Abstract
Alternate wetting and drying (AWD) irrigation is widely adopted to save water in rice production. AWD practice shifts lowland paddy fields from being continuously anaerobic to being alternately anaerobic and aerobic, thus affecting nitrogen (N) transformations in paddy field soils. Using the barometric process separation technique, a large number of soil cores sampled from lowland paddy field soil profiles were measured for gross nitrification and denitrification rates under different temperature and soil moisture conditions. The gross nitrification and denitrification rates vary with rice growth stages and range between 1.18–30.8 and 0.65–13.54 mg N m−3 h−1, respectively. Results indicate that both gross nitrification and denitrification rates increased with the increase in temperature in all three studied soil layers. Gross nitrification rates significantly decrease with increasing soil moisture while denitrification rates increase, and different soil layers demonstrated different rates of variation to the increase in soil moisture. Gross nitrification rates in the cultivated horizon layer decreased more sharply with the increase in soil moisture. High soil water content is favorable to denitrification of all soil layers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is cultivated in paddy fields under a wide range of climate, soil, and water regime (Li and Barker 2004). Water and nitrogen (N) are two of the most important inputs for high grain yields in rice production (Bouman et al. 2007; Zhu and Chen 2002). Inorganic N fertilizers are increasingly applied for rice, but the overall N use efficiency is often low, with reports at about 30% (Zhu and Chen 2002). Gaseous losses, leaching losses and runoff losses of N accounted for about 40, 0–19 and 0–11% of the applied N fertilizer, respectively (Zhu and Chen 2002). The agricultural gaseous N emissions from paddy fields in the form of nitrous oxide, N2O, and ammonia, NH3, which are greenhouse gases, may influence regional and global atmospheric chemistry and cause globe warming (Peng et al. 2011a; Ussiri and Lal 2013), while inorganic N (NH4+–N and NO3−–N) solutes contribute greatly to eutrophication risk in water bodies.
An appreciable part of both native and applied N in flooded rice soils is lost by nitrification–denitrification (Arth and Frenzel 2000; Buresh et al. 2008). NO3−–N, which is the product of nitrification of NH4+–N in the localized aerobic zone of the submerged paddy soils, enters the anaerobic zone by mass flow and diffusion, and can be rapidly denitrified (Reddy and Patrick 1986; Sahrawat 1980). Because of the flooded condition of rice paddy fields, the NH4+–N diffusion and oxygen availability for nitrification determines the processes of nitrification–denitrification (Patrick and Reddy 1975). The alternate drying and flooding improves oxygen availability and nitrification to form NO3−–N to be denitrified, thus the N losses increase (Reddy and Patrick 1986). However, there was limited quantitative information identifying the effects of soil water regime in paddy fields on nitrification and denitrification rates, as many factors affect the N transformations and it is hard to measure their rates. Benefiting from the non-disturbance and related low cost of the barometric process separation (BaPS) technique (Breuer et al. 2002; Ingwersen et al. 1999, 2008), it is possible to rapidly measure and compare soil gross nitrification, denitrification and respiration rates under different soil conditions.
The plow pan layer (PPL) formed by puddling is a highly compacted soil layer with low hydraulic conductivity in lowland paddy fields, normally found at 20–30 cm depth. The PPL retards water movement and solution transport from upper soil to deep soil, causing highly variable soil water profile and vertical N distribution (Bouman et al. 2007; Tan et al. 2013). Soil structure dynamics caused by puddling results in changes to microhabitats (Eickhorst and Tippkötter 2009). Kögel-Knabner et al. (2010) comprehensively reviewed the biogeochemistry of paddy fields and emphasized the layer effects on organic matter decomposition and N transformations. Various researches about biochemical processes regarding N cycling in lowland paddy fields show large N losses in different soil layers (Bhandral et al. 2007; Colbourn and Dowdell 1984; Dhondt et al. 2004; Ishii et al. 2011), but N transformation rates in different soil layers are rarely measured for understanding pathways of N losses.
As water is becoming increasingly scarce, techniques of water saving irrigation (WSI) are widely adopted in global rice production systems. Different from the continuously anaerobic paddy field under traditional flooding irrigation, WSI, e.g., alternate wetting and drying irrigation (AWD) (Belder et al. 2005; Cabangon et al. 2001; Li and Barker 2004; Tan et al. 2013), control irrigation (Peng et al. 2011a, b), results in a frequent change in soil water regime and induces the paddy field under alternate aerobic and anaerobic condition. Hence, the N transformations in paddy field soils have been greatly changing due to WSI practices (Buresh and Haefele 2010; Peng et al. 2006). Although several soil modules were proposed to simulate the biomass, N and water dynamics under alternation or transition of soil environments (Gaydon et al. 2012; Jing et al. 2010; Ridolfi et al. 2003), little work has been done to examine the relationship between N transformation rates of paddy soils and soil moisture. Since the field water regime variation evidently also brings about the soil temperature change (Alberto et al. 2011), this paper presents the gross nitrification and denitrification rates measured from soil cores with different temperature and soil moisture using the BaPS system.
Materials and methods
Measuring sites and soil sampling
Intact soil core samples were taken during the rice growing season in 2011 from experimental rice paddy fields at Tuanlin Hubei, China (30°49′N, 112°10′E), which were intensively researched for WSI including AWD (Tan et al. 2013, 2014, 2015). The local average altitude and temperature is 90 m and 16 °C, respectively. The site belongs to the zone of subtropical monsoon climate in terms of climatic regionalization. The rainfall and pan evaporation amounts to 700–1100 and 1300–1800 mm, respectively. On average, nearly 60% of the yearly rainfall occurs during the rice growing season (May to September). The soil texture was silty clay loam. Based on vertical differences in soil physical characteristics analyzed (Tan et al. 2013, 2014, 2015), the paddy soil profile can be divided into three layers, i.e., cultivated horizon layer (CHL, 1–18 cm), PPL (18–33 cm) and illuvial horizon layer (IHL, 33 cm and below) based on the soil characteristics (Table 1). N fertilizer (urea) was applied in three splits following a prescribed fertilizer schedule as local farmers and previous field experiments applied (Tan et al. 2013, 2014, 2015). These three splits are 90 kg ha−1 basal fertilization (Jun-04-2011, before rice being transplanted), 60 kg ha−1 early-tillering fertilization (June-30-2011), and 30 kg ha−1 panicle-initiation fertilization (Jul-22-2011).
Lowland paddy fields are characterized by heavy soils and the existence of a PPL which are requirements for successful AWD irrigation for rice production with reduced water inputs without a significant impact on yield (Bouman et al. 2007). The experimental paddy fields are representative lowland paddy fields in China that are, in total 12 million ha in area, adopted with AWD irrigation (Li and Barker 2004). Because of the representative field soils and climate conditions, successful AWD practices obtained from long-term experiments that were conducted in our experimental fields (e.g., Belder et al. 2004, 2005; Bouman and Tuong 2001; Bouman et al. 2007; Cabangon et al. 2001, 2004; Tan et al. 2013, 2014) are adopted by many Chinese (and some other Asian countries’) lowland paddy fields for increasing water productivity (Li and Barker 2004; Bouman et al. 2007). Studies about the effects of AWD on N regimes and balances in experimental fields are follow-ups of these researches for integrated management of water and N in AWD paddy fields (Belder et al. 2005; Cui et al. 2004; Tan et al. 2013, 2015).
A soil domain of 1 × 1 × 1 m was excavated on the day of rice growth stage of early-vegetative (Jun-07-2011 and Jun-14-2011), early-tillering (Jul-04-2011 and Jul-12-2011), panicle-initiation (Jul-28-2011 and Aug-06-2011) on the different site but in the same paddy field for the investigation of possible differences in gross nitrification and denitrification rates of the paddy soil resulting from the hypothetical difference in the abundance and/or community structure of microbial nitrifier and denitrifier communities. Due to the difference in root depth and the N fertilization and utilization during different rice growth stages, the oxygen availability and substrate concentration for nitrification and denitrification processes may also be different. Undisturbed intact soil cores (steel cutting ring, 100 ml) were taken from each soil layer after appropriate drainage that allowed soil dry enough to be sampled intactly. The soil profile was vertically even sampled with an interval of 10 cm, and soil cores from the same layer were combined to be put in the measuring chamber of BaPS system as seven replicates were required by BaPS system for each measurement. Soil cores were stored immediately after collection in a cold room at 4 °C and transported to the soil analysis laboratory at Wuhan University, Wuhan, China in an insulated box and then frozen until the commencement of N transformation measurement. Disturbed soils were also sampled for each layer on all three sampling days and used for the soil N and organic matter (OM) analysis. The disturbed soil samples were extracted with 2 mol l−1 KCl (soil-to-solution ratio 1:10), and the extracts were measured for NH4+–N, and NO3−–N by the indophenol blue method and disulfonic acid phenol method using a UV-2800 spectrophotometer (SEPA 2002). The soil total N (TN) content was determined as well by the Kjeldahl method (Bremner 1960), while the OM content was determined by loss on ignition at 500 °C for 24 h.
Nitrification and denitrification rates measurement using BaPS system
Nitrification and denitrification rates were measured with the automatic BaPS measuring system made by Umweltanalytische Mess-Systeme (UMS) GmbH (Munich, Germany). The BaPS technique has been widely used in the research of the soil nitrification, denitrification, and respiration (Breuer et al. 2002; Chen and Huang 2006; Geng et al. 2005; Muller et al. 2004; Rosenkranz et al. 2006), as BaPS technique allows the determination of gross nitrification, denitrification, and respiration rates without destroying the original soil structure and does not need to add labeled substrates to the soil as 15N-isotope pool dilution technique (Davidson et al. 1991). The BaPS method works well in acidic to weakly acidic soils, but it cannot determine the N transformation of anaerobic soils as, among others, methane is produced (Ingwersen et al. 2008).
Details about the theoretical considerations, mathematical procedures of the BaPS technique, as well as the calibration and measurement result analysis can be found in the literature (e.g., Breuer et al. 2002; Ingwersen et al. 1999; Ingwersen et al. 2008). The BaPS technique is based on the determination of the CO2, O2, and total gas balances inside an isothermal, gas tight soil system. Biological processes, i.e., nitrification, denitrification and respiration are responsible for gas pressure changes inside such a system. By measuring the net changes of CO2, O2, as well as the pressure change, the production of gaseous N-compounds (NxOy) via denitrification can be calculated. Thus, the gross nitrification, denitrification, and respiration rates can be obtained.
Temperature and moisture treatments
In order to investigate the temperature and moisture dependence of gross nitrification and denitrification rates of paddy field soils, the collected soil cores were grouped and, respectively, incubated in the BaPS system at four temperatures × four soil moistures over 2 weeks. The temperature was set to 20, 25, 30, and 35 °C, corresponding to the seasonal range of air temperatures observed at the climate station near the experimental paddy field. Temperatures were controlled by the thermostat of the BaPS system. Deionized water was randomly dripped onto the soil surface via syringe. 12 h after each moisture addition, the soil core was assumed to be homogeneous to the new moisture condition and was subsequently transferred for measurement using the BaPS system. At the end of each measurement, volumetric soil moisture content was measured after drying for 24 h at 105 °C. Since the BaPS system cannot measure N transformation rates for anaerobic soils, BaPS measurement results for saturated or almost saturated soils (anaerobic) were not included in further analyses. In case of anaerobic soils, another aerobic measurement was additionally conducted to substitute the anaerobic ones.
Since there were 48 measurements (4 temperatures × 4 moistures × 3 soil layers) to be conducted for each rice growth stage, approximately 3 weeks (typically 7–12 h for each measurement) were required to measure the rates of nitrification and denitrification under different temperature and moisture conditions. It was not possible to have replicates to be measured for all treatments in less than about 1 month (length of the rice growth stage) as we just have one BaPS system. Moreover, rates of nitrification and denitrification may change significantly after 1 month, so soil cores collected were typically needed to be analyzed in 1 month to ensure that measured rates of nitrification and denitrification represent the in situ rates (Ingwersen et al. 1999, 2008; Shi et al. 2010; Sun et al. 2009). Three replicate measurements for the same soil core and condition showed that the rate differences of gross nitrification and denitrification between them were within 10%. Moreover, every measurement has already combined seven soil cores. Therefore, no replicate measurements were conducted for each condition.
Statistical analysis
All statistical analyses were performed with SPSS 17 (SPSS Inc., USA). Because variables were not normally distributed (Breuer et al. 2002; Jahangir et al. 2012), the nonparametric Mann–Whitney test was used (1) to identify differences between soil cores from the three soil layers and (2) to distinguish temperature and soil moisture treatment on gross nitrification and denitrification rates, and (3) to compare seasonal means of nitrification and denitrification rates. Simple linear regressions (stepwise) analyses were performed to test relationships between gross nitrification and denitrification rates and soil temperature, and moisture as well. A statistical probability of p < 0.05 was considered significant for all tests. Analysis of variance (ANOVA) with soil moisture as the main factor and temperature as the sub-factor was also conducted on data for each combined treatment (4 temperatures × 4 moistures) from different soil layers and rice growth stages.
Results
N distribution in paddy soil profile
OM and N contents, as well as the pH in paddy soil profile are shown in Table 2. The pH values ranged 5.3–6.1, which indicated that the BaPS approach is applicable to accurately measure the nitrification and denitrification rates of these paddy soil cores as BaPS works well in acidic to weakly acidic soils (Ingwersen et al. 2008). OM and N contents in deep soils (IHL) were significantly lower than that in upper soils (CHL and PPL) in all rice growth seasons. OM contents in IHL were only 30.6% of those in CHL and PPL on average. The total N and NH4+–N content in IHL was 50.5 and 63.1% of that in CHL and PPL, respectively, while NO3−–N in IHL was only 21.1% of that in CHL and PPL. In paddy fields fertilized with urea like this experimental paddy, NH4+–N concentration in soil water is very large compared to NO3−–N concentration (Cui et al. 2004). Soil usually readily adsorbs available NH4+–N at cation exchange points. However, paddy soils are not adsorbable to NO3−–N and NO3−–N in deep soils could be easily denitrified as a result of the deep anaerobic environment. Consequently, the NO3−–N content in IHL was significantly lower than that in CHL and PPL. The OM and N content in PPL was slightly lower (less than 8%) than that in CHL, in that NO3−–N content in PPL was even 5.9% higher than that in CHL. Because of the downward water and solute transport after fertilization, average gross nitrification rate at rice growth stage of early-tillering was 34.8, 12.2, and 45.9% higher than those at the stage of early-vegetative, and 17.3, 6.6, and 26.7% higher than those at the stage of panicle-initiation, respectively. NO3−–N gradually decreased after rice transplanting because of leaching losses and/or plant water uptake.
Temperature effects on nitrification and denitrification of paddy soils
Table 3 shows the statistical gross nitrification and denitrification rates under different temperature. The high standard deviations were caused by the different soil moisture levels. The gross nitrification and denitrification rates ranged between 1.18–30.8 and 0.65–13.54 mg N m−3 h−1, respectively. These N transformation rates were notably (approximate 1–2 order) lower than that of previously reported values, e.g., gross nitrification rates of 20–1400 μg N kg−1 soil h−1 and denitrification rates of 0–280 μg N kg−1 soil h−1 (Breuer et al. 2002; Chen and Huang 2006; Geng et al. 2005). In these studies, the soil samples were from very thin (less than 10 cm) surface soil as they assumed that soil respiration and N transformations mostly happens in surface soil. However, N losses by nitrification–denitrification were contributed by the whole soil profile and the deep soil also had high N2O concentration in paddy fields (Onishi et al. 2012; Xing et al. 2002; Zhu et al. 2003), which was also indicated by the N transformation rates of deep soil (Table 3). Sampled soil cores from surface soil in these experiments were mixed and represented by the mean N transformation rates, so the N transformation rates were low.
Both gross nitrification and denitrification rates slightly increased with the increase in the temperature with great significance in all three soil layers, although the effect of soil moisture was more evident. Since soil moisture induced large variations in gross nitrification and denitrification rates that change with temperature, there was no definable regression relationship between gross nitrification and denitrification rates and temperature, although the increasing trend was significant. Through ANOVA, the effects of soil moisture and temperature, and their interaction on gross nitrification and denitrification rates are all statistically significant at the significance level of 0.01.
On average of the three rice growth stages and three soil layers, the gross nitrification and denitrification rates increased 36.0 and 42.0%, respectively, as temperature increased from 20 to 35 °C. The gross nitrification rate of IHL was only 15.6 and 24.7% of that of CHL and PPL on average, respectively, while the PPL (5.1 mg N m−3 h−1) had the highest denitrification rate averaged on three rice growth stages, compared to CHL (4.6 mg N m−3 h−1) and IHL (3.2 mg N m−3 h−1). The variations in both gross nitrification and denitrificaiton rates in three rice growth season were also significant. Average gross nitrification rate at the stage of early-tillering (12.2 mg N m−3 h−1) was 71.8 and 16.2% higher than that at the early-vegetative stage (7.1 mg N m−3 h−1) and the panicle-initiation stages (10.5 mg N m−3 h−1), respectively. The average denitrification rate at the stage of early-tillering (5.0 mg N m−3 h−1) was 25.0 and 16.3% higher than that at early-vegetative (4.0 mg N m−3 h−1) and panicle-initiation (4.3 mg N m−3 h−1) averaged on three soil layers, respectively. The variations in these by soil layer and rice growth are also indicated in Figs. 1 and 2.
Moisture effects on nitrification and denitrification of paddy soils
Mean nitrification and denitrification rates at different soil moisture conditions which were represented by the volumetric soil water content are shown in Figs. 1 and 2. The standard deviations show the variations caused by temperature. Soil water content ranged from 18.4 to 37.3% which was mostly lower than the corresponding field capacity of three layers (Table 1). These simulated soil water contents were in the range of soil moisture on paddy fields under AWD irrigation during the dry period (Peng et al. 2011a, b; Tan et al. 2013). The gross nitrification rates decreased with the increase in soil water content, while denitrification rates significantly increased.
There was no significant variance of denitrification rates between rice growth stages at all soil layers, while the nitrification rates in the rice growth stage of early-vegetative (7.1 mg N m−3 h−1) was significantly (41.0 and 32.2%) lower than that of early-tillering (12.2 mg N m−3 h−1) and panicle-initiation (10.5 mg N m−3 h−1) averaged on three soil layers. There were great variations in both gross nitrification and denitrification rates between soil layers. Gross nitrification rate of IHL (2.6 on mg N m−3 h−1) was only 15.6 and 24.7% of that of CHL (16.7 mg N m−3 h−1) and PPL (10.5 mg N m−3 h−1) averaged over rice growth stages, respectively. However, the paddy soils of PPL had the highest denitrification rate of 5.1 mg N m−3 h−1 which is 10.9 and 59.3% higher than that of CHL (4.6 mg N m−3 h−1) and IHL (3.2 mg N m−3 h−1), respectively. These results were in agreement with the findings that soil compaction caused a significant increase in N losses by denitrification (Bhandral et al. 2007; Torbert and Wood 1992).
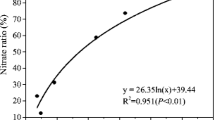
As nitrification and denitrification rates were intensively associated with the soil water content, the correlations between mean nitrification and denitrification rates and soil water content are shown in Fig. 3. These correlations varied greatly with the soil layers, which was caused by the different soil properties (Tables 1, 2), microbial communities and possibly also the oxygenation. The linear correlation r ranged 0.87–0.93. Because of the relative low nitrification rate of IHL in the growth stage of early-vegetative (Fig. 2), the gross nitrification rate of IHL did not decrease very significantly with the increasing soil water content (Fig. 3). Gross nitrification and denitrification rates of the surface soil layer (CHL) were mostly sensitive to the soil water content. To our knowledge, no other experiments on the soil depth dependency of gross nitrification or denitrification rates, especially for paddy soils, have been published so far. However, the data of Chen and Huang (2006) suggest a strong depth dependency of gross nitrification and denitrification in wheat field soil profiles (20 cm). Dhondt et al. (2004) found that denitrification rates in the soil profile (210 cm) were associated with the accumulation of buried OM. The lower OM content (Table 2) in IHL resulted in lower denitrification rates compared to CHL and PPL.
Discussions
Factors influencing the rate of nitrification and denitrification
Even though gross rates of nitrification and denitrification in rice paddy fields which are man-made wetlands were seldom investigated, there are some studies about the effects of soil temperature, moisture and physicochemical properties on the rate of nitrification and denitrification in natural wetlands. There were large spatial variations in western USA in terms of the optimum temperature for nitrification (Mahendrappa et al. 1966), since nitrification of soils from northern regions was faster at 20 and 25 °C than at 35 and 40 °C while the reverse was true for the southern soils which nitrified fastest at 35 °C. Malhi and McGill (1982) found that the optimum temperature for nitrification in soils of a grassland wetland in central Alberta, Canada was 20 °C and at 30 °C nitrification activity had almost ceased. Corre et al. (2002) found that in a grassland wetland in the USA, the gross nitrification was significantly, negatively related to soil moisture represented by water-filled pore space (WFPS) with a correlation coefficient of r = − 0.79 which is similar to our results (r = − 0.74 to −0.91 by WFPS converted from r = − 0.83 to −0.92 by volumetric soil water content), and positively related to temperature with a coefficient of r = 0.55. These studies indicate that nitrification microbial communities in soils from different wetlands have adapted to different temperature ranges.
The denitrification process could occur in a wide range of temperature (5–70 °C), and the rate of denitrification increases with the temperature, although extremely low (< 15 °C) or high (> 40 °C) temperature inhibits denitrification (Stanford et al. 1975; Maag and Vinther 1996). Since the denitrification occurs under anaerobic conditions, soil moisture indirectly affects the rate of denitrification through changing the oxygenation condition (Ruser et al. 2006; Ryden et al. 1987). Generally, rates of denitrification are low in soils with low moisture (< 20%), and high in soils with high moisture (> 30%) (Ryden et al. 1987; del Prado et al. 2006), which are consistent with our results.
The substrate concentration is another important factor that is related to the rate of nitrification and denitrification. High amount of N2O (product of the nitrification–denitrification process) fluxes was associated with high-level fertilizer application for paddy fields (Zhang et al. 2014). Malhi and McGill (1982) examined that Michaelis–Menten kinetics, Eq. (1), is appropriate to describe the relationship between rates of nitrification and substrate concentration under constant temperature and soil moisture.
where v is the rate of nitrification or denitrification; Vmax represents the maximum rate achieved by the system at maximum (saturating) substrate concentrations; the Michaelis constant Km is the substrate concentration at which the reaction rate is half of Vmax. We fitted the average rates of nitrification and denitrification of each soil layer and corresponding NH4+–N (Fig. 4a) and total N (TN) (Fig. 4b) concentrations to Eq. (1). There were significant relationships between the rate of nitrification and denitrification and the substrate concentration that follows the Michaelis–Menten kinetics. Note that scatter dots in Figs. 4a, b are average values from different temperature and soil moisture conditions, so it is not appropriate to estimate Vmax and Km from these least-squares fittings. The difference in the rates of nitrification and denitrification between different soil layers in paddy fields could be likely attributed to the difference in substrate concentration between them.
In order to predict the rate of denitrification and denitrification based on known temperature, soil moisture, pH and substrate concentration, Parton et al. (1996) proposed a generalized model to estimate rates of nitrification and denitrification based on experimental data from drylands. However, due to specific relationships between influencing factors and rates of nitrification and denitrification for different soils, more studies should be conducted for soils from different lowland paddy fields to promote the N fertilizer utilization.
Effects of AWD irrigation on nitrification and denitrification
Since temperature and soil moisture are correlated with the nitrification and denitrification processes, the AWD irrigation, which changes the field environment compared to the continuously flooded (CF) irrigation, likely leads to the change in N pathway in lowland paddy fields. Alberto et al. (2009) shows that AWD paddy fields had 48% more sensible heat flux than CF paddy fields, indicating that more radiation was used for warming the surrounding air and soils. The soil temperature of paddy fields typically ranged 20–32 °C (Alberto et al. 2009, 2011; Tan et al. 2015), and the soil temperature of AWD paddy fields is significantly higher (lower) than that of the CF paddy fields during daytime (nighttime) by about 1.0 (0.5) °C (Alberto et al. 2009). Since the rate of nitrification and denitrification increases with the temperature, this temperature difference could result in more intensified nitrification–denitrification process in AWD paddy fields that lead to higher NxOy emissions and lower N use efficiency. However, Liu et al. (2015) found that warming alone did not affect the abundance or community structure of ammonia oxidizing archaea and bacteria in the rice rhizosphere of paddy fields at any growth stage. Again, given the specific effects of temperature on the N transformation and microbial communities, further studies for diverse soils would be required to obtain a sound understanding of potential changes in N cycling and rice productivity under warming soils in AWD paddy fields.
Compared to temperature differences between AWD and CF paddy fields, the soil moisture difference is more notable. The soil moisture in the AWD paddy fields where soil sampled for this study ranged from 17% cm3 cm−3 to saturated water content (Tan et al. 2013, 2014, 2015). If we suppose that a continuously, extremely low and high soil water content in paddy fields was 25 and 45%, the corresponding total N nitrified (denitrified) during the rice growth season would be 121.3 and 29.3 kg N ha−1 (52.5 and 161.1 kg N ha−1) in soil domain with 1 m depth, respectively. The extremely low (high) temperature corresponds to the frequent condition in AWD (CF) paddy fields. The hypothetical total N transformed per unit area (ha), NT (kg ha−1), in each soil layer are estimated by Eq. (2) using the rates of transformation that are estimated from the linear model shown in Fig. 3 with the soil volume for that soil layer and the time of the rice growth season
where rT is the rate of nitrification or denitrification (mg N m−3 d−1); ds is the depth of a soil layer (m). The total N transformed in 1 m depth soil was the summation of NT in three soil layers. Although this estimate does not consider the change in substrate concentration and soil moisture in paddy fields, the differences in N transformed under low and high temperature indicate that AWD paddy fields potentially dominate with NO3−–N rather than NH4+–N that is easily absorbed by rice (Tan et al. 2013), because of the high rates of nitrification in AWD fields. The product of nitrification NO3−–N increases the substrate concentration for denitrification in the days with high soil moisture in AWD paddy fields. Therefore, more NxOy emissions would occur in AWD paddy fields than in CF paddy fields, which is also shown in the results of experiments (Peng et al. 2011a, c; Yang et al. 2012) and simulations (Tan et al. 2015). In this sense, decreasing the number of wetting and drying cycles is likely and alternative to inhibiting nitrification–denitrification processes and decreasing N losses. On the other hand, NH4+–N-dominated CF paddy fields have higher volatilization NH3 losses than NO3−–N-dominated AWD paddy fields. Therefore, it is hard to estimate the differences in total gaseous losses (NH3 + NxOy) between AWD and CF paddy fields.
Due to complicated N transformation and transport processes in paddy fields, limited simulation studies (Li et al. 2015; Tan et al. 2015) for N transport and balance generally used sequential first-order decay chain reactions to describe the N transformation. Parameters of the first-order decay reactions were calibrated by field regimes of NH4+–N and NO3−–N. The rates of nitrification and denitrification estimated in this study that are associated with soil moisture cannot be converted to parameters of the first-order decay reactions. However, in the future work, N transformation models can be updated to include Michaelis–Menten kinetics for incorporating measured rates of transformation and Michaelis parameters which are associated with soil temperature and moisture, or other soil physicochemical properties. Thus, modeling studies could promote N management in AWD fields through adjusting irrigation schedule to increase N use efficiency and reduce greenhouse gas emissions.
Conclusions
Since the BaPS technique allows rapid analysis of the soil respiration and N transformations, a large number of sampled soil cores from lowland paddy field soil profiles were measured for detecting gross nitrification and denitrification rates. The gross nitrification and denitrification rates varied at different rice growth stages and ranged 1.18–30.8 and 0.65–13.54 mg N m−3 h−1, respectively. Using the BaPS technique, the effects of temperature and soil moisture on gross nitrification and denitrification of paddy soil from different soil layers were also examined. Both gross nitrification and denitrification rates increased with the increase in the temperature in all three soil layers. Gross nitrification rates significantly decreased with the increasing soil moisture while denitrification rates increased, although different soil layers demonstrated varied rates of variation to the soil moisture increase. Gross nitrification of CHL decreased more sharply with the increase in soil moisture while high soil water content was favorable to denitrification of all three soil layers.
N management in lowland paddy fields under AWD irrigation should take account of the effects of soil moisture and soil layer on nitrification–denitrification transformation, as AWD irrigation, compared to CF irrigation, increases the number of wetting and drying cycles during the rice growth season that facilitate the applied NH4+–N to be lost via nitrification during the drying phase and denitrification during the subsequently wetting phase. However, the N losses by nitrification–denitrification transformation in paddy fields with AWD environment during the whole rice growing season should be further explored through coupling the transformation rate with the field water and N regimes. Models for simulating water and N processes in AWD paddy fields require incorporating a moisture-dependent N transformation module for representing the effects of changes in soil moisture on the N transport and transformation. Thus, simulations can be applied to optimize the AWD irrigation schedule and N fertilizer application methods to increase N use efficiency while decrease greenhouse gases emission.
References
Alberto MCR, Wassmann R, Hirano T, Miyata A, Kumar A, Padre A, Amante M (2009) CO2/heat fluxes in rice fields: comparative assessment of flooded and non-flooded fields in the Philippines. Agric For Meteorol 149:1737–1750
Alberto MCR, Wassmann R, Hirano T, Miyata A, Hatano R, Kumar A, Padre A, Amante M (2011) Comparisons of energy balance and evapotranspiration between flooded and aerobic rice fields in the Philippines. Agric Water Manag 98:1417–1430
Arth I, Frenzel P (2000) Nitrification and denitrification in the rhizosphere of rice: the detection of processes by a new multi-channel electrode. Biol Fertil Soils 31:427–435
Belder P, Bouman BAM, Cabangon R, Guoanc L, Quilang EJP, Li Y, Spiertz JHJ, Tuong TP (2004) Effect of water-saving irrigation on rice yield and water use in typical lowland conditions in Asia. Agric Water Manag 65:193–210
Belder P, Spiertz J, Bouman B, Lu G, Tuong T (2005) Nitrogen economy and water productivity of lowland rice under water-saving irrigation. Field Crop Res 93:169–185
Bhandral R, Saggar S, Bolan N, Hedley M (2007) Transformation of nitrogen and nitrous oxide emission from grassland soils as affected by compaction. Soil Tillage Res 94:482–492
Bouman BAM, Tuong TP (2001) Field water management to save water and increase its productivity in irrigated lowland rice. Agric Water Manag 49:11–30
Bouman B, Humphreys E, Tuong T, Barker R (2007) Rice and water. Adv Agron 92:187–237
Bremner JM (1960) Determination of nitrogen in soil by the Kjeldahl method. J Agric Sci 55:11–33
Breuer L, Kiese R, Butterbach-Bahl K (2002) Temperature and moisture effects on nitrification rates in tropical rain-forest soils. Soil Sci Soc Am J 66:834–844
Buresh RJ, Haefele SM (2010) Changes in paddy soils under transition to water-saving and diversified cropping systems. In: Paper to be presented at world congress of soil science, Brisbane, Australia, 1–6 August 2010, pp 9–12
Buresh RJ, Reddy LR, van Kessel C (2008) Nitrogen transformations in submerged soils. In: Schepers JS, Raun WR (eds) Nitrogen in agricultural systems. American Society of Agronomy, Madison, pp 401–436
Cabangon R, Castillo E, Bao L, Lu G, Wang G, Cui Y, Tuong T, Bouman B, Li Y, Chen C (2001) Impact of alternate wetting and drying irrigation on rice growth and resource-use efficiency. In: Proceedings of an international workshop Wuhan, China. International Water Management Institute, pp 55–79
Cabangon RJ, Tuong TP, Castillo EG, Bao LX, Lu G, Wang G, Cui Y, Bouman BAM, Li Y, Chen C, Wang J (2004) Effect of irrigation method and N-fertilizer management on rice yield, water productivity and nutrient-use efficiencies in typical lowland rice conditions in China. Paddy Water Environ 2:195–206
Chen S, Huang Y (2006) Determination of respiration, gross nitrification and denitrification in soil profile using BaPS system. J Environ Sci 18:937–943
Colbourn P, Dowdell RJ (1984) Denitrification in field soils. Plant soil 76:213–226
Corre MD, Schnabel RR, Stouta WL (2002) Spatial and seasonal variation of gross nitrogen transformations and microbial biomass in a Northeastern US grassland. Soil Biol Biochem 34:445–457
Cui Y, Li Y, Lu G, Sha Z (2004) Nitrogen movement and transformation with different water supply for paddy rice. Adv Water Sci 15:280–285 (in Chinese with English abstract)
Davidson EA, Hart SC, Shanks CA, Firestone MK (1991) Measuring gross nitrogen mineralization, and nitrification by 15N isotopic pool dilution in intact soil cores. J Soil Sci 42:335–349
del Prado A, Merino P, Estavillo JM, Pinto M, González-Murua C (2006) N2O and NO emissions from different N sources and under a range of soil water contents. Nutr Cycl Agroecosyst 74:229–243
Dhondt K, Boeckx P, Hofman G, Cleemput O (2004) Temporal and spatial patterns of denitrification enzyme activity and nitrous oxide fluxes in three adjacent vegetated riparian buffer zones. Biol Fertil Soils 40:235–251
Eickhorst T, Tippkötter R (2009) Management-induced structural dynamics in paddy soils of south east China simulated in microcosms. Soil Tillage Res 102:168–178
Gaydon DS, Probert ME, Buresh RJ, Meinke H, Suriadi A, Dobermann A, Bouman B, Timsina J (2012) Rice in cropping systems-Modelling transitions between flooded and non-flooded soil environments. Eur J Agron 39:9–24
Geng S, Ning W, Peng L (2005) Soil N pools and transformation rates under different land uses in a subalpine forest-grassland ecotone. Pedosphere 15:52–58
Ingwersen J, Butterbach-Bahl K, Gasche R, Richter O, Papen H (1999) Barometric process separation: new method for quantifying nitrification, denitrification, and nitrous oxide sources in soils. Soil Sci Soc Am J 63:117–128
Ingwersen J, Schwarz U, Stange CF, Ju X, Streck T (2008) Shortcomings in the commercialized barometric process separation measuring system. Soil Sci Soc Am J 72:135–142
Ishii S, Ikeda S, Minamisawa K, Senoo K (2011) Nitrogen cycling in rice paddy environments: past achievements and future challenges. Microbes Environ 26:282–292
Jahangir MMR, Khalil MI, Johnston P, Cardenas LM, Hatch DJ, Butler M, Barrett M, O’flaherty V, Richards KG (2012) Denitrification potential in subsoils: a mechanism to reduce nitrate leaching to groundwater. Agric Ecosyst Environ 147:12–23
Jing Q, Keulen HV, Hengsdijk H (2010) Modeling biomass, nitrogen and water dynamics in rice–wheat rotations. Agric Syst 103:433–443
Kögel-Knabner I, Amelung W, Cao Z, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kölbl A, Schloter M (2010) Biogeochemistry of paddy soils. Geoderma 157:1–14
Li Y, Barker R (2004) Increasing water productivity for paddy irrigation in China. Paddy Water Environ 2:187–193
Li Y, Šimůnek J, Zhang Z, Jing L, Ni L (2015) Evaluation of nitrogen balance in a direct-seeded-rice field experiment using Hydrus-1D. Agric Water Manag 148:213–222
Liu Y, Zhou H, Wang J, Liu X, Cheng K, Li L, Zheng J, Zhang X, Zheng J, Pan G (2015) Short-term response of nitrifier communities and potential nitrification activity to elevated CO2 and temperature interaction in a Chinese paddy field. Appl Soil Ecol 96:88–98
Maag M, Vinther FP (1996) Nitrous oxide emission by nitrification and denitrification in different soil types and at different soil moisture contents and temperatures. Appl Soil Ecol 4:5–14
Mahendrappa MK, Smith RL, Christiansen AT (1966) Nitrifying organisms affected by climatic region in western United States. Soil Sci Soc Am J 30:60–62
Malhi SS, McGill WB (1982) Nitrification in three Alberta soils: effect of temperature, moisture and substrate concentration. Soil Biol Biochem 14:393–399
Muller C, Abbasi MK, Kammann C, Clough TJ, Sherlock RR, Stevens RJ, Jager HJ (2004) Soil respiratory quotient determined via barometric process separation combined with nitrogen-15 labeling. Soil Sci Soc Am J 68:1610–1615
Onishi T, Nakamura K, Horino H, Adachi T, Mitsuno T (2012) Evaluation of the denitrification rate of terraced paddy fields. J Hydrol 436–437:111–119
Parton WJ, Mosier AR, Ojima DS, Valentine DW, Schimel DS, Weier K, Kulmala AE (1996) Generalized model for N2 and N2O production from nitrification and denitrification. Global Biogeochem Cycl 10:401–412
Patrick WH, Reddy KR (1975) Nitrification-denitrification reactions in flooded soils and water bottoms: dependence on oxygen supply and ammonium diffusion. J Environ Qual 5:469–472
Peng S, Buresh R, Huang J, Yang J, Zou Y, Zhong X, Wang G, Zhang F (2006) Strategies for overcoming low agronomic nitrogen use efficiency in irrigated rice systems in China. Field Crop Res 96:37–47
Peng S, Hou H, Xu J, Mao Z, Abudu S, Luo Y (2011a) Nitrous oxide emissions from paddy fields under different water managements in southeast China. Paddy Water Environ 9:403–411
Peng S, Yang S, Xu J, Luo Y, Hou H (2011b) Nitrogen and phosphorus leaching losses from paddy fields with different water and nitrogen managements. Paddy Water Environ 9:333–342
Peng S, Yang S, Xu J, Gao H (2011c) Field experiments on greenhouse gas emissions and nitrogen and phosphorus losses from rice paddy with efficient irrigation and drainage management. Sci China Technol Sci 54:1581–1587
Reddy K, Patrick W (1986) Denitrification losses in flooded rice fields. Nutr Cycl Agroecosyst 9:99–116
Ridolfi L, D’Odorico P, Porporato A, Rodriguez-Iturbe I (2003) The influence of stochastic soil moisture dynamics on gaseous emissions of NO, N2O, and N2. Hydrol Sci J 48:781–798
Rosenkranz P, Brüggemann N, Papen H, Xu Z, Horváth L, Butterbach-Bahl K (2006) Soil N and C trace gas fluxes and microbial soil N turnover in a sessile oak (Quercus petraea (Matt.) Liebl.) forest in Hungary. Plant Soil 286:301–322
Ruser R, Flessa H, Russow R, Schmidt G, Buegger F, Munch J (2006) Emission of N2O, N2 and CO2 from soil fertilized with nitrate: effect of compaction, soil moisture and rewetting. Soil Biol Biochem 38:263–274
Ryden J, Skinner J, Nixon D (1987) Soil core incubation system for the field measurement of denitrification using acetylene-inhibition. Soil Biol Biochem 19:753–757
Sahrawat K (1980) Soil and fertilizer nitrogen transformations under alternate flooding and drying moisture regimes. Plant Soil 55:225–233
SEPA (2002) Water and wastewater monitoring methods. Chinese Environmental Science Publishing House, Beijing
Shi F, Chen H, Wu Y, Wu N (2010) Effects of livestock exclusion on vegetation and soil properties under two topographic habitats in an alpine meadow on the eastern Qinghai-Tibetan Plateau. Pol J Ecol 58:125–133
Stanford G, Dzienia S, Vander Pol RA (1975) Effect of temperature on denitrification rate in soils. Soil Sci Soc Am J 39:867–870
Sun G, Luo P, Wu N, Qiu P, Gao Y, Chen H, Shi F (2009) Stellera chamaejasme L. increases soil N availability, turnover rates and microbial biomass in an alpine meadow ecosystem on the eastern Tibetan Plateau of China. Soil Biol Biochem 41:86–91
Tan X, Shao D, Liu H, Yang F, Xiao C, Yang H (2013) Effects of alternate wetting and drying irrigation on percolation and nitrogen leaching in paddy fields. Paddy Water Environ 11:381–395
Tan X, Shao D, Liu H (2014) Simulating soil water regime in lowland paddy fields under different water managements using HYDRUS-1D. Agric Water Manag 132:69–78
Tan X, Shao D, Gu W, Liu H (2015) Field analysis of water and nitrogen fate in lowland paddy fields under different water managements using HYDRUS-1D. Agric Water Manag 150:67–80
Torbert HA, Wood CW (1992) Effects of soil compaction and water-filled pore space on soil microbial activity and N losses. Commun Soil Sci Plant 23:1321–1331
Ussiri D, Lal R (2013) Nitrous oxide emissions from rice fields. In: Ussiri D, Lal R (eds) Soil emission of nitrous oxide and its mitigation. Springer, Dordrecht, pp 213–242
Xing G, Cao Y, Shi S, Sun G, Du L, Zhu J (2002) Denitrification in underground saturated soil in a rice paddy region. Soil Biol Biochem 34:1593–1598
Yang S, Peng S, Xu J, Luo Y, Li D (2012) Methane and nitrous oxide emissions from paddy field as affected by water-saving irrigation. Phys Chem Earth 53–54:30–37
Zhang X, Yin S, Li Y, Zhuang H, Li C, Liu C (2014) Comparison of greenhouse gas emissions from rice paddy fields under different nitrogen fertilization loads in Chongming Island, Eastern China. Sci Total Environ 472:381–388
Zhu ZL, Chen DL (2002) Nitrogen fertilizer use in China—contributions to food production, impacts on the environment and best management strategies. Nutr Cycl Agroecosyst 63:117–127
Zhu J, Liu G, Han Y, Zhang Y, Xing G (2003) Nitrate distribution and denitrification in the saturated zone of paddy field under rice/wheat rotation. Chemosphere 50:725–732
Acknowledgements
This study was granted by the National Natural Science Foundation of China (Nos. 51439006 and 51379150) and the State Key Laboratory of Water Resources and Hydro-power Engineering at the Wuhan University (2017NSG01). The first author was financially supported by the China Scholarship Council (CSC), Henry Kroeger Memorial Graduate Scholarship and the University of Alberta. The authors are grateful to Hua Zhang and Gang Pan for their excellent technical assistance during the field work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, X., Shao, D. & Gu, W. Effects of temperature and soil moisture on gross nitrification and denitrification rates of a Chinese lowland paddy field soil. Paddy Water Environ 16, 687–698 (2018). https://doi.org/10.1007/s10333-018-0660-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10333-018-0660-0