Abstract
The 2011 Great East Japan earthquake and tsunami led to dispersal of radionuclides into the surrounding area from the Tokyo Electric Power Company’s Fukushima Daiichi Nuclear Power Plant. Before agricultural activities can safely resume after this accident, it is necessary to evaluate the dynamics of radiocesium in irrigation water in the area. We measured the concentrations of total and dissolved radiocesium in irrigation water and analyzed radiocesium concentrations in rice and soil samples, the soil to brown rice transfer factor (TF) in two decontaminated paddy fields located within the zone where paddy rice culture was prohibited, and radiocesium/water balance in a paddy field in 2014. Our key findings were as follows: (1) about 85% of the radiocesium in the irrigation water did not drain away but accumulated in the paddy field; (2) in comparison with the amount of the radiocesium deposited in the fields, the amount of radiocesium that accumulated during irrigation was approximately 0.076%; (3) the outflow of total radiocesium from paddy field was 13.0% of the inflow to a paddy field (irrigation water and fallout); (4) the soil to brown rice TF was 0.0015–0.0068 in the decontaminated paddy fields where soil improvement was performed to increase the content of exchangeable potassium to 200 (mg K)/kg soil before the conventional application; and (5) the concentration of radiocesium in brown rice was about 2% of the standard limit in Japan (100 Bq/kg), and the impact on brown rice from radiocesium in the irrigation water was limited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
On March 11, 2011, the Great East Japan earthquake and tsunami caused an accident at the Tokyo Electric Power Company’s Fukushima Daiichi Nuclear Power Plant (FDNPP). The radionuclides released and dispersed by this accident were deposited mainly in Fukushima Prefecture (Endo et al. 2012; MEXT 2011). The total area of farmland contaminated with radiocesium concentrations > 5000 Bq/kg in the plow layer (the upper 15 cm of soil) was estimated at approximately 8900 ha (MAFF 2012a). Paddy field cropping restrictions were imposed in the area in 2011 to limit the transfer of emitted radionuclides to agricultural crops (MAFF 2014b).
Various decontamination measures, such as topsoil removal, the use of inverting plows, and decontamination using soil puddling with water, were implemented in the paddy fields and irrigation and drainage canals (MAFF 2012b). The decontamination work of the government’s planned agricultural land (31,060 ha) was completed at 99.9% as of February 2018 (MOE 2018).
Tsukada et al. (2002) found that the geometric mean of transfer factor of 137Cs from paddy soil to polished rice was 0.0016, its 95% confidence interval was 0.00021–0.012, and the transfer factors of 137Cs decreased with increasing potassium concentration in the paddy soil. Analysis of the factors affecting the transfer of radiocesium from the paddy field soil to rice crop in 2011 revealed that the content of exchangeable potassium in the soil played an important role (Kato et al. 2015). Potassium fertilization effectively reduced radiocesium uptake due to the antagonism between potassium and radiocesium, with little or no effect on rice production (Eguchi et al. 2015; Fujimura et al. 2016a). Therefore, soil improvement to increase the content of exchangeable potassium to 200 (mg K)/kg soil before the conventional application was proposed as a countermeasure against radiocesium absorption (Kato et al. 2015).
Recommended measures to control of radiocesium absorption (for example, by potassium fertilization) contributed to reduction of the concentration of radiocesium in brown rice (Ota 2014). The results of the monitoring studies revealed that, at several sites, the radiocesium concentration in environmental water (river, lake, and groundwater) since the FDNPP accident was less than the quantification limits or 1 Bq/L (MAFF 2013a; MOE 2012a, b). To date, in areas where cropping has recommenced, there have been no confirmed reports of the effect of radiocesium in irrigation water on crops.
However, before farming can recommence in areas such as the restricted residence zone, the impact of radiocesium on farming must be investigated. In particular, there are concerns about the transfer of radiocesium to irrigation water sources such as rivers and ponds via surface runoff and the consequent emergence of new hot spots of contamination in agricultural irrigation facilities (Kubota et al. 2014; Yoshikawa et al. 2014).
Radiocesium in environmental water exists not only as dissolved ions, but also as suspended particulates, which are adsorbed or fixed by suspended organic and inorganic particles (MAFF 2014a). Tsukada et al. (2017) reported the geometric mean of Kd, determined from 54 irrigation waters in 2014 in the Fukushima Prefecture, was 110,000 over one order of magnitude higher than that reported by IAEA (2010) from 29,000 in freshwater ecosystems determined by field experiments. A higher Kd is associated with the higher the distribution of radiocesium primarily in the solid phase in the equilibrium state; i.e., at the same radiocesium concentration, the concentration of dissolved radiocesium is lower. Suspended radiocesium is not easily dissolved; therefore, its absorption by rice in paddy fields is low. In contrast, dissolved radiocesium can be directly absorbed by plants and is thought to transfer easily to brown rice (MAFF 2014c; Suzuki et al. 2015). On the other hand, Shiozawa (2012) suggested that the contamination of brown rice in 2011 mainly occurred because of fallout deposited on the organic matter covering the paddy fields and not via irrigation water. It is important to elucidate the impact of radiocesium in irrigation water on brown rice before agricultural activities recommence. This task involves clarifying the changes over time in the radiocesium concentration, quantifying the concentration of radiocesium, and determining the behavior of radiocesium in irrigation water in paddy fields.
In the present study, we conducted trials in decontaminated paddy fields at a site in a zone with restricted cropping. Our objectives were to (1) quantify the mass balance of radiocesium in a paddy field by analyzing the water balance; (2) investigate the behavior of radiocesium in irrigation water in decontaminated paddy fields; and (3) clarify the effect of the radiocesium in irrigation water on the radiocesium concentration in brown rice.
Materials and methods
Study area and sampling sites
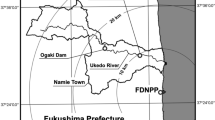
We conducted our research in two decontaminated paddy fields at site K which was in the “restricted residence zone,” designated in March 2014, and located ca. 40-km northwest of FDNPP in Fukushima Prefecture, Japan (Fig. 1).
In 2012, the Ministry of Agriculture, Forestry and Fisheries (MAFF) performed topsoil removal at the site K as a test case, and decontamination at this site K was confirmed. From 2012 to 2014, paddy field rice and vegetables were grown in cultivation trials to measure the transfer of radiocesium to crops through irrigation water after decontamination (MAFF 2012c, 2013b). In the present study, we assessed the quality of the irrigation water and analyzed the results of cultivation trials conducted in 2014. To increase the content of exchangeable potassium to at least 200 (mg K)/kg soil before the conventional application was performed, potassium chloride fertilizer equivalent to 274 (mg K)/kg soil was applied to the paddy fields before the cultivation trials.
In this study, it is assumed that the radiocesium that flowed into the two paddy fields from irrigation water, rainfall, fallout did not evaporate or permeate at site K. Therefore, we performed the evaluation according to the water balance between inflow through rainfall and irrigation water and outflow through drainage water. We cultivated paddy rice to quantify the behavior and balance of radiocesium in the paddy fields [both approximately 0.29 hectare (100 m × 29 m)]. The first paddy field, located in the southern part of the site, was irrigated with water from a pond; hereafter, this paddy field is denoted as KS and its irrigation water as KP. The other paddy field, located in the northern part of the site, received river water that pumped through a measuring weir (220 cm × 40 cm × 40 cm) using a pump (KR-80; Koshin Co. Ltd., Kyoto, Japan). The inner diameter of the pump tube was 76.2 mm and the maximum flow rate was 950-L/min; hereafter, this paddy field is denoted as KN and its irrigation water as KR (Fig. 2).
The soil in site K is classified as a Gray Lowland soil (NIAES 2001). The Gray Lowland soil occupies approximately half of the paddy fields in Fukushima Prefecture (Cultivated Soil Classification Committee 1995). Some of the river’s tributaries upstream of KN contain ponds; therefore, water from these ponds might have affected our results. In addition, the water irrigating KS passes through a farm ditch approximately 550 m long between the pond and the paddy. Consequently, the irrigation water might be affected by the soil at the bottom of the farm ditch.
Survey methods
To assess temporal changes in the concentrations of the different forms of radiocesium in the irrigation water, we obtained irrigation water samples 2–4 times per month during the sampling period in 2014. Water was sampled using buckets, plastic containers, pumps, and hoses. To analyze the water balance and the concentration of radiocesium in the water samples (irrigation, ponded, and drainage) in the paddy field, we installed an automatic water sampler (3700; Teledyne ISCO Co. Ltd., Nebraska, USA) and a water gauge (S&DLmini; OYO Corporation, Tokyo, Japan) at the inlet of site KN to obtain 1-L samples every 10 min when irrigation occurred. We installed an automatic water sampler, a Parshall flume flow meter produced according to Japanese Industrial Standard (JIS) B7553 (2-inch type; Uizin, Tokyo, Japan), and a water gauge at the water outlet at site KN to sample 1-L samples every 20 min when drainage occurred. We installed a rain gauge to obtain rainfall at site K. We did not install equipment to measure the amount of irrigation water at site KS.
The irrigation volume from the inlet at site KN was calculated with reference to the performance curve of the pump (discharge rate when the total pump head is 5 m = 900 L/min) and the height from the river to the inlet (Fig. 2). The irrigation time was the time during which the water level increased, according to the water gauge set on the measuring weir. The measurement was started after transplantation; therefore, the irrigation volume for puddling was not measured.
To analyze behavior and changes in the total (suspended plus dissolved) and dissolved radiocesium in the irrigation water flowing into the paddy fields, we measured the concentrations of radiocesium in the surface water of the paddy fields at sites KN and KS at various distances between the inlet and outlet. We blocked the outlet at a time when there was almost no surface water in the paddy field after ponding water was released in KN (September 4) and KS (August 21–22); we subsequently irrigated KN and KS at 2 h and 3 h, respectively. We obtained surface water samples from KN and KS at 5.5 h and 20 h, respectively, after the start of irrigation (Table 1). No rain fell during the sampling period of the surface water in the paddy fields. Sampling pumps and hoses with an inner diameter of 8 mm were carefully set up outside of the paddy fields to avoid disturbing the surface water and mixing the soil. We obtained 50-L samples for total and dissolved radiocesium (10-L × 1 and 20-L × 2) at a flow rate of 4 L/min at 1 m, 6 m, 15 m, 40 m, and 90 m from the inlet. (The sample obtained at x m is termed KN − x, KS − x, respectively; Fig. 2).
To estimate the impact of radiocesium fallout from rain and dust, we installed a stainless steel basin with a surface area of 0.5 m2 at site K. We collected samples once or twice per month during the sampling period, using a silicon spatula and a plastic container. We filled the stainless steel basin with distilled water up to the 5-cm level to trap the air dust after installation and sampling.
We obtained soil and brown rice samples from near the inlet, at the center of the paddy, and near the outlet (indicated, for example, as KN − I, KN − C, and KN − O, respectively) at approximately 5–6 m from the farm ditch by using quadrat sampling on 3 Oct. In site KS, the outlet was close to the inlet; therefore, the KS − O sample was obtained at a distance of approximately 100 m from the inlet. The inlet, outlet, and sampling sites are shown in Fig. 3.
The rice samples were taken from 50 hills from each quadrat, which consisted of 3–4 rows. Soil samples were obtained from the plow layer (the upper 15 cm of soil) using a soil sample collector with 5 cm radius. We combined soil samples taken from four places within each quadrat.
Radiocesium analyses
We defined the total radiocesium as the concentration in the raw water of the sample, and the dissolved radiocesium as the concentration in the liquid passed through a membrane filter with 0.45-µm pores. We collected the water samples in 0.5-L and 2-L polypropylene plastic containers and 10-L and 20-L low-density polyethylene plastic containers. We transported these containers to the laboratory and performed the pre-processing procedures shown in Fig. 4. We separated the water samples into three fractions of total radiocesium (2–10 L), dissolved radiocesium (20–40 L), and suspended solids (0.5 L). We analyzed 134Cs and 137Cs in all the samples.
To analyze total radiocesium, we used the evaporative concentration method in a draft chamber to gradually reduce the sample volume from 10 L to approximately 2 L by using 2-L beakers and a hot plate. The radiocesium concentration in the sample was measured using a 2-L Marinelli beaker. We analyzed the concentration for fallout samples in the stainless steel basin after pre-processing them in the same way as the samples used to analyze the total radiocesium concentration.
To process solid–liquid separation, we filtered the 20–40-L samples by using a glass fiber filter with a 150 mm diameter and pore diameter of 1.0 μm (GA-100; Advantec Co. Ltd., Tokyo, Japan) and a membrane filter with a 142 mm diameter and pore diameter of 0.45 μm (A045A; Advantec Co. Ltd.). Samples for which the volume collected by the automatic water sampler was small (approximately 2 L) were filtered using a 47-mm-diameter filter. The 1.0-μm and 0.45-μm filters of 47 mm diameter were used to measure the suspended radiocesium concentration.
To analyze dissolved radiocesium of the samples, we reduced the pre-processing and measurement time by adapting a method that used a nonwoven fabric cartridge filter impregnated with potassium zinc ferrocyanide (CS-13ZN; Japan Vilene Co. Ltd., Tokyo, Japan), developed by Yasutaka et al. (2015). We used a dissolved radiocesium concentration measurement device (AFC-550; Fujiwara Scientific Co. Ltd., Tokyo, Japan) developed by the National Institute of Advanced Industrial Science and Technology (Tsuji et al. 2014). The dissolved radiocesium was collected on the fabric within the cartridge. We used a nondestructive method (ICTRM 2015; Yasutaka et al. 2015) to calculate the concentration of dissolved radiocesium (Bq/L).
The radiocesium concentrations of the water, brown rice, and soil samples were determined with high-purity germanium (HPGe) detectors with relative efficiencies of 25% and 40%, respectively (GC4020-7500SL or GC2520-7500SL; Canberra Japan Co. Ltd., Tokyo, Japan). For all the samples, we measured to an accuracy of 10% or less of the relative standard deviation and applied attenuation compensation on November 1, 2014, for comparison.
Analyses of suspended solids and exchangeable potassium
To analyze the concentration of suspended solids, we filtered 0.5-L samples through a glass fiber filter with a pore size of 0.4 μm (Fig. 4). We selected brown rice samples by using a 1.8-mm mesh. Following radiocesium measurements, we applied a 15% moisture correction using the method for calculating brown rice weights in Japanese rice production guidelines (MAFF 2016). Soil samples were ground after natural drying and passed through a 2-mm mesh sieve. After radiocesium measurement, we corrected the concentration on an oven-dry basis (105 °C).
We measured the content of exchangeable potassium in the soil at the quadrat sampling locations at each site after cultivation trials. The exchangeable potassium was extracted using 1 M ammonium acetate (soil: solution = 1:10, 1-h shaking) at the room temperature, and potassium concentrations in the solution were determined using an atomic absorption spectrophotometer (ZA3000; Hitachi High-Technologies Corporation, Tokyo, Japan).
Calculation of the transfer factor (TF)
The TF of radiocesium from soil to brown rice varies depending on the soil, fertilizer, and other conditions (Fujimura et al. 2016b). We calculated the TF of radiocesium from soil to brown rice as follows (Eq. 1).
where the brown rice has a 15% moisture correction after radiocesium measurement.
Consideration of the solid/liquid distribution coefficient
Radionuclides exist in a suspended or dissolved form in fresh water. The distribution of the dissolved and suspended forms of radiocesium is expressed by the solid/liquid distribution coefficient, Kd (IAEA 2010). The Kd of radiocesium in suspended environmental water is defined as the ratio of the radiocesium concentration in suspended solid form to the dissolved radiocesium concentration (JAERI 1995; Yamaguchi et al. 2012). In this study, the Kd for irrigation water was calculated using (Eq. 2), which modifies the suspended radiocesium based on the equation from Yamaguchi et al. (2012).
where T is the total radiocesium concentration (Bq/L), D is the dissolved radiocesium concentration (Bq/L), and SS is the suspended solids concentration (kg/L).
Results
Temporal changes in the radiocesium concentration in irrigation and drainage water
The total and dissolved radiocesium concentrations at KN were lower in drainage water than in irrigation water (Fig. 5). The average ratios (dissolved/total) for the radiocesium concentrations of the two irrigation water samples were 40.3% for KN (KR and KN − I ratio) and 13.6% for KS (KP ratio) (Figs. 5, 6).
Spatial behavior and form changes of radiocesium in irrigation water
The total and dissolved radiocesium concentrations in the surface water in the both paddy fields, measured in the manner of Table 1 and Fig. 2, tended to decrease with increasing distance from the inlet (Fig. 7). However, at some sampling points located far from the inlet, the total concentrations of radiocesium were higher than those at sampling points located closer to the inlet (e.g., KS − 15 compared with KS − 6) (Fig. 7).
Radiocesium concentration in fallout, soil, and brown rice
The time-weighted average fallout density during the entire sampling period was 0.125 Bq/(m2 day), and the total deposit was 17.8 Bq/m2. The time-weighted average 134Cs/137Cs ratio was 0.30 in atmospheric depositions (Table 2).
The content of exchangeable potassium (mg K)/kg in the soil and the concentration of radiocesium in the soil and brown rice are shown in Table 3. The soil to brown rice transfer factor (TF) at each sampling points was 0.0015–0.0068.
Quantification limits of 134Cs and 137Cs
Our measurements were to an accuracy of 10% or less of the relative standard deviation. The quantification limits of 134Cs were 0.0013–0.25 Bq/L, 0.00080–0.043 Bq/(m2 day), 0.16–1.05 Bq/kg, and 13.45–86.68 Bq/kg for water, fallout, brown rice, and soil samples, respectively. Similarly, the quantification limits of 137Cs were 0.0013–0.17 Bq/L, 0.00092–0.052 Bq/(m2 day), 0.17–1.14 Bq/kg, and 11.19–66.19 Bq/kg for water, fallout, brown rice, and soil samples, respectively.
Quantification of radiocesium in the irrigation and drainage water
From May 20 to October 17, 2014, the irrigation volume from the inlet at site KN was 362 mm, and the inflow volumes of total and dissolved radiocesium were 95.3 Bq/m2 and 37.2 Bq/m2, respectively (Table 4). During the same period, the drainage volume from the outlet was 150 mm, and the outflow volumes of total and dissolved radiocesium were 14.7 Bq/m2 and 3.2 Bq/m2, respectively (Table 4). The rainfall at site K from May 16 to October 17 was 1010 mm, and the flux of water that flowed into KN during this period was approximately 1372 mm. The surface water level at KN from August 13 to 16 drying in the survey period was decreased 7.7 mm/day.
Ratio of solid/liquid radiocesium based on water source
The Kd ranged from 1.7 to 9.1 and 2.0 to 12.1 (105 L/kg) at KN and KS, respectively (Table 5). Furthermore, the average Kd value was approximately 1.3 times larger for KS than for KN.
Discussion
Quantification of the radiocesium balance in the paddy fields
Shin et al. (2015) reported that the amount of radiocesium inflow in irrigation water for a decontaminated paddy field was 0.031% of the amount deposited by scattering of radiocesium at the time of the FDNPP accident in difficult-to-return zone from July to October 2013. However, the behavior of radiocesium in brown rice, fallout, and soil in decontaminated paddy fields remains unclear. Miyazu et al. (2016) reported that total outflow of 137Cs from the fields was 0.003–0.028% during puddling and 0.001–0.011% during midsummer drainage of already existing 137Cs in the paddy field.
At site KN, the total annual balance of total radiocesium and dissolved radiocesium in irrigation water was estimated to be 80.6 Bq/(m2 sampling period) and 34.0 Bq/(m2 sampling period), respectively (Table 6). The outflow of total radiocesium [drainage water: 14.7 Bq/(m2 sampling period)] was 13.0% of the inflow to the paddy field [irrigation water: 95.3 Bq/(m2 sampling period) plus fallout: 17.8 Bq/(m2 sampling period)] at site KN (Table 6).
The balance of total radiocesium [98.4 Bq/(m2 sampling period)] was 0.076% of the already existing radiocesium in the decontaminated paddy field, located ca. 40 km from FDNPP [if apparent specific gravity (dry bulk density) of a Gray Lowland soil was 0.93 Mg/m3 of measured value of KN. The amount of the plow layer (the upper 15 cm of soil) was 139.5 kg/m2, and the amount of radiocesium in the KN plow layer was 130 kBq/m2 (932 Bq/kg × 139.5 kg/m2)]. At all sampling points in site K, the soil and brown rice transfer factor (TF) value ranged from 0.0015 to 0.0068 and was within levels recorded before the FDNPP accident (0.00021 to 0.012) by Tsukada et al. (2002). Gravitational irrigation could possibly increase the flux of inflow of the pump irrigation at KN. As the amount of irrigation increases, it could affect concentration of radiocesium in brown rice. If the ratio of discharge to amount of irrigation assumed to be constant, the balances were estimated at 111–311 Bq/(m2 sampling period) at an irrigation volume of 500–2000 mm (Table 6).
Behavior of radiocesium in the surface water of paddy fields
The average Kd value was approximately 1.3 times larger for KS than for KN (Table 5). We believed that radiocesium in the irrigation water from ponds was more likely to be adsorbed to the solid phase than radiocesium from rivers. However, because the difference in Kd was small, further studies are required to completely elucidate the behavior of the transfer of radiocesium from irrigation water sources of multiple sites.
The amount of dissolved radiocesium, which can be absorbed by crops, declined by 60% and 80% within 15 m and 40 m of the inlet, respectively, and total radiocesium also tended to decrease toward outlet (Fig. 7). This finding indicates that the dissolved radiocesium was either directly absorbed by rice, algae, and other plants, or it was adsorbed onto soil particles, and the suspended substances including radiocesium in irrigation water sedimented in the paddy field during nonrainfall.
Radionuclides in soil–water environments migrate vertically in solution, as colloids with infiltration water flow, or attached to fine soil particles. Transport of radiocesium in solution by infiltration is slower than the water flow because of sorption–desorption and fixation on soil particles (Konoplev et al. 2016). Considering the short time span (5.5–22.7 h) between irrigation and sampling and assuming the radiocesium concentration in irrigation water and the amount of adsorption to the soil (IAEA 2010) are in equilibrium, the reduction of the concentration of dissolved radiocesium was probably caused by adsorption to the soil with decreasing water flow after flowing into paddy fields with a lot of soil particles. However, it is necessary to evaluate the adsorption to soil particles by residence time of surface water and the contact time settled on the bottom of the paddy in future studies.
Origin of radiocesium
After the FDNPP accident, most of the radiocesium fallout was retained in the soil. The Kd value in the area was 104−6 L/kg (Yoshimura et al. 2015; Tsukada et al. 2017), and the Kd measured in this study was 1.7 × 105–1.2 × 106 L/kg. Dry and windy weather conditions were expected to result in further contamination, as wind-blown particles of soil containing radiocesium were scattered to new locations (Watanabe 2014).
The inflow volume of radiocesium by atmospheric deposition was 0.755 Bq/(m2 day) on August 1–21, 2014 (Table 2). This value was higher than that during the other investigated periods. In August 2014, typhoon 11 hit Japan, blowing strong winds across the country (JMA 2014) and possibly causing further fallout.
The average radiocesium fallout density (including rainfall) from rain and dust during the entire sampling period was 0.125 Bq/(m2 day), and the total deposit was 17.8 Bq/(m2 sampling period) (Table 2). Therefore, the effect of the atmospheric fallout on paddy soil was expected to be negligible, because the volume was 0.012% of the amount of radiocesium in the decontaminated paddy field at KN. It is possible that radiocesium from the fallout directly adhered to rice plants and was taken up by the brown rice. However, the effect of the atmospheric fallout on rice at the sampled site was limited in this study.
The radiocesium of the sampling sites was thought to be derived from the accident, considering the half-life (134Cs: 2.1 year, 137Cs: 30.2 year) of radiocesium, because 134Cs was detected in all samples in the present study.
Impact on the radiocesium concentration in brown rice
In contrast to the concentration of radiocesium in the surface water in the paddy field, the concentration in the soil was different despite being adjacent between the investigated KN and KS (Table 3). This variability might be attributed to the difference in removal efficiency of radiocesium during the official decontamination program (MAFF 2013b). On the other hand, the radiocesium concentration in the brown rice did not differ much and it was low. These low concentrations were much lower than the standard limit in Japan (100 Bq/kg) (MHLW 2012).
The content of exchangeable potassium was > 200 (mg K)/kg after cultivation trials (Table 3), potassium fertilization was suspected of suppressing radiocesium uptake in brown rice due to the antagonism between potassium and radiocesium, and this information, together with the low variability between samples, suggests that the radiocesium concentration in the soil and irrigation water had little effect on the radiocesium concentration in brown rice when there was enough potassium in paddy soil.
Conclusions
In the present study, we quantitatively analyzed the balance of radiocesium and monitored its behavior at two decontaminated paddy fields located ca. 40-km northwest of FDNPP in 2014.
Our study yielded several key findings. First, at the investigated site K at the 40-km zone, the total and dissolved concentrations of radiocesium flowing into a decontaminated paddy field via irrigation water were at least 95 Bq/(m2 sampling period) and 37 Bq/(m2 sampling period), respectively, in 2014.
Second, about 85% of the radiocesium in the irrigation water did not drain away but accumulated in the paddy field. However, in comparison with the amount of the radiocesium deposited in the fields, the amount of radiocesium that accumulated due to irrigation was approximately 0.076%. The outflow of total radiocesium from the paddy field was 13.0% of that in the inflow to the paddy field (irrigation water and fallout) at site KN.
Third, the concentration of total and dissolved radiocesium in the irrigation water tended to decline rapidly with the increasing distance from the inlet in the paddy fields. Moreover, accumulation was higher near the inlets than at the outlets. Radiocesium that settled in the paddy field was likely adsorbed onto soil particles.
Fourth, the soil to brown rice transfer factor (TF) was from 0.0015 to 0.0068 in the decontaminated paddy fields in which soil improvement was conducted to increase the content of exchangeable potassium to 200 (mg K)/kg soil before the conventional application in 2014.
Finally, at all the sampling point, the concentration of radiocesium in brown rice was about 2% of the standard limit in Japan (100 Bq/kg), and the impact on brown rice from radiocesium in the irrigation water was limited.
It may be possible for farming to recommence in regions where decontamination of agricultural land has been completed, but continuous monitoring will be required.
References
Cultivated Soil Classification Committee (1995) Classification of cultivated soils in Japan (3rd approximation). Misc Publ Natl Inst Agro-Environ Sci 17:1–79 (in Japanese)
Eguchi T, Ohta T, Ishikawa T, Matsunami H, Takahashi Y, Kubo K, Yamaguchi N, Kihou N, Shinano T (2015) Influence of the nonexchangeable potassium of mica on radiocesium uptake by paddy rice. J Environ Radioact 147:33–42
Endo S, Kimura S, Takatsuji T, Nanasawa K, Imanaka T, Shizuma K (2012) Measurement of soil contamination by radionuclides due to the Fukushima Dai-ichi nuclear power plant accident and associated estimated cumulative external dose estimation. J Environ Radioact 111:18–27
Fujimura S, Yoshioka K, Ota T, Ishikawa T, Sato M, Satou M (2016a) The inhibitory effects of potassium chloride versus potassium silicate application on 137Cs uptake by rice. J Environ Radioact 153:188–194
Fujimura S, Eguchi T, Matsunami H, Ota T, Murakami T, Ishikawa T, Makino T, Akahane I, Kamiya T, Aono K, Naka T, Okushima S (2016b) Transfer factors of radiocesium to brown rice in the decontaminated paddy field. Jpn J Crop Sci 85(2):211–217 (in Japanese)
International Atomic Energy Agency (IAEA) (2010) Handbook of parameter values for the prediction of radionuclide transfer in terrestrial and freshwater environments. Tech Rep Ser 472:25–38
Investigative Committee of Technical Report on Monitoring Radio-Caesium in Water (ICTRM) (2015) Technical report: a comparison of the radio caesium pretreatment and analytical methods in water for environmental radioactivity monitoring. http://staff.aist.go.jp/t.yasutaka/PDF/AIST-GREEN-Cswater-TD201509.pdf. Accessed 27 Dec 2018 (in Japanese)
Japan Atomic Energy Research Institute (JAERI) (1995) Methodologies for measurement and application of distribution coefficient. http://jolissrch-inter.tokai-sc.jaea.go.jp/pdfdata/JAERI-Review-95-009.pdf. Accessed 22 Jan 2019 (in Japanese)
Japan Meteorological Agency (JMA) (2014) Heavy rainfall and strong winds by fronts from typhoon number 11 and 12. http://www.data.jma.go.jp/obd/stats/data/bosai/report/2014/20140730/jyun_sokuji20140730-0811.pdf. Accessed 27 Dec 2018 (in Japanese)
Kato N, Kihou N, Fujimura S, Ikeba M, Miyazaki N, Saito Y, Eguchi T, Itoh S (2015) Potassium fertilizer and other materials as countermeasures to reduce radioactive cesium levels in rice: results of urgent experiments in 2011 responding to the Fukushima Daiichi Nuclear Power Plant accident. Soil Sci Plant Nutr. https://doi.org/10.1080/00380768.2014.995584
Konoplev A, Golosov V, Laptev G, Nanba K, Onda Y, Takase T, Wakiyama Y, Yoshimura K (2016) Behavior of accidentally released radiocesium in soil–water environment: looking at Fukushima from a Chernobyl perspective. J Environ Radioact 151:568–578
Kubota T, Taruya H, Tanaka Y, Hamada K (2014) Feature and future tasks of sedimentation of radioactive cesium in irrigation canal system. Water Land Environ Eng 82(3):33–37 (in Japanese)
Ministry of Agriculture, Forestry and Fisheries (MAFF) (2012a) About creating the distribution map of radioactive material concentration in farmland soil. http://www.affrc.maff.go.jp/docs/map/h24/120323.htm. Accessed 27 Dec 2018 (in Japanese)
Ministry of Agriculture, Forestry and Fisheries (MAFF) (2012b) FY2011 annual report on food, agriculture and rural areas in Japan. http://www.maff.go.jp/j/wpaper/w_maff/h23/pdf/e_all.pdf. Accessed 27 Dec 2018
Ministry of Agriculture, Forestry and Fisheries (MAFF) (2012c) Results of cultivation trials public enterprise of demonstration of measures for farmland decontamination. http://www.maff.go.jp/j/nousin/seko/josen/pdf/sakutuke.pdf. Accessed 27 Dec 2018 (in Japanese)
Ministry of Agriculture, Forestry and Fisheries (MAFF) (2013a) Regarding the situation and measures of radioactive substances in ponds in Fukushima Prefecture. http://www.maff.go.jp/j/kanbo/joho/saigai/pdf/tyukan.pdf. Accessed 27 Dec 2018 (in Japanese)
Ministry of Agriculture, Forestry and Fisheries (MAFF) (2013b) Technical handbook of measures for farmland decontamination (Fourth reference materials edition). http://www.maff.go.jp/j/nousin/seko/josen/pdf/sankou.pdf. Accessed 27 Dec 2018 (in Japanese)
Ministry of Agriculture, Forestry and Fisheries (MAFF) (2014a) Basic edition of technical manual of measures for radioactive substances in ponds. http://www.maff.go.jp/j/press/nousin/saigai/pdf/141119-02.pdf. Accessed 27 Dec 2018 (in Japanese)
Ministry of Agriculture, Forestry and Fisheries (MAFF) (2014b) Response to radionuclide contamination in foods after the nuclear power plant accident. http://www.maff.go.jp/e/quake/pdf/140812_response_to_emergenc.pdf. Accessed 27 Dec 2018
Ministry of Agriculture, Forestry and Fisheries (MAFF) (2014c) Regarding factors causing high radioactive cesium concentration in rice and its measures. http://www.maff.go.jp/j/kanbo/joho/saigai/pdf/youin_kome2.pdf. Accessed 27 Dec 2018 (in Japanese)
Ministry of Agriculture, Forestry and Fisheries (MAFF) (2016) Rice production guidelines. http://www.maff.go.jp/j/seisan/kankyo/hozen_type/h_sehi_kizyun/attach/pdf/akita01-12.pdf. Accessed 19 Oct 2018 (in Japanese)
Ministry of Education, Culture, Sports, Science and Technology-Japan (MEXT) (2011) Results of the third airborne monitoring survey by MEXT. http://radioactivity.nsr.go.jp/en/contents/5000/4182/24/1304797_0708e.pdf. Accessed 27 Dec 2018
Ministry of Health, Labour and Welfare (MHLW) (2012) New standard limits for radionuclides in foods. http://www.mhlw.go.jp/english/topics/2011eq/dl/new_standard.pdf. Accessed 27 Dec 2018
Ministry of the Environment, Government of Japan (MOE) (2012a) FY2012 radioactive material monitoring surveys of the water environment. http://www.env.go.jp/en/water/rmms/result_pw-fy2011.html. Accessed 27 Dec 2018
Ministry of the Environment, Government of Japan (MOE) (2012b) Results of radioactive material monitoring of groundwater (report No. 7). http://www.env.go.jp/en/water/rmms/attach/result_gw-fy2011/Report11_07.pdf. Accessed 27 Dec 2018
Ministry of the Environment, Government of Japan (MOE) (2018) Environmental remediation in Japan. http://josen.env.go.jp/en/pdf/progressseet_progress_on_cleanup_efforts.pdf. Accessed 19 Oct 2018
Miyazu S, Yasutaka T, Yoshikawa N, Tamaki S, Nakajima K, Sato I, Nonaka M, Harada N (2016) Measurement and estimation of radiocesium discharge rate from paddy field during land preparation and mid-summer drainage. J Environ Radioact 155–156:23–30
National Institute for Agro-Environmental Sciences (NIAES) (2001) Soil information browsing system. https://soil-inventory.dc.affrc.go.jp/figure.html?lat=37.6943&lng=140.748&zoom=15. Accessed 27 Dec 2018 (in Japanese)
Ota T (2014) Suppressing countermeasures for radioactive cesium uptake in paddy rice. Jpn J Soil Sci Plant Nutr 85(2):90–93 (in Japanese)
Shin M, Kubota T, Hamada K, Hitomi T, Ota T (2015) Dynamic analysis of radioactive cesium in decontaminated paddy fields. J Water Environ Technol 13(5):383–394
Shiozawa S (2012) Radioactive contamination of paddy field and its transfer to rice. Water Land Environ Eng 80(7):15–18 (in Japanese)
Suzuki Y, Yasutaka T, Fujimura S, Yabuki T, Sato M, Yoshioka K, Inubushi K (2015) Effect of the concentration of radioactive cesium dissolved in irrigation water on the concentration of radioactive cesium in brown rice. Soil Sci Plant Nutr. https://doi.org/10.1080/00380768.2014.1003192
Tsuji H, Kondo Y, Suzuki Y, Yasutaka T (2014) Development of a method for rapid and simultaneous monitoring of particulate and dissolved radioactive cesium in water with nonwoven fabric cartridge filters. J Radioanal Nucl Chem 299(1):139–147
Tsukada H, Hasegawa H, Hisamatsu S, Yamasaki S (2002) Transfer 137Cs and stable Cs from paddy soil to polished rice in Aomori, Japan. J Environ Radioact 59:351–363
Tsukada H, Nihira S, Watanabe T, Takeda S (2017) The 137Cs activity concentration of suspended and dissolved fractions in irrigation waters collected from the 80 km zone around TEPCO’s Fukushima daiichi nuclear power station. J Environ Radioact 178–179:354–359
Watanabe A (2014) Long-term variation of atmospheric concentrations and drop amount of radioactive material. J Jpn Soc Atmos Environ. https://doi.org/10.11298/taiki.49.a89 (in Japanese)
Yamaguchi N, Takata Y, Hayashi K, Ishikawa S, Kuramata M, Eguchi S, Yoshikawa S, Sakaguchi A, Asada K, Wagai R, Makino T, Akahane I, Hiradate S (2012) Behavior of radiocesium in soil-plant systems and its controlling factor: a review. Bull Natl Inst Agro-Environ Sci 31:75–129 (in Japanese)
Yasutaka T, Tsuji H, Kondo Y, Suzuki Y, Takahashi A, Kawamoto T (2015) Rapid quantification of radioactive cesium dissolved in water by using nonwoven fabric cartridge filters impregnated with potassium zinc ferrocyanide. J Nucl Sci Technol. https://doi.org/10.1080/00223131.2015.1013071
Yoshikawa N, Obara H, Ogasa M, Miyazu S, Harada N, Nonaka M (2014) 137Cs in irrigation water and its effect on paddy fields in Japan after the Fukushima nuclear accident. Sci Total Environ 481:252–259
Yoshimura K, Onda Y, Sakaguchi A, Yamamoto M, Matsuura Y (2015) An extensive study of the concentrations of particulate/dissolved radiocaesium derived from the Fukushima Dai-ichi Nuclear Power Plant accident in various river systems and their relationship with catchment inventory. J Environ Radioact 139:370–378
Acknowledgements
This study presents part of the results from the project “Development of Decontamination Technologies for Radioactive Substances in Agricultural Land (task name: Elucidation of transfer dynamics of radiocesium in decontaminated paddy fields; task number, 31107)” commissioned by the Ministry of Agriculture, Forestry and Fisheries. This study was supported partly by a grant-in-aid from the Kurita Water and Environment Foundation (15A012 and 16K004) and JSPS KAKENHI Project (16H01791). We gratefully acknowledge the help of many people during our research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, M., Kubota, T., Manpuku, Y. et al. Behavior of radiocesium in decontaminated paddy fields in Fukushima Prefecture, Japan. Paddy Water Environ 17, 703–714 (2019). https://doi.org/10.1007/s10333-019-00694-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10333-019-00694-6