelly R (eds) Avian Ecology and Conservation in an Urbanizing World. Kluwer, Norwell, pp 19–47
Miller MW (2006) Apparent effects of light pollution on singing behavior of American robins. Condor 108(1):130–139
Murphy MT, Sexton K, Dolan AC, Redmond LJ (2008) Dawn song of the eastern kingbird: an honest signal of male quality? Anim Behav 75(3):1075–1084. doi:10.1016/j.anbehav.2007.08.020
Navara KJ, Nelson RJ (2007) The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res 43(3):215–224. doi:10.1111/j.1600-079X.2007.00473.x
Nordt A, Klenke R (2013) Sleepless in town–drivers of the temporal shift in dawn song in urban European blackbirds. PLoS ONE 8(8):e71476. doi:10.1371/journal.pone.0071476
Ockendon N, Davis S, Toms M, Mukherjee S (2009) Eye size and the time of arrival of birds at garden feeding stations in winter. J Ornithol 150(4):903–908. doi:10.1007/s10336-009-0412-4
Partecke J, Gwinner E (2007) Increased sedentariness in European blackbirds following urbanization: a consequence of local adaptation? Ecology 88(4):882–890. doi:10.1890/06-1105
Partecke J, Van’t Hof TJ, Gwinner E (2005) Underlying physiological control of reproduction in urban and forest-dwelling European blackbirds Turdus merula. J Avian Biol 36(4):295–305. doi:10.1111/j.0908-8857.2005.03344.x
Poesel A, Kunc HP, Foerster K, Johnsen A, Kempenaers B (2006) Early birds are sexy: male age, dawn song and extrapair paternity in blue tits, Cyanistes (formerly Parus) caeruleus. Anim Behav 72:531–538. doi:10.1016/j.anbehav.2005.10.022
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass-size residuals: validating body condition indices. Ecology 86(1):155–163. doi:10.1890/04-0232
Schwartz A, Henderson RW (1991) Amphibians and reptiles of the West Indies: descriptions, distributions, and natural history. University of Florida Press, Gainsville
Shochat E (2004) Credit or debit? Resource input changes population dynamics of city-slicker birds. Oikos 106(3):622–626
Stearns SC (1976) Life-history tactics: a review of the ideas. Q Rev Biol 51:3–47
Stephan B (1999) Die Amsel. Die Neue Brehm-Bücherei Bd. 95. 2 edn. Westarp Wissenschaften, Hohenswarsleben
Strohbach MW, Haase D, Kabisch N (2009) Birds and the city: urban biodiversity, land use, and socioeconomics. Ecol Soc 14(2):31
Thomas RJ, Széskely T, Cuthill IC, Harper DGC, Newson SE, Frayling TD, Wallis PD (2002) Eye size in birds and the timing of song at dawn. Proc R Soc Lond B 269(1493):831–837. doi:10.1098/rspb.2001.1941
Wood SN (2004) Stable and efficient multiple smoothing parameter estimation for generalized additive models. J Am Stat Assoc 99:673–686
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We thank Diana Höhlig, Stephanie Ibsen, and Pia Schmitz for obtaining Blackbird observations, and Simon Dietzel, Sarah Effertz, Daniela Dunger, Diana Höhlig, Terezia Lucenicova, and Rebecca Thier-Lange, for their help in mistnetting. Without the support of these field assistants this work would not have been possible. Stephanie Ibsen and two anonymous reviewers provided useful comments on an earlier draft and considerably improved the manuscript. The project was funded by the Federal Ministry of Education and Research (BMBF, http://www.bmbf.de/en/index.php, FKZ: 033L038E), additional financial support was provided by Helmholtz Impulse and Networking Fund through Helmholtz Interdisciplinary Graduate School for Environmental Research (http://www.higrade.ufz.de). Bird handling and observations were carried out under the permission of the relevant German agencies.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Russ, A., Rüger, A. & Klenke, R. Seize the night: European Blackbirds (Turdus merula) extend their foraging activity under artificial illumination. J Ornithol 156, 123–131 (2015). https://doi.org/10.1007/s10336-014-1105-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-014-1105-1